Abstract
More than 50 years after spironolactone has come on the market its mechanism of action continues to expand. In this issue of Circulation Research, Good et al. document the discovery that spironolactone is not only an inhibitor of the mineralocorticoid receptor, but also inhibits pannexin 1 channels.
Subject codes: Hypertension, Treatment, Mechanisms, Cell Signaling
Keywords: Spironolactone, mineralocorticoid receptor, pannexin channel, alpha adrenergic vasoconstriction, phenylephrine, purinergic signaling
Resistant hypertension (RH), defined as blood pressure >140/90 mm Hg despite the use of three antihypertensive agents, is well recognized and not rare1. For drug treatment of RH, first-line drug choices for blood pressure control include (1) a diuretic (should be included unless not tolerated), (2) either an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker and (3) a calcium channel blocker and in certain patients a beta blocker2. Based on several smaller clinical trials, guidelines recommend to add the mineralocorticoid receptor (MR) antagonists spironolactone or eplerenone when blood pressure remains uncontrolled2. Recently the randomized controlled PATHWAY-2 trial showed a superior beneficial effect of spironolactone in patients on optimal three drug regimen in a double-blind crossover setting over the alternative drugs bisoprolol (beta receptor blocker) and doxazosin (alpha receptor blocker)3. Results showed that spironolactone lowered systolic blood pressure by −8.7 mmHg, while bisoprolol and doxazosin were also effective, but inferior with −4.5 mmHg and −4.0 mmHg reduction respectively.
Spironolactone’s well recognized mode of action is the antagonism of the MR in the distal tubule of the kidney. Activation of the MR e.g. by aldosterone leads to its translocation into the nucleus and subsequent changes in gene expression. The net effect on ion and water homeostasis is sodium retention, volume increase and potassium excretion. Antagonizing the MR has therefore the opposite effect, making spironolactone a potassium sparing diuretic.
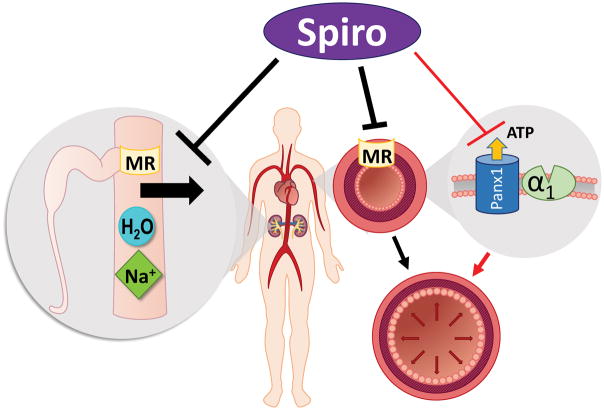
While there seems to be a clear benefit of spironolactone in RH, the mechanistic basis for this has not been established. One common hypothesis is that by adding another diuretic, more effective diuresis/natriuresis is achieved than with one diuretic alone (Fig. 1). The MR is also expressed in the vessel wall in both endothelial cells and SMCs and its actions has been associated with effects on blood pressure4, 5. Other “off target” effects of spironolactone may play a role as well, but clear demonstration that such effects contribute significantly to lower blood pressure has not been presented.
Fig. 1.
Multiple proposed actions of spironolactone (spiro) to lower blood pressure. The primary target is the mineralocorticoid receptor (MR) in the kidney which reduces sodium re-uptake from the distal tubule lumen causing increased sodium and water excretion (left). More recently MR inhibition in the vasculature is increasingly recognized to reduce vasoconstriction (middle), but has also been associated with additional benefits including e.g. anti-inflammatory and anti-fibrotic effects not depicted in the scheme. The new pathway proposed by Good et al. is marked with red arrows (right). Spironolactone blocks pannexin channels (Panx1) in SMCs which reduces ATP release during alpha adrenergic stimulation (α1). This subsequently diminishes alpha adrenergic vasoconstriction resulting in lower vascular tone.
