Abstract
MicroRNA (miRNA) regulation of gene expression is becoming an increasingly recognized mechanism by which host immune responses are governed following microbial infection. miRNAs are short, non-coding RNAs that repress translation of target genes, and have been implicated in a number of activities that modulate host immune responses, including the regulation of immune cell proliferation, survival, expansion, differentiation, migration, polarization, and effector function. This review highlights several examples in which mammalian-encoded miR-155 influences immune responses following viral infection of the CNS.
Keywords: microRNAs, miR-155, neurotropic viruses, neuropathogenesis, neuroinflammation
2. Introduction
2.1 MicroRNAs (miRNAs)
miRNAs are short, non-coding RNA molecules that function to regulate gene expression at the post-transcriptional level by binding complimentary 3′ untranslated region (3′ UTR) sequences of target mRNAs, thereby repressing gene expression (1). miRNAs were first reported in the 1990s as regulatory sequences involved in C. elegans development (2); however, they have since been further characterized as gene-repression elements that affect gene-expression profiles in more than 100 animal species (3).
The majority of miRNAs are encoded within intron regions of genomes, and are transcribed by RNA polymerase II into primary transcripts referred to as pri-miRNAs. In the canonical miRNA pathway, pri-miRNAs are cleaved by an RNAase III-Drosha complex in order to yield pre-miRNAs. Alternatively, miRNA transcripts called mirtrons are produced independently of the RNAase III-Drosha complex (4). Pre-miRNAs and mirtrons are transported from the nucleus by Exportin 5 into the cytoplasm and processed by Dicer into short (~22 nucleotides), double-stranded miRNA/miRNA molecules that subsequently form an RNA-induced silencing complex (RISC) with Argonaute and other proteins. In the RISC complex, one strand of the miRNA duplex functions to bind complementary sequences in the 3′UTR and thereby repress target genes, while the other strand is degraded (5). miRNAs are regulated in part by RNA-binding proteins that help determine the context in which miRNAs are available for target-gene repression (6).
Because miRNAs have been shown to target many important signaling proteins and transcription factors that govern immune processes and differentiation (7, 8), it is not surprising that these molecules have important roles during immune responses to microbial infections, including those that affect the CNS. Infection of the CNS results in significant changes in miRNA expression profiles, many of which facilitate various aspects of immune processes (9, 10). It should be noted that there is a growing body of literature that discusses miRNAs encoded by viruses that influence viral pathogenesis; however, they are beyond the scope of this review. One miRNA that has gained considerable attention in recent years is mammalian-encoded miR-155, which numerous reports have implicated in regulating immune responses, including to neurotropic viruses. Here we provide a discussion of several examples in which miR-155 regulates neuroinflammation during viral infection of the CNS.
2.2. miR-155
While miR-155 was originally identified as an oncogene in chicken lymphomas (11), subsequent work has revealed that it has myriad roles in regulating immune responses. miR-155 is overexpressed in some mammalian hematopoietic cancers and is expressed by and functions within a variety of activated immune cell types, including B cells, macrophages, various T cell populations, NK cells, and dendritic cells (12–16) to regulate cytokines, chemokines, and transcription factors important for mounting an optimal immune response. For example, miR-155 expression leads to increased production of IFN-γ and diminished expression of IL-2 by T cells (17–19), augments IFN-γ-dependent CD4+ and CD8+ T cell responses to tumors (20), contributes to the development of T regulatory cells (21, 22), and alters the CD8+ T cell memory:effector ratio by skewing CD8+ T cells toward an effector memory phenotype (23). Within immune cells, miR-155 represses a variety of immunoregulatory proteins that include signaling molecules such as SHIP1 (24) and SOCS1 (25), as well as transcriptional regulators such as Jarid2 (26), Ets1 (27, 28), PU.1 (29) and Fosl2 (30). Importantly, Moore et al. (31) showed that miR-155 drives myeloid cells toward an M1, or proinflammatory, phenotype. Several studies suggests that expression of miR-155 by microglia is important in regulating expression of proinflammatory genes that subsequently influence neuroinflammation, primarily though the inhibition of SOCS1 and genes involved in microglial polarization such as IL-13R, SMAD2, and CEBPβ (10, 32–34). These and other studies have demonstrated that miR-155 is an important regulator of immune cell development and function.
There is increasing evidence that miR-155 influences neuroinflammatory diseases such as the human demyelinating disease multiple sclerosis. miR-155 was initially shown to influence neuroinflammation through the induction of myelin-reactive Th17 cells following induction of experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis (MS) (35, 36). Alejandro et al. (37) discovered that miR-155 is upregulated in neurovascular units in active MS lesions compared to normal-appearing white matter in MS patients. In addition, the group used the EAE model to show that miR-155 expression is dramatically increased in mice with hind-limb paralysis during the recovery phase, and that miR-155 regulates blood-brain-barrier (BBB) function. The latter finding is consistent with a study by Lopez-Ramirez (37) showing that miR-155 negatively regulates blood-brain-barrier permeability by targeting the cell-cell complex molecules annexin-2 and claudin1, as well as the adhesion components DOCK-1 and syntenin-1. In a recent study, Cerutti et al. (38) also demonstrated that miR-155 regulates blood-brain barrier function by targeting adhesion molecules VCAM1 and ICAM1, thereby affecting monocyte and T cell adhesion to the brain endothelium. Roles for miR-155 during neuroinflammation have also been demonstrated in models of Parkinson’s Disease (39), Alzheimer’s Disease (40), alcohol-induced neuroinflammation (41), and amyotrophic lateral sclerosis (ALS) (42).
Not surprisingly, multiple reports identify miR-155 as important in mediating host responses to microbial diseases (43), including viral infections with members of the Herpesviridae, Coronaviridae, Arenaviridae, Flaviviridae, and Retroviridae families (discussed further below) (44–55). Recently, miR-155 has been shown to tailor immune responses in models of viral-induced neurologic disease, and numerous mechanisms by which miR-155 controls immune responses following viral infection have been identified. For example, multiple studies have demonstrated that T cell responses are impaired in the absence of miR-155 during infection with certain neurotropic viruses (21, 47–51, 55). Below, we highlight several examples in which miR-155 influences inflammatory responses following viral infection of the CNS (Table 1).
Table 1.
Mechanisms of miR-155-mediated regulation of neuroinflammation.
| Virus | Mechanisms of miR-155-mediated regulation of neuroinflammation |
|---|---|
| Herpes simplex virus | Modulates disease severity and viral load in CNS Regulates accumulation of CD8+ T cells Affects number of CD8+ T cells that produce TNF-α and/or IFN-γ Necessary for optimal expression of homing molecules VLA-4 and CD44 Important for Th1 and Th17 cell accumulation in lymph nodes and spleens Promotes CD4+ T cell proliferation Regulates expression of IL-1β, IL-6, IFN-γ, and IL-16 Regulates levels of SHIP1 and IFN-γRα in activated CD4+ T cells |
| Cytomegalovirus | Critical for expansion of effector NK cells Regulates survival of NK cells Necessary for optimal memory NK cell generation Targets Noxa and SOCS1 |
| Lymphocytic choriomeningitis virus | Necessary for optimal CD4+ and CD8+ T cell responses Necessary for optimal CD8+ T cell proliferation Necessary for optimal CD8+ T cell survival by interfering with PI3/Akt pathway Regulates cytokine signaling by targeting SOCS1 Targets SOCS1 to regulate expansion of virus-specific NK cells Targets SOCS1 to regulate maintenance of virus-specific CD8+ T cells during chronic infection |
| Japanese encephalitis virus | Mediates disease severity Regulates expression of IFN-β, TNF-α, IL-10, MCP-1, IL-6, IRF-3/7, IRF-8, CFH, and TBK-1 Negatively regulates SHIP1 expression Contributes to JEV-induced microglial activation and neuronal death |
| Human immunodeficiency virus | Regulates HIV post-entry infectivity in macrophages Decreases expression of ADAM10, Nup153, and LEDGF/p57 Targets TRIM32 to decrease NF-κB activation Decreases expression of DC-SIGN; blocks gp120 binding to dendritic cells |
| Murine hepatitis virus | Regulates disease severity and virus clearance Necessary for optimal CD4+ and CD8+ T cell accumulation Necessary for optimal T cell production of IFN-γ Regulates CXCR3 expression on CD8+ T cells Necessary for optimal CTL activity Necessary for optimal CD4+ and CD8+ T cell migration to CNS |
3. miR-155 and neuroinflammation following CNS viral infection
3.1 Herpes simplex virus (HSV)
HSV infections generally result in surface lesions on skin, mucosa, and eyes. After primary infection, HSV establishes a life-long latent infection in neuronal tissues, although latent virus is periodically reactivated. While this process is not thoroughly defined, factors such as fever, UV exposure, increased viral load, stress, and host genetics have been implicated in HSV reactivation (56). Occasionally, HSV spreads to the brain and causes a rare but life-threatening condition called herpes simplex encephalitis (HSE) (57). In adults, HSE is generally a result of reactivated infection with HSV-1 and results in focal hemorrhagic necrosis in the temporal lobe, whereas, in infants, HSE is more often the result of primary HSV-2 infection and manifests as diffuse brain involvement or multifocal lesions, often without hemorrhage (58). Lesions in the brain are believed to be caused by both the infection and the immune response to the infection (59). Ocular infection of susceptible mice results in a T cell-mediated lesion in the cornea that can lead to encephalitis, and provides a useful model for studying the neuroinflammatory response to HSV infection (60).
Bhela et al. (48) showed that miR-155−/− mice were significantly more susceptible to HSE than WT animals following ocular HSV-1 infection, and that this was concomitant with higher viral titers in brains, but not corneas. In addition, the degree of astrocytosis in WT animals was higher than in miR-155−/− mice. Numbers of virus-specific CD8+ T cells in draining lymph nodes were reduced in miR-155−/− mice compared to WT mice (48), and transferring HSV-immune CD8+ T cells from HSV-specific TCR transgenic mice into miR-155−/− mice rescued the knockout animals from lethal herpetic encephalitis, indicating that the increased disease susceptibility of miR-155−/− mice was due to impaired CD8+ T cell responses (48). The number of CD8+ T cells from HSV-infected miR-155−/− mice that produced TNF-α and/or IFN-γ in response to HSV-1 infection was reduced compared to CD8+ T cells from WT mice. Furthermore, expression levels of homing molecules VLA-4 and CD44 on CD8+ T cells from HSV-1-infected miR-155−/− mice were significantly reduced compared to cells from WT mice.
In a subsequent study, Bhela et al. (47) showed that after ocular infection with HSV-1, CD4+ T cell accumulation in corneas was significantly decreased in miR-155−/− mice compared to WT mice, which corresponded with reduced lesion severity in either miR-155−/− mice or WT mice treated subconjunctively with a miR-155 antagonist (antagomir-155). In addition, HSV-infected miR-155−/− mice exhibited decreased frequencies of Th1 and Th17 cells in lesions and lymphoid organs compared to infected WT mice, and this was likely due to miR-155-mediated promotion of CD4+ T cell proliferation. Local antagomir-155 treatment after HSV-1 infection resulted in decreased CD4+ T cell and neutrophil infiltration to corneas, in addition to reduced expression of IL-1β, IL-6, IFN-γ, and IL-16. Further, the group showed that expression levels of SHIP1 and IFN-γRα, which regulate IFN-γ expression and Th1 differentiation, respectively, were higher in activated CD4+ T cells from miR-155−/− mice compared to cells from WT mice during HSV-1 infection. These studies demonstrate an important role for miR-155-mediated regulation of T cell responses during HSV-1 infection.
3.2 Cytomegalovirus (CMV)
CMV is a common virus that infects persons of all ages. It is generally asymptomatic, but can cause disease in people with weakened immune systems and fetuses infected with the virus in utero. Infants born after congenital CMV infection can have neurological defects, including microcephaly, cerebral palsy, ocular problems, seizures, hearing loss, and cognitive deficiencies (61–65). Intraperitoneal inoculation of newborn mice with murine CMV (MCMV) provides a model that recapitulates the major characteristics of human CMV infection with regard to route of neuroinvasion, neuropathology, and immunopathology (66). NK cells are important in controlling immune responses to viruses, including MCMV (67). After infection, NK cells produce cytokines and lytic molecules that help control viral replication (68, 69). NK cells that express the Ly49H receptor, which recognizes glycoproteins on MCMV virions, secrete perforin and granzymes to specifically kill virally infected cells (70, 71). In addition, they proliferate to produce large numbers of virus-specific effector NK cells, and a small population of memory NK cells remains after the infection is cleared (67, 72, 73).
Zawislak et al. (55) showed that NK cell populations in naïve miR-155−/− mice demonstrated a more mature phenotype than NK populations in WT mice, indicating that miR-155 regulates NK cell maturation. The group generated mixed-bone-marrow-chimeric mice by reconstituting irradiated mice with equal numbers of bone marrow cells from miR-155−/− (CD45.1) and WT (CD45.2) mice. The percentage of miR-155−/− NK cells in the peripheral blood was significantly lower than that of WT NK cells by 11 weeks post reconstitution, suggesting that NK cell homeostasis is impaired in the absence of miR-155. The group co-transferred WT and miR-155−/− NK cells into Rag2−/− × Il2rg−/− mice (deficient in NK, B, and T cells) and showed that while there were no differences in proliferation rate, miR-155−/− NK cells showed decreased survival compared to WT NK cells. Seven days post intraperitoneal (i.p.) MCMV infection of mixed-bone-marrow-chimeric mice, miR-155−/− NK cells were significantly reduced compared to WT NK cells, indicating that miR-155 is important for the expansion of effector NK cells during MCMV infection. In addition, upon adoptive transfer of equal numbers of purified WT or miR-155−/− Ly49H+ NK cells into Ly49H-deficient mice, the percentage of Ly49H+ NK cells in each group at defined points post-MCMV infection was determined. There were significantly higher numbers of long-lived, memory WT NK cells compared to miR-155−/− NK cells, and the ratio of WT:miR-155−/− NK cells increased steadily from 15 days post-infection (p.i.) to 45 days p.i., suggesting that miR-155 is necessary for robust memory NK cell generation in the context of MCMV infection. The group further showed that miR-155 targets Noxa and SOCS1 to regulate NK cell responses to MCMV infection. These findings suggest that miR-155 is important in shaping NK cell responses during MCMV infection.
3.3 Lymphocytic choriomeningitis virus (LCMV)
LCMV is a rodent-borne arenavirus that causes mild meningitis or, rarely, meningoencephalitis in adults and more severe complications in fetuses and neonates, including acute hydrocephalus, fetal demise, birth defects such as microcephaly and intracranial calcifications, and chorioretinitis. Infections with LCMV are not generally fatal; however, post-infection neurological damage is possible. As with many diseases, LCMV disease is caused by the combination of the viral infection and the host immune response to the virus (74).
Dudda et al. (50) infected WT or miR-155−/− mice intravenously (i.v.) with LCMV and demonstrated that the levels of total and virus-specific CD8+ T cells were drastically reduced in miR-155−/− mice compared to WT mice at the peak of response. The group used adoptive transfer experiments to show that during LCMV infection, miR-155−/− CD8+ T cells exhibited decreased proliferation and increased levels of the apoptosis-marker AnnexinV compared to cells from WT mice. SOCS1 transcript and protein levels were inversely related to the cellular levels of miR-155 in CD8+ T cells. Furthermore, stimulation of effector CD8+ T cells isolated 8 days after LCMV infection resulted in limited phosphorylation of STAT5 in miRNA-155-ablated cells compared to WT cells, demonstrating impaired cytokine signaling in response to IL-2, IL-7, or IL-15 stimulation. This study identified a novel role for miR-155 in regulating cytokine production through SOCS1.
Lind et al. (51) showed that after i.v. LCMV infection of WT or miR-155−/− mice, there was a dramatic reduction of virus-specific CD8+ T cells in splenocytes from miR-155−/− mice compared to WT mice. As multiple studies have demonstrated that miR-155 is required for optimal CD8+ T cell accumulation, the group hypothesized that the phenomenon could be due to interference in PI3K/Akt signaling. The PI3K/Akt signaling pathway is a highly conserved, tightly regulated signaling cascade that relays signals from activated cell-surface receptors, such as receptor tyrosine kinases and cytokine receptors, to downstream effectors that regulate transcription, protein synthesis, metabolism, growth, proliferation, and survival (75). Previous studies have shown that miR-155 targets multiple steps in this survival pathway (76). Lind et al. demonstrated that anti-CD3-stimulated CD8+ T cells isolated from WT mice demonstrated an increase in phosphorylated Akt Ser473; however, there was no increase in Akt Ser473 phosphorylation in stimulated CD8+ T cells from miR-155−/− mice (51), indicating that miR-155 mediates T cell survival by regulating the PI3/Akt signaling pathway.
Consistent with previous studies, Lu et al. (21) used bone marrow chimeras to show that miR-155 is necessary for optimal CD4+ and CD8+ T cell accumulation after i.v. infection with LCMV-Armstrong, which results in acute disease, and that the effect was independent of miR-155 repression of SOCS1; however, the group demonstrated that SOCS1 repression by miR-155 was necessary for the expansion of virus-specific NK cells, as well as the maintenance of virus-specific CD8+ T cell levels after i.p. infection with LCMV Clone 13, which results in chronic infection. These results suggest that miR-155-mediated regulation of T cell expansion is context-specific. Taken together, these studies indicate that miR-155 influences multiple aspects of LCMV-mediated neuroinflammation.
3.4 Japanese encephalitis virus (JEV)
JEV is a mosquito-borne Flavivirus that targets the CNS and causes encephalitis, with the degree of virus-mediated neuroinflammation inversely correlated with positive clinical outcome. Japanese encephalitis has a high mortality rate (up to 30%) and is associated with moderate to severe post-infection sequelae in the majority of patients that survive the disease, including permanent cognitive deficits, behavioral changes, and neurological problems such as paralysis, recurrent seizures, and aphasia (77, 78).
Work by Pareek et al. (79) assessed the effects of miR-155 on JEV-associated inflammation in vitro. The authors overexpressed miR-155 in an immortalized microglial cell line, CHME3, and showed that concomitant with reduced JEV replication, expression levels of IFN-β, interferon-stimulated genes, TNF-α, and IL-10 were decreased in cells overexpressing miR-155. In addition, interferon regulatory factor 8 (IRF8), complement factor H (CFH), and JEV-induced NF-κB downstream gene expression was attenuated in cells overexpressing miR-155. These findings suggest that miR-155 is important for regulating levels of cytokines and other pro-inflammatory molecules in response to JEV infection in microglia.
Thounaojam et al. (80) reported that miR-155 is upregulated within the CNS of mice infected i.v. with JEV, and subsequent in vitro experiments demonstrated that microglial cells are a source of miR-155. Locked nucleic acid (LNA)-mediated repression of miR-155 minimized inflammatory responses to JEV infection by promoting increased SHIP1 expression and thereby decreasing downstream expression of the inflammatory cytokines and chemokines IFN-β, TNF-α, MCP-1, and IL-6 in mouse brains. In addition, repressing miR-155 led to the downregulation of TBK-1, IRF-3/7, and NF-κB phosphorylation. Furthermore, the group showed that inhibition of miR-155 resulted in decreased JEV-induced microglial activation and neuronal death, as well as improved survival and clinical symptoms, indicating that miR-155 may be a therapeutic target for virus-mediated inflammation. It is unclear why this study yielded seemingly different results from the previous study with regard to cytokine production; however, it is possible that the parameters of their model systems were responsible for miR-155 having different effects under varying conditions. For example, the study by Pareek et al. employed CHEM3 cells in vitro, while Thounaojam et al. studied inflammatory responses in vivo in the CNS of mice. Because miR-155 is known to mediate inflammatory mechanisms in a variety of cell types, it is likely that its net effect on JEV-induced inflammation is context dependent.
3.5 Human immunodeficiency virus type-1 (HIV-1)
HIV-1 enters the CNS shortly after infection and causes demonstrable CNS pathology within a few months in untreated individuals, most often manifested as minor neurocognitive or neuromotor impairment as assessed by neurological testing (81–84). Autopsy studies of recently infected and presymptomatic HIV-positive individuals demonstrate encephalopathic changes within the brain, characterized by subtle gliosis, perivascular lymphocytic cuffing, microglial activation, perivascular macrophage accumulation, and multinucleated giant cells (85–87). The spectrum of HIV-1-induced CNS disease, referred to as HIV-associated neurocognitive disorder (HAND), includes encephalitis, metabolic encephalopathy, motor disorders, neurocognitive dysfunction, and dementia [reviewed in (88)]. HAND is thought to be the result of persistent HIV-1 infection of the CNS with the release of toxic viral products, including gp120, Tat, and VPR, in addition to immune activation of CNS-resident microglia and macrophages. Rarely, HIV-1 infected individuals may also experience T-cell-mediated immune reconstitution inflammatory syndrome (IRIS) in the CNS following initiation of antiretroviral therapy. Although the incidence of HIV-associated dementia has decreased with the introduction of effective anti-retroviral therapy, the majority of HIV-1-infected individuals will experience clinically evident neurologic dysfunction during the course of the illness (81, 89).
Within the CNS, HIV-1 primarily infects microglial cells and perivascular macrophages, leading to the establishment of a reservoir for persistent virus production. Recent studies have demonstrated that miR-155 alters HIV-1 replication by targeting both host and viral factors involved in HIV pathogenesis. Swaminathan et al. (90) showed that miR-155 is upregulated in macrophages in response to TLR3 stimulation, concomitant with a post-entry block to infection with HIV-1, as measured by increased late reverse transcription products and decreased integrated proviral DNA. The miR-155-mediated HIV-1 post-entry block was associated with decreased expression of factors involved in trafficking and/or nuclear import of HIV-1 pre-integration complexes, such as ADAM10, Nup153, and LEDGF/p75, all of which are targets of miR-155.
Ruelas et al. (91) showed that miR-155 contributes to transcriptional silencing of HIV-1 in T cells by targeting host factor TRIM32, an E3 ubiquitin ligase that activates NF-kB and drives HIV-1 transcription and associated inflammatory responses. Recent reports suggest that miR-155 is able to interfere with HIV-1 spread within the CNS by regulating expression of DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) on monocytes and monocyte-derived dendritic cells. DC-SIGN has been shown in a number of studies to be involved in HIV-1 spread through a mechanism known as trans-infection (92), and is also expressed on both macrophages and microglial cells (93–95). Martinez-Nunez et al. (53) demonstrated that overexpression of miR-155 leads to the downregulation of cell-membrane levels of DC-SIGN by repressing the transcription factor PU.1, thereby decreasing the ability of the HIV-1 surface glycoprotein gp120 to bind the surface of dendritic cells. This study is supported by research by Napuri, et al. (54), who showed that cocaine-mediated miR-155 repression resulted in increased infectivity in monocyte-derived dendritic cells, concomitant with increased expression of DC-SIGN.
In addition to regulating cellular factors involved in HIV-1-mediated inflammatory processes, miR-155 has a direct impact on HIV-1 infection of CNS target cells. Whisnant and colleagues (96) reported that miR-155 binds to the HIV-1 genome in the region of the viral infectivity factor (vif) gene and is capable of decreasing viral gene expression in experimental systems. Beyond the mechanisms described above, it is likely that miR-155 controls HIV neuroinflammation via other pathways. As discussed previously, miR-155 plays a role in the polarization of macrophages and microglial cells to the classically proinflammatory or M1 phenotype (31). M1-polarized macrophages are refractory to HIV-1 infection (97) and are associated with decreased viral production (98), so it stands to reason that miR-155 activity will prove to be important in controlling multiple aspects of HIV-1 infectivity and innate immune responses to the virus.
3.7 Mouse Hepatitis Virus
Intracerebral inoculation of the neurotropic JHM strain of mouse hepatitis virus (JHMV) provides a model for examining host immune responses that control viral replication and modulate neuroinflammation within distinct cell lineages present in the brain (99, 100). CD4+ and CD8+ T cell infiltration controls viral replication during acute infection (101–103); however, virus clearance is incomplete, and animals that survive the acute disease develop an immune-mediated demyelinating disease that is governed by both T cells and macrophages (104–110).
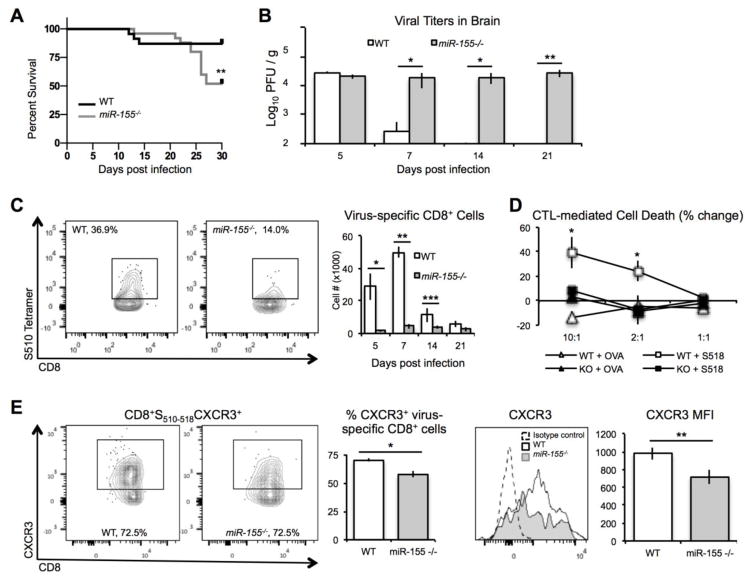
Dickey et al. (49) recently reported that genetic silencing of miR-155 in JHMV-infected mice results in increased disease severity concomitant with increased mortality and decreased ability to clear virus (Figures 1A and B). CNS infiltration of both total and virus-specific CD4+ T cells and CD8+ T cells of infected miR-155−/− mice was reduced compared to WT mice (Figure 1C). T cell antiviral function was also dramatically impaired in miR-155−/− mice infected i.p. with MHV. IFN-γ secretion by CD4+ and CD8+ T cells in response to viral peptides was significantly reduced in infected miR-155−/− mice compared to infected control animals (49). In addition, CTL activity was diminished in miR-155−/− CD8+ T cells compared to those WT cells, arguing that miR-155 has a role in influencing antiviral T cell responses (Figure 1D) (49). Adoptive transfer experiments revealed that virus-specific CD4+ and CD8+ T cells from miR-155−/− mice exhibited impaired migration to the CNS of JHMV-infected RAG-1−/− mice. Previous studies have shown that expression of both CXCR3 and CCR5 promote migration of virus-specific T cells into the CNS of JHMV-infected mice (111–114). Surprisingly, Dickey et al. (49) showed that surface expression of CXCR3 was decreased on CD8+ T cells isolated from miR-155−/− mice compared to those from WT mice (Figure 1E); however, there were no differences in CXCR3 expression on CD4+ T cells between WT or miR-155−/− mice, nor were there differences in homing receptor CCR5 on WT or miR-155−/− T cells. These findings suggest that regulation of homing receptor expression by miR-155 likely occurs via more than one mechanism. Taken together, these studies show that miR-155 is important in mediating T cell responses to virally-induced demyelinating disease.
Figure 1. miR-155 regulates T cell accumulation and antiviral activity in response to JHMV infection.
(A) JHVM-infected miR-155−/− mice demonstrated greater mortality than WT mice, concomitant with impaired ability to clear virus from brain (B). (C) Virus-specific CD8+ T cell accumulation in brains of JMHV infected mice was impaired in miR-155−/− mice. Virus-specific miR-155−/− CD8+ T cells exhibited diminished CTL activity in response to peptide stimulation (D), as well as decreased levels of CXCR3 expression (E).
4. Conclusions
This review highlights various mechanisms by which miR-155 regulates inflammatory processes in response to viral infections in the CNS. Currently, miR-155 is known to influence virally induced neuroinflammation by regulating CD4+ and CD8+ T cell accumulation, NK cell maturation and expansion, T cell cytokine production, CD8+ T cell-mediated cytotoxicity, astrogliosis, macrophage polarization, expression of receptors necessary for viral entry, and expression of viral proteins. As miRNA-virus pathogenesis interactions are an emerging concept in neuroimmunology, it is likely that in the coming years, many additional mechanisms by which miR-155 regulates virally induced CNS inflammation will be discovered.
Highlights.
MicroRNAs can silence the expression of target genes
MicroRNA 155 is important for immune cell homeostasis and effector functions
MicroRNA 155 regulates many aspects of immune responses to viral infections of CNS
Acknowledgments
Funding
This work was supported by NIH grant R01 NS041249 to TEL and R01 AG047956 to RMO. LLD is supported by Postdoctoral Fellowship FG20105A1 from the National Multiple Sclerosis Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 2.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 5.Dai R, Ahmed SA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl Res. 2011;157:163–179. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11:644–656. doi: 10.1038/nrc3107. [DOI] [PubMed] [Google Scholar]
- 7.O’Connell RM, Baltimore D. MicroRNAs and hematopoietic cell development. Current topics in developmental biology. 2012;99:145–174. doi: 10.1016/B978-0-12-387038-4.00006-9. [DOI] [PubMed] [Google Scholar]
- 8.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 9.Dahm T, Rudolph H, Schwerk C, Schroten H, Tenenbaum T. Neuroinvasion and Inflammation in Viral Central Nervous System Infections. Mediators Inflamm. 2016;2016:8562805. doi: 10.1155/2016/8562805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso AL, Guedes JR, de Lima MC. Role of microRNAs in the regulation of innate immune cells under neuroinflammatory conditions. Curr Opin Pharmacol. 2016;26:1–9. doi: 10.1016/j.coph.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Tam W, Ben-Yehuda D, Hayward WS. bic, a novel gene activated by proviral insertions in avian leukosis virus-induced lymphomas, is likely to function through its noncoding RNA. Mol Cell Biol. 1997;17:1490–1502. doi: 10.1128/mcb.17.3.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haasch D, Chen YW, Reilly RM, Chiou XG, Koterski S, Smith ML, Kroeger P, McWeeny K, Halbert DN, Mollison KW, Djuric SW, Trevillyan JM. T cell activation induces a noncoding RNA transcript sensitive to inhibition by immunosuppressant drugs and encoded by the proto-oncogene, BIC. Cell Immunol. 2002;217:78–86. doi: 10.1016/s0008-8749(02)00506-3. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee A, Schambach F, DeJong CS, Hammond SM, Reiner SL. Micro-RNA-155 inhibits IFN-gamma signaling in CD4+ T cells. Eur J Immunol. 2010;40:225–231. doi: 10.1002/eji.200939381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das LM, Torres-Castillo MD, Gill T, Levine AD. TGF-beta conditions intestinal T cells to express increased levels of miR-155, associated with down-regulation of IL-2 and itk mRNA. Mucosal Immunol. 2013;6:167–176. doi: 10.1038/mi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gracias DT, Stelekati E, Hope JL, Boesteanu AC, Doering TA, Norton J, Mueller YM, Fraietta JA, Wherry EJ, Turner M, Katsikis PD. The microRNA miR-155 controls CD8(+) T cell responses by regulating interferon signaling. Nat Immunol. 2013;14:593–602. doi: 10.1038/ni.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huffaker TB, Hu R, Runtsch MC, Bake E, Chen X, Zhao J, Round JL, Baltimore D, O’Connell RM. Epistasis between microRNAs 155 and 146a during T cell-mediated antitumor immunity. Cell Rep. 2012;2:1697–1709. doi: 10.1016/j.celrep.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu LF, Gasteiger G, Yu IS, Chaudhry A, Hsin JP, Lu Y, Bos PD, Lin LL, Zawislak CL, Cho S, Sun JC, Leslie CS, Lin SW, Rudensky AY. A Single miRNA-mRNA Interaction Affects the Immune Response in a Context- and Cell-Type-Specific Manner. Immunity. 2015;43:52–64. doi: 10.1016/j.immuni.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J Immunol. 2009;182:2578–2582. doi: 10.4049/jimmunol.0803162. [DOI] [PubMed] [Google Scholar]
- 23.Almanza G, Fernandez A, Volinia S, Cortez-Gonzalez X, Croce CM, Zanetti M. Selected microRNAs define cell fate determination of murine central memory CD8 T cells. PLoS One. 2010;5:e11243. doi: 10.1371/journal.pone.0011243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. miR-155 regulates IFN-gamma production in natural killer cells. Blood. 2012;119:3478–3485. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang P, Hou J, Lin L, Wang C, Liu X, Li D, Ma F, Wang Z, Cao X. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226–6233. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa R, Leyland R, Meyer-Hermann M, Lu D, Turner M, Arbore G, Phan TG, Brink R, Vigorito E. MicroRNA-155 controls affinity-based selection by protecting c-MYC+ B cells from apoptosis. J Clin Invest. 2016;126:377–388. doi: 10.1172/JCI82914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, Guo Z, Liu J, Wang Y, Yuan W, Qin Y. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Hu R, Huffaker TB, Kagele DA, Runtsch MC, Bake E, Chaudhuri AA, Round JL, O’Connell RM. MicroRNA-155 confers encephalogenic potential to Th17 cells by promoting effector gene expression. J Immunol. 2013;190:5972–5980. doi: 10.4049/jimmunol.1300351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, Smith KG, Rada C, Enright AJ, Toellner KM, Maclennan IC, Turner M. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu R, Kagele DA, Huffaker TB, Runtsch MC, Alexander M, Liu J, Bake E, Su W, Williams MA, Rao DS, Moller T, Garden GA, Round JL, O’Connell RM. miR-155 promotes T follicular helper cell accumulation during chronic, low-grade inflammation. Immunity. 2014;41:605–619. doi: 10.1016/j.immuni.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore CS, Rao VT, Durafourt BA, Bedell BJ, Ludwin SK, Bar-Or A, Antel JP. miR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann Neurol. 2013;74:709–720. doi: 10.1002/ana.23967. [DOI] [PubMed] [Google Scholar]
- 32.Freilich RW, Woodbury ME, Ikezu T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS One. 2013;8:e79416. doi: 10.1371/journal.pone.0079416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61:91–103. doi: 10.1002/glia.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su W, Aloi MS, Garden GA. MicroRNAs mediating CNS inflammation: Small regulators with powerful potential. Brain Behav Immun. 2016;52:1–8. doi: 10.1016/j.bbi.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murugaiyan G, Beynon V, Mittal A, Joller N, Weiner HL. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2213–2221. doi: 10.4049/jimmunol.1003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, Kay O, de Vries HE, Hirst MC, Sharrack B, Baker D, Male DK, Michael GJ, Romero IA. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J. 2014;28:2551–2565. doi: 10.1096/fj.13-248880. [DOI] [PubMed] [Google Scholar]
- 38.Cerutti C, Soblechero-Martin P, Wu D, Lopez-Ramirez MA, de Vries H, Sharrack B, Male DK, Romero IA. MicroRNA-155 contributes to shear-resistant leukocyte adhesion to human brain endothelium in vitro. Fluids Barriers CNS. 2016;13:8. doi: 10.1186/s12987-016-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thome AD, Harms AS, Volpicelli-Daley LA, Standaert DG. microRNA-155 Regulates Alpha-Synuclein-Induced Inflammatory Responses in Models of Parkinson Disease. J Neurosci. 2016;36:2383–2390. doi: 10.1523/JNEUROSCI.3900-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guedes JR, Custodia CM, Silva RJ, de Almeida LP, Pedroso de Lima MC, Cardoso AL. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum Mol Genet. 2014;23:6286–6301. doi: 10.1093/hmg/ddu348. [DOI] [PubMed] [Google Scholar]
- 41.Lippai D, Bala S, Csak T, Kurt-Jones EA, Szabo G. Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice. PLoS One. 2013;8:e70945. doi: 10.1371/journal.pone.0070945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parisi C, Arisi I, D’Ambrosi N, Storti AE, Brandi R, D’Onofrio M, Volonte C. Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis. 2013;4:e959. doi: 10.1038/cddis.2013.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng FR, Tang LJ, He Y, Garcia RC. An update on the role of miRNA-155 in pathogenic microbial infections. Microbes Infect. 2015;17:613–621. doi: 10.1016/j.micinf.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Yao Y, Nair V. Role of virus-encoded microRNAs in Avian viral diseases. Viruses. 2014;6:1379–1394. doi: 10.3390/v6031379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaluzna EM. MicroRNA-155 and microRNA-196b: promising biomarkers in hepatitis C virus infection? Rev Med Virol. 2014;24:169–185. doi: 10.1002/rmv.1785. [DOI] [PubMed] [Google Scholar]
- 46.Gottwein E. Roles of microRNAs in the life cycles of mammalian viruses. Curr Top Microbiol Immunol. 2013;371:201–227. doi: 10.1007/978-3-642-37765-5_8. [DOI] [PubMed] [Google Scholar]
- 47.Bhela S, Mulik S, Gimenez F, Reddy PB, Richardson RL, Varanasi SK, Jaggi U, Xu J, Lu PY, Rouse BT. Role of miR-155 in the pathogenesis of herpetic stromal keratitis. Am J Pathol. 2015;185:1073–1084. doi: 10.1016/j.ajpath.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhela S, Mulik S, Reddy PB, Richardson RL, Gimenez F, Rajasagi NK, Veiga-Parga T, Osmand AP, Rouse BT. Critical role of microRNA-155 in herpes simplex encephalitis. J Immunol. 2014;192:2734–2743. doi: 10.4049/jimmunol.1302326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickey LL, Worne CL, Glover JL, Lane TE, O’Connell RM. MicroRNA-155 enhances T cell trafficking and antiviral effector function in a model of coronavirus-induced neurologic disease. J Neuroinflammation. 2016;13:240. doi: 10.1186/s12974-016-0699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dudda JC, Salaun B, Ji Y, Palmer DC, Monnot GC, Merck E, Boudousquie C, Utzschneider DT, Escobar TM, Perret R, Muljo SA, Hebeisen M, Rufer N, Zehn D, Donda A, Restifo NP, Held W, Gattinoni L, Romero P. MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity. 2013;38:742–753. doi: 10.1016/j.immuni.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lind EF, Elford AR, Ohashi PS. Micro-RNA 155 is required for optimal CD8+ T cell responses to acute viral and intracellular bacterial challenges. J Immunol. 2013;190:1210–1216. doi: 10.4049/jimmunol.1202700. [DOI] [PubMed] [Google Scholar]
- 52.Lu C, Huang X, Zhang X, Roensch K, Cao Q, Nakayama KI, Blazar BR, Zeng Y, Zhou X. miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood. 2011;117:4293–4303. doi: 10.1182/blood-2010-12-322503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez-Nunez RT, Louafi F, Friedmann PS, Sanchez-Elsner T. MicroRNA-155 modulates the pathogen binding ability of dendritic cells (DCs) by down-regulation of DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) J Biol Chem. 2009;284:16334–16342. doi: 10.1074/jbc.M109.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Napuri J, Pilakka-Kanthikeel S, Raymond A, Agudelo M, Yndart-Arias A, Saxena SK, Nair M. Cocaine enhances HIV-1 infectivity in monocyte derived dendritic cells by suppressing microRNA-155. PLoS One. 2013;8:e83682. doi: 10.1371/journal.pone.0083682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zawislak CL, Beaulieu AM, Loeb GB, Karo J, Canner D, Bezman NA, Lanier LL, Rudensky AY, Sun JC. Stage-specific regulation of natural killer cell homeostasis and response against viral infection by microRNA-155. Proc Natl Acad Sci U S A. 2013;110:6967–6972. doi: 10.1073/pnas.1304410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roizman B, Whitley RJ. An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol. 2013;67:355–374. doi: 10.1146/annurev-micro-092412-155654. [DOI] [PubMed] [Google Scholar]
- 57.Whitley RJ, Soong SJ, Linneman C, Jr, Liu C, Pazin G, Alford CA. Herpes simplex encephalitis. Clinical Assessment. JAMA. 1982;247:317–320. [PubMed] [Google Scholar]
- 58.Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–148. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Wang JP, Bowen GN, Zhou S, Cerny A, Zacharia A, Knipe DM, Finberg RW, Kurt-Jones EA. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J Virol. 2012;86:2273–2281. doi: 10.1128/JVI.06010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lundberg P, Ramakrishna C, Brown J, Tyszka JM, Hamamura M, Hinton DR, Kovats S, Nalcioglu O, Weinberg K, Openshaw H, Cantin EM. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J Virol. 2008;82:7078–7088. doi: 10.1128/JVI.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varani S, Landini MP. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae. 2011;2:6. doi: 10.1186/2042-4280-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57(Suppl 4):S178–181. doi: 10.1093/cid/cit629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, de Lima Isaac M, de Carvalho e Oliveira PF, Boppana S, Britt WJ. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis. 2009;49:522–528. doi: 10.1086/600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol. 2006;35:216–220. doi: 10.1016/j.jcv.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Anderson KS, Amos CS, Boppana S, Pass R. Ocular abnormalities in congenital cytomegalovirus infection. J Am Optom Assoc. 1996;67:273–278. [PubMed] [Google Scholar]
- 66.Slavuljica I, Kvestak D, Huszthy PC, Kosmac K, Britt WJ, Jonjic S. Immunobiology of congenital cytomegalovirus infection of the central nervous system-the murine cytomegalovirus model. Cell Mol Immunol. 2015;12:180–191. doi: 10.1038/cmi.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei H, Nash WT, Makrigiannis AP, Brown MG. Impaired NK-cell education diminishes resistance to murine CMV infection. Eur J Immunol. 2014;44:3273–3282. doi: 10.1002/eji.201444800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 70.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 72.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med. 2011;208:357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonthius DJ. Lymphocytic choriomeningitis virus: an underrecognized cause of neurologic disease in the fetus, child, and adult. Semin Pediatr Neurol. 2012;19:89–95. doi: 10.1016/j.spen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto M, Kondo E, Takeuchi M, Harashima A, Otani T, Tsuji-Takayama K, Yamasaki F, Kumon H, Kibata M, Nakamura S. miR-155, a Modulator of FOXO3a Protein Expression, Is Underexpressed and Cannot Be Upregulated by Stimulation of HOZOT, a Line of Multifunctional Treg. PLoS One. 2011;6:e16841. doi: 10.1371/journal.pone.0016841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, Ginsburg AS. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89:766–774. 774A–774E. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ooi MH, Lewthwaite P, Lai BF, Mohan A, Clear D, Lim L, Krishnan S, Preston T, Chieng CH, Tio PH, Wong SC, Cardosa J, Solomon T. The epidemiology, clinical features, and long-term prognosis of Japanese encephalitis in central sarawak, malaysia, 1997–2005. Clin Infect Dis. 2008;47:458–468. doi: 10.1086/590008. [DOI] [PubMed] [Google Scholar]
- 79.Pareek S, Roy S, Kumari B, Jain P, Banerjee A, Vrati S. MiR-155 induction in microglial cells suppresses Japanese encephalitis virus replication and negatively modulates innate immune responses. J Neuroinflammation. 2014;11:97. doi: 10.1186/1742-2094-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thounaojam MC, Kundu K, Kaushik DK, Swaroop S, Mahadevan A, Shankar SK, Basu A. MicroRNA 155 regulates Japanese encephalitis virus-induced inflammatory response by targeting Src homology 2-containing inositol phosphatase 1. J Virol. 2014;88:4798–4810. doi: 10.1128/JVI.02979-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lentz MR, Kim WK, Lee V, Bazner S, Halpern EF, Venna N, Williams K, Rosenberg ES, Gonzalez RG. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–1472. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- 85.Bell JE. Report of a workshop on diagnostic pathology services in Bosnia-Herzegovina held in Sarajevo in May 1996. Brain Pathol. 1996;6:521–524. doi: 10.1111/j.1750-3639.1996.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 86.Anthony IC, Crawford DH, Bell JE. B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain. 2003;126:1058–1067. doi: 10.1093/brain/awg118. [DOI] [PubMed] [Google Scholar]
- 87.Tomlinson GS, Simmonds P, Busuttil A, Chiswick A, Bell JE. Upregulation of microglia in drug users with and without pre-symptomatic HIV infection. Neuropathol Appl Neurobiol. 1999;25:369–379. doi: 10.1046/j.1365-2990.1999.00197.x. [DOI] [PubMed] [Google Scholar]
- 88.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13:976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 90.Swaminathan G, Rossi F, Sierra LJ, Gupta A, Navas-Martin S, Martin-Garcia J. A role for microRNA-155 modulation in the anti-HIV-1 effects of Toll-like receptor 3 stimulation in macrophages. PLoS Pathog. 2012;8:e1002937. doi: 10.1371/journal.ppat.1002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruelas DS, Chan JK, Oh E, Heidersbach AJ, Hebbeler AM, Chavez L, Verdin E, Rape M, Greene WC. MicroRNA-155 Reinforces HIV Latency. J Biol Chem. 2015;290:13736–13748. doi: 10.1074/jbc.M115.641837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 93.Soilleux EJ, Morris LS, Rushbrook S, Lee B, Coleman N. Expression of human immunodeficiency virus (HIV)-binding lectin DC-SIGNR: Consequences for HIV infection and immunity. Hum Pathol. 2002;33:652–659. doi: 10.1053/hupa.2002.124036. [DOI] [PubMed] [Google Scholar]
- 94.Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, Coleman N, Lee B. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 95.Garcia-Vallejo JJ, Ilarregui JM, Kalay H, Chamorro S, Koning N, Unger WW, Ambrosini M, Montserrat V, Fernandes RJ, Bruijns SC, van Weering JR, Paauw NJ, O’Toole T, van Horssen J, van der Valk P, Nazmi K, Bolscher JG, Bajramovic J, Dijkstra CD, t Hart BA, van Kooyk Y. CNS myelin induces regulatory functions of DC-SIGN-expressing, antigen-presenting cells via cognate interaction with MOG. J Exp Med. 2014;211:1465–1483. doi: 10.1084/jem.20122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Whisnant AW, Bogerd HP, Flores O, Ho P, Powers JG, Sharova N, Stevenson M, Chen CH, Cullen BR. In-depth analysis of the interaction of HIV-1 with cellular microRNA biogenesis and effector mechanisms. MBio. 2013;4:e000193. doi: 10.1128/mBio.00193-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cassol E, Cassetta L, Rizzi C, Alfano M, Poli G. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J Immunol. 2009;182:6237–6246. doi: 10.4049/jimmunol.0803447. [DOI] [PubMed] [Google Scholar]
- 98.Cassetta L, Kajaste-Rudnitski A, Coradin T, Saba E, Della Chiara G, Barbagallo M, Graziano F, Alfano M, Cassol E, Vicenzi E, Poli G. M1 polarization of human monocyte-derived macrophages restricts pre and postintegration steps of HIV-1 replication. AIDS. 2013;27:1847–1856. doi: 10.1097/QAD.0b013e328361d059. [DOI] [PubMed] [Google Scholar]
- 99.Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lane TE, Hosking MP. The pathogenesis of murine coronavirus infection of the central nervous system. Crit Rev Immunol. 2010;30:119–130. doi: 10.1615/critrevimmunol.v30.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marten NW, Stohlman SA, Bergmann CC. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 2001;14:1–18. doi: 10.1089/08828240151061329. [DOI] [PubMed] [Google Scholar]
- 102.Phares TW, Stohlman SA, Hwang M, Min B, Hinton DR, Bergmann CC. CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis. J Virol. 2012;86:2416–2427. doi: 10.1128/JVI.06797-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Plaisted WC, Weinger JG, Walsh CM, Lane TE. T cell mediated suppression of neurotropic coronavirus replication in neural precursor cells. Virology. 2014;449:235–243. doi: 10.1016/j.virol.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheever FS, Daniels JB, Pappenheimer AM, Bailey OT. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. Journal of Exerimental Medicine. 1949;90:181–194. doi: 10.1084/jem.90.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perlman SR, Lane TE, Buchmeier MJ. Coronaviruses: Hepatitis, peritonitis, and central nervous system disease. In: Cunningham MW, Fujinami RS, editors. Effects of Microbes on the Immune System. Vol. 1. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 331–348. [Google Scholar]
- 106.Stohlman SA, Ramakrishna C, Tschen SI, Hinton DR, Bergmann CC. The art of survival during viral persistence. J Neurovirol. 2002;8(Suppl 2):53–58. doi: 10.1080/13550280290167884. [DOI] [PubMed] [Google Scholar]
- 107.Wang FI, Stohlman SA, Fleming JO. Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J Neuroimmunol. 1990;30:31–41. doi: 10.1016/0165-5728(90)90050-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sorensen O, Perry D, Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980;37:478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- 109.Houtman JJ, Hinze HC, Fleming JO. Demyelination induced by murine coronavirus JHM infection of congenitally immunodeficient mice. Adv Exp Med Biol. 1995;380:159–163. doi: 10.1007/978-1-4615-1899-0_26. [DOI] [PubMed] [Google Scholar]
- 110.Houtman JJ, Fleming JO. Dissociation of demyelination and viral clearance in congenitally immunodeficient mice infected with murine coronavirus JHM. J Neurovirol. 1996;2:101–110. doi: 10.3109/13550289609146543. [DOI] [PubMed] [Google Scholar]
- 111.Glass WG, Lane TE. Functional analysis of the CC chemokine receptor 5 (CCR5) on virus-specific CD8+ T cells following coronavirus infection of the central nervous system. Virology. 2003;312:407–414. doi: 10.1016/S0042-6822(03)00237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Glass WG, Lane TE. Functional expression of chemokine receptor CCR5 on CD4(+) T cells during virus-induced central nervous system disease. J Virol. 2003;77:191–198. doi: 10.1128/JVI.77.1.191-198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glass WG, Liu MT, Kuziel WA, Lane TE. Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology. 2001;288:8–17. doi: 10.1006/viro.2001.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stiles LN, Hosking MP, Edwards RA, Strieter RM, Lane TE. Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. Eur J Immunol. 2006;36:613–622. doi: 10.1002/eji.200535509. [DOI] [PubMed] [Google Scholar]