Abstract
High dose melphalan followed by autologous stem cell transplantation remains standard of care for eligible patients with multiple myeloma, but disease response and toxicity, including severe mucositis, varies among patients. Our randomized trial investigated duration of cryotherapy (2 and 6 hours) for reduction of mucositis prevalence and severity and explored factors associated with variability in pharmacokinetics and outcomes from melphalan therapy. The results demonstrate 2-hour is at least as effective as 6-hour cryotherapy in decreasing severe mucositis. From a population pharmacokinetic model, we identified fat free mass, hematocrit, and creatinine clearance were significant covariates, as had been reported previously. Furthermore, we observed the rs4240803 SLC7A5 polymorphism was significantly associated with pharmacokinetic variability, and pharmacokinetics was associated with both mucositis and neutropenia. However, melphalan exposure was not associated with progression-free or overall survival in our dataset. These findings contribute to ongoing efforts to personalize melphalan dosing in transplant patients.
Keywords: melphalan, multiple myeloma, autologous stem cell transplant, cryotherapy, pharmacokinetics
INTRODUCTION
Autologous hematopoietic stem cell transplant (HSCT) for patients with multiple myeloma (MM) is currently standard of care and highly effective, with approximately 5,000 transplants performed annually in the United States (1–4). Melphalan, an interstrand DNA cross-linking agent, remains the standard chemotherapy regimen for HSCT in MM, and patients are treated with either 140 mg/m2 or 200 mg/m2 depending on the presence or absence of significant medical co-morbidities (5). Treatment with high dose melphalan (HDM) is associated with variable durations and severities of oral mucositis, enteritis, and neutropenia.
Severe oral mucositis following HDM is a primary source of patient discomfort and is associated with several unfavorable outcomes, including longer duration of febrile neutropenia, doubling of infectious risk, two to three additional days of total parenteral nutrition (TPN) use, intravenous narcotic use, longer length of hospital stay, and a 3.9-fold increase in 100-day mortality; factors leading to an increase of $25,405 (US) in hospital charges (6). Numerous strategies to decrease the incidence of mucositis have been employed, including routine oral care, saline rinses, palifermin, and artificial saliva (Caphosol®). However, the use of oral cryotherapy remains superior (7–11). It has been previously shown that 6 hours of oral cryotherapy significantly reduces mucositis (8), although patients often find this duration to be quite uncomfortable, and many do not adhere to the cryotherapy regimen during the full 6 hours. While the minimal necessary duration of cryotherapy required to reduce the incidence of severe mucositis has not been identified, other studies suggest that shorter durations including 1 hour (10) and less than 2 hours (12) result in tolerability and outcomes that may be comparable to 6 hours. Despite these reports, no prospective randomized trial has been completed that confirms cryotherapy duration shorter than 6 hours leads to similar patient outcomes, reduced length of hospitalization, and decreased incidence of bacteremia (8, 10, 11).
Despite improvements in supportive care, many HSCT patients continue to experience significant toxicities following HDM. It is presently unclear why certain patients tolerate treatment better than others, although inter-patient variability in melphalan pharmacokinetics and inherent sensitivity to the cytotoxic effects of melphalan are suspected to be important factors. Pharmacokinetic (PK) analysis has identified pre-treatment hematocrit, fat free mass, and estimated creatinine clearance as significant sources of inter-patient variability (13). Additionally, pharmacodynamic (PD) characteristics, such as induction of ex vivo apoptosis, modulation of p53 expression in PBMCs, and the ability to repair double-stand breaks in primary myeloma cells have been identified as factors associated with response and toxicity (14, 15). Similarly, a single nucleotide polymorphism (rs4240803) in SLC7A5, a gene that encodes L-type amino acid transporter 1 (LAT1), which is a transporter that mediates melphalan uptake into cells (16, 17), has been associated with TPN requirement following development of HDM-related gastrointestinal toxicity (18). Collectively, these findings suggest that intrinsic PK and PD variables can be used to better understand and potentially predict the in vivo activity and an individual’s sensitivity to melphalan.
