ABSTRACT
Adult female acne is a chronic inflammatory, immune-mediated disease that affects the pilosebaceous unit in women in their 20s to 40s, and is considered different from acne vulgaris. Propionibacterium acnes is recognized by TLR-2, resulting in activation of this receptor and an inflammatory response through the NFκ B pathway. This therapeutic, interventional, open, randomized, evaluator-blinded and comparative trial included 38 adult women with moderate facial acne and 10 age-matched controls, all aged between 26 and 44 years. Two treatments were performed over six months: 15% azelaic acid gel (AA) bid (n = 18) and oral contraceptive (COC) drospirenone 3 mg/ethinylestradiol .02 mg (n = 20). Biopsies were taken at baseline (control, lesion, perilesional) and at the conclusion (lesion and perilesional) of the study to evaluate TLR-2 expression by immunohistochemistry. Lesion count and blind photographic evaluation were used for efficacy. The groups were homogeneous: 70% of lesions were located in the submandibular area, 95% of participants had inflammatory lesions; of these, 50% had persistent and 50% had late-onset acne. The mean ages were 33.7 ± 5.5 and 33.1 ± 5.3 years (COC and AA group, respectively). A moderate clinical improvement was observed in both groups. No difference in TLR-2 expression in the lesion or perilesional areas was observed; however, reduced TLR-2 expression was seen in the control group. A significant reduction in expression was observed after both treatments, with no difference between the groups. This finding suggests an anti-inflammatory effect of COCs and AA in adult female acne, via modulation of the TLR-2 receptor.
KEYWORDS: Acne, toll-like receptor 2, combined oral contraceptive, azelaic acid, sebaceous glands
Introduction
Acne vulgaris is a common skin disease in adolescents, with a prevalence of 80 to 90%. During the previous 20 years, an increase of acne prevalence in adults, particularly in women over 25 years of age, has been described.1 The reasons for this are not fully understood; some authors postulate that hormonal factors related to modern life, increases in the search for dermatological care, and increased medical attention to this problem may be involved.2-5 Acne in adult women may have persisted from adolescence, present as late onset,6 or recur in adult life after onset during puberty and a disease-free period.7
Adult female acne is a chronic inflammatory and immune-mediated disease that affects the pilosebaceous unit in active, working women in their 20s to 40s, associated or unassociated with hyperseborrhoea and alterations in serum sexual hormone concentrations (although the majority show no hormonal abnormalities). This condition is characterized by mild or moderate inflammatory lesions that worsen during the pre-menstrual period in 80% of the women affected.8 Results from a study of 374 adults with acne showed that in adult women the lesion distribution may be similar to that seen in adolescents, or be predominant in the mandibular and neck regions.9 The skin of adult women with acne is more sensitive than that of teenagers and prone to irritation with anti-acne topical products. Adult acne is experienced as stressful and negatively impacts quality of life. Affected women frequently present with a severe skin-picking habit, which results in post-inflammatory hyperpigmentation and scarring. This disease is highly refractory to treatment, with frequent recurrences.8-11
Evidence supports the role of inflammation in all stages of acne, including subclinically and prior to comedo formation.12,13 The underlying pathways are complex and incompletely elucidated, but may involve Propionibacterium acnes (P. acnes) and inflammatory mediators and their receptors, including cytokines, defensins, peptidases, sebum lipids, and neuropeptides.14
The toll-like receptors (TLRs) are trans-membrane receptors related to the innate immune response. They are present in immune, epithelial and endothelial cells, and are involved in the pathogenesis of many dermatoses.15,16 The sebaceous gland is a skin appendage that expresses many receptors, including TLRs.17 TLR-2 is expressed in keratinocytes, sebocytes, dendritic and inflammatory cells, as well as in monocytes and macrophages, in acne patients.18,19 It plays a crucial role in comedogenesis and inflammation via activation of transcriptional nuclear factors, such as nuclear factor kappa B (NFƙB), through triggering the production of several cytokines (including IL-1α, IL-8, IL-12) and via activation of other cells, such as macrophages, natural-killer (NK) and neutrophils.20,21 Additionally, the adaptive immune response is stimulated.15,16 Several in vivo and in vitro studies have demonstrated that P. acnes exhibits PAMPs (pathogen associated molecular patterns) that are recognized by TLR-2, which leads to over-expression and to an inflammatory response.20,22-25 It is possible to investigate the expression of TLR-2 in skin cells using rabbit polyclonal antibodies.24 TLRs can also up-regulate the production of microorganism peptides, such as β defensins 1 and 2, by epithelial cells.16
