Abstract
Background and Purpose
Symptomatic carotid artery disease is associated with significant morbidity and mortality. The pathophysiologic mechanisms of cerebral ischemia among patients with carotid occlusion remain underexplored.
Methods
We conducted a prospective observational cohort study of patients hospitalized within seven days of ischemic stroke (IS) or transient ischemic attack (TIA) due to ≥50% carotid artery stenosis or occlusion. Transcranial Doppler (TCD) emboli detection was performed in the middle cerebral artery ipsilateral to the symptomatic carotid. We describe the prevalence of microembolic signals (MES), characterize infarct topography, and report clinical outcomes at 90-days.
Results
Forty-seven patients, 19 with carotid occlusion and 28 with carotid stenosis, had complete TCD recordings and were included in the final analysis. MES were present in 38%. There was no difference in MES between those with carotid occlusion (7/19, 37%) compared to stenosis (11/28, 39%; p=0.87). In patients with radiographic evidence of infarction (n=39), 38% had a watershed pattern of infarction, 41% had a non-watershed pattern, and 21% had a combination. MES were present in 40% of patients with an exclusively watershed pattern of infarction. Recurrent cerebral ischemia occurred in 9 patients (19%; 6 with TIA, 3 with IS). There was no difference in the rate of recurrence in those with compared to without MES.
Conclusion
Cerebral embolization plays an important role in the pathophysiology of ischemia in both carotid occlusion and stenosis, even among patients with watershed infarcts. The role of aggressive antithrombotic and/or antiplatelet therapy for symptomatic carotid occlusions may warrant further investigation given our findings.
Keywords: Carotid Occlusive Disease, Embolization, Transcranial Dopper, Carotid Stenosis
Subject Headings: Clinical Studies, Cerebrovascular Disease, Pathophysiology, Stenosis
Introduction
Symptomatic carotid disease is associated with a high-risk of recurrent cerebral ischemia.1–4 This risk is higher in the setting of carotid occlusion compared to carotid stenosis, both in terms of early and late ischemic events.5, 6 Proposed mechanisms of infarction among patients with large vessel disease include cerebral hypoperfusion,7, 8 artery-to-artery embolization,9, 10 as well as a complementary interaction between the two via reduced perfusion limiting the ability of the bloodstream to wash out emboli lodged in distal vessels.11, 12 However, the frequency of cerebral embolization and its association with different radiographic patterns of infarction among patients with recently symptomatic carotid artery occlusion has not been thoroughly explored.13–15 This area of research is of particular importance as emerging evidence suggests that external or cortical border zone (CBZ) type watershed infarctions, classically thought to be due to cerebral hypoperfusion, are often embolic in etiology.16–19
Embolization into the cerebral vasculature can be studied using transcranial Doppler (TCD) to detect microembolic signals (MES). Among patients with carotid occlusion, emboli from the distal portion of the occluded vessel,20 the proximal portion of the occlusion through external carotid artery collaterals (the original “stump emboli” hypothesis),21 or from vasculature contralateral to the occlusion have all been reported.22 Detection of MES has been associated with increased risk of future ischemia in both asymptomatic23 and symptomatic24, 25 carotid stenosis in some studies, highlighting the clinical importance of embolization and the validity of intracranial MES detection using TCD to identify debris or thrombus coming from more proximal carotid plaque. Clarifying the pathophysiology of cerebral ischemia in symptomatic carotid occlusion has the potential to guide treatment decisions. Whereas interventions to improve cerebral perfusion such as liberalizing blood pressure goals may be useful if hypoperfusion is the underlying mechanism of ischemia, aggressive antithrombotic therapy may be of greater benefit if embolization is the primary mechanism. Some studies have indeed suggested improved outcome among symptomatic carotid occlusion patients treated with anticoagulation.26
In this study, we compare the clinical characteristics, MES frequency, and index cerebral infarction pattern between patients with symptomatic carotid occlusion and carotid stenosis.
Methods
Subjects
We conducted a prospective single-center observational study at the Hospital of the University of Pennsylvania of patients hospitalized following an ischemic stroke or transient ischemic attack (TIA) attributed to large vessel atherosclerotic carotid disease by the treating vascular neurologist. Patients admitted to the Vascular Neurology consult and inpatient services were screened from 12/1/2011 to 10/1/2015 for study enrollment. The diagnosis of ischemic stroke required focal symptoms or signs persisting ≥24 hours or radiographic evidence of infarction;27 TIA was defined as a transient episode of neurological dysfunction caused by focal brain or retinal ischemia without radiographic evidence of acute infarction.28 Patients were eligible for enrollment if they were ≤7 days from symptom onset and had ≥ 50% carotid stenosis or carotid occlusion ipsilateral to cerebral infarct or TIA symptomatology as confirmed on vascular imaging. Patients with a known high-risk source of cardioembolism as defined in the TOAST trial29 or who were treated with therapeutic anticoagulation were excluded. Clinical, laboratory, and relevant radiographic data were collected using a standardized case report form. The presence or absence of ≥ 50% stenosis in the contralateral carotid artery on vascular imaging studies done at the time of study enrollment was also recorded.
Recurrent cerebral ischemia, including both TIA and ischemic stroke, revascularization procedures, and mortality were recorded during index hospitalization and at 90-day follow-up by staff blinded to TCD results. Events within 48 hours of carotid endarterectomy (CEA) or carotid artery stenting (CAS) were considered peri-procedural. IRB approval was obtained prior to study commencement and written consent was obtained from all participants.
Imaging
All index infarctions were categorized as watershed or non-watershed in appearance by a single vascular neurologist (AL) blinded to TCD results using a previously described methodology.30 Watershed infarcts were defined as one lesion >1.5cm or more than one lesion <1.5cm in either the cortical border zone (CBZ) or the internal border zone (IBZ). The CBZ was defined as the junction between the anterior, middle and posterior cerebral artery territories, and the IBZ as the junction of the anterior, middle, and posterior cerebral arteries with the Hubner, lenticulostriate, and anterior choroidal artery territories.19 Infarcts were classified as non-watershed if there were one or more ischemic lesions in a non-watershed territory, or an isolated single small lesion (<1.5cm) in a possible watershed territory.18
Data Acquisition
TCD MES detection was performed using 2-MHz pulse-wave digital TCD (DWL Doppler Box, Compumedics, Singen, Germany). Insonation of the middle cerebral artery (MCA) ipsilateral to the cerebral infarction or TIA symptomatology at a depth of 45–65 mm was performed for 60 minutes using a standard head-frame. Offline manual review of the full duration of TCD recording for detection of MES was performed by an experienced reader (BC) blinded to clinical and radiographic data using standard criteria to identify MES.31
Statistical Analysis
The primary outcome measure was the percentage of patients with ≥ 1 MES on TCD recording (MES+). Recurrent cerebral ischemia and mortality within 90 days were secondary outcomes. Parametric and non-parametric comparisons of categorical and continuous variables were made using chi-squared, Fisher, student t-test, and Mann-Whitney U tests as appropriate. All significance tests were two-sided. We considered type-1 errors < 5% (p<0.05) to be statistically significant. All calculations were done using SPSS (IBM Corp Released 2014, Version 23.0, Armonk, NY).
Results
A total of 68 patients consented to participate; 48 had completed TCD recordings. Of the 20 patients without completed TCD recordings, 17 did not have temporal windows. One patient was excluded after enrollment due to identification of a high-risk cardiac source in addition to carotid disease. The study cohort therefore consisted of 47 patients, 19 with carotid occlusion and 28 with carotid stenosis (Figure 1).
Figure 1.
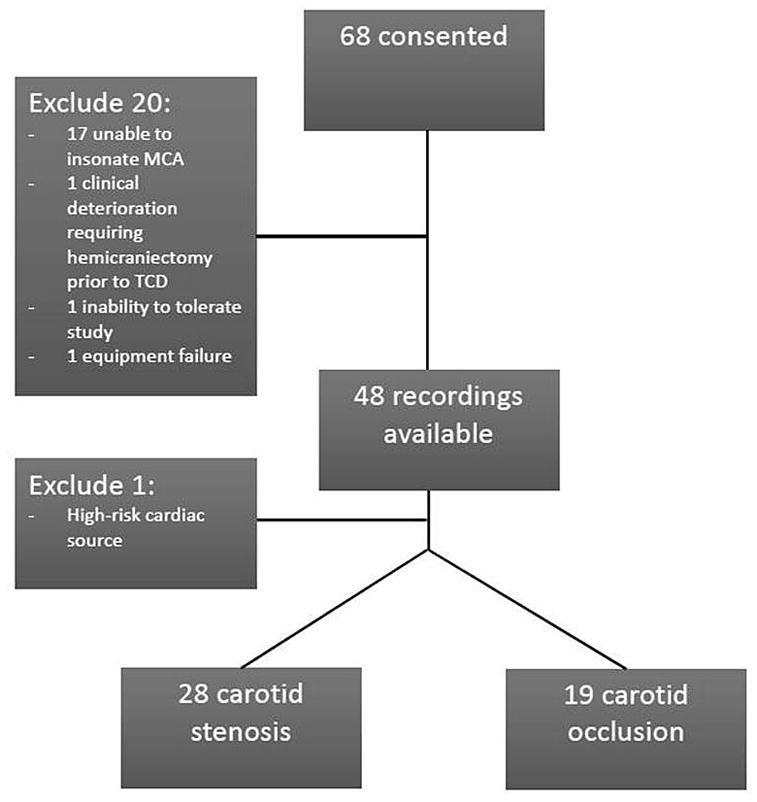
Study Flow Diagram
Patient characteristics are shown in Table 1. Patients with carotid occlusion were younger, less often had a history of diabetes, and more often had a history of prior stroke or TIA than those with carotid stenosis. TCD recordings were obtained a median of 4 days after symptom onset. TCD monitoring characteristics were similar among those with carotid stenosis and occlusion (Table 2).
Table 1.
Demographic, Clinical, and Outcome Data in Study Cohort
| Total (n=47) | Carotid Occlusion (n= 19) | Carotid Stenosis (n= 28) | P-value | |
|---|---|---|---|---|
| Mean Age, [SD] | 66 [10] | 62 [9] | 69 [10] | .02 |
| Women, (%) | 19 (40) | 7 (35) | 12 (41) | .68 |
| Black, (%) | 12 (25) | 5 (26) | 7 (25) | .92 |
| Past Medical History | ||||
| Hypertension, (%) | 38 (79) | 15 (79) | 22 (79) | .98 |
| Hyperlipidemia, (%) | 34 (72) | 15 (79) | 19 (68) | .40 |
| Coronary artery disease, (%) | 7 (15) | 2 (11) | 5 (18) | .49 |
| Diabetes, (%) | 16 (34) | 2 (11) | 14 (50) | .01 |
| Current or former smoker, (%) | 34 (72) | 16 (84) | 18 (64) | .24 |
| Prior stroke or transient ischemic attack, (%) | 13 (28) | 9 (47) | 4 (14) | .01 |
| Peripheral vascular disease, (%) | 3 (6) | 0 | 3 (11) | .14 |
| Clinical Presentation | ||||
| Ischemic stroke, (%) | 39 (81) | 18 (95) | 21 (75) | .17 |
| Median admission NIH stroke scale [IQR] |
6 [2–12] | 7 [5–12] | 4 [2–13] | .44 |
| Contralateral carotid stenosis, (%) | 10 (21) | 2 (11) | 8 (29) | .14 |
| Medications Prior to Admission | ||||
| No anti-platelet, (%) | 23 (49) | 9 (47) | 14 (50) | .86 |
| Single anti-platelet, (%) | 21 (45) | 8 (42) | 13 (46) | .77 |
| Dual anti-platelet, (%) | 3 (6) | 2 (11) | 1 (4) | .34 |
| Statin therapy, (%) | 26 (55) | 10 (53) | 16 (57) | .76 |
| Radiographic Infarct Pattern | ||||
| Any watershed infarct, (%) | 22 (47) | 10 (53) | 12 (43) | .72 |
| Any non-watershed infarct, (%) | 24 (51) | 13 (68) | 11 (39) | .08 |
| Medications at the Time of Transcranial Doppler | ||||
| Single anti-platelet, (%) | 37 (79) | 13 (68) | 24 (86) | .16 |
| Dual anti-platelet, (%) | 6 (13) | 3 (16) | 3 (10) | .61 |
| Clinical Outcomes | ||||
| Mean duration of hospitalization (days) [SD] | 7 [4] | 6 [4] | 7 [4] | .12 |
| Carotid revascularization procedure, (%) | 20 (43) | 1(5)* | 19(68) | <.001 |
| Recurrent cerebral ischemia at ninety days, (%) | 9 (19) | 4 (21) | 5 (18) | .78 |
| Death at ninety days, (%) | 1 (2) | 0 | 1 (3) | .41 |
One occlusion patient was noted to have partial vessel recanalization at 1 month and then underwent a carotid endarterectomy
Table 2.
Transcranial Doppler Monitoring Results
| Total (n=47) | Carotid Occlusion (n=19) | Carotid Stenosis (n=28) | P-value | |
|---|---|---|---|---|
| Mean time from symptom onset to transcranial doppler, [SD] (hours) | 92 [60] | 103[79] | 85 [43] | .89 |
| Mean recording time, [SD] (min) | 54 [14.3] | 55 [12] | 51 [16] | .42 |
| Any microembolic signal, (%) | 18 (38) | 7 (37) | 11 (39) | .87 |
| Median number of microembolic signals [IQR] | 2 [1–3] | 2 [2–3] | 1 [1–5] | .63 |
Overall, 18/47 (38%) patients were MES+ on TCD. There were no differences in MES detection rates between those with carotid occlusion (7/19, 37%) as compared to carotid stenosis (11/28, 39%; Table 2). MES+ patients were less likely to have hypertension compared to MES− patients, but otherwise there were no significant differences between those with and without MES (Table 3).
Table 3.
Comparison of Those with Microembolic Signals (MES+) Versus Those Without (MES−)
| MES− (n=29) | MES+ (n=18) | P-value | |
|---|---|---|---|
| Mean Age, [SD] | 66 [10] | 65 [9] | .84 |
| Women, (%) | 12 (41) | 7 (37) | .89 |
| Black, (%) | 8 (28) | 4 (21) | .95 |
| Past Medical History | |||
| Hypertension, (%) | 26 (90) | 11 (61) | .02 |
| Hyperlipidemia, (%) | 20 (69) | 14 (78) | .51 |
| Coronary Artery Disease, (%) | 5 (17) | 2 (11) | .57 |
| Diabetes, (%) | 11 (38) | 5 (28) | .48 |
| Current or former smoker (%) | 22 (76) | 12 (63) | .73 |
| Prior stroke or TIA, (%) | 8 (28) | 5 (28) | .99 |
| Peripheral vascular disease, (%) | 3 (10) | 0 | .16 |
| Ischemic stroke, (%) | 23 (79) | 16 (89) | .65 |
| Contralateral carotid stenosis, (%) | 6 (21) | 4 (22) | .90 |
| Medications Prior to Admission | |||
| No anti-platelet, (%) | 15 (52) | 8 (44) | .63 |
| Single anti-platelet, (%) | 11 (38) | 10 (56) | .24 |
| Dual anti-platelet, (%) | 3 (10) | 0 | .16 |
| Statin therapy, (%) | 16 (55) | 10 (56) | .98 |
| Medications at the Time of Transcranial Doppler | |||
| Single anti-platelet, (%) | 24 (83) | 13 (72) | .39 |
| Dual anti-platelet, (%) | 3 (10) | 3 (17) | .53 |
| Statin therapy, (%) | 27 (93) | 16 (89) | .62 |
| Outcomes | |||
| Recurrent cerebral ischemia at ninety days, (%) | 6 (21) | 3(17) | .73 |
| Death at ninety days, (%) | 0 | 1 (6) | .83 |
All 47 patients had neuroimaging available for review; 27 (57%) MRI and 20 (43%) CT. All patients who presented with ischemic stroke (n=39) had evidence of infarction on imaging. An exclusively watershed pattern of infarction was present in 15/39 (38%) patients, an exclusively non-watershed pattern in 16/39 (41%), and 8/39 (21%) had evidence of both watershed and non-watershed infarctions (Figure 2). Among those with evidence of any watershed infarction, 9/23 (39%) were found to be MES+. In the subgroup of patients with exclusively watershed infarcts, 6/15 (40%) were MES+. The percentage of patients with MES was similar in those with exclusively watershed infarctions compared to those with any other pattern of infarction (40% vs. 42%, p=0.92). Similarly, no significant difference was found comparing those with an exclusively IBZ pattern of watershed infarction to those with any other infarct pattern (20% vs. 44% MES+, p=0.63). Numerically more patients with exclusively CBZ infarcts had MES than those with an exclusively IBZ pattern, but this was not significant (71% vs. 20%, p=0.24; Figure 2). Patterns of cerebral infarction were similar among patients with carotid occlusion compared to stenosis (Table 1).
Figure 2.
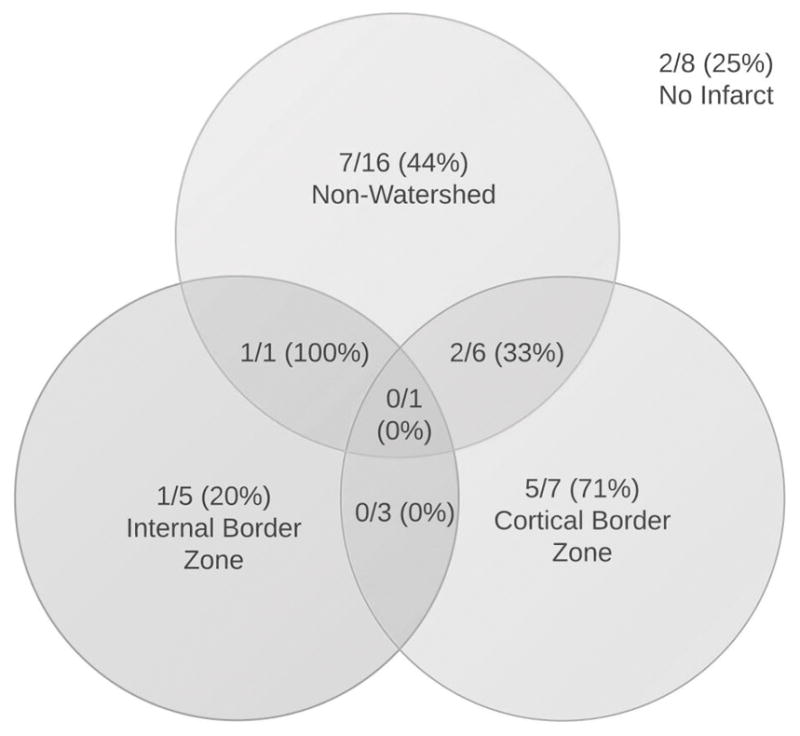
Presence of Microembolic Signals (+MES) by Infarct Topography
Recurrent cerebral ischemia occurred in 9 patients (19%; 6 with TIA, 3 with stroke) within the 90-day follow up period. One of the strokes was peri-procedural following a CEA. Most ischemic events occurred early, with 8/9 (89%) cerebral ischemic events occurring during index hospitalization. One patient died from surgical complications of coronary bypass surgery during index hospitalization (Table 1). There were no differences in clinical outcome between patients with MES compared to those without (Table 3).
Discussion
We found that nearly a third of recently symptomatic carotid occlusion patients had MES on TCD monitoring, with a similar MES+ rate between those with carotid stenosis and carotid occlusion. A significant portion of patients with watershed (IBZ and CBZ) infarctions on imaging were MES+, suggesting that embolization may play an important role even in this subgroup.
Our results are concordant with earlier studies that have compared subjects with carotid occlusion to those with stenosis and noted similar rates of MES detection between the two groups (Table 4).32–35 We are aware of three prior studies which describe MES frequency and neuroimaging results among stroke/TIA patients.13–15 Only one of these studies contains detailed data on index cerebral infarction patterns. In this study, which examined 30 patients with middle cerebral artery stenosis, MES were more frequently seen in those with watershed infarction than in those without watershed infarction (50% vs. 14%); differentiation between CBZ and IBZ was not reported.15 Our finding that MES are common among those with watershed infarcts is in agreement with this prior study and extends their observation to patients with extracranial carotid disease. We thus provide further support for the concept that even watershed infarcts may be linked to impaired embolic washout in patients with large vessel stenosis or occlusion.12
Table 4.
Relevant Prior Literature
| Lead study author | Carotid occlusion with Microembolic signals detected, (%) | Carotid stenosis with Microembolic signals detected, (%) | Time from symptom onset | Study limitations |
|---|---|---|---|---|
| Eicke, BM et al32 | 5/13 (38) | 7/42 (17) | - Not reported | - Symptomatic & asymptomatic cases in each group |
| Babikian, VL et al33 | 4/23 (17) | 22/76 (29) | < 6 months | |
| Droste, DW et al34 | 4/10 (40) | 17/41 (41) | 0 to 3,474 days | -Among those with MES, two had competing cardiac sources |
| Orlandi, G et al35 | 0/8 (0) | 14/33 (42) | < 120 days |
The rate of recurrent cerebral ischemia seen in our cohort overall is consistent with that of prior data.5, 6, 36 However, unlike some prior studies, we did not see a difference in clinical outcome between MES+ and MES− patients.23–25 Currently, there are no surgical and few targeted therapeutic options for those with symptomatic carotid occlusion in contrast to carotid stenosis.37 Given the frequency of microembolization we observed, prior trial results demonstrating MES reduction with dual antiplatelet therapy, and possible clinical benefit of anticoagulation in patients with stroke due to large vessel disease, further investigation of aggressive antithrombotic regimens among patients with symptomatic carotid occlusions may be warranted.25, 26, 29, 38, 39
Our study has a number of important limitations. First, we likely significantly underestimated the true rate of microembolization in our patients. TCD recordings were done for only 1 hour; more prolonged monitoring would probably have increase the detection rate of MES. Our MES detection rate may also have been higher had we performed TCDs earlier after symptoms onset as MES detection rates vary inversely with time from symptom onset.10 In addition, as is typical with TCD, a minority of our patients did not have temporal bone windows and were not included; often these patients are older and may have been more likely to show emboli. We also excluded patients treated with therapeutic anticoagulation. It is possible that patients at highest risk for early ischemic recurrence may have been more likely than others to be treated with anticoagulation and therefore also potentially more likely to have MES.25, 33, 40 Second, we are limited in the generalizability of our findings by our relatively small sample size, single-center design, and our selective inclusion and exclusion criteria. Our institutional practices may be unique. For example, all of our included patients received aggressive stroke unit care and over half of our patients reported pre-admission statin use which may significantly lower the risk of early stroke recurrence in patients with carotid disease.41 Some patients in our study were treated with dual antiplatelet therapy which may also reduce both the rate of microembolizaton and recurrent ischemic events.25 Third, we did not capture data on systemic or cerebral hemodynamics in our patients which limits our ability to assess the relative interaction between cerebral hypoperfusion and embolization. We also lack data on the patterns of collateral cerebral blood flow in our patients; this may have prevented us from detecting MES in subjects with atypical collateral flow patterns since we performed TCD only at the MCA ipsilateral to the symptomatic carotid.20–22 Finally, while all our patients were acutely symptomatic, we cannot be certain whether our patients had acute or chronic carotid occlusion, which may have different outcomes and mechanisms of ischemia.5
Conclusion
We found a similarly high rate of microembolization among patients with recently symptomatic carotid occlusion as compared to those with carotid stenosis. MES were found with similar frequency in all infarct patterns, including watershed infarctions. These findings suggest that embolization plays a major role in the mechanism of injury in symptomatic large vessel carotid disease, including carotid occlusion. Future investigations of aggressive antithrombotic, antiplatelet, or other medical therapy among patients with symptomatic carotid occlusion may be warranted given the current lack of targeted therapeutic options for this high-risk group.
Acknowledgments
Funding: This study was supported by grant 1U10NS086474 (Dr. Liberman) from the National Institute of Neurological Disorders and Stroke as well as an unrestricted educational grant from Bristol Myers Squib (Dr. Cucchiara).
Footnotes
Disclosures: None
References
- 1.Weimar C, Mieck T, Buchthal J, Ehrenfeld CE, Schmid E, Diener HC, et al. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol. 2005;62:393–397. doi: 10.1001/archneur.62.3.393. [DOI] [PubMed] [Google Scholar]
- 2.Ay H, Gungor L, Arsava EM, Rosand J, Vangel M, Benner T, et al. A score to predict early risk of recurrence after ischemic stroke. Neurology. 2010;74:128–135. doi: 10.1212/WNL.0b013e3181ca9cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaghi S, Rostanski SK, Boehme AK, Martin-Schild S, Samai A, Silver B, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2015.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty ML, Flemming KD, McClelland R, Jorgensen NW, Brown RD., Jr Population-based study of symptomatic internal carotid artery occlusion: Incidence and long-term follow-up. Stroke. 2004;35:e349–352. doi: 10.1161/01.STR.0000135024.54608.3f. [DOI] [PubMed] [Google Scholar]
- 6.Burke MJ, Vergouwen MD, Fang J, Swartz RH, Kapral MK, Silver FL, et al. Short-term outcomes after symptomatic internal carotid artery occlusion. Stroke. 2011;42:2419–2424. doi: 10.1161/STROKEAHA.111.615278. [DOI] [PubMed] [Google Scholar]
- 7.Grubb RL, Jr, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055–1060. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- 8.Bogousslavsky J, Regli F. Borderzone infarctions distal to internal carotid artery occlusion: Prognostic implications. Ann Neurol. 1986;20:346–350. doi: 10.1002/ana.410200312. [DOI] [PubMed] [Google Scholar]
- 9.Pessin MS, Hinton RC, Davis KR, Duncan GW, Roberson GH, Ackerman RH, et al. Mechanisms of acute carotid stroke. Ann Neurol. 1979;6:245–252. doi: 10.1002/ana.410060311. [DOI] [PubMed] [Google Scholar]
- 10.Wijman CA, Babikian VL, Matjucha IC, Koleini B, Hyde C, Winter MR, et al. Cerebral microembolism in patients with retinal ischemia. Stroke. 1998;29:1139–1143. doi: 10.1161/01.str.29.6.1139. [DOI] [PubMed] [Google Scholar]
- 11.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 12.Caplan LR, Wong KS, Gao S, Hennerici MG. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc Dis. 2006;21:145–153. doi: 10.1159/000090791. [DOI] [PubMed] [Google Scholar]
- 13.Muller M, Reiche W, Langenscheidt P, Hassfeld J, Hagen T. Ischemia after carotid endarterectomy: Comparison between transcranial doppler sonography and diffusion-weighted mr imaging. AJNR Am J Neuroradiol. 2000;21:47–54. [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura K, Minematsu K, Koga M, Arakawa R, Yasaka M, Yamagami H, et al. Microembolic signals and diffusion-weighted mr imaging abnormalities in acute ischemic stroke. AJNR Am J Neuroradiol. 2001;22:1037–1042. [PMC free article] [PubMed] [Google Scholar]
- 15.Wong KS, Gao S, Chan YL, Hansberg T, Lam WW, Droste DW, et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: A diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250. [DOI] [PubMed] [Google Scholar]
- 16.Bergui M, Castagno D, D’Agata F, Cicerale A, Anselmino M, Maria Ferrio F, et al. Selective vulnerability of cortical border zone to microembolic infarct. Stroke. 2015;46:1864–1869. doi: 10.1161/STROKEAHA.114.008194. [DOI] [PubMed] [Google Scholar]
- 17.Momjian-Mayor I, Baron JC. The pathophysiology of watershed infarction in internal carotid artery disease: Review of cerebral perfusion studies. Stroke. 2005;36:567–577. doi: 10.1161/01.STR.0000155727.82242.e1. [DOI] [PubMed] [Google Scholar]
- 18.Mangla R, Kolar B, Almast J, Ekholm SE. Border zone infarcts: Pathophysiologic and imaging characteristics. Radiographics. 2011;31:1201–1214. doi: 10.1148/rg.315105014. [DOI] [PubMed] [Google Scholar]
- 19.Yong SW, Bang OY, Lee PH, Li WY. Internal and cortical border-zone infarction: Clinical and diffusion-weighted imaging features. Stroke. 2006;37:841–846. doi: 10.1161/01.STR.0000202590.75972.39. [DOI] [PubMed] [Google Scholar]
- 20.Delcker A, Diener HC, Wilhelm H. Source of cerebral microembolic signals in occlusion of the internal carotid artery. J Neurol. 1997;244:312–317. doi: 10.1007/s004150050093. [DOI] [PubMed] [Google Scholar]
- 21.Barnett HJ, Peerless SJ, Kaufmann JC. “Stump” on internal carotid artery--a source for further cerebral embolic ischemia. Stroke. 1978;9:448–456. doi: 10.1161/01.str.9.5.448. [DOI] [PubMed] [Google Scholar]
- 22.Georgiadis D, Grosset DG, Lees KR. Transhemispheric passage of microemboli in patients with unilateral internal carotid artery occlusion. Stroke. 1993;24:1664–1666. doi: 10.1161/01.str.24.11.1664. [DOI] [PubMed] [Google Scholar]
- 23.Markus HS, King A, Shipley M, Topakian R, Cullinane M, Reihill S, et al. Asymptomatic embolisation for prediction of stroke in the asymptomatic carotid emboli study (aces): A prospective observational study. Lancet Neurol. 2010;9:663–671. doi: 10.1016/S1474-4422(10)70120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altaf N, Kandiyil N, Hosseini A, Mehta R, MacSweeney S, Auer D. Risk factors associated with cerebrovascular recurrence in symptomatic carotid disease: A comparative study of carotid plaque morphology, microemboli assessment and the european carotid surgery trial risk model. J Am Heart Assoc. 2014;3:e000173. doi: 10.1161/JAHA.113.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: The clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (caress) trial. Circulation. 2005;111:2233–2240. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 26.Klijn CJ, Kappelle LJ, Algra A, van Gijn J. Outcome in patients with symptomatic occlusion of the internal carotid artery or intracranial arterial lesions: A meta-analysis of the role of baseline characteristics and type of antithrombotic treatment. Cerebrovasc Dis. 2001;12:228–234. doi: 10.1159/000047708. [DOI] [PubMed] [Google Scholar]
- 27.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the american heart association/american stroke association stroke council; council on cardiovascular surgery and anesthesia; council on cardiovascular radiology and intervention; council on cardiovascular nursing; and the interdisciplinary council on peripheral vascular disease. The american academy of neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 29.Low molecular weight heparinoid, org 10172 (danaparoid), and outcome after acute ischemic stroke: A randomized controlled trial. The publications committee for the trial of org 10172 in acute stroke treatment (toast) investigators. JAMA. 1998;279:1265–1272. [PubMed] [Google Scholar]
- 30.Massaro A, Messe SR, Acker MA, Kasner SE, Torres J, Fanning M, et al. Pathogenesis and risk factors for cerebral infarct after surgical aortic valve replacement. Stroke. 2016;47:2130–2132. doi: 10.1161/STROKEAHA.116.013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basic identification criteria of doppler microembolic signals. Consensus committee of the ninth international cerebral hemodynamic symposium. Stroke. 1995;26:1123. [PubMed] [Google Scholar]
- 32.Eicke BM, von Lorentz J, Paulus W. Embolus detection in different degrees of carotid disease. Neurol Res. 1995;17:181–184. doi: 10.1080/01616412.1995.11740309. [DOI] [PubMed] [Google Scholar]
- 33.Babikian VL, Wijman CA, Hyde C, Cantelmo NL, Winter MR, Baker E, et al. Cerebral microembolism and early recurrent cerebral or retinal ischemic events. Stroke. 1997;28:1314–1318. doi: 10.1161/01.str.28.7.1314. [DOI] [PubMed] [Google Scholar]
- 34.Droste DW, Dittrich R, Kemeny V, Schulte-Altedorneburg G, Ringelstein EB. Prevalence and frequency of microembolic signals in 105 patients with extracranial carotid artery occlusive disease. J Neurol Neurosurg Psychiatry. 1999;67:525–528. doi: 10.1136/jnnp.67.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlandi G, Parenti G, Bertolucci A, Murri L. Silent cerebral microembolism in asymptomatic and symptomatic carotid artery stenoses of low and high degree. Eur J Neurol. 1997;38:39–43. doi: 10.1159/000112900. [DOI] [PubMed] [Google Scholar]
- 36.Schneider J, Sick B, Luft AR, Wegener S. Ultrasound and clinical predictors of recurrent ischemia in symptomatic internal carotid artery occlusion. Stroke. 2015;46:3274–3276. doi: 10.1161/STROKEAHA.115.011269. [DOI] [PubMed] [Google Scholar]
- 37.Powers WJ, Clarke WR, Grubb RL, Jr, Videen TO, Adams HP, Jr, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: The carotid occlusion surgery study randomized trial. JAMA. 2011;306:1983–1992. doi: 10.1001/jama.2011.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams HP, Jr, Bendixen BH, Leira E, Chang KC, Davis PH, Woolson RF, et al. Antithrombotic treatment of ischemic stroke among patients with occlusion or severe stenosis of the internal carotid artery: A report of the trial of org 10172 in acute stroke treatment (toast) Neurology. 1999;53:122–125. doi: 10.1212/wnl.53.1.122. [DOI] [PubMed] [Google Scholar]
- 39.Wang QS, Chen C, Chen XY, Han JH, Soo Y, Leung TW, et al. Low-molecular-weight heparin versus aspirin for acute ischemic stroke with large artery occlusive disease: Subgroup analyses from the fraxiparin in stroke study for the treatment of ischemic stroke (fiss-tris) study. Stroke. 2012;43:346–349. doi: 10.1161/STROKEAHA.111.628347. [DOI] [PubMed] [Google Scholar]
- 40.Valton L, Larrue V, le Traon AP, Massabuau P, Geraud G. Microembolic signals and risk of early recurrence in patients with stroke or transient ischemic attack. Stroke. 1998;29:2125–2128. doi: 10.1161/01.str.29.10.2125. [DOI] [PubMed] [Google Scholar]
- 41.Merwick A, Albers GW, Arsava EM, Ay H, Calvet D, Coutts SB, et al. Reduction in early stroke risk in carotid stenosis with transient ischemic attack associated with statin treatment. Stroke. 2013;44:2814–2820. doi: 10.1161/STROKEAHA.113.001576. [DOI] [PMC free article] [PubMed] [Google Scholar]