To investigate whether a shorter duration of cryotherapy would be at least as effective as a standard 6-hour regimen for minimization of severe oral mucositis (grade 3 or higher), our group performed a prospective randomized non-inferiority trial investigating 2 versus 6 hours of cryotherapy in 146 patients with MM. Additionally, we collected patient samples to further explore sources of inter-patient variability in melphalan PK and outcomes from therapy. Our results establish 2 hours of cryotherapy as the new standard of care for mucositis prophylaxis, and we have further explored within this cohort of patients, factors that explain portions of melphalan inter-patient PK variability and associations of PK with clinical outcomes.
RESULTS
Patients
A total of 146 patients consecutively admitted for HSCT were consented, registered and randomized to receive either 2- or 6-hours of cryotherapy (Table 1). Each treatment arm included 73 patients, with all patients receiving treatment as randomized. Overall, the median age was 59 (range 35 – 72), 62% (90/146) were male, and 87% (127/146) were White, 61% (89/146) had standard risk disease, and 36% (53/146) had either intermediate- or high-risk disease defined by cytogenetic analysis of diagnostic bone marrow samples (19). The majority of patients were treated with a single dose of melphalan 200 mg/m2 (84%, 123/146) with an actual median melphalan dose of 392 mg (range: 278 – 486 mg). The remaining patients (16%, 23/146) were treated with 140 mg/m2 (range: 192 – 350 mg). The mean CD34+ stem cell dose infused on day 0 was 4.68 × 106 CD34+ cells/kg (range: 1.9 – 15.7 × 106 cells/kg).
Table 1.
Baseline patient characteristics
| Characteristic | 2 hour cryotherapy (n = 73) |
6 hour cryotherapy (n = 73) |
|
|---|---|---|---|
| Age | |||
| Mean (sd) | 58.3 (8.1) | 58.1 (7.2) | |
| Median (min – max) | 60 (38 – 71) | 59 (35 – 72) | |
| < 60 | 35 (48.0) | 39 (53.4) | |
| 60 – 65 | 23 (31.5) | 27 (37.0) | |
| > 65 | 15 (20.5) | 7 (9.6) | |
|
| |||
| Gender | |||
| Male (%) | 46 (63.0) | 44 (60.3) | |
| Female (%) | 27 (37.0) | 29 (39.7) | |
|
| |||
| Race | |||
| White (%) | 66 (90.4) | 61 (83.6) | |
| Other (%) | 7 (9.6) | 12 (16.4) | |
|
| |||
| Risk group | |||
| Standard (%) | 44 (60.3) | 45 (61.6) | |
| Intermediate (%) | 13 (17.8) | 21 (28.8) | |
| High (%) | 13 (17.8) | 6 (8.2) | |
| Missing (%) | 3 (4.1) | 1 (1.4) | |
|
| |||
| Melphalan dose | |||
| 140 mg/m2 (%) | 12 (16.4) | 11 (15.1) | |
| 200 mg/m2 (%) | 61 (83.6) | 62 (84.9) | |
|
| |||
| CD34+ stem cell dose (106 cells/kg) | |||
| Mean (sd) | 4.6 (2.2) | 4.7 (2.1) | |
| Median (min – max) | 4.2 (2.0 – 15.7) | 4.1 (1.9 – 11.7) | |
|
| |||
| Creatinine Clearance (mL/min) | |||
| Mean (sd) | 87.4 (42.5) | 87.6 (40.7) | |
| Median (min – max) | 85.2 (7.39 – 196.5) | 90.6 (0.2 – 168.7) | |
|
| |||
| Hemoglobin (g/dL) | |||
| Mean (sd) | 11.0 (1.7) | 10.9 (1.6) | |
| Median (min – max) | 10.9 (7.1 – 14.8) | 10.9 (7.0 – 14.7) | |
|
| |||
| Fat Free Mass (kg) | |||
| Mean (sd) | 57.7 (13.3) | 58.3 (11.9) | |
| Median (min – max) | 61.5 (31.3 – 81.9) | 60.0 (35.2 – 80.4) | |
|
| |||
| SLC7A5 genotype (rs4240803) | |||
| MAF (p value) | 0.31 (0.996) | 0.3 (0.908) | |
| M/M (%) | 24 (32.9) | 26 (35.6) | |
| M/m (%) | 21 (28.8) | 25 (34.2) | |
| m/m (%) | 5 (6.8) | 4 (5.5) | |
| Missing (%) | 23 (31.5) | 18 (24.7) | |
Risk groups were defined by cytogenetic analysis of diagnostic bone marrow samples as defined by Mikhael and colleagues (19). For SLC7A5 genotyping, MAF, minor allele frequency; M, major allele; m, minor allele; Two-tailed χ2 tests were used to analyze the differences between the actual and predicted (Hardy-Weinberg equilibrium) genotype frequency, and the p-values for these are presented.