Adult female acne usually requires a specific therapeutic approach.26 Hormonal therapy with combined oral contraceptives (COCs) may be considered the first line of treatment when there are no contraindications and no desire for pregnancy.27,28 These hormones decrease the liberation of gonadotrophins, control androgen production, block androgenic receptors and reduce free testosterone by increasing sexual binding hormone globulins (SHBG) secretion by the liver.29-31 This is a safe and effective therapeutic option for women with or without hormonal abnormalities, including hyperandrogenism caused by polycystic ovary syndrome (PCOS).27 A systematic review published by the Cochrane Library that analysed comparative studies about the use of various COCs for acne concluded that they were effective in the reduction of inflammatory and non-inflammatory lesions, with a mean reduction of 60% in the lesion count, with no difference seen between different combinations.32 The side effects of this treatment included nausea, headache, breast pain, spotting and decreased libido.31,32 In addition tothe reduction in acne, the COCs exert a protection against ovarian and endometrial cancer, and reduce dysmenorrhoea, anaemia (caused by iron deficiency) and the risk of inflammatory bowel disease.31 Concerning the risk of venous thromboembolism, the incidence is low in young women and there is a small increase (.05% to .1%) related to COC use.33 Nevertheless, the risk of a thrombotic event after delivery is .5%, and during pregnancy is .1%.34 The indication of COC therefore requires a detailed history including contraindications such as smoking and a familial history or risk for deep venous thrombosis and cardiovascular disease.31
Azelaic acid (AA) is a saturated dicarboxylic acid and is a topical acne treatment option.35,36 It normalizes follicular hyperkeratinization, reduces P. acnes colonization and controls inflammation. This is related to the activation of the nuclear receptor PPARγ and inhibition of the NF-kB pathway.37,38 AA used twice daily for six months demonstrates an efficacy similar to .1% adapalene in maintenance treatment of acne in adult women.39 The aim of this study is to investigate the possible modulation of TLR-2 expression in sebaceous glands by COC or AA in adult female acne.
Patients and methods
The study design was a therapeutic, interventional, open, randomized, evaluator-blinded and comparative trial with parallel groups. The participants were 38 healthy adult women with moderate facial acne and 10 age-matched controls, aged between 26 and 44 years, who were included after protocol approval by the Institutional Review Board. Informed consent was obtained from participants. Inclusion criteria were the presence of facial acne with no other signs or symptoms of hyperandrogenism. Exclusion criteria were treatment of acne with oral antibiotics and COCs (during the previous three months), oral isotretinoin (during the previous two years), topical products (during the previous three months), and having a contraindication for COC use. The intervention was: At baseline, two 3-mm punch biopsies were performed under local anaesthesia from the submandibular region (inflammatory lesion and perilesional area). A randomization list was obtained through a computational program to create two treatment groups for a period of six months: AA (n = 18), who would use 15% AA gel bid, and COC (n = 20), who would use COC (drospirenone 3 mg/ethinylestradiol .02 mg). A 3-mm punch biopsy was also performed under local anaesthesia from the submandibular region in participants from the control group. The efficacy parameters included: the lesion count; clinical evaluation by two independent dermatologists by comparing baseline and post-treatment photos; immunohistochemistry using TLR-2 rabbit polyclonal antibody (Abcam, code ab24192, dilution1/150) followed by histomorphometric analysis using the software ImageJ 64 (100x one field). The statistical analysis comprised: Student's t-test of mean ± standard deviation scores (age, TLR-2 expression), Fischer´s exact test (smoking habit, localization of lesions, photographic evaluation), analysis of variance (ANOVA) with repeated measures (lesion count, TLR-2 expression). SPSS® version 16.0 (SPSS® Inc; Illinois, EUA) was used. Results were deemed significant for a p value of less than 5% (p < .05).
