Abstract
B cells play an important role in type 1 diabetes (T1D) development. However, the role of B cell activation-induced cytidine deaminase (AID) in diabetes development is not clear. We hypothesized that AID is important in the immunopathogenesis of T1D. To test this hypothesis, we generated AID-deficient (AID–/–) NOD mice. We found that AID–/–NOD mice developed accelerated T1D, with worse insulitis and high levels of anti-insulin autoantibody in the circulation. Interestingly, neither maternal IgG transferred through placenta, nor IgA transferred through milk affected the accelerated diabetes development. AID–/–NOD mice showed increased activation and proliferation of B and T cells. We found enhanced T-B cell interactions in AID–/–NOD mice, with increased T-bet and IFN-γ expression in CD4+ T cells in the presence of AID–/– B cells. Moreover, excessive lymphoid expansion was observed in AID–/–NOD mice. Importantly, antigen-specific BDC2.5 CD4+ T cells caused more rapid onset of diabetes when cotransferred with AID–/– B cells than when cotransferred with AID+/+ B cells. Thus, our study provides insights into the role of AID in T1D. Our data also suggest that AID is a negative regulator of immune tolerance and ablation of AID can lead to exacerbated islet autoimmunity and accelerated T1D development.
Keywords: Autoimmunity, Immunology
Keywords: Adaptive immunity, Autoimmune diseases, Diabetes
Activation-induced cytidine deaminase deficiency breaks B cell tolerance and promotes type 1 diabetes development in NOD mice.
Introduction
Type 1 diabetes (T1D) is an organ-specific autoimmune disease characterized by the destruction of insulin-secreting β cells in the pancreas (1). Although the disease is mainly driven by diabetogenic T cells, B lymphocytes also play an important role in disease development. B cell–deficient (μMT–/–) NOD mice rarely develop diabetes (2, 3), and temporarily depleting B cells with specific antibody can prevent and reverse T1D in NOD mice (3–7). Treatment with rituximab (anti-human CD20) can also reduce the loss of C-peptide in patients with T1D (8). B cells contribute to islet autoimmunity, both as secretors of pathogenic autoantibodies and through the activation of autoreactive T cells (9). Patients with T1D display defective central and peripheral B cell tolerance, as shown by the accumulation of self-reactive, mature naive B cells in the peripheral blood (10).
Activation-induced cytidine deaminase (AID), an important enzyme for B cell function, is responsible for the initiation of the somatic hypermutation and class-switch recombination. This facilitates development of Ig diversity and specificity for the recognition of intruding pathogens through humoral immunity governed by B cells (11–14). Furthermore, AID is critical for the maintenance of B cell tolerance through its removal of pathogenic B cells through apoptosis in both bone marrow and germinal centers (GCs), and its ablation results in the accumulation of large numbers of autoreactive B cells in the sera of AID-deficient (AID–/–) patients (15, 16).
B cells with AID mutations fail to have normal Ig class-switching and can only secrete IgM, and this leads to hyper-IgM syndrome in mice and humans (17). Approximately 25% of patients with hyper-IgM syndrome have presented with different autoimmune manifestations (10, 18, 19). In mice, AID deficiency is also correlated with autoimmune diseases (11, 20, 21). Conversely, a study by Jiang et al. showed that AID deficiency correlated with a reduction in autoreactive B cells, a decrease in T cell activation, and a decrease in kidney lymphocytic infiltration in a lupus mouse model (JHT.mIg.MRL/lpr), where the mice had mIg only but were unable to secrete any Igs (22). The authors suggested that AID acts as an important contributor to the antibody-independent role of B cells in autoimmunity. These observations demonstrate that AID plays an important role, either to promote or prevent the development of autoimmune diseases. However, the role of AID in T1D remains unclear.
Given the importance of B cells in T1D development, we hypothesized that AID contributes to the immunopathogenesis of the disease. To test our hypothesis, we generated AID–/– NOD mice. Unlike the results in a very recent report (23), we found that AID–/–NOD mice developed accelerated T1D with increased anti-insulin antibody in the circulation and enhanced insulitis in the pancreatic islets. Further mechanistic studies demonstrated significantly increased expansion and activation of autoimmune B cells in the absence of AID. The activated B cells effectively facilitated activation of T cells in vitro, enhanced diabetogenic T-B cell interaction, and accelerated diabetes development in vivo.
Results
AID deficiency accelerates T1D development in NOD mice.
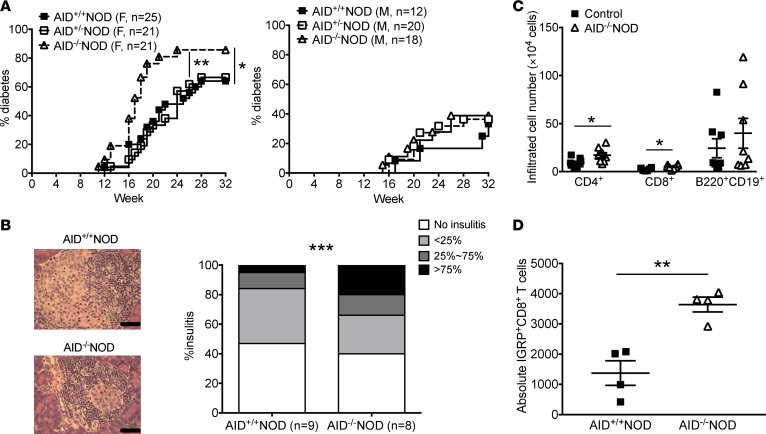
To study whether AID plays a role in the development of T1D, we backcrossed AID–/–C57BL/6 mice onto the NOD genetic background for more than 10 generations. The purity of the NOD genetic background, including the known Idd loci, was verified by Illumina SNP chip (DartMouse, data not shown). Diabetes development was significantly accelerated in female AID–/–NOD mice, with a higher disease incidence, compared with female AID-sufficient littermates that were AID+/+NOD or AID+/–NOD, which developed similar diabetes incidence to each other (Figure 1A). No statistical differences in the diabetes development were seen in male AID–/–NOD mice compared with AID+/+NOD mice (Figure 1A). Consistent with the acceleration of diabetes development, more islets with severe insulitis (infiltration >75%) were observed in AID–/–NOD mice compared with AID+/+NOD mice (21.4% vs. 4.9%), and the percentage of infiltrated islets was also higher in the AID–/–NOD group (60.6% vs. 53.0%), as evaluated by insulitis score (Figure 1B). We analyzed the infiltrated lymphocytes from the islets by flow cytometry and found that both CD4+ and CD8+ T cells were significantly more abundant in the islets from AID–/–NOD mice, compared with control mice (AID-sufficient mice, including both AID+/+NOD and AID+/–NOD mice, as no difference was found between them, in either diabetes development or other phenotypic characteristics) (Figure 1C). Further staining with islet-specific glucose-6-phosphatase catalytic subunit–related protein (IGRP, an autoantigen in both NOD and human T1D) tetramer demonstrated a significant increase in both the frequency and absolute number of IGRP-autoreactive CD8+ T cells in the islet infiltrates of AID–/–NOD mice compared with control mice (Figure 1D and Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.95882DS1). AID deficiency does not affect pancreatic islet development, as AID–/–NOD mice had similar numbers of islets compared with control mice (Supplemental Figure 1B). Our data demonstrated that AID plays an important role in immune tolerance to islet β cells and AID deficiency promotes autoreactive cell infiltration into islets and exacerbates T1D development.