In this issue of Circulation Research, Isakson, Ravichandran and coworkers report a novel target of spironolactone6. The investigators used a cellular assay to identify small molecule inhibitors of pannexin 1 channels and discovered that spironolactone and several of its metabolites block pannexin channels. Could this be one of the suspected “off target” effects of spironolactone?
Pannexin 1 is ubiquitously expressed in multiple tissue and cell-types including endothelial and smooth muscle cells (SMCs) of the vasculature. Prior work from the Isakson group established that pannexin channels amplify vasoconstriction in response to alpha-adrenergic stimulation via purinergic signaling7. Consistent with the earlier study, here the authors demonstrate that spironolactone blunted the response to the alpha1 agonist phenylephrine in isolated human and mouse arteries and thus acts as an apparent alpha adrenoceptor antagonist. They elegantly utilize genetic strategies to show that this inhibition is independent of MR inhibition, but dependent on pannexin channels in SMCs. The data are intriguing, did pannexin inhibition contribute all along unrecognized to the blood pressure lowering effects of spironolactone?
Then again, Good et al. do not provide direct evidence that pannexin channels are inhibited by spironolactone in RH patients and it seems premature to assume that this is the case. Foremost it is uncertain if pannexin inhibition is broadly achieved with routinely administered doses of spironolactone, e.g. in PATHWAY-2 patients received typical doses of 25–50 mg p.o. per day3. Directly corresponding plasma and tissue levels of spironolactone are not available, but plasma levels of total spironolactone metabolites after eight days of 100 mg spironolactone p.o. daily reach peak values that are just about approaching the IC50 of 18.9 μmole/L for pannexin inhibition in isolated arteries8. In plasma spironolactone is almost entirely bound to plasma protein, which limits diffusion. Spironolactone concentrations in the arterial vessel wall have apparently not been investigated and might rely on surrounding tissue. Spironolactone and its metabolites enrich most notable in the intestine, the liver and the kidney, but e.g. in the brain and skeletal muscle tissue concentrations remain below plasma concentrations9. If we take the primary target of spironolactone, the MR, the IC50 is around 24 nmole/L, i.e. spironolactone is close to 1000 fold more potent inhibiting the MR than pannexin channels10. Altogether, it requires additional evidence that pannexin channels in resistance arteries are inhibited by typical doses of spironolactone.
It should also be highlighted that Good et al. analyzed acute blood pressure changes within 45 min after i.p. injection of spironolactone. While that further emphasizes that the observed effects are independent of MR inhibition, it does not allow for conclusions about long-term blood pressure regulation. If pannexins in SMCs are inhibited, then pannexins in other cell types e.g. in endothelial cells and erythrocytes would be inhibited as well, which might reduce vasodilatory ATP release into the vessel lumen11. It is also noteworthy that the role of pannexin channels in alpha adrenergic stimulation has been challenged12. Last but not least, the study by Good et al. utilizes only male mice and recruited only male donors for human arteries. Therefore, strictly, results may not directly apply to the female sex.
At this point there are also ongoing controversies about some of the properties of the pannexin 1 channel that require clarification in the future13, 14. Briefly, for pannexin 1 two open channel conformations have been proposed, one low and one large conductance state that is dependent on the mode of activation13. Voltage stimulation in the absence of other stimuli induces the chloride selective, low conductance conformation, while other stimuli e.g. high K+ induce the large pore that is permeable to ATP. In Good et al. the large conductance channel is never observed, consistent with previous reports from this group, while ATP release is reported separately in apoptotic Jurkat cells. While not of major concern for the conclusion of the study, independent confirmation of the inhibitory effect of spironolactone on the large conductance state of pannexin 1 and the corresponding IC50 might be useful.
Despite these open questions, the discovery that spironolactone inhibits pannexin channels brings the pannexin field one step closer to an inhibitor that is in widespread clinical use. Spironolactone might be a new useful tool to probe the divers and exciting emerging roles of pannexin 1 channels. The results presented in Good et al. are compelling to suggest that pannexin channel inhibition is a potential new treatment modality for RH. Whether or not pannexin inhibition already contributes to long-term blood pressure lowering in hypertensive patients receiving spironolactone remains to be elucidated.
Acknowledgments
We thank Dr. Tanja Dudenbostel for critical reading of the manuscript and Grace Salzer and Isaac Shamblin for assistance with literature search and help with the figure.
SOURCES OF FUNDING
This work was supported by grants from the NIH RO1 HL128044 and HL128563 and University of Alabama at Birmingham IMPACT funds to S.H.
ABBREVIATIONS
- ATP
adenosinetriphosphate
- i.p
intraperitoneal
- IC50
half-maximal inhibitory concentration
- mmHg
millimeter of mercury
- MR
mineralcorticoid receptor
- p.o
by mouth, taken orally
- SMC
smooth muscle cell
Footnotes
DISCLOSURES
None.
References
- 1.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–19. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Sr, Williamson JD, Wright JT., Jr ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017 [Google Scholar]
- 3.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ British Hypertension Society’s PSG. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068. doi: 10.1016/S0140-6736(15)00257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen XN, Phan VK, Chau VM, Bui HT, Nguyen XC, Vu KT, Hoang le TA, Jo SH, Jang HD, Kwon YI, Kim YH. A new monoterpenoid glycoside from Myrica esculenta and the inhibition of angiotensin I-converting enzyme. Chem Pharm Bull (Tokyo) 2010;58:1408–10. doi: 10.1248/cpb.58.1408. [DOI] [PubMed] [Google Scholar]
- 5.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–33. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Good ME, Chiu YH, Poon IK, Medina CB, Butcher JT, Mendu SK, DeLalio LJ, Lohman AW, Leitinger N, Barrett EJ, Lorenz UM, Desai BN, Jaffe IZ, Bayliss D, Isakson BE, Ravichandran KS. Pannexin 1 Channels as an Unexpected New Target of the Anti-Hypertensive Drug Spironolactone. Circ Res. 2017 doi: 10.1161/CIRCRESAHA.117.312380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE. Pannexin1 regulates alpha1-adrenergic receptor-mediated vasoconstriction. Circ Res. 2011;109:80–5. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho PC, Bourne DW, Triggs EJ, Smithurst BA. Comparison of plasma levels of canrenone and metabolites after base hydrolysis in young and elderly subjects following single and multiple doses of spironolactone. Eur J Clin Pharmacol. 1984;27:435–9. doi: 10.1007/BF00549591. [DOI] [PubMed] [Google Scholar]
- 9.Karim A. Spironolactone: disposition, metabolism, pharmacodynamics, and bioavailability. Drug Metab Rev. 1978;8:151–88. doi: 10.3109/03602537808993782. [DOI] [PubMed] [Google Scholar]
- 10.Fagart J, Hillisch A, Huyet J, Barfacker L, Fay M, Pleiss U, Pook E, Schafer S, Rafestin-Oblin ME, Kolkhof P. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem. 2010;285:29932–40. doi: 10.1074/jbc.M110.131342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006;103:7655–9. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angus JA, Betrie AH, Wright CE. Pannexin-1 channels do not regulate alpha1-adrenoceptor-mediated vasoconstriction in resistance arteries. Eur J Pharmacol. 2015;750:43–51. doi: 10.1016/j.ejphar.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Ambrosi C, Qiu F, Jackson DG, Sosinsky G, Dahl G. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci Signal. 2014;7:ra69. doi: 10.1126/scisignal.2005431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu YH, Schappe MS, Desai BN, Bayliss DA. Revisiting multimodal activation and channel properties of Pannexin 1. J Gen Physiol. 2018;150:19–39. doi: 10.1085/jgp.201711888. [DOI] [PMC free article] [PubMed] [Google Scholar]