Abstract
To generate new beta-cells after birth is a key focus of regenerative medicine that could greatly aid the major health burden of diabetes. Beta-cell regeneration has been described using four different approaches: 1) the development of beta-cells from putative precursor cells of the adult pancreas termed neogenesis, 2) replication of existing beta-cells, 3) differentiation from embryonic or induced pluripotent stem cells, and 4) reprogramming of non-beta to beta-cells. Studies from our laboratory have shown that beta-cell reprogramming can be achieved by transduction of adult pancreatic tissues with viral constructs containing the three developmentally important transcription factors Pdx1, Ngn3, and MafA. This protocol outlines the generation of a polycistronic construct containing the three transcription factors, the expansion and purification of the polycistronic virus and in vivo transduction for acinar to beta-cell reprogramming in adult mice. The ultimate goal is to generate beta-like cells that resemble endogenous beta-cells in phenotype and function as closely as possible for potential translational applications.
Keywords: Acinar to beta-cell reprogramming, transcription factors, polycistronic viral construct, adenoviral transfection, diabetes
INTRODUCTION
Cellular reprogramming is a process in which one differentiated cell type is converted to another differentiated cell type (Zhou and Melton, 2008). In terms of beta-cell regeneration, acinar to beta-cell reprogramming can be achieved by transduction of the three developmentally important transcription factors Pdx1, Ngn3, and MafA, which results in a fate switch from an acinar to beta-cell like cell (Zhou et al., 2008). Reprogramming success by polycistronic viral constructs containing all three transcription factors has been proven superior to monocistronic viral constructs carrying each single transcription factors (Li et al., 2014a). Within the polycistronic construct, separate transcription factors are separated by 2A peptides. These 2A peptides mediate translational “skipping”, which allow generation of multiple proteins from a single transcript (Szymczak and Vignali, 2005).
Here, we present a protocol for polycistronic viral construct generation, expansion, purification, and transduction of the viral construct by in vivo intra-pancreatic injection, which yields robust and reproducible reprogramming of acinar to beta-cells. First, we describe the generation of the polycistronic construct (see Basic Protocol 1). Second, the adenoviral production and tittering are detailed in order to generate a purified preparation suitable for in vivo transduction in mice (see Basic Protocol 2). This is important as reprogramming success depends to a significant extent on high viral titers during viral transfection. Lastly, we describe the surgical procedure for intra-pancreatic viral injection (see Basic Protocol 3). The adult pancreas appears to have the right niche facilitating epigenetic and genetic changes needed for adoption of a beta-cell like phenotype and function.
The use of this protocol to achieve successful acinar to beta-cell reprogramming in vivo facilitates optimization of reprogramming conditions. For example, normoglycemia has been identified as a critical physiological factor for reprogramming success (Cavelti-Weder et al., 2015). Also, a reproducible reprogramming protocol is prerequisite for the study of long-term behavior of reprogrammed beta-cell like cells. Thus, maturation of reprogrammed cells has been shown to gradually occur in an in vivo environment with first occurrence of epigenetic changes, followed by alterations in the gene-expression profile and eventually acquisition of glucose-dependent insulin secretion (Li et al., 2014a). Detailed characterization of transcription factor induced reprogrammed cells showed a close resemblance to endogenous beta-cells, although not being absolutely identical (Li et al., 2014a). For simplicity, we refer to these reprogrammed cells as “beta-cells”.
In summary, successful acinar to beta-cell reprogramming brings about a deepened understanding about factors facilitating and mechanisms involved in acinar to beta-cell fate switch. This will eventually help to translate this promising approach to a future clinical application.
BASIC PROTOCOL 1
MAKING POLYCISTRONIC CONSTRUCT
This protocol describes the production of the viral construct containing the three transcription factors Pdx1, Ngn3, and MafA as well as the mCherry as a marker for infected cells.
NOTE: The virus production and handling should be performed under BL2- conditions.
Materials
Gateway pENTR2B vector (Invitrogen, San Diego, CA)
Polycistronic sequence (oligos synthesized by IDT, Coralville, IA) TCGACACTAGTGCCACGAACTTCTCTCTGTTAAAGCAAGCAGGAGATGTTGAAGAA AACCCCGGGCCTGGATCCGAGGGCAGAGGAAGTCTTCTAACATGCGGTGACGTG GAGGAGAATCCCGGCCCTATCGATCAGTGTACTAATTATGCTCTCTTGAAATTGGC TGGAGATGTTGAGAGCAACCCAGGTCCCGC
pAd/CMV/V5-DEST™ Gateway Vector Kit (Invitrogen)
Vector: pENTR2B (Invitrogen/ ThermoScientific)
Agarose gel electrophoresis station
Qiaex ii gel extraction kit
Heating block or incubator
Restriction enzymes and corresponding buffers
KAPA High Fidelity DNA polymerase
T4 dna ligase
Access to DNA sequencing service
Making polycistronic construct
Follow basic molecular biology protocols. As template for the murine transcription factors, cDNA from reverse transcribed newborn mouse pancreas RNA is a good source. Primer sequences are available upon request.
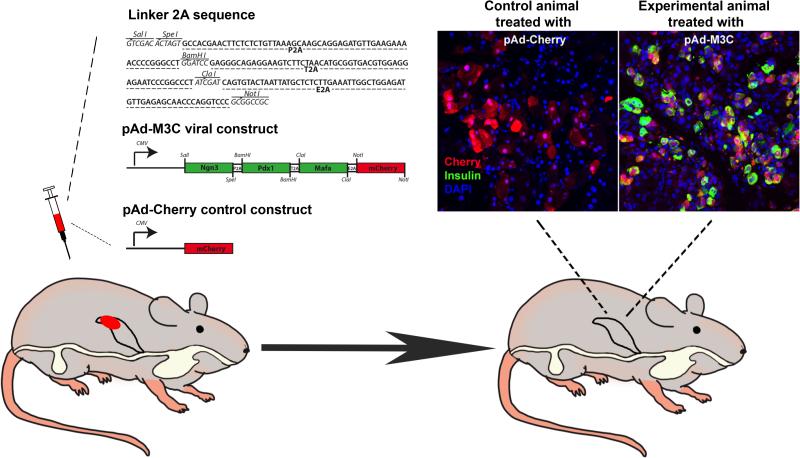
Create a new multiple cloning site containing suitable restriction sites and three different 2A sequences. Therefore, anneal forward and reverse DNA oligonucleotide (shown in Figure 1) and ligate the double-strand linker into pENTR 2B vector digested by Sal I and Not I. This construct is named pENTR-linker 2A.
- Then, sequentially amplify and clone the mouse cDNA of three reprogramming factors and mCherry into the pENTR-linker 2A:
- Ngn3 by Sal and SpeI
- Pdx1 by BamH I (check correct orientation by digest or sequencing)
- Mafa by Cla I (check correct orientation by digest or sequencing)
- mCherry by Not I (check correct orientation by digest or sequencing)
- Sequence the whole construct and confirm correct sequence forming a single open reading frame.
- This construct is named pENTR-linker2A-M3C.
As control, mCherry is cloned into the pENTR-linker2A by SalI and SpeI (pENTR-linker2A-Cherry).
Figure 1. Adenovirus mediated reprogramming of pancreatic acinar to beta-like cells in adult mice.
Schematic representation of virus injection of polycistronic viral construct into the pancreas of adult mice, which leads to appearance of Cherry/ insulin-double positive cells in the experimental animals treated with pAd-M3C viral construct. In contrast, no insulin-positivity cannot be found in control animals injected with the pAd-Cherry viral construct. Three different 2A peptides used: P2A (porcine teschovirus-1 2A), T2A (Thosea asigna virus 2A), and E2A (equine rhinitis A virus 2A). CMV: cytomegalovirus promoter.
BASIC PROTOCOL 2
VIRAL PRODUCTION AND PURIFICATION
Once the viral construct has been made, it needs to be expanded in adenoviral packing cell line and the resulting viral particles purified.
Materials
ViraPower Adenoviral Expression System (Invitrogen), containing pAd/CMV/V5-DEST™ adenoviral vector and an optimized 293A Cell Line
Vivapure Adenopack 100 (Sartorius, Bohemia, NY)
Opti-MEM I medium (Gibco, Grand Island, NY)
Lipofectamine 2000 (Invitrogen)
293A cell line complete growth medium
Phenol/chloroform, sterile water or QIAEX II Gel extraction kit (Qiagen)
Storage buffer
6-well and 24-well cell culture plates
Dry ice/ ethanol bath
37° C water bath
Adenoviral vector preparation
- Clone the M3C fragment on the pENTR-linker2A-M3C (or pENTR-linker2A-Cherry as control) further into a pAd-V5 DEST vector through Gateway cloning following manufacturer's instructions. Due to large insert size and large adenoviral genome, expect only few colonies after gateway reaction. This construct is named pAd-M3C (or pAd-Cherry for the control, respectively).The adenoviral construct is also available at Addgene, ID 61041.
- Digest about 5 ug of pAd-M3C or pAd-Cherry plasmids by Pac I to expose the left and right inverted terminal repeats (ITRs) for the adenovirus packaging and replication. Purify the digested pAd-M3C or pAd-Cherry further by phenol/chloroform extraction and finally elute by sterile water to a concentration of 1.0 ug/ul.Instead of phenol/chloroform extraction, we successfully used QIAEX II Gel extraction kit (Qiagen) to isolate the digested vector.
Transfection and crude adenoviral lysate preparation
-
3.
Plate 5 × 105 293A cells with 2 ml complete growth medium into each well of a 6-well cell culture plate the day before the transfection.
-
4.
On the day of transfection, replace the cell culture medium with 1.5 ml Opti-MEM I medium containing serum without antibiotics. Then incubate 2 ug linearized, purified pAd-M3C or pAd-Cherry (from step 2) with 250 ul Opti-MEM I medium without serum for 5 minutes at room temperature. In parallel, add 6 ul Lipofectamine 2000 into another 250 ul Opti-MEM I medium without serum and incubate for 5 minutes at room temperature.
-
5.
After incubation, mix the medium containing pAd-M3C or pAd-Cherry and the medium containing Lipofectamine 2000, and incubate at room temperature for 20 minutes. Distribute this transfection mix drop-wise onto the 293A cell in the 6-well plate. Swirl the plate gently to ensure even distribution of transfection mix over cells.
-
6.
On the day after transfection, replace the medium with complete growth medium.
-
7.
48 hours after the transfection, mCherry expression from transient expression should be detectable by fluorescence microscopy.
-
8.
Trypsinize the cells and transfer them into a 10 cm tissue culture plate in 10 ml 293A complete growth medium.
-
9.
Replace culture medium with fresh, complete culture medium every 2-3 days until first regions of cytopathic effect (CPE) are observed (typically 7-10 days post-transfection).
-
10.
Subsequently, replenish medium (ca. 1ml/ day) and allow infections to proceed until approximately 80% CPE is observed (typically 10-13 days post-transfection).
-
11.
Harvest adenovirus-containing cells by squirting cells off the plate with a 10 ml tissue culture pipette. Transfer cells and media to a sterile 15 ml capped tube.
-
12.
Prepare crude viral lysate by 3 cycles of freeze/thaw: Freeze cells in dry ice/ethanol bath, then thaw in 37° C water bath. Make sure cells are deep-frozen (leave in dry ice/ethanol for at least 15 minutes or transfer to −80° C freezer for 30 minutes) and do not extend thawing in water-bath > 15 minutes. Repeat 2x.
-
13.
Centrifuge the cell lysate in a table-top centrifuge at 3000 rpm for 15 minutes at room temperature to pellet the cell debris.
-
14.Collect the supernatant as crude viral lysate and store at −80° Celsius.For storage and later amplification purposes, we found that aliquots of 1 ml are optimal for the crude lysate.
Virus amplification
-
15.
Expand 293A cells to ten 15 cm plates at 80% confluency.
-
16.
Add 200 ul of the crude viral lysate into each plate to infect the cells.
-
17.
Harvest the virus when 90% of the cells have rounded up, which usually takes 2 to 3 days. Virus is present in supernatant, but the majority of the virus is still within the cells.
-
18.
Concentrate adenovirus using Vivapure Adenopack 100 kit.
-
19.Perform a buffer exchange into storage buffer using the supplied centrifugal Vivaspin concentrators. Store the purified virus in aliquots at −80° Celsius.For storage purposes, we found that aliquots of 100 ul are optimal for the purified adenovirus. For titer determination, set aside smaller aliquot.
Viral titer determination
As the mCherry marker enables a direct estimate of infection, fluorescence microscopy or FACS-based methods can be used to determine functional virus titer.
-
20.
Plate 1 × 105 293A cells per well in a 24-well plate. Per concentration tested, prepare triplicate wells. Incubate cells 16 to 24 hours.
-
21.
Thaw the purified virus on ice. Make serial 10-fold dilutions of the virus down to 1:107, using complete growth medium.
-
22.
Add 1ul of the 1:105 – 1:107 dilutions per well to infect cells on the 24-well plate.
-
23.24-36 hours post infection, count the mCherry-positive cells under a fluorescence microscope.Virus titer (pfu/ml) = mCherry-positive cell number × dilution factor × 103. Use average from the three infection replicates. We routinely get virus titers between 2 - 15 × 1010 pfu/ml. Hence, a 10 × 1010 pfu/ml virus would yield 1000 infected cells at the 1:105, 100 infected cells at the 1:106 dilution and 10 infected cells at the 1:107 dilution.
BASIC PROTOCOL 3
SURGICAL PROCEDURE
After viral production and purification, the viral construct can be used to transduce pancreatic tissue in vivo. The virus has to be administered directly into the pancreatic tail of mice by the surgical procedure described below.
We routinely use Rag1−/− mice (B6.129S7-Rag1<tm1Mom>/J), lacking adaptive immunity. We have also tested NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice and have obtained similar results as in Rag1−/−. We assume that every immune-compromised mouse lacking adaptive T cell responses might be suitable host.
NOTE: Make sure you have institutional and animal welfare authority approval to perform the in vivo experiments.
Materials
Before procedure
70% EtOH
Anesthesia for survival surgery
18G and 27G needles (BD, Franklin Lakes, NJ), 1ml syringes for injection Shaver
Alcohol preps (Kendall, Mansfield, MA) and betadine solution (Santa Cruz, Dallas, TX)
Virus injection
Betadine solution (Santa Cruz)
Storage buffer
Syringes (for virus injection 3/10cc insulin syringes (BD), for anesthesia 1ml syringe) and needles: 27G + 18G (BD)
Surgical gloves (sterile) and facemasks
Warming pads and Delta Phase operating board (Braintree Scientific, Braintree MA)
Dissecting microscope for surgery (Leica stereo zoom 7, Leica, Germany)
Blue sterile tissues (IMCO, Daytona Beach, FL)
Surgery tools (Stapler/ staples/ small scissors and forceps), all autoclaved
Suture (5-0 Chromic gut) (Butler Schein, Dublin, OH)
Sterile drape (IMCO)
Bead sterilizer (Fine Science Tool, Foster City, CA)
After procedure
Banamine (Merck, Whitehouse Station, NJ)
Heating lamp
Before surgical procedure
Wipe surfaces with 70% alcohol preps for cleaning purposes.
Weigh all animals as anesthesia will be weight-adapted.
Animals are anesthetized for survival surgery.
Once asleep, shave animals’ left side and clean the skin three times alternately with alcohol preps and betadine solution.
Virus handling
-
5.
Keep adenovirus at −80° Celsius for long-term storage, and prevent freezing and thawing for more than 3 times.
-
6.
When filling the syringe with virus, be careful not to create bubbles.
-
7.
Dilute the virus with storage buffer to the final injection titer of 2 × 1010 pfu/ml. Use 100 ul of the diluted virus for each animal.
Virus injection
-
8.
The animal lies on its right side. Palpate the left costal arch and make a small incision about 0.5 cm distally of the costal arch with sharp scissors.
-
9.
Separate the skin from the subcutaneous tissue with scissors.
-
10.
Move the incision you made so that the red-colored spleen can be seen shining through the peritoneum.
-
11.
Lift up the peritoneum and cut a little incision.
-
12.
Enlarge the incision above the spleen.
-
13.
From now on use a microscope for surgery. Pop out the spleen by slight pressure.
-
14.
With the forceps in your left hand, get hold of the tail of the pancreas where it is attached to the spleen. If necessary clear the pancreas from the mesentery, which appears slightly more glossy than the pancreas.
-
15.
Inject 100 ul of purified virus in 1-2 loci with a 3/10 ml insulin syringe. A deposit forms at the injection site if virus is injected successfully.
-
16.
Put the pancreas and spleen back into the abdomen. Suture the peritoneum and staple the skin.
After surgical procedure
-
17.
Put betadine solution on the wound.
-
18.
Inject analgesia (banamine, Merck) intramuscularly into the opposite rear leg of anesthesia injection.
-
19.
Place the animals under a heating lamp until anesthesia recovery.
REAGENTS AND SOLUTIONS
Anesthesia for survival surgery
Mix 1.0 ml Ketamine (Putney,100mg/ml), 0.2 ml Xylazine (Lloyd, 100mg/ml) and 5.4 ml saline 0.9%. Inject a weight-adapted dose intraperitoneally (Ketamine 90-100 mg/kg, Xylazine 10 mg/kg, e.g. for a 20 g mouse inject 0.1 ml of anesthesia solution).
293A cell line complete growth medium
DMEM (4.5 g D-glucose/L) with 10% heat inactivated FBS, 2 mM L-glutamine, and 1% penicillin/streptomycin (Sigma)
Storage buffer
20 mM Tris/HCl, 25 mM NaCl, 2.5% Glycerol (w/v), pH=8.0
COMMENTARY
Background Information
The term “direct reprogramming” describes direct cell fate conversion from one differentiated cell type into another without going through a multipotent or pluripotent stage (Graf, 2011; Vierbuchen and Wernig, 2011). One of the earliest examples of direct reprogramming was the induction of myogenesis by the myogenic master regulator MyoD with the finding that ectopic expression of MyoD directed differentiation of fibroblasts into muscle cells in vitro (Davis et al., 1987). Since then, a variety of cell types has been generated by direct reprogramming such as neurons, cardiomyocytes, vascular cells, and beta-cells among others (Al-Hasani et al., 2013; Baeyens et al., 2014; Caiazzo et al., 2011; Ginsberg et al., 2012; Guo et al., 2014; Ieda et al., 2010; Lee et al., 2013; Song et al., 2012; Su et al., 2014; Thorel et al., 2010; Vierbuchen et al., 2010; Zhou et al., 2008).
In terms of beta-cells, one of the first non-beta to beta-cell reprogramming attempts used systemic injection of the transcription factor Pdx1 to direct liver cells towards insulin-producing cells (Ferber et al., 2000). Since then, generation of insulin-positive cells has been reported from various cell populations including pancreatic acinar cells, pancreatic duct cells, pancreatic endocrine alpha- and delta-cells, liver cells, and cells of the gastrointestinal system, thus mostly with starting cell populations of endodermal lineages due to the close developmental relationship to beta-cells (Ariyachet et al., 2016; Chen et al., 2014; Chera et al., 2014; Ferber et al., 2000; Sancho et al., 2014; Sumazaki et al., 2004; Talchai et al., 2012; Thorel et al., 2010; Zhou et al., 2008). Manipulation of transcription factors has become the dominant method to promote cell fate conversion toward beta-cells.
Among all non-beta to beta-cell reprogramming approaches, acinar to beta-cell reprogramming has been especially successful in generating new beta-like cells. The first report showed that transient expression of the three transcription factors Ngn3, Pdx1, and Mafa in adult mouse pancreas rapidly converts acinar cells to beta-like cells within 10 days (Zhou et al., 2008). Lineage tracing confirmed that mature acinar cells were the predominant target cells converted into insulin-expressing cells after transcription factor delivery. In the reprogramming process, the three reprogramming factors play different roles (Li et al., 2014b): Ngn3 induces delta-specification, thus promoting establishment of a generic endocrine state at the onset of reprogramming by suppressing acinar fate-regulators and activating islet endocrine genes. The primary function of Mafa and Pdx1, in contrast, appears to promote beta-cell specification while simultaneously suppressing alternative cell fates including those of delta- and alpha-cells. Thus, co-expression of all three factors Pdx1, Ngn3, and Mafa is necessary to induce formation of beta-like cells (Li et al., 2014b). In a more recent study, a polycistronic viral construct containing the three transcription factors was used for achieving long-term stability and development of induced insulin-positive cells from acinar cells in vivo (Li et al., 2014a). Over time, reprogrammed insulin-producing cells aggregated to islet-like clusters and persisted throughout the observation period up to 13 months. Detailed analyses over time revealed that DNA methylation changes occurred mainly within 10 days of induction, whereas full maturation as evidenced by DNA network remodeling and acquisition of regulated insulin release required at least two months to resemble endogenous beta-cells (Li et al., 2014a). In addition to the necessary role of transcription factors, environmental cues such as normoglycemia have been identified as beneficial for reprogramming success (Cavelti-Weder et al., 2015).
Besides transcription factors, other means have been described in order to achieve exocrine to beta-cell reprogramming, such as treatment of adult mice with EGF (epidermal growth factor) and CNTF (ciliary neurotrophic factor) through implanted mini-osmotic pumps leading to the appearance of numerous beta-like cells in the pancreas of mice made diabetic with alloxan treatment (Baeyens et al., 2014). At least some of the new insulin-producing cells were shown to derive from acinar cells. Another study used pancreatic duct ligation (PDL) and Ptf1a as lineage tracing, which demonstrated that Ptf1a-positive acinar cells converted into a ductal phenotype upon PDL, re-expressed Ngn3 and subsequently differentiated into endocrine cells at low frequency, expressing mature beta-cell markers such as Pdx1, Nkx6.1 and Mafa (Pan et al., 2013).
Critical Parameters and Troubleshooting
Harvesting lysate
Large-scale viral production for animal injection should always begin with crude lysate and not the purified virus, the latter yielding reasonable titers but generally poor beta-cell induction. The reason for this is not clear.
Estimating the proper time for harvesting the 293A cells infected by crude lysate is crucial as harvesting the cells too early or too late will result in a poor viral titer. The best infection status for harvesting is when 100% of the 293A cells express mCherry and 90% of the cells have rounded up or float in the medium.
If the infected cells are lysed very slowly (more than 7 days) after adding the crude lysate, it might be due to a too small amount of the crude lysate. In that case, the cells should be prepared again, and the infection with more crude lysate repeated (for example 5 times higher than before). If the results are still poor, re-titer the virus to exclude a potential mistake in calculation or check the passage number of the 293A cells. Less than 15 passages of 293A cells should be used for the virus production as too many passages may yield a poor viral titer.
Storing, freezing and thawing
Adenovirus in storage buffer is unstable once thawed. As frequent freezing and thawing of the virus will decrease the viral titer, no more than 3 cycles of freezing and thawing should be performed for the crude lysate. For animal induction experiments, only freshly thawed purified virus should be used. Leftovers may be refrozen and used one more time, but expect a possible significant reduction in beta-cell induction.
Anticipated Results
Reprogramming success can be assessed by different methods. For instance, reprogrammed cells can be characterized phenotypically such as by immunohistochemistry, flow cytometry or insulin content.
In addition, reprogrammed cells can be tested functionally. The function of reprogrammed cells can be distinguished from endogenous beta-cells in animals that have been depleted of their endogenous beta-cells prior to beta-cell reprogramming, e.g. by the beta-cell toxin streptozocin (STZ). However, as normoglycemia has been found to be beneficial for optimal reprogramming success (Cavelti-Weder et al., 2015), we have previously established a model using islet cell transplantation under the kidney capsule (Cavelti-Weder et al., 2014). After an initial period of normoglycemica where maturation of reprogrammed cells occurs, transplanted islet cells can be removed by nephrectomy for functional assessment of reprogrammed cells (Cavelti-Weder et al., 2014). After nephrectomy, blood glucose levels are solely under the control of the induced beta-cells residing in the pancreas and can be tested by glucose monitoring or glucose and insulin tolerance tests.
Time Considerations
Production of the viral construct takes 2 to 3 weeks.
Virus production from generation of linearized adenoviral construct, transfection and crude lysate generation takes approximately 2 weeks.
Virus amplification, purification and tittering can be done within 7 days.
For the in vivo part of the study, reprogrammed cells can be found very soon after viral transduction (see Table 1). However, the longer the reprogrammed cells are in a normoglycemic in vivo environment, the more they will come to resemble endogenous beta-cells.
Table 1.
Time-dependent changes of key acinar and beta-cell factors during the reprogramming process
| Mist1 | Ptf1A | Nr5a2 | Pax6 | SYP | Ins | Nkx6.1 | Ucn3 | |
|---|---|---|---|---|---|---|---|---|
| d1 | lost! | |||||||
| d2 | lost | lost | lost | trace | ||||
| d4 | lost | lost | lost | trace | trace | |||
| d5 | lost | lost | lost | 50 | 10 | trace | ||
| d7 | lost | lost | lost | 70 | 30 | |||
| d10 | lost | lost | lost | 70 | 90 | 70 | 20 | |
| d15 | lost | lost | lost | 90 | 10 | |||
| d20 | lost | lost | lost | 90 |
We routinely analyzed acinar and beta-cell factors to evaluate the efficiency of the initial reprogramming process. Mist1, Ptf1a, and Nr5a2 are key acinar cell determination factors, which are down-regulated rapidly after reprogramming factor delivery. Pax6, Synaptophycin (Syp) are pan-endocrine factors. Insulin and Nkx6.1 are beta-cell specific factors, whereas Ucn3 labels mature murine beta-cells. Detailed marker expression by immunofluorescence 1-20 days after M3Cherry infection is shown. Numbers represent percentile of infected cells (Cherry+) that are also positive for the specific markers assayed.
Significance Statement.
Creating new beta-cells is a key focus of regenerative medicine with the aim to alleviate if not reverse diabetes-associated symptoms and complications. Four approaches of beta-cell regeneration have been described in recent years: neogenesis, beta-cell replication, beta-cell differentiation from embryonic or induced pluripotent stem cells and reprogramming of differentiated non-beta to beta-cells. Here, we provide a detailed and robust protocol for inducing pancreatic acinar to functional beta-cell reprogramming in adult mice by an adenoviral construct expressing three beta-cell master regulators (Ngn3, Pdx1, and MafA). The reprogramming approach may be further developed into a safe, reliable and easy protocol of beta-cell reprogramming that could potentially be translated into clinics.
ACKNOWLEDGEMENT
These studies are supported by NIH U01 DK089536 to Q. Z. C. C-W. and A. Z. were supported by postdoctoral fellowships from the Swiss Science Foundation (SNF) and the Swiss Foundation for Grants in Biology and Medicine (SFGBM). W. L. was supported by a postdoctoral fellowship from the Juvenile Diabetes Research Foundation (JDRF).
REFERENCES
- Al-Hasani K, Pfeifer A, Courtney M, Ben-Othman N, Gjernes E, Vieira A, Druelle N, Avolio F, Ravassard P, Leuckx G, et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell. 2013;26:86–100. doi: 10.1016/j.devcel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Ariyachet C, Tovaglieri A, Xiang G, Lu J, Shah MS, Richmond CA, Verbeke C, Melton DA, Stanger BZ, Mooney D, et al. Reprogrammed Stomach Tissue as a Renewable Source of Functional beta Cells for Blood Glucose Regulation. Cell Stem Cell. 2016;18:410–421. doi: 10.1016/j.stem.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens L, Lemper M, Leuckx G, De Groef S, Bonfanti P, Stange G, Shemer R, Nord C, Scheel DW, Pan FC, et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol. 2014;32:76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Cavelti-Weder C, Li W, Weir GC, Zhou Q. Direct lineage conversion of pancreatic exocrine to endocrine Beta cells in vivo with defined factors. Methods Mol Biol. 2014;1150:247–262. doi: 10.1007/978-1-4939-0512-6_17. [DOI] [PubMed] [Google Scholar]
- Cavelti-Weder C, Li W, Zumsteg A, Stemann-Andersen M, Zhang Y, Yamada T, Wang M, Lu J, Jermendy A, Bee YM, et al. Hyperglycaemia attenuates in vivo reprogramming of pancreatic exocrine cells to beta cells in mice. Diabetologia. 2015 doi: 10.1007/s00125-015-3838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Finkbeiner SR, Weinblatt D, Emmett MJ, Tameire F, Yousefi M, Yang C, Maehr R, Zhou Q, Shemer R, et al. De novo formation of insulin-producing “neo-beta cell islets” from intestinal crypts. Cell reports. 2014;6:1046–1058. doi: 10.1016/j.celrep.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, et al. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature. 2014 doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- Ginsberg M, James D, Ding BS, Nolan D, Geng F, Butler JM, Schachterle W, Pulijaal VR, Mathew S, Chasen ST, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9:504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sugiyama T, Liu Y, Wang J, Gu X, Lei J, Markmann JF, Miyazaki S, Miyazaki J, Szot GL, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. eLife. 2013;2:e00940. doi: 10.7554/eLife.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cavelti-Weder C, Zhang Y, Clement K, Donovan S, Gonzalez G, Zhu J, Stemann M, Xu K, Hashimoto T, et al. Long-term persistence and development of induced pancreatic beta cells generated by lineage conversion of acinar cells. Nat Biotechno. 2014a;l32:1223–1230. doi: 10.1038/nbt.3082. [DOI] [PubMed] [Google Scholar]
- Li W, Nakanishi M, Zumsteg A, Shear M, Wright C, Melton DA, Zhou Q. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. eLife. 2014b;3:e01846. doi: 10.7554/eLife.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, Wright CV. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140:751–764. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R, Gruber R, Gu G, Behrens A. Loss of Fbw7 reprograms adult pancreatic ductal cells into alpha, delta, and beta cells. Cell Stem Cell. 2014;15:139–153. doi: 10.1016/j.stem.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nature communications. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin Biol Ther. 2005;5:627–638. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet. 2012;44:406–412. S401. doi: 10.1038/ng.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat Biotechnol. 2011;29:892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Melton DA. Extreme makeover: converting one cell into another. Cell Stem Cell. 2008;3:382–388. doi: 10.1016/j.stem.2008.09.015. [DOI] [PubMed] [Google Scholar]