Figure 1. AID deficiency promotes the development of T1D in NOD mice.
(A) Diabetes incidence in AID–/–NOD mice and their AID+/–NOD and AID+/+NOD littermates. Data were pooled from 3 independent experiments. n = 21–25 mice/female group and n = 12–20 mice/male group. (B) Insulitis in 8-week-old nondiabetic female AID–/–NOD and AID+/+NOD mice. At least 100 islets were examined from 8–9 mice/group. Scale bars: 50 μm. (C) Summary of the absolute cell number of infiltrating CD4+ T cells, CD8+ T cells, and B220+CD19+ B cells in the islets of 10- to 12 week-old female AID–/–NOD and control NOD mice (mixed AID+/–NOD and AID+/+NOD). Data are expressed as mean ± SEM and were pooled from 2 independent experiments. (D) Absolute IGRP+CD8+ T cells. Islet infiltrates from 10- to 12 week-old female AID–/–NOD and control mice were stained with IGRP-tetramer, CD45, CD8, and TCRβ followed with FACS analysis. n = 4 mice/group. *P < 0.05; **P < 0.01; ***P < 0.001, Gehan-Breslow-Wilcoxon survival test (A), χ2 test (B), and Student’s t test (C and D).
Maternal class-switched Igs are dispensable to T1D development.
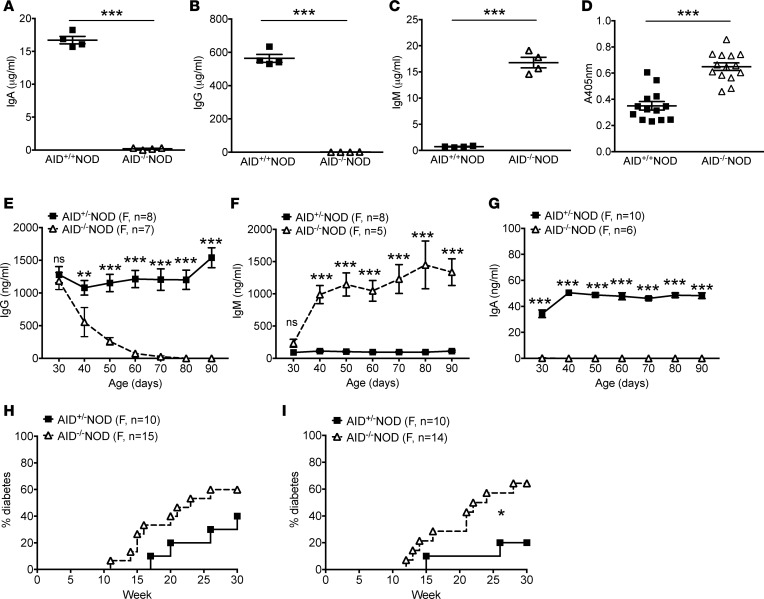
B cells deficient in AID fail to undergo class-switch recombination and produce class-switched Ig isotypes, especially IgG and IgA. These two major class-switched Ig isotypes were undetectable in the circulation of AID–/–NOD mice, and as expected, AID–/–NOD mice showed hyper-IgM in the sera compared with AID+/+NOD mice (Figure 2, A–C). Next, we examined anti-insulin autoantibody (IAA) levels in the circulation, an important indicator of humoral autoimmune responses of T1D. In line with the accelerated disease phenotype, we found significantly increased levels of IAA in the sera of female AID–/– NOD mice compared with AID+/+NOD counterparts (Figure 2D). All the IAAs in AID–/–NOD mice were of the IgM isotype (Supplemental Figure 2).
Figure 2. Early exposure to maternal IgG is dispensable for T1D development in AID–/–NOD mice.
(A–C) Serum Igs from 2-month-old nondiabetic female AID–/–NOD mice and AID+/+NOD littermates were measured by ELISA. (A) IgA; (B) IgG; (C) IgM. Data are shown as mean ± SEM from 1 of at least 2 independent experiments. n = 4 mice/group. (D) Total anti-insulin Ig (IgH+L) measured by ELISA using sera from 2-month-old nondiabetic female AID–/–NOD mice and AID+/+NOD littermates. Data are presented as OD of 405 nm and shown as mean ± SEM by pooling 3 independent experiments. n = 13–14 mice/group. (E–G) Dynamic changes in serum Igs. Sera were taken from 30-day-old female AID–/– and AID+/– NOD mice and then every 10 days thereafter followed by Ig measurement by ELISA. Data are shown as mean ± SEM and were pooled from 2 independent experiments. n ≥ 5 mice/group. (H) Diabetes incidence in female progeny of female AID+/–NOD and male AID–/–NOD breeding. (I) Diabetes incidence of female progeny of female AID–/–NOD and male AID+/–NOD breeding. Data were pooled from 2 independent experiments. n ≥ 10 mice/group. *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test (A–G) and Gehan-Breslow-Wilcoxon survival test (H and I).
It is known that maternal IgG can cross the placenta to the fetus and maternal milk is also rich in class-switched Igs, especially IgA, all of which contribute to the circulating Igs in neonates and provide protection from infections in early life (24). Although adult AID–/–NOD mice have impaired production of IgA and IgG, the AID–/–NOD neonates could still receive these class-switched Igs early in life from their mothers. To test for the dynamic change in serum Igs in AID–/–NOD mice, AID+/–NOD female breeders were used to breed with male AID–/–NOD mice to generate AID+/–NOD and AID–/–NOD offspring. We then examined the circulating IgG, IgA, and IgM in AID+/– and AID–/–NOD littermates from two sets of breeders. It is interesting that AID–/–NOD progeny had similar amounts of circulating IgG and IgM at a very young age (day 30) compared with their AID+/– littermates (Figure 2, E and F). However, a sharp decrease in IgG and an increase in IgM were observed in AID–/–NOD mice after day 30, and the reduction in IgG and increase in IgM in AID–/–NOD mice occurred with age (Figure 2, E and F). However, both IgG and IgM levels remained relatively stable in AID+/–NOD littermates (Figure 2, E and F). Unlike IgG, it is interesting that AID–/–NOD progeny had undetectable serum IgA, even at a very young age, and IgA continued to be absent (Figure 2G). In AID+/– littermates, serum IgA increased between 30 and 40 days of age, and the levels remained steady afterward (Figure 2G). In contrast, there was a significant decline of serum IgG in AID–/–NOD mice during this period (Figure 2E). These data suggest that AID–/–NOD pups received maternal IgG through the placenta and possibly also from breast feeding but gradually lost these antibodies with age, especially after weaning (before 30 days). At the same time as the reduction of circulating IgG, AID–/–NOD mice showed a marked increase in serum IgM, and the levels of serum IgM continued to be high (Figure 2F). To test whether early exposure to maternal IgG affected diabetes development, we set up additional breeding pairs by breeding female AID+/–NOD mice with male AID–/–NOD mice or female AID–/–NOD mice with male AID+/–NOD mice. We observed the female progeny of AID+/–NOD and AID–/–NOD littermates from these two sets of breeding for diabetes development. Interestingly, AID–/–NOD mice in both cohorts developed a higher incidence of diabetes than their AID+/–NOD littermates (Figure 2, H and I), indicating that early exposure to maternal IgG does not affect accelerated T1D development in AID–/–NOD mice.