Results
All participants in the COC group completed the study; in the AA group, four participants dropped out due to personal reasons. At baseline, the groups were homogeneous for all clinical and biographic data, such as smoking habit (90% did not smoke); lesion distribution (70% in the submandibular region); lesion type (95% inflammatory, i.e., moderate acne), acne onset (50% persistent and 50% late). The mean ages were 33.7 ± 5.5 years (COC group) and 33.1 ± 5.3 years (AA group). All patients showed moderate clinical improvement and no severe adverse effects were detected.
TLR-2 expression in the sebaceous glands was observed in sebocytes cytoplasm (Fig. 1). No difference in acne lesions were observed between the groups at baseline (p = .266; Fig. 2). A significant difference in TLR-2 expression was seen between lesion and control group at baseline (p < .001), estimated as 31.0 ± 6.4 (95% CI 17.9, 44.0), as well as between the perilesional area and control group (p < .001), estimated at 27.3 (95% CI 16.3, 38.2; Figs. 3 and 4). Using the ANOVA with repeated measures, the treatment groups were homogeneous in relation to TLR-2 expression at baseline, with no difference between them; this was also seen for the lesion and perilesional area. When comparing TLR-2 expression at baseline and after treatment, no interaction between group and time was demonstrated (p = .554); the groups were homogeneous (p = .192). Nevertheless, a time effect was observed for both groups (p < .001), with a decrease in TLR-2 expression after six months of either treatment; there was no difference seen between the treatments (Fig. 5).
Figure 1.
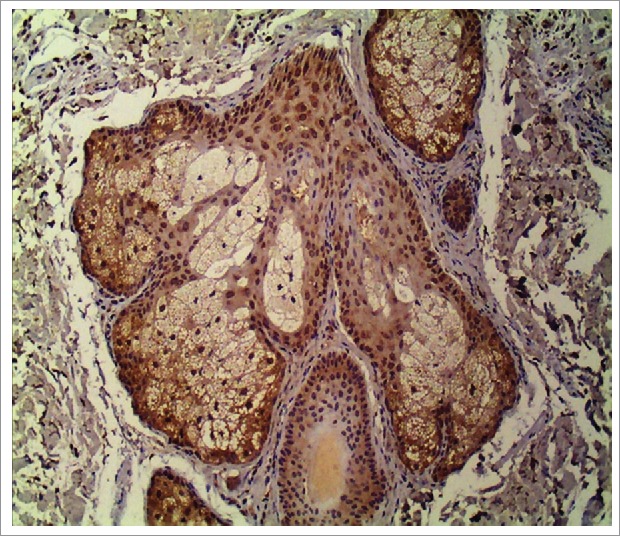
TLR-2 expression in sebocyte cytoplasm in a sebaceous gland (400x).
Figure 2.
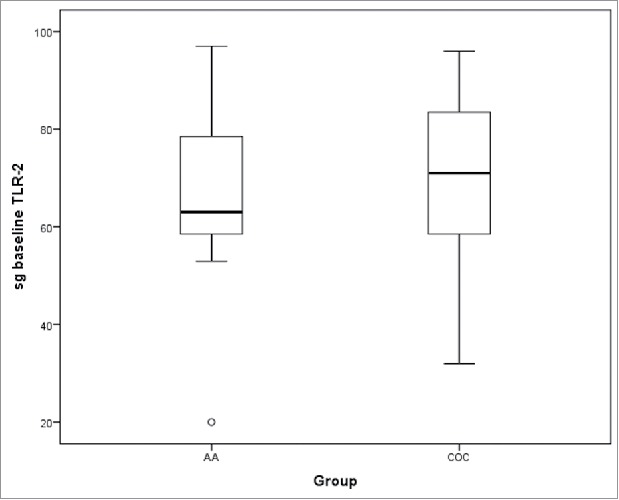
TLR-2 expression in sebaceous glands in acne lesions at baseline (sg-baseline) between the two treatment groups.
Figure 3.
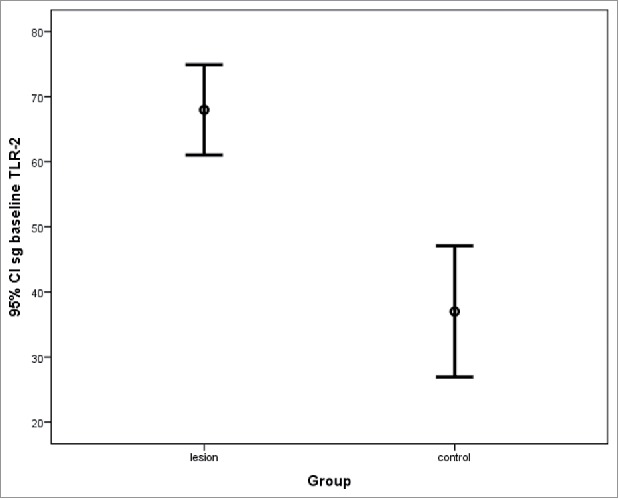
TLR-2 expression in acne lesions at baseline and in the control area in sebaceous glands (sg_baseline; mean ± standard deviation).
Figure 4.
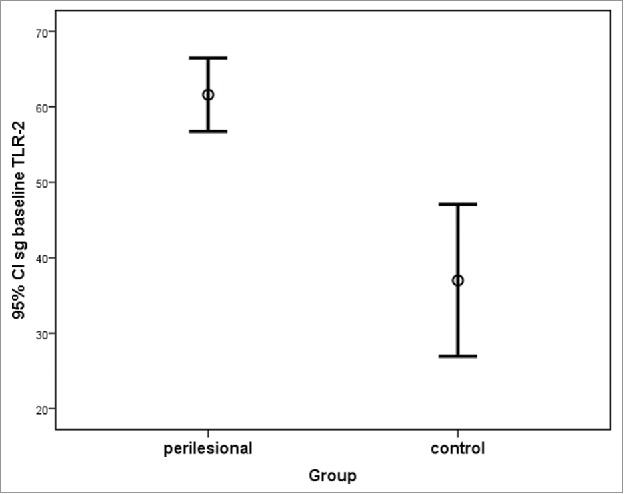
TLR-2 expression in perilesional areas at baseline and control areas in sebaceous glands (sg_baseline; mean ± standard deviation).
Figure 5.
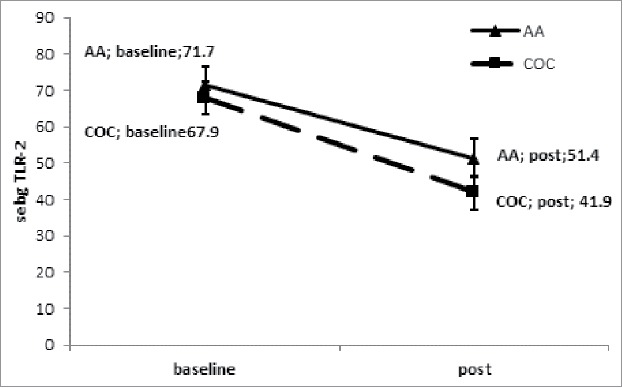
Reduction of TLR-2 expression in sebaceous glands in an acne lesion pre- and post-treatment with COC and AA.
Discussion
Adult female acne is a chronic, multifactorial and recalcitrant disease. Many researchers consider its clinical features different to those of acne vulgaris.4,8-10,40,41 New insights showing that the innate immune response, especially inflammation, is mediated by TLRs, have contributed to a better understanding of several acne treatments.23,42 The prevalence of this disease has varied in different populational studies, depending on study design. The majority have been retrospective, for which the presence of acne is declared by the patient (sometimes by telephone interview), and which may have introduced a possible overestimation. In contrast, the frequent use of COCs may lead to an underestimation of the number of cases.3,8-11 The population of women included in this study showed a homogenous distribution of TLR-2 in the two treatment groups (COCs versus AA), supporting adequate randomization and therefore a lower risk of bias. The mean age was 33 years, demonstrating the chronicity of the disease, corroborating previous studies that showed a higher prevalence between 30 and 40 years of age.3,9 There is no consensus in the literature about the minimum and maximum age of participants in studies concerning adult female acne.10 The low number of women who smoked may reflect the results of repeated campaigns in Brazil regarding the risks of tobacco. The similar prevalence for persistent and late-onset acne differed from the majority of studies, which have emphasized the predominance of persistent acne.8-10 This may be explained by the rigorous exclusion of patients with hirsutism and/or signs of hyperandrogenism. The lesions were mostly inflammatory and located in the lower face (“U” type), according to the predominant data from the literature.8,10,11,40,41 A recent study did not confirm this lesion distribution, probably because 93% of the women presented with comedones beyond inflammatory lesions.9 In our study, the acne severity was moderate. It is difficult to compare this finding with other studies, as the first standardization for acne severity scoring in female adults was published in 2016.43 Clinical evaluation, comparing pre- and post-treatment photos and lesion counts, showed that at least 80% of patients treated with a COC experienced an improvement greater than 50%, supporting the findings of previous studies.29,30,32 The group treated with AA also showed a significant decrease in the presence of lesions, as has elsewhere been reported.39 The authors did not expect the complete clearance of lesions since, according to the study design, the patients only received monotherapy. A recent study from our group demonstrated that even a moderate amelioration of acne has a positive impact on sleep quality and in turn on a woman's quality of life.44 There is an unquestionable consensus that inflammatory acne needs combined therapy for a satisfactory result.45
Immunohistochemistry here revealed cytoplasmic expression of TLR-2 in the sebocytes of the infundibular area of the pilosebaceous unit, with a predominance in the periphery of sebaceous glands in the acne lesion and perilesional regions, as have previously been reported.24 An over-expression of TLR-2 in keratinocytes and sebocytes in acne lesions was here observed. The increased expression in the perilesional area, compared to the control group, corroborates the hypothesis that inflammation occurs prior to the development of clinical lesions.13,21,24 This observation supports the current recommendation to apply topical anti-acne products to the entire compromised area, not only on the isolated lesions.45 In this study, a significant decrease in TLR-2 expression after six months of both treatments was observed, which was parallel to clinical improvement. Previous studies have shown modulation of TLR-2 in keratinocytes in vitro with adapalene,23 as well as in monocytes with oral isotretinoin.42 No data exists regarding TLR-2 changes induced by AA or COCs in acne treatment. Therefore, the modulation of TLR-2 may be a novel anti-acne mechanism of these drugs. The possible anti-inflammatory effect of COCs associated with a reduction of androgenic stimulus in the sebaceous glands may explain their positive effect on the treatment of acne in women after puberty, even for those with no hormonal abnormalities.10,27,45 Despite the limitation of sample size in this study, it was possible to demonstrate the cytoplasmic location and distribution of TLR-2 in sebaceous glands, which was over-expressed in both the lesion and perilesional areas in the acne group compared to the control group. Histomorphometric analysis showed a reduction in TLR-2 expression accompanied by clinical improvement of acne lesions. This finding may suggest an anti-inflammatory effect of COCs and AA in adult female acne. Future studies investigating the treatment of acne targeting inflammation should aim to determine the modulation of TLR-2 and TLR-4, as well as cytokines such as IL-17.46-48
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
Fleury Institute; Bayer, Brazil (donation of study medications) Clinicaltrials.gov NCT01850095.
Funding
Support Fund to Dermatology – Sao Paulo Regional of Brazilian, Society of Dermatology – FUNADERSP.
References
- [1].Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474-85. https://doi.org/ 10.1111/bjd.12149 [DOI] [PubMed] [Google Scholar]
- [2].Albuquerque RG, Rocha MA, Bagatin E, Tufik S, Andersen ML. Could adult female acne be associated with modern life? Arch Dermatol Res. 2014;306(8):683-8. https://doi.org/ 10.1007/s00403-014-1482-6 [DOI] [PubMed] [Google Scholar]
- [3].Collier CN, Harper JC, Cafardi JA, Wang W, Foster KW. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-9. https://doi.org/ 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- [4].Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11(6):708-13 [PubMed] [Google Scholar]
- [5].Willians C, Layton AM. Persistent acne in women. Am J Clin Dermatol. 2006;7(5):281-90. https://doi.org/ 10.2165/00128071-200607050-00002 [DOI] [PubMed] [Google Scholar]
- [6].Dumont-Wallon G, Dreno B. Specificity of acne in women older than 25 years. Presse Med. 2008;37(pt1):585-91. https://doi.org/ 10.1016/j.lpm.2007.07.014 [DOI] [PubMed] [Google Scholar]
- [7].Preneau S, Dreno B. Female acne – a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26(3):277-82. https://doi.org/ 10.1111/j.1468-3083.2011.04214.x [DOI] [PubMed] [Google Scholar]
- [8].Dreno B, Layton A, Zouboulis CC, López-Estebaranz JL, Zalewska-Janowska A, Bagatin E, Zampeli VA, Yutskovskaya Y, Harper JC. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27(9):1063-70. https://doi.org/ 10.1111/jdv.12061 [DOI] [PubMed] [Google Scholar]
- [9].Dreno B, Thiboutot D, Layton AM, Berson D, Perez M, Kang S. On behalf of the Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-106. https://doi.org/ 10.1111/jdv.12757 [DOI] [PubMed] [Google Scholar]
- [10].Zeichner JA, Baldwin HE, Cook-Bolden FE, Eichenfield LF, Fallon-Friedlander S, Rodriguez DA. Emerging Issues in Adult Female Acne. J Clin Aesthet Dermatol. 2017;10(1):37-46. [PMC free article] [PubMed] [Google Scholar]
- [11].Holzmann R, Shakery K. Postadolescent Acne in Females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8. https://doi.org/ 10.1159/000354887 [DOI] [PubMed] [Google Scholar]
- [12].Tanghetti EA. The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol. 2013;6(9):27-35. [PMC free article] [PubMed] [Google Scholar]
- [13].Rocha MA, Costa CS, Bagatin E. Acne vulgaris: an inflammatory disease even before the onset of clinical lesions. Inflamm Allergy Drug Targets. 2014;13(3):162-7. https://doi.org/ 10.2174/1871528113666140606110024 [DOI] [PubMed] [Google Scholar]
- [14].Kurokawa I, Danby WJ, Ju Q, Wang X, Xiang LF, Xia L, Chen W, Nagy I, Picardo M, Suh DH, et al.. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-32. https://doi.org/ 10.1111/j.1600-0625.2009.00890.x [DOI] [PubMed] [Google Scholar]
- [15].Lancaster IG, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M. The physiological regulation of toll-like receptor expression and function in humans. J Physiol. 2005;563(3):945-55. https://doi.org/ 10.1113/jphysiol.2004.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005;125(1):1-8. https://doi.org/ 10.1111/j.0022-202X.2004.23459.x [DOI] [PubMed] [Google Scholar]
- [17].Zouboulis CC. Sebaceous gland receptors. Dermato-Endocrinol. 2009;1(2):77-80. https://doi.org/ 10.4161/derm.1.2.7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pivarcsi A, Koreck A, Bodai L, Szell M, Szeg C, Belso N, Kenderessy-Szabo A, Bata-Csorgo Z, Dobozy A, Kemeny L. Differentiation-regulated expression of Toll-like receptors 2 and 4 in HaCaT keratinocytes. Arch Dermatol Res. 2004;296:120-4. https://doi.org/ 10.1007/s00403-004-0475-2 [DOI] [PubMed] [Google Scholar]
- [19].Fathy A, Mohamed RW, Ismael NA, El-Akhras MA. Expression of toll-like receptors on peripheral monocytes of patients with inflammatory and noninflammatoy acne vulgaris. Egypt J Immunol. 2009;16(1):127-34. [PubMed] [Google Scholar]
- [20].Kim J. Review of the innate response in acne vulgaris: activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193-98. https://doi.org/ 10.1159/000087011 [DOI] [PubMed] [Google Scholar]
- [21].Selway JL, Kurczab T, Kealey T, Langlands K. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatology. 2013;13:10. https://doi.org/ 10.1186/1471-5945-13-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, Dreno B. Induction of toll-like receptor by Propionibacterium acnes. Br J Dermatol. 2005;153(6):1105-13. https://doi.org/ 10.1111/j.1365-2133.2005.06933.x [DOI] [PubMed] [Google Scholar]
- [23].Tenaud I, Khammari A, Dréno B. In vitro modulation of TLR-2, CD1d and IL-10 by adapalene on normal human skin and acne inflammatory lesions. Exp Dermatol. 2007;16:500-6. https://doi.org/ 10.1111/j.1600-0625.2007.00552.x [DOI] [PubMed] [Google Scholar]
- [24].Bakry OA, Samaka RM, Sebika H. Toll-lke receptor 2 and P. acnes: Do They trigger initial acne vulgaris lesions? Anal Quant Cytopathol Histpathol. 2014;36(2):100-10. [PubMed] [Google Scholar]
- [25].Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21(3):323-33. [DOI] [PubMed] [Google Scholar]
- [26].Vera N, Patel N, Cardwell LA, Saleem M, Feldman SR. Chemical pharmacotherapy options for managing adult acne. Expert Opin Pharmacotherapy. 2017;18(3):263. https://doi.org/ 10.1080/14656566.2017.1282460 [DOI] [PubMed] [Google Scholar]
- [27].Katsambas AD, Dessinioti C. Hormonal therapy for acne: why not as first line therapy? Facts and controversies. Clin Dermatol. 2010;28(1):17-23. https://doi.org/ 10.1016/j.clindermatol.2009.03.006 [DOI] [PubMed] [Google Scholar]
- [28].Lakshmi C. Hormonal Therapy in acne. Indian J Dermatol Venereol Leprol. 2013;79:322-37. https://doi.org/ 10.4103/0378-6323.110765 [DOI] [PubMed] [Google Scholar]
- [29].Lucky AW, Koltun W, Thiboutot D, Niknian M, Sampson-Landers C, Korner P, Marr J. A combined oral contraceptive containing 3-mg drospirenone/ 20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82(2):143-50. [PubMed] [Google Scholar]
- [30].Maloney JM, Dietze P Jr, Watson D, Niknian M, Lee-Rugh S, Sampson-Landers C, Korner P. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen: a randomized controlled trial. Obstet Gynecol. 2008;112(4):773-81. https://doi.org/ 10.1097/AOG.0b013e318187e1c5 [DOI] [PubMed] [Google Scholar]
- [31].Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68(6):1022-9. https://doi.org/ 10.1016/j.jaad.2012.11.018 [DOI] [PubMed] [Google Scholar]
- [32].Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7 Art. No.: CD004425. https://doi.org/ 10.1002/14651858.CD004425.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous tromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-44. https://doi.org/ 10.1097/AOG.0b013e31825194ca [DOI] [PubMed] [Google Scholar]
- [34].Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ. Trends in the incidence of venous tromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143:697-706. https://doi.org/ 10.7326/0003-4819-143-10-200511150-00006 [DOI] [PubMed] [Google Scholar]
- [35].Thiboutot D. Versatility of azelaic acid 15% gel in treatment of inflammatory acne vulgaris. J Drugs Dermatol. 2008;7(1):13-6. [PubMed] [Google Scholar]
- [36].Bagatin E, Holzmann R, Shakery K, Sieber MA, Hegel JKE, Vargas-Diez E, Hofmann MA, Bravo B, Malgazhdarovag KAO, Yutzcovskaya Y. Adult female acne. Azelaic acid in the treatment of acne in adult female. Skin Pharmacol Physiol. 2014;27(Suppl 1):1-2. https://doi.org/ 10.1159/000354886 [DOI] [Google Scholar]
- [37].Mastrofrancesco A, Ottaviani M, Aspite N, Cardinali G, Izzo E, Graupe K, Zouboulis CC, Camera E, Picardo M. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPAR gamma activation. Exp Dermatol. 2010;19(9):813-20. https://doi.org/ 10.1111/j.1600-0625.2010.01107.x [DOI] [PubMed] [Google Scholar]
- [38].Briganti S, Flori E, Mastrofrancesco A, Kovacs D, Camera E, Ludovici M, Cardinali G, Picardo M. Azelaic acid reduced senescence-like phenotype in photo-irradiated human dermal fibroblasts: possible implication of PPARγ. Exp Dermatol. 2013;22(1):41-7. https://doi.org/ 10.1111/exd.12066 [DOI] [PubMed] [Google Scholar]
- [39].Thielitz A, Lux A, Wiede A, Kropf S, Papakonstantinou E, Gollnick H. A randomized investigator-blind parallel-group study to assess efficacy and safety of azelaic acid 15% gel vs. adapalene 0.1% gel in the treatment and maintenance treatment of female adult acne. J Eur Acad Dermatol Venereol. 2015;29(4):789-96. https://doi.org/ 10.1111/jdv.12823 [DOI] [PubMed] [Google Scholar]
- [40].Choi CW, Lee DH, Kim HS, Kim BY, Park KC, Youn SW. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25(4):454-61. https://doi.org/ 10.1111/j.1468-3083.2010.03813.x [DOI] [PubMed] [Google Scholar]
- [41].Capitanio B, Sinagra JL, Bordignon V, Cordiali Fei P, Picardo M, Zouboulis CC. Understimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63:782-8. https://doi.org/ 10.1016/j.jaad.2009.11.021 [DOI] [PubMed] [Google Scholar]
- [42].Dispenza MC, Wolpert EB, Gilliland KL, Dai JP, Cong Z, Nelson AM, Thiboutot DM. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132(9):2198-205. https://doi.org/ 10.1038/jid.2012.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Auffret N, P. Claudel J-P, Leccia M-T, Poli F, Farhi D, B. Dreno B. AFAST – Adult Female Acne Scoring Tool: an easy-to-use tool for scoring acne in adult females. J Eur Acad Dermatol Venereol. 2016;30:824-8. https://doi.org/ 10.1111/jdv.13518 [DOI] [PubMed] [Google Scholar]
- [44].Albuquerque RG, Rocha MAD, Hirotsu C, Hachul H, Bagatin E, Tufik S, Andersen ML. A randomized comparative trial of a combined oral contraceptive and azelaic acid to assess their effect on sleep quality in adult female acne patients. Arch Dermatol Res. 2015;307(10):905-915. https://doi.org/ 10.1007/s00403-015-1600-0 [DOI] [PubMed] [Google Scholar]
- [45].Zaenglein AL, Pathy AL, Bethanee J., Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, Bowe WP, Graber EM MD, Harper JC, et al.. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-73. https://doi.org/ 10.1016/j.jaad.2015.12.037 [DOI] [PubMed] [Google Scholar]
- [46].Su Q, Grabowski M, Weindl G. Recognition of Propionibacterium acnes by human TLR2 heterodimers. Int J Med Microbiol. 2017;307(2):108-12. https://doi.org/ 10.1016/j.ijmm.2016.12.002 [DOI] [PubMed] [Google Scholar]
- [47].Ozlu E, Karadag AS, Ozkanli S, Oguztuzun S, Kilic M, Zemheri E, Akbulak O, Akdeniz N. Comparison of TLR-2, TLR-4, and antimicrobial peptide levels in different lesions of acne vulgaris. Cutan Ocul Toxicol. 2016 Dec;35(4):300-9. https://doi.org/ 10.3109/15569527.2015.1120742 [DOI] [PubMed] [Google Scholar]
- [48].Thiboutot DM, Layton AM, Eady EA. IL-17: a key player in the P. acnes inflammatory cascade? J Invest Dermatol. 2014;134(2):307-10. https://doi.org/ 10.1038/jid.2013.400 [DOI] [PubMed] [Google Scholar]