Safety and toxicities
Overall, 62.3% (n=91) of patients had grade 1 – 3 oral mucositis, and no patient experienced a grade 4 event. In total, 45.2% (66/146) of patients had at worst grade 1 mucositis, 13.7% (20/146) had at worst grade 2, and 3.4% (5/146) had at worst grade 3 events. The rate of severe mucositis, of at least grade 3, was not different (non-inferior) between the randomized groups. Severe mucositis was observed in 2.7% (n=2) and 4.1% (n=3) of patients receiving 2 or 6 hours of cryotherapy, respectively (difference in risk: −1.4%, 90% CI: −6.3%, 3.6%). Moreover, patients who received 2 hours of cryotherapy did not have a higher rate of mucositis (of any grade) (58.9% in the 2-hour cohort versus 65.8% in the 6-hour cohort, risk difference: −6.8%, 90% CI: −20.0, 6.3%) or of grade 2 or above (2-hour: 16.4% (n=12), 6-hour: 17.8% (n=13), risk difference: −1.4%, 90% CI: −11.6%, 8.9%) (Table 2). These differences were well within the 15% non-inferiority margin set during the design stage. Additionally, in patients who experienced mucositis of any grade, the onset of worst grade symptoms was not significantly different between patients treated in the 2-hour cohort (mean (sd): 9.4 days (3.0)) and those in the 6-hour cohort (8.5 days (3.6), difference: 0.85 days, 95% CI: −0.53, 2.24) (Supplementary Figure 1).
Table 2.
Clinically relevant patient outcomes
| Characteristic | 2 hour cohort | 6 hour cohort | |
|---|---|---|---|
| Worst per patient grade mucositis | |||
| None | 30 (41.1) | 25 (34.2) | |
| Grade 1 | 31 (42.5) | 35 (47.9) | |
| Grade 2 | 10 (13.7) | 10 (13.7) | |
| Grade 3 | 2 (2.7) | 3 (4.1) | |
|
| |||
| Bacteremia | |||
| Mean day of first positive culture | 7 (1.6) | 7 (2.5) | |
| Gram positive | 10 (50.0) | 12 (48.0) | |
| Gram negative | 8 (40.0) | 8 (32.0) | |
| Gram positive and negative | 2 (10.0) | 5 (20.0) | |
|
| |||
| Length of Stay | |||
| Mean (sd) | 14.7 days (2.9) | 14.9 days (2.8) | |
| Median (min – max) | 14 days (10 – 21) | 14 days (11 – 21) | |
Mucositis and bacteremia data are presented as number of patients (%), except for mean day of first positive culture, which is presented as days (standard deviation). Differences between cohorts were evaluated by two-sample t-test (day of first positive culture, length of stay) or by Fisher’s exact test (worst grade of mucositis and gram positive cultures). No statistically significant differences were observed, with all p-values > 0.05.
There were also no significant differences in the incidence of bacteremia or length of hospitalization between randomized groups (Table 2). Forty-five patients had bacteremia that occurred on a median of 7 days post-transplant in both groups (27.4% of patients in the 2-hour cohort (n=20) and 34.2% of patients in the 6-hour cohort (n=25), risk difference: 6.8%, 95% CI: −21.8%, 8.1%). Patient length of hospitalization was on average 14.8 days (14.7 (2.9) versus 14.9 (2.8) days in the 2- and 6-hour cohorts, respectively, difference: 0.29 days, 95% CI: −1.22, 0.65). Adjusted for age and randomized group, the incidence of bacteremia or the length of hospital stay was not strongly associated with grade of mucositis (adjusted incidence of bacteremia: 36.0% (mucositis grade 2–3) vs. 29.8% (mucositis grade 0–1), difference 7.6%, 95% CI: −13.5, 28.7; Adjusted length of stay: 15.8 days (mucositis grade 2–3) vs. 14.6 days (mucositis grade 0–1), difference 0.90 days, 95% CI: −0.33, 2.14).
SLC7A5 genotyping
Genomic DNA extracted from pre-transplant baseline PBMCs was available from 105 patients and was used to determine SLC7A5 rs4240803 genotype. The SLC7A5 rs4240803 homozygous minor allele genotype (AA) was observed in 9 patients, the heterozygous allele (AG) in 47 patients, and homozygous major allele (GG) in 50 patients (Table 1). Overall, the genotype distribution of SLC7A5 rs4240803 in this cohort of patients follows the Hardy-Weinberg equilibrium. The minor allele frequency (MAF) obtained in this study, 30.7%, is comparable to the reported allele frequencies in the NCBI Short Genetic Variation Database (dbSNP) of the ethnicity of our study population (34.1%, EUR population).
Population PK modeling
Plasma pharmacokinetic data was obtained from 118 patients. A population PK model was established utilizing PK data, data from PBMCs, and clinical data obtained prior to and during melphalan treatment to investigate which factors significantly contributed to PK variability among patients. Inter-patient variability parameters (IPV) for CL and V2 were strongly associated (r ≅ 0.8), so a shared IPV parameter was used with an estimated scale factor applied to the IPV for V2 (i.e. IPVV2 = THETA(n)×IPVCL, where THETA(n) is the estimated scale factor).
Covariate adjusted models were developed using a step-wise selection procedure with forward addition and backward elimination. Creatinine clearance (normalized to body surface area of 1.73 m2) and hematocrit on CL, SLC7A5 polymorphism on V2, and allometric-scaled fat free mass were chosen for the final PK model (Supplementary Table 1A and 1B). Notably, while backward elimination is often done with a p-value cutoff of 0.01, we chose a cut-off of 0.05, which allowed us to retain hematocrit, which had been reported previously by Nath et al. (13). The inclusion of these four covariates in the final model decreased the overall IPV of CL/V2 (shared IPV), V1, and Q compared to the structural model by 16.9%, 7.8%, and 5.2%, respectively, in which percent difference was calculated by . The parameter estimates in the structural and final model are listed in Table 3. The objective function value (OFV) in the final model was reduced by 98.6 compared to the structural model, indicating a significant improvement in the model fitting based on the log likelihood ratio test.
Table 3.
Population pharmacokinetic parameter estimates of the structural model and the final covariate model
| Structural model | Covariate model | |||
|---|---|---|---|---|
|
|
||||
| Parameters | Estimate | RSE (%) | Estimate | RSE (%) |
| CL (L/hr) | 26.5 | 3.9 | 29.0 | 3.4 |
| V1 (L) | 17.7 | 5.8 | 19.5 | 6.1 |
| Q (L/hr) | 28.3 | 10.1 | 28.6 | 9.5 |
| V2 (L) | 18.3 | 5.8 | 20.9 | 5.7 |
| Shared ETA Scale Factor | 1.17 | 11.5 | 1.31 | 9.8 |
| IPVCL (CV%) | 33.8 | 14.9 | 28.1 | 16.7 |
| IPVV1 (CV%) | 29.4 | 22.6 | 27.1 | 33.1 |
| IPVQ (CV%) | 57.4 | 23.4 | 54.4 | 26.0 |
| ε (proportional) | 0.061 | 14.5 | 0.0569 | 15.3 |
Estimate, population mean estimate; RSE, relative standard error; IPV, Inter-Patient Variability; CV, coefficient variation; CL, clearance; V1, volume of distribution into the central compartment; Q, inter-compartmental clearance; V2, volume of distribution into the peripheral compartment; ε, proportional residual error.