AID deficiency promotes systemic lymphocytic expansion and enhanced T cell activation and inflammatory cytokine production.
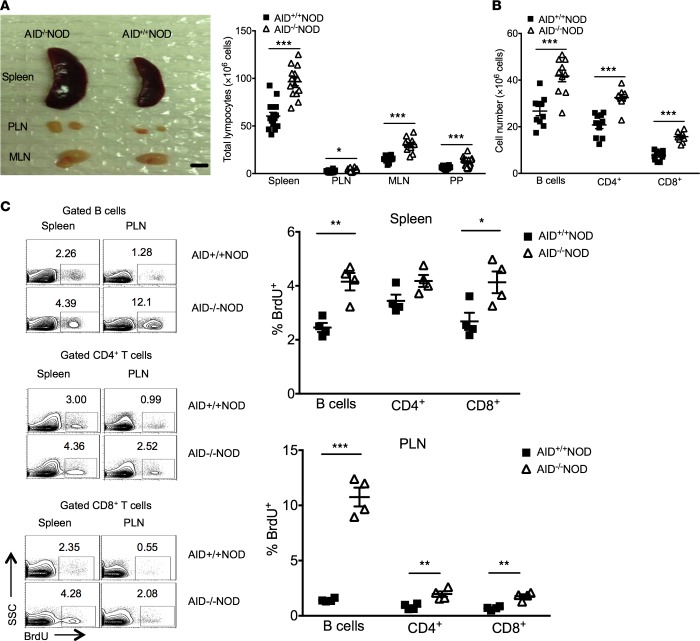
To uncover the mechanism by which AID deficiency promotes autoimmune diabetes, we first characterized different lymphocytic compartments in AID–/–NOD mice. Notably, compared with AID+/+NOD mice, AID–/–NOD mice had enlarged spleens, pancreatic lymph nodes (PLNs), mesenteric lymph nodes (MLNs), and Peyer’s patches (PPs), with significantly increased lymphocyte counts (Figure 3A). In line with these findings, AID–/–NOD mice also had significantly (P < 0.001) more CD4+ and CD8+ T cells as well as B cells in the spleen, PLNs, MLNs, and PPs in comparison with AID+/+NOD mice (Figure 3B and data not shown). To investigate whether the lymphocytic expansion was correlated with enhanced cell proliferation, we performed in vivo BrdU incorporation assays. There was significantly increased proliferation in CD4+ and CD8+ T cells as well as B cells in spleens and PLNs of AID–/–NOD mice compared with AID+/+NOD mice (Figure 3C). However, there was no difference in either cell numbers or the proliferation of different thymic T cell subsets between AID–/–NOD and AID+/+NOD mice (data not shown).
Figure 3. AID deficiency promotes the lymphoid expansion and proliferation in spleen and lymph nodes.
(A) Enlargement of lymphoid organs and expansion of lymphocytes. Images of representative spleens, PLN, and MLN of 8-week-old female AID–/–NOD and AID+/+NOD mice and the summary of total lymphocyte count are shown. Scale bar: 6 mm. (B) Total numbers of B220+CD19+ B cells, CD4+ T cells, and CD8+ T cells in spleens of 8-week-old female AID–/–NOD and AID+/+NOD mice analyzed by flow cytometry. (A and B) Data were pooled from 3 independent experiments and are shown as mean ± SEM. n ≥ 9 mice/group. (C) BrdU incorporation was performed in spleen and PLN cells in 8-week-old female AID–/–NOD and AID+/+NOD mice. The representative flow cytometric plots and the summarized percentage of BrdU+ lymphocytes are shown. Individual data and mean ± SEM of 1 of the 2 independent experiments are shown. n = 4 mice/group. *P < 0.05; **P < 0.01; ***P < 0.001, multiple t test. PLN, pancreatic lymph nodes; MLN, mesenteric lymph nodes; PP, Peyer’s patches; SSC, side scatter.
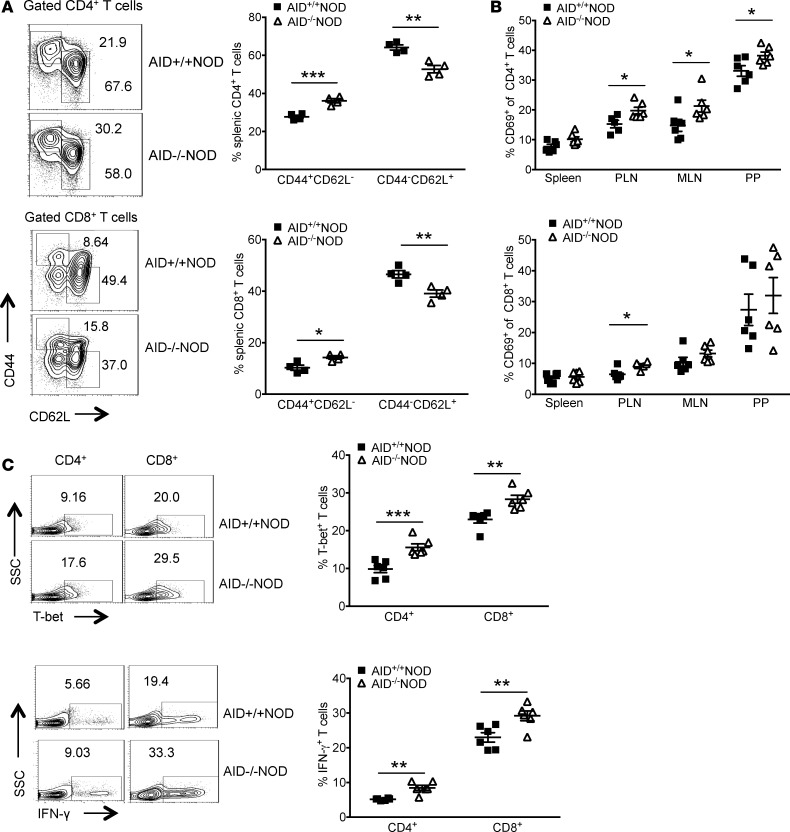
We further investigated the phenotype and function of T cells in AID–/–NOD and AID+/+NOD mice. We found a significantly higher proportion of effector/memory (CD44hiCD62Llo) but a significant reduction in naive (CD44loCD62Lhi) splenic CD4+ and CD8+ T cells in AID–/–NOD mice compared with AID+/+NOD mice (Figure 4A). T cells were more activated (CD69 expressing) in PLNs, MLNs, and PPs in AID–/–NOD mice compared with AID+/+NOD mice (Figure 4B). Moreover, splenic T cells had significantly higher T-bet and expressed more IFN-γ after stimulation (Figure 4C). Interestingly, there was no difference in the frequency, CTLA4 expression, or CD44hiCD62Llo subset of Foxp3+ Tregs in the spleen, PLNs, MLNs, and PPs between these two strains (Supplemental Figure 3, A–C). We also tested the suppressive function of the Tregs and found a similar suppressive ability of Tregs from AID–/–NOD or AID+/+NOD mice (Supplemental Figure 3D).
Figure 4. Enhanced T cell activation and inflammatory cytokine production in AID–/–NOD mice.
(A) Flow cytometric plots of splenocytes from 8-week-old female AID–/–NOD and AID+/+NOD mice are shown for CD44 and CD62L expression in gated CD4+ or CD8+ T cells. The summary of effector/memory CD44hiCD62lo (shown as CD44+CD62L–) and naive CD44loCD62Lhi (shown as CD44–CD62L+) T cells in CD4+ or CD8+ T cells is shown. One of four independent experiments is shown as mean ± SEM. n = 4 mice/group. (B) Summary of the percentage of CD4+ and CD8+ T cells expressing the activation marker CD69 in spleen, PLN, MLN, and PP of 8-week-old female AID–/–NOD and AID+/+NOD mice. Data are shown as mean ± SEM and were pooled from 2 independent experiments. n = 9 mice/group. (C) CD4+ and CD8+ splenic T cells from 8-week-old female AID–/–NOD and AID+/+NOD mice were stained for the transcription factor T-bet and intracellular IFN-γ and analyzed by flow cytometry. Representative flow cytometric plots of splenic IFN-γ– and T-bet–expressing cells are shown as well as the summary of T-bet and IFN-γ expression, shown as mean ± SEM. This experiment was repeated 3 times. n = 6 mice/group. *P < 0.05; **P < 0.01; ***P < 0.001, multiple t test. PLN, pancreatic lymph nodes; MLN, mesenteric lymph nodes; PP, Peyer’s patches; SSC, side scatter.
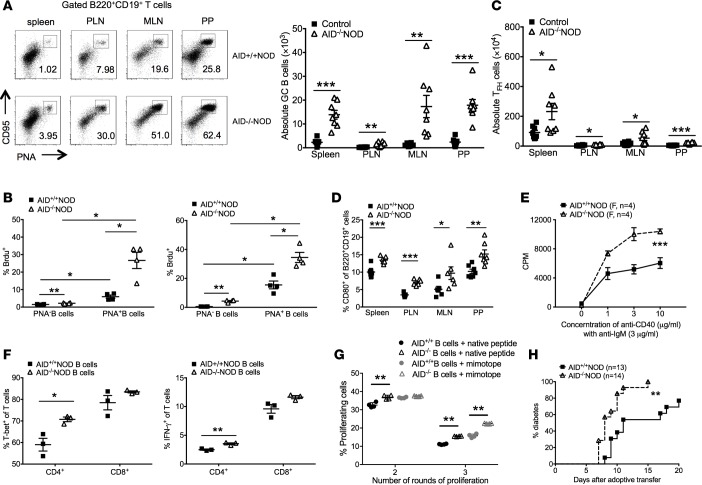
Enhanced B cell activation and B-T cell interaction and increased B cell pathogenesis in AID–/–NOD mice.
Since AID is predominantly expressed in GC B cells, we next examined the effect of AID deficiency on GC B cells. A highly significant increase in GC B cells (peanut agglutinin, PNA+CD95+) was observed in spleens, PLNs, MLNs, and PPs in AID–/–NOD mice compared with AID+/+NOD mice (Figure 5A and Supplemental Figure 4A). Furthermore, BrdU incorporation assay demonstrated that more GC B cells were proliferating in both spleens and PLNs compared with non-GC B cells, regardless of AID expression (Figure 5B). Importantly, however, the proliferation of GC B cells, as assessed by Brdu incorporation, in AID–/–NOD mice, was greater than in their AID+/+NOD mouse counterparts (Figure 5B). This difference in proliferation was also seen between the non-GC B cells in these two strains (Figure 5B). These results indicate that the expansion of B cells seen in AID–/–NOD mice was mainly due to the enhanced proliferation of GC B cells. We also observed significantly increased T follicular-like CD4+ T cells (Tfh, TCRβ+CD4+CXCR5+PD-1+) in spleens, PLNs, MLNs, and PPs in AID–/–NOD mice compared with AID+/+NOD mice (Figure 5C), and there was also a modest increase of Tfh cells in the spleen (Supplemental Figure 4B). These data suggest that the expansion of GC B cells in AID–/–NOD mice was most likely through the interaction of Tfh and B cells. We did not find enhanced CD1d/CD5-expressing B regulatory cells in AID–/–NOD mice (data not shown), but we found that B cells in AID–/–NOD mice were more activated, with a higher percentage of the B cells expressing the costimulatory molecule CD80 compared with AID+/+NOD mice (Figure 5D). The percentage of cells expressing CD86 was also increased in AID–/–NOD mice (data not shown). Furthermore, purified splenic B cells from AID–/–NOD mice exhibited significantly stronger responses to B cell stimulation by anti-IgM and anti-CD40 compared with AID+/+NOD mice (Figure 5E). Taken together, our data suggest that, not only does AID deficiency promote the proliferation of B cells, but the B cells are also more activated. Although we did not find enhanced expression of CD28 or CTLA4 on T cells (data not shown), the increased B cell activation could facilitate the activation and function of T cells. To test this hypothesis, we cocultured purified CD4+ or CD8+ T cells from WT NOD mice with B cells from AID–/–NOD mice or AID+/+NOD mice in the presence of a low concentration of anti-CD3. As shown in Figure 5F, a significantly increased number of CD4+ T cells cocultured with AID–/–NOD B cells expressed T-bet and IFN-γ, compared with those cocultured with AID+/+NOD B cells, while AID did not have a marked effect on the expression of T-bet or IFN-γ in CD8+ T cells in the in vitro assay (Figure 5F). Next, we tested if AID–/–NOD B cells more potently promoted diabetes in vitro and in vivo. In vitro, we cocultured purified CD4+ BDC2.5 T cells, which were labeled with CFSE, with B cells from either AID–/–NOD or AID+/+NOD mice in the presence of BDC2.5 native (25, 26) or mimotope peptide. The results showed that B cells from AID–/–NOD mice promoted significantly more BDC2.5 T cell proliferation (Figure 5G). In vivo, we cotransferred purified CD4+ BDC2.5 T cells with B cells from either AID–/–NOD or AID+/+NOD mice to immune-deficient hosts (Rag2–/–NOD or NOD.scid). In line with the in vitro data, immune-deficient mice receiving cotransferred AID–/–NOD B cells developed significantly accelerated diabetes and an overall higher incidence compared with those that received cotransferred AID+/+ B cells (Figure 5H). Our data suggest that, in the absence of AID, B cells are more activated and more effective at promoting diabetogenic CD4+ T cell activation and proliferation and enhancing the action of autoantigen-specific T cells.
Figure 5. Enhanced B cell activation and B-T cell interaction and increased B cell pathogenicity in AID–/–NOD mice.
8- to 10 week-old female AID–/–NOD and control mice were used for A–F. (A) FACS plots of CD95 and PNA coexpression in B cells and summary of total GC B cells of spleen, PLN, MLN, and PP. n = 8 mice/group. (B) BrdU incorporation in PNA+ B cells from spleen and PLNs. (C) Summary of total Tfh cells (PD-1+CXCR5+CD4+TCRβ+) from spleen, PLN, MLN, and PP. n = 8 mice/group. (D) CD80 expression in gated B220+CD19+ B cells. n = 8 mice/group. (E) Cell proliferation of sorted splenic B cells (5 × 104 cells/well) from female AID–/–NOD and AID+/+NOD mice, cultured with anti-IgM (3 μg/ml) and different concentrations of anti-CD40 for 4 days. (F) T-bet and IFN-γ –expressing T cells. Purified splenic B cells (2 × 106 cells/well) were cocultured with purified NOD splenic CD4+ or CD8+ T cells (T/B = 1:1) with anti-CD3 antibody (1:300 dilution) for 4 days. Cells were then stained for IFN-γ and T-bet, analyzed by FACS. (G) Purified splenic B cells (106 /ml) from AID–/– or AID+/+NOD mice were cocultured with CFSE-labeled CD4+ T cells (106 /ml) from BDC2.5 NOD mice in the presence of native hybrid peptide (insulin/ChgA) or mimotope(10 ng/ml). Dilution (number of round of proliferation) of BDC2.5 CD4+ T cells was examined. The difference in percentage of proliferating BDC2.5 T cells in the second or third peaks of proliferation was compared. **P < 0.01, multiple t tests with FDR correction. (H) Purified splenic B cells (5 × 106 ) were cotransferred with 3 × 106 purified BDC-2.5 CD4+ T cells into Rag2–/–NOD or NOD.scid mice (4–5 weeks old), followed by observation for diabetes development. Data were pooled from at least 2 independent experiments (A, C, D, and G) or 1 of over 3 individual experiments (B, E, and F). *P < 0.05; **P < 0.01; ***P < 0.001, multiple testing with Bonferroni correction (A, D, and F), 2-tailed Student’s t test (B and C), 2-way ANOVA (E), and Gehan-Breslow-Wilcoxon survival test (G). Data are shown as mean ± SEM.
Discussion
In our study, we have shown that the AID deficiency accelerated and increased autoimmune diabetes in the NOD mouse. In the absence of AID, NOD mice also developed a lymphoproliferative phenotype in different lymphoid tissues. Moreover, both T and B cells were more activated and inflammatory. The B cells lacking functional AID were also more autoreactive, as shown by the excessive production of IAA in the circulation and by the enhanced facilitation of diabetogenic T cells that cause T1D when cotransferred to immunodeficient hosts. Thus, our results suggest that under normal circumstances, in addition to the role in antibody class-switching, AID is important in maintenance of immune tolerance to islet β cell autoimmunity.
A number of lines of evidence suggest that B cells may play multiple roles in the development of diabetes. B cell–deficient NOD mice do not develop diabetes (3, 6), and impaired signaling of the B cell receptor protects NOD mice from diabetes development as well (27). The antigen-presenting function of B cells is also important for T1D development (2, 28). We have previously shown that B cell–deficient μMT NOD mice, which have transgenic expression of membrane-bound IgM (mIg) but are unable to secrete any switched isotypes of Igs, restored the B cell compartment to a normal level, as found in NOD mice (3). The expression of mIg also restored diabetes development in 16% of the mice, compared with B cell–deficient μMT NOD mice, which had only 1.5% incidence of diabetes (3). However, the incidence of diabetes in mIg μMT NOD mice was much lower than that seen in WT normal NOD mice, indicating that antibody production by B cells and a broad B cell repertoire may also be important for T1D development.
Autoantibody production is a characteristic of many autoimmune diseases, including lupus, rheumatoid arthritis, and autoimmune hepatitis. Autoantibodies contribute to the pathology of the targeted tissues via activation of complement system, formation and deposition of immune complexes, stimulation of phagocytes, and induction of inflammation (29, 30). Although the role of the autoantibodies in T1D is not completely understood, autoantibodies against GAD, IA2, and insulin are important biomarkers for diagnosis and prediction of T1D development (31–33). AID is an essential enzyme for the production of all class-switched antibodies, and, in the absence of AID, B cells in both mice and humans can only produce IgM. As expected in the AID–/– NOD mice, IgM was increased, whereas IgA and IgG were virtually undetectable. By preventing the generation of IgG, lack of AID negates the normal negative feedback mediated by IgG interaction with the inhibitory IgG Fc receptor expressed principally on B cells, FcγRIIB (34, 35). This removes a natural brake on immune tolerance, and this may be an important contributor to the increase in autoantibodies. While autoantibodies are usually of the IgG isotype, we found that, in the absence of AID, there was enhanced production of IgM insulin autoantibodies. The ability to transmit autoantibodies from mother to the offspring has previously been shown to be important in inducing diabetes (36). It is known that IgM cannot transmit through the placenta, and yet the incidence of diabetes in offspring of mothers that could not transmit antibodies was not different to the diabetes incidence in the offspring of the mothers that were able to transmit class-switched antibodies. To test whether the IgM has a causal role in accelerating diabetes, we infused sera from AID–/–NOD mice into Rag–/–NOD recipients, 24 hours prior to adoptively transferring diabetogenic BDC2.5 CD4+ T cells into the Rag–/–NOD hosts. Hyper-IgM did not affect the diabetes development induced by BDC2.5 CD4+ T cells (Supplemental Figure 5). Thus, the accelerated diabetes development in AID–/–NOD mice is most likely a reflection of the loss of tolerance due to the AID deficiency.
The effect of AID deficiency on other B cell subsets was not obvious, except that there was an increased number of follicular B cells (Supplemental Figure 6, A–C). The increase of follicular B cells is in line with an even greater increase of GC B cells in the absence of AID. In GCs, B cells undergo affinity maturation that leads to the production of high-affinity, antigen-specific antibodies via mutations in the Ig locus controlled by AID (37). AID deficiency results in impaired GC reactions, which leads to the expansion of self-reactive B cell clones (38, 39). Furthermore, AID deficiency may also enhance the escape of these pathogenic B cell clones from GCs of peripheral lymphoid tissues by evading the selection pressures and circulating to target the systemic host organs (39). AID–/–B6 mice exhibited larger numbers of GC B cells compared with AID+/+ controls, due to reduced B cell death in AID–/–B6 B cells (15). We also found a significant increase in B220+CD19+ B cells and PNA+ GC B cells, accompanied by increased B cell proliferation in the spleens and PLNs in vivo by BrdU incorporation and in vitro by BCR stimulation in AID–/–NOD mice, compared with AID+/+NOD mice. However, we found increased B cell apoptosis in AID–/–NOD mice in response to both anti-IgM and LPS stimulation, compared with AID+/+NOD mice (data not shown). This suggested that the NOD genetic background also affects the balance of B cell homeostasis and leads to the accumulation of B cells, including autoreactive B cells, within and outside of GCs.
Although AID is highly transcribed in GC B cells, especially plasma and memory B cells (40), one study reported that AID is also expressed in other cells, including CD4+ T cells (41). We did not find AID gene expression in sorted CD4+ T cells (data not shown); however, we observed significantly activated, proliferated CD4+ T cells and CD8+ T cells in AID–/–NOD mice in vivo and in vitro. This suggests that the enhanced T cell activation and proliferation in AID–/–NOD mice may not be caused by an intrinsic T cell defect in the absence of AID, but it is most likely to be associated with the loss of immune tolerance due to hyperactivated B cells when AID is ablated. Indeed, we identified increased Th1 cytokine secretion from AID+/+NOD T cells when cocultured with AID–/–NOD B cells. Moreover, we found that Tfh cells were also significantly increased in AID–/–NOD mice, further supporting the expansion of PNA+ GC B cells through T-B interaction. This enhanced T-B interaction in the absence of AID also increased antigen-specific proliferation of diabetogenic BDC2.5 CD4+ T cells in vitro, and these cells were more efficient in facilitating islet β cell destruction in vivo. Thus, our data provide an insight into the role of AID in T-B cell interaction. It is interesting that although B cells showed enhanced expression of costimulatory molecules CD80 and CD86, the counterpart receptors CD28 and CTLA-4 were not upregulated. It is not yet clear through what molecular mechanism the enhanced T-B interaction occurs in the AID–/–NOD mice. The autoaggressive phenotype in our AID–/–NOD mice was not related to any changes in the proportion and the suppressive function of B regulatory cells, as shown in a very recent report (23), in which the results obtained could be due to the different approach used for genetic targeting and a different environment. Foxp3+ Tregs were also not affected here by AID deficiency in our model system. It is possible that a third party, including dendritic cells, might be involved in the accelerated autoimmunity in the absence of AID. Further investigation is required to examine this possibility.
In summary, we have reported that AID ablation accelerated T1D development in NOD mice by loss of B cell tolerance, together with increased B cell activation, proliferation, and enhanced autoantibody production. This increased the ability to promote Th1 T cells in vitro and facilitated the diabetogenic T cells in islet β cell destruction in vivo. Importantly, maternally transmitted class-switched Igs do not affect diabetes development. Taken together, the finding from the current study adds knowledge to our understanding the role of AID in autoimmune diabetes and provides insight into the possible therapeutic targets for the prevention or treatment of T1D in the future.
Methods
Mice.
NOD/Caj mice were originally obtained from The Jackson Laboratory and have been maintained at Yale University for over 30 years. AID–/–NOD mice were generated by backcrossing AID–/–B6 mice (a gift from Tasuku Honjo, Kyoto University, Kyoto, Japan) to the NOD/Caj genetic background for more than 10 generations. The purity of the NOD genetic background was examined by whole-genome single nucleotide polymorphism scan (DartMouse). BDC2.5 NOD, Rag2–/–NOD, and NOD.scid mice were all originally obtained from The Jackson Laboratory. All the mice were kept in specific pathogen–free conditions in a 12-hour-dark/light cycle and housed in individually ventilated filter cages with autoclaved food in the Yale University animal facility.
Antibodies and reagents.
All the fluorochrome-conjugated mAbs used in this study were purchased from Biolegend except anti-Foxp3 (150D), which was from eBioscience. They include anti-B220 (RA3-6B2), anti-BrdU (3D4), anti-CD4 (GK1.5), anti-CD8 (5.3-6.7), anti-CD19 (6D5), anti-25 (PC61), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-CD69 (H1.2F3), anti-CD80 (16-10A1), anti-CD86 (GL-1), anti-CD95 (SA367H8), anti-CXCR5 (L138D7), anti-ICOS (C398.4A), anti-IFN-γ (XMG1.2), anti-Foxp3 (150D), anti-PD-1 (29F.1A12), anti-T-bet (4B10), and anti-TCRβ (H57-597). PE-conjugated IGRP-tetramer (H-2DK) was obtained from the NIH Tetramer Core facility in Emory University. Alkaline phosphatase–conjugated (AP-conjugated) goat anti-mouse IgG, IgM, IgA, and IgH+L antibodies for ELISA were purchased from Southern Biotechnology. Hybridoma supernatants containing mAbs, used for cell purification or stimulation, were provided by the late Charles Janeway Jr. (Yale University). Magnetic beads conjugated with goat anti-mouse IgG, goat anti-mouse IgM, or goat anti-rat IgG were purchased from QIAGEN. The BrdU kit was purchased from MilliporeSigma. The B cell purification kit was purchased from STEMCELL Technologies. RPMI-1640 medium and heat-inactivated FCS were purchased from Invitrogen and Gemini, respectively.
Natural history of diabetes development.
AID–/–NOD and AID+/+NOD littermates from the intercross of AID+/–NOD mice were observed for spontaneous diabetes development. To study the effect of maternal class-switched Igs on T1D development, we also bred female AID+/–NOD mice with male AID–/–NOD mice or AID–/–NOD female mice with AID+/–NOD male mice, respectively. All breeders were >7 weeks old and nondiabetic. Diabetes development was also observed in the offspring. Mice were tested for glycosuria weekly, and diabetes was confirmed by blood glucose ≥250 mg/dl (13.9 mmol/l). For some mechanistic studies, we bred AID+/–NOD female mice with AID+/–NOD male mice to generate AID+/–NOD and AID+/+NOD mice for experiments. For later studies, we also bred AID+/–NOD female mice with AID–/–NOD male mice to generate AID–/–NOD and AID+/–NOD mice for experiments. As there was no difference in either diabetes development or phenotype between AID+/+NOD and AID+/–NOD mice, in some experiments, we used both genotypes as AID–/– controls.
Histology of pancreatic tissues.
Pancreatic tissues were fixed with 10% buffered formalin, embedded in paraffin, sectioned at a thickness of 5 μm, and then stained with hematoxylin and eosin and examined under light microscopy. Insulitis was scored under light microscopy with the following grading scale: 0, no infiltration; 1, <25% infiltration of the islet; 2, 25%–75% infiltration of the islet; and 3, >75% islet infiltration. More than 100 islets were scored for insulitis in each group (n = 8–9 mice/group).
Isolation of infiltrated immune cells from pancreatic islets.
Pancreata were removed from the mice, placed in 1 mg/ml collagenase solution, and digested at 37°C in a water bath for 8–10 minutes. Islets were handpicked under a microscope after the enzymatic digestion. The islet-infiltrating cells were harvested from the handpicked islets after further dissociation with Cell Dissociation Solution Non-Enzymatic (MilliporeSigma), followed by filtering using nylon membranes. The infiltrated cells were then stained with different antibodies for flow cytometric analysis.
Serum Ig detection.
Serum samples were obtained from 2-month-old female AID–/–NOD mice and AID+/+NOD mice from AID+/–NOD intercross breeding. Serum samples were also collected from female AID+/–NOD and AID–/–NOD mice, from AID+/–NOD female × AID–/–NOD male breeding, on day 30 after birth and every 10 days thereafter. Serum Igs were quantitated by ELISA. Briefly, the plates were coated with goat anti-mouse IgH+L (10 μg/ml, SouthernBiotech) antibody overnight. After washing and blocking the plates, diluted serum samples were added to the plates and assessed against known concentrations of the standard. The plates were incubated at room temperature for 2 hours followed by adding AP-conjugated goat anti-mouse IgG, IgM, or IgA. The detection of different Ig isotypes was determined by enzymatic reaction of AP and phosphate substrate (MilliporeSigma). The plates were read with a microplate spectrophotometer (Perkin Elmer) under OD at 405 nm, and the concentrations of different isotypes were determined by standard curves for each isotype.
Serum IAA detection.
Serum samples from 2-month-old female AID–/–NOD, AID+/+NOD, and AID+/–NOD mice were tested for anti-insulin antibody. The plates were coated with human insulin (10 μg/ml, Lilly) overnight, and serum samples diluted at 1:100 were tested for anti-insulin total Ig, IgM, and IgG by ELISA, with AP-conjugated goat anti-mouse IgH+L, IgM, IgG, and phosphate substrate. The plates were read with a microplate spectrophotometer at OD 405nm.
Intracellular cytokine assay.
Splenocytes (5 × 106 cells/ml) from 8-week-old female AID–/–NOD and AID+/+NOD mice were stimulated with PMA (50 ng/ml, MilliporeSigma) and ionomycin (500 ng/ml, MilliporeSigma) in the presence of Golgi-plug (1 μl/ml, eBioscience), in complete medium, for 4 hours. Intracellular cytokine staining was performed according to the protocol provided with Intracellular Fixation & Permeabilization Buffer kits from eBioscience. The cells were stained with surface markers before fixation and permeabilization. After blocking with Fc-blocking antibody (2.4G2), the cells were stained with the recommended amount of fluorochrome-labeled antibodies to detect intracellular cytokines.
Cell purification.
CD4+ or CD8+ T cells were purified from the spleens of 8-week-old female AID–/–NOD and AID+/+NOD mice by negative selection with magnetic beads according to the manufacturer’s instructions (QIAGEN). The purity was routinely 90%–95%, as verified by flow cytometry. Splenic B cells were purified with the B cell isolation kit (STEMCELL Technology) or sorted by flow cytometry for B220+CD19+ cells.
B-T cell interaction assay.
B cell isolation kit–purified splenic B cells (2 × 106 cells/well) from 8-week-old female AID–/–NOD and AID+/+NOD mice were cocultured with bead-purified AID+/+NOD splenic CD4+ or CD8+ T cells (T/B = 1:1) in a 12-well plate with a low dose of anti-CD3 antibody (1:300 dilution of 2C11 hybridoma supernatant) for 3 days. Cells were then stained for intracellular IFN-γ and T-bet.
BrdU incorporation assay.
8-week-old female AID–/–NOD and AID+/+NOD mice were injected i.p. with BrdU (10 mg/ml, 200 μl/mice, Biolegend) and sacrificed 24 hours later. Immune cells isolated from spleens, PLNs, and thymi were stained with fluorochrome-labeled anti-BrdU and analyzed by flow cytometry.
Proliferation assay.
Flow cytometrically sorted splenic B cells (stained for B220+CD19+) from 8-week-old female AID–/–NOD and AID+/+NOD mice were seeded at 5 × 104 cells/well and cultured with polyclonal goat anti-mouse IgM (3 μg/ml, MilliporeSigma) and different concentrations of monoclonal rat anti-mouse CD40 (FGK4.5, BioXcell). 3H-thymidine was added to the coculture during the last 16 hours of the 96-hour culture. Proliferation was determined by 3H-thymidine incorporation using a β counter and presented as CPM. Proliferation was also performed by CFSE dilution assay. Purified splenic BDC2.5 CD4+ T cells were labeled with CFSE and cocultured with purified splenic B cells from AID–/–NOD and AID+/+NOD mice in the presence of antigenic peptide (10 ng/ml). Proliferation of BDC2.5 CD4+ T cells was determined by percentage of CFSE dilution (= number of round of proliferation).
Adoptive transfer experiments.
Purified splenic BDC2.5 CD4+ T cells and B cells were used in the adoptive transfers. Three million BDC2.5 CD4+ T cells and 5 × 106 B cells from either 8-week-old female AID+/+NOD or AID–/–NOD mice were injected i.v. into 4- to 5-week-old and sex-matched Rag2–/–NOD or NOD.scid mice. In one set of adoptive transfer experiments, Rag–/–NOD mice were first infused with serum, at different dilutions from AID–/–NOD mice, 24 hours prior to transferring BDC2.5 CD4+ T cells (3 × 106/mouse). All the recipients were screened for diabetes development by testing for glycosuria daily, and the experiments were terminated 20–32 days after the cell transfer unless the mice developed diabetes, which was confirmed by blood glucose ≥250 mg/dl (13.9 mmol/l).
Statistics.
Statistical analysis was performed using GraphPad Prism software version 6.0. Diabetes incidence was compared using the Gehan-Breslow-Wilcoxon test. In vitro assays were analyzed with the 2-tailed Student’s t test, multiple t tests with FDR or Bonferroni correction, or ANOVA. P < 0.05 was considered significant.
Study approval.
The use of the animals and the procedures applied in this study were reviewed and approved by the Institutional Animal Care and Use Committee of Yale University.
Author contributions
LW conceived the project. LW, QT, NT, and FSW designed the experiments. QT, NT, YL, JP, and SP performed the experiments. QT, NT, YL, JP, FSW, and LW analyzed the data. QT, NT, FSW, and LW wrote the manuscript. ZZ provided scientific input.
Supplementary Material
Acknowledgments
We thank Xiao-jun Zhang for taking care of the mice used in this study and Jian Peng for genotyping all the mice used in this study. This work was supported by grants from the NIH (DK092882, DK100500, and P30 DK945735 to LW), the American Diabetes Association (1-14-BS-222 to LW), the Chinese Student and Scholar Council (2011637093 to QT), and the Shanghai Pujiang Program (16PJ1408300 and National Natural Science Foundation of China 81600627 to QT). JP is a JDRF postdoctoral fellow (PDF-2016-197).
Version 1. 01/11/2018
Electronic publication
Version 2. 01/11/2018
The order of author affiliations was updated.
Version 3. 01/11/2018
Zhiguang Zhou's affiliation address has been updated.
Version 4. 01/16/2018
The first affiliation address was updated.
Funding Statement
Fundings from NIH and ADA to Li Wen.Fundings from Chinese Student and Scholar Council and Shanghai Pujiang Program to Qiyuan Tan
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: JCI Insight. 2018;3(1):e95882. https://doi.org/10.1172/jci.insight.95882.
Contributor Information
Qiyuan Tan, Email: qiyuantan0602@sina.com.
Ningwen Tai, Email: ningwen.tai@yale.edu.
James Pearson, Email: james.pearson@yale.edu.
Sean Pennetti, Email: Sean.Pennetti@quinnipiac.edu.
Zhiguang Zhou, Email: zhouzg@hotmail.com.
F. Susan Wong, Email: wongfs@cardiff.ac.uk.
Li Wen, Email: li.wen@yale.edu.
References
- 1.Jahromi MM, Eisenbarth GS. Cellular and molecular pathogenesis of type 1A diabetes. Cell Mol Life Sci. 2007;64(7-8):865–872. doi: 10.1007/s00018-007-6469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161(8):3912–3918. [PubMed] [Google Scholar]
- 3.Wong FS, et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes. 2004;53(10):2581–2587. doi: 10.2337/diabetes.53.10.2581. [DOI] [PubMed] [Google Scholar]
- 4.Hu CY, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117(12):3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiu Y, et al. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol. 2008;180(5):2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 6.Serreze DV, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med. 1996;184(5):2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endo Y, et al. Expression of activation-induced cytidine deaminase in human hepatocytes via NF-kappaB signaling. Oncogene. 2007;26(38):5587–5595. doi: 10.1038/sj.onc.1210344. [DOI] [PubMed] [Google Scholar]
- 8.Pescovitz MD, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong FS, Hu C, Xiang Y, Wen L. To B or not to B--pathogenic and regulatory B cells in autoimmune diabetes. Curr Opin Immunol. 2010;22(6):723–731. doi: 10.1016/j.coi.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Menard L, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121(9):3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Guo L, Tian J, Zheng B, Han S. Deficiency in activation-induced cytidine deaminase promotes systemic autoimmunity in lpr mice on a C57BL/6 background. Clin Exp Immunol. 2010;159(2):169–175. doi: 10.1111/j.1365-2249.2009.04058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham P, Bransteitter R, Petruska J, Goodman MF. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424(6944):103–107. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri J, Khuong C, Alt FW. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature. 2004;430(7003):992–998. doi: 10.1038/nature02821. [DOI] [PubMed] [Google Scholar]
- 14.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102(5):553–563. doi: 10.1016/S0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 15.Zaheen A, Boulianne B, Parsa JY, Ramachandran S, Gommerman JL, Martin A. AID constrains germinal center size by rendering B cells susceptible to apoptosis. Blood. 2009;114(3):547–554. doi: 10.1182/blood-2009-03-211763. [DOI] [PubMed] [Google Scholar]
- 16.Kuraoka M, et al. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proc Natl Acad Sci USA. 2011;108(28):11560–11565. doi: 10.1073/pnas.1102571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2) Cell. 2000;102(5):565–575. doi: 10.1016/S0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 18.Quartier P, et al. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to activation-induced cytidine deaminase deficiency. Clin Immunol. 2004;110(1):22–29. doi: 10.1016/j.clim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Durandy A, Revy P, Imai K, Fischer A. Hyper-immunoglobulin M syndromes caused by intrinsic B-lymphocyte defects. Immunol Rev. 2005;203:67–79. doi: 10.1111/j.0105-2896.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 20.Hase K, Takahashi D, Ebisawa M, Kawano S, Itoh K, Ohno H. Activation-induced cytidine deaminase deficiency causes organ-specific autoimmune disease. PLoS ONE. 2008;3(8):e3033. doi: 10.1371/journal.pone.0003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, et al. Critical role of activation induced cytidine deaminase in experimental autoimmune encephalomyelitis. Autoimmunity. 2013;46(2):157–167. doi: 10.3109/08916934.2012.750301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang C, Zhao ML, Waters KM, Diaz M. Activation-induced deaminase contributes to the antibody-independent role of B cells in the development of autoimmunity. Autoimmunity. 2012;45(6):440–448. doi: 10.3109/08916934.2012.682668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratiu JJ, et al. Genetic and small molecule disruption of the AID/RAD51 axis similarly protects nonobese diabetic mice from type 1 diabetes through expansion of regulatory B lymphocytes. J Immunol. 2017;198(11):4255–4267. doi: 10.4049/jimmunol.1700024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van de Perre P. Transfer of antibody via mother’s milk. Vaccine. 2003;21(24):3374–3376. doi: 10.1016/S0264-410X(03)00336-0. [DOI] [PubMed] [Google Scholar]
- 25.Delong T, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351(6274):711–714. doi: 10.1126/science.aad2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiles TA, et al. An insulin-IAPP hybrid peptide is an endogenous antigen for CD4 T cells in the non-obese diabetic mouse. J Autoimmun. 2017;78:11–18. doi: 10.1016/j.jaut.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendall PL, Moore DJ, Hulbert C, Hoek KL, Khan WN, Thomas JW. Reduced diabetes in btk-deficient nonobese diabetic mice and restoration of diabetes with provision of an anti-insulin IgH chain transgene. J Immunol. 2009;183(10):6403–6412. doi: 10.4049/jimmunol.0900367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161(3):1163–1168. [PubMed] [Google Scholar]
- 29.Rose NR. Predictors of autoimmune disease: autoantibodies and beyond. Autoimmunity. 2008;41(6):419–428. doi: 10.1080/08916930802031686. [DOI] [PubMed] [Google Scholar]
- 30.Eggert M, Zettl UK, Neeck G. Autoantibodies in autoimmune diseases. Curr Pharm Des. 2010;16(14):1634–1643. doi: 10.2174/138161210791164144. [DOI] [PubMed] [Google Scholar]
- 31.Baekkeskov S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 32.Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994;13(5):505–514. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- 33.Tiittanen M, Knip M, Vaarala O. Anti-insulin activity in IgG-fractions from children with newly-diagnosed type 1 diabetes and negative for insulin autoantibodies. Autoimmunity. 2004;37(1):45–49. doi: 10.1080/0887044032000158929. [DOI] [PubMed] [Google Scholar]
- 34.Su K, et al. Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol. 2007;178(5):3272–3280. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Catalán D, et al. B cells from rheumatoid arthritis patients show important alterations in the expression of CD86 and FcgammaRIIb, which are modulated by anti-tumor necrosis factor therapy. Arthritis Res Ther. 2010;12(2):R68. doi: 10.1186/ar2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greeley SA, et al. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat Med. 2002;8(4):399–402. doi: 10.1038/nm0402-399. [DOI] [PubMed] [Google Scholar]
- 37.Wolniak KL, Shinall SM, Waldschmidt TJ. The germinal center response. Crit Rev Immunol. 2004;24(1):39–65. doi: 10.1615/CritRevImmunol.v24.i1.20. [DOI] [PubMed] [Google Scholar]
- 38.Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J Exp Med. 1995;182(6):1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaheen A, Martin A. Activation-induced cytidine deaminase and aberrant germinal center selection in the development of humoral autoimmunities. Am J Pathol. 2011;178(2):462–471. doi: 10.1016/j.ajpath.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasqualucci L, et al. Expression of the AID protein in normal and neoplastic B cells. Blood. 2004;104(10):3318–3325. doi: 10.1182/blood-2004-04-1558. [DOI] [PubMed] [Google Scholar]
- 41.Qin H, et al. Activation-induced cytidine deaminase expression in CD4+ T cells is associated with a unique IL-10-producing subset that increases with age. PLoS One. 2011;6(12):e29141. doi: 10.1371/journal.pone.0029141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.