Malaria is an infectious disease caused by the Plasmodium parasite, and transmitted by Anopheles mosquitoes. In 2016, a staggering 216 million cases of malaria and 445,000 deaths were recorded, mostly in Africa, although half of the world’s population in 91 countries is at risk of the disease (1). Malaria prevention methods include control of the mosquito with insecticide-treated bed nets and indoor residual spraying of insecticides. Prompt diagnosis through the use of rapid diagnostic tests is also key. Although there is a malaria vaccine, RTS,S/AS01, it shows limited efficacy and has yet to be used widely. However, the frontline against malaria is antimalarial drugs, in particular artemisinin-based combination therapies (ACTs), which are mixtures of artemisinin and its derivatives from the Chinese sweet wormwood herb, with drugs such as piperaquine. Alarmingly, the parasite is now resistant to most drugs that have been developed (see the figure). It is imperative that we identify new inhibitors if progress in reducing malaria is to be sustained. On page 191 of this issue, Cowell et al. (2) present a major step forward, revealing new antimalarial drug targets and their possible resistance mechanisms.
Figure. P. falciparum endemicity and the spread of drug resistance.
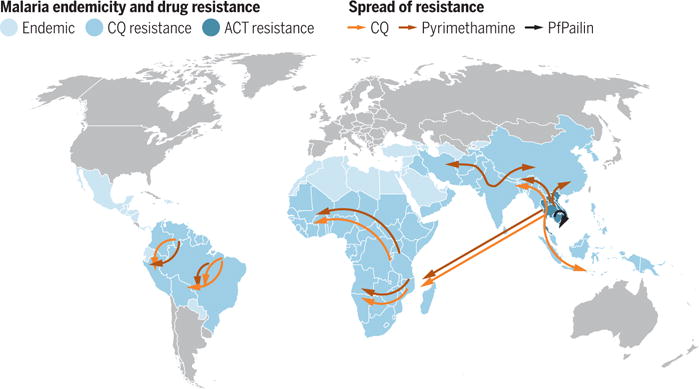
Malaria is endemic in the countries highlighted in light blue. Resistance to antimalarial drugs such as chloroquine (CQ) and pyrimethamine is widespread (darker blue), while resistance to artemisinin-based combination therapies (ACTs) is spreading. A new P. falciparum strain, PfPailin, that is resistant to artemisinin plus piperaquine, has been found. Data presented in the map are adapted from (7, 8).
The Plasmodium parasite is a formidable eukaryotic microbe, an ancient organism that has shaped the history, politics, and evolution of its human host. Almost 500 species have been identified that infect mammals, birds, and reptiles; however, only five routinely infect humans, including Plasmodium falciparum, the deadliest, and Plasmodium vivax, the most geographically widespread (3). Because of its virulence and ease of in vitro culture, research has focused on P. falciparum, and molecular and cell biology techniques have been developed for its interrogation, such as genome editing (4, 5), and high-throughput analyses, such as metabolomics (6).
These are worrisome times for the battle against malaria. In addition to the parasite being resistant to almost every single-drug antimalarial regime (7), the spread of a multidrug-resistant P. falciparum lineage (PfPailin) in the Greater Mekong area of Southeast Asia was recently reported (8). This strain contains a dominant mutation in Pfkelch13 (mutations in this gene are associated with artemisinin resistance) and is also resistant to piperaquine. The spread of this evolutionarily “fit” multidrug-resistant malaria parasite between endemic countries is extremely concerning.
Cowell et al. used experimental evolution to identify new P. falciparum drug targets while also anticipating their possible resistance mechanisms. Experimental evolution is the maintenance of populations of organisms in controlled environments where changes in genotype and phenotype can be evaluated over thousands of generations; it is most often applied to microbes because of their rapid generation times and small sizes (9). In these studies, environmental conditions, such as changes in nutrient availability or antimicrobial drugs, can be manipulated to explore adaptation of the organisms being studied. Probably the most widely known example of experimental evolution is the “Escherichia coli long-term evolution experiment” (LTEE) that has been tracking genetic changes in 12 originally identical populations of E. coli maintained in continuous culture since 1988 (10).
Experimental evolution is not new to malaria research; the first evolution experiments on malaria were carried out in the 1940s, using a species of bird malaria parasite that was propagated in laboratory chickens (11). In later decades, researchers generated drug-resistant strains of rodent malaria parasites in laboratory mice that, in the absence of high-throughput sequencing methods, were subsequently investigated by genetic crossing experiments and linkage mapping (12). Such studies provided important data regarding the single-gene or multigenic nature of the induced drug resistance, as well as identifying resistance loci and their alleles. More recently, the technique has been used to identify resistance loci in the rodent malaria species Plasmodium chabaudi (13) and P. falciparum (14) that were subjected to artemisinin. Although these evolution experiments can take years, they may provide immense scientific reward, as evidenced by the eventual identification of Pfkelch13 as an artemisinin resistance–associated locus after a 5-year evolution experiment (14).
Whereas these previous studies evaluated the response to known antimalarials, or focused on mutations in one target gene, Cowell et al. examined the parasite’s genetic response to a large number of new inhibitors. Three well-studied P. falciparum laboratory clonal isolates were individually subjected to experimental evolution over 3 to 6 months in vitro using 37 publicly available compounds with proven antimalarial activity to various stages of the P. falciparum life cycle. Comparison of the genomes of >200 genetically stable, compound-resistant clones revealed a remarkable enrichment of mutations, including single-nucleotide variations, in just 57 genes, and 150 copy number variations. These loci are informative as either genes involved in resistance (because a particular gene was mutated repeatedly in response to multiple compounds), or as potential drug targets, although in many cases the authors were unable to discern between these two possibilities. One interesting example of a resistance gene is a putative ABC (ATP-binding cassette) transporter, Pfabc13, that harbored point mutations and was involved in 12 different amplification events with four separate compounds. Four target-inhibitor pairs were also singled out as possible new antimalarial drug targets, identified because of their enzymatic function that enabled docking and homology modeling. Ultimately, one gene (either a new drug target or a new drug resistance locus) was identified for each compound examined.
This rich data set increases our understanding of the biology and evolution of P. falciparum, providing a powerful contribution toward basic research for malaria elimination. In the end, designing “resistance-proof” drugs may be the best strategy for controlling malaria. There have been several developments toward this goal (7), including targeting host factors required for parasite growth (15), although the field has some way to mature.
References
- 1.World Health Organization. World Malaria Report 2016. 2016 [Google Scholar]
- 2.Cowell AN, et al. Science. 2018;359:191. doi: 10.1126/science.aan4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins SL, et al. J Parasitol. 2014;100:11. doi: 10.1645/13-362.1. [DOI] [PubMed] [Google Scholar]
- 4.Ghorbal M, et al. Nat Biotechnol. 2014;32:819. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- 5.Wagner JC, et al. Nat Methods. 2014;11:915. doi: 10.1038/nmeth.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olszewski KL, et al. Cell Host Microbe. 2009;5:191. doi: 10.1016/j.chom.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blasco B, et al. Nat Med. 2017;23:917. doi: 10.1038/nm.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imwong M, et al. Lancet Infect Dis. 2017;17:1022. doi: 10.1016/S1473-3099(17)30524-8. [DOI] [PubMed] [Google Scholar]
- 9.Barrick JE, Lenski RE. Nat Rev Genet. 2013;14:827. doi: 10.1038/nrg3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddamsetti R, et al. Genome Biol Evol. 2017;9:1072. doi: 10.1093/gbe/evx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson J, Lourie EM. Ann Trop Med Parasitol. 1947;41:278. [PubMed] [Google Scholar]
- 12.Carlton JM, et al. Trends Parasitol. 2001;17:236. doi: 10.1016/s1471-4922(01)01899-2. [DOI] [PubMed] [Google Scholar]
- 13.Hunt P, et al. BMC Genomics. 2010;11:499. doi: 10.1186/1471-2164-11-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariey F, et al. Nature. 2014;505:50. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zumla A, et al. Lancet Infect Dis. 2016;16:e47. doi: 10.1016/S1473-3099(16)00086-4. [DOI] [PubMed] [Google Scholar]