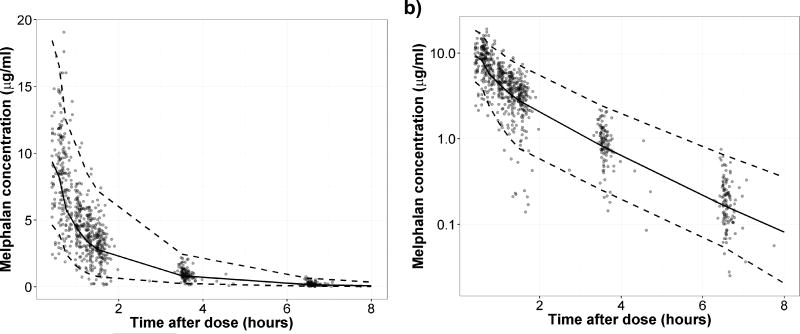
Bias and stability of the final model was assessed by 95% confidence intervals of all predicted parameters utilizing the bootstrap method (Table 4). The prediction performance of the final model was evaluated by the visual predictive check (VPC) from 1,000 simulation runs, in which the observed melphalan concentration data was compared with the 25th, 50th, and 97.5th percentiles of the predicted values (Figure 1).
Table 4.
Parameter estimates from the final covariate model and 1,000 bootstrap iterations
| Parameters | Estimate | Bootstrap median (95% CI) |
|---|---|---|
| CL (L/hr) | 29.0 | 28.8 (27.0 − 31.2) |
| V1 (L) | 19.5 | 19.6 (17.2 − 24.1) |
| Q (L/hr) | 28.6 | 28.2 (20.4 − 34.2) |
| V2 (L) | 20.9 | 20.6 (18.3 − 23.2) |
CL, clearance; V1, volume of distribution into the central compartment; Q, inter-compartmental clearance; V2, volume of distribution into the peripheral compartment.
Figure 1. VPC plot of the final model simulated data versus observed data.
Data are presented in a) linear and b) log scales. Dots, the observed data; black dashed line, 2.5th and 97.5th percentiles of the simulated data; black solid line, the median of the simulated data.
Relationship among PK, PD biomarker, and clinical outcomes
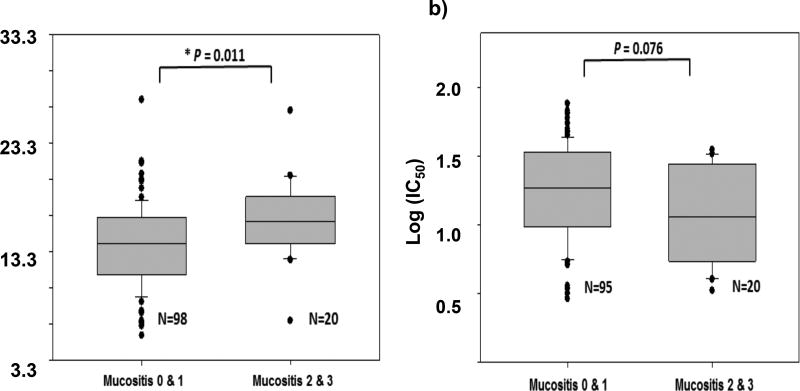
Using pre-treatment PBMCs obtained from 118 enrolled patients, we investigated whether PD biomarkers could be a measure of myeloma cell responsiveness to melphalan and explain portions of inter-subject variability in outcomes. Following treatment of patients’ PBMCs with melphalan, we measured the cytotoxicity (IC50) of melphalan in PBMCs with the WST-1 proliferation assay (n=115). The mean ex vivo IC50 was 21.40 ± 15.43 µM (sd), equivalent to 6.53 ± 4.71 µg/mL, suggesting high variability in patients’ sensitivity to ex vivo melphalan (Supplementary Figure 2). Interestingly, the log (base 10) transformed ex vivo IC50 values were marginally associated with mucositis grade (Wilcoxon rank sum test p=0.076) (Figure 2). Melphalan ex vivo potency appeared to be higher in patients with intermediate or severe mucositis (grades 2 and 3) compared to patients with no or mild mucositis (grades 0 and 1) (Table 5).
Figure 2. Comparison of mucositis grade versus melphalan AUC and ex vivo log (IC50).
Panel a) shows the relationship between melphalan AUC (N=118), and panel b) shows the relationship between log IC50 (N=115) relative to mucositis grade. The association between AUC or log IC50 values and worst mucositis grade (0 & 1 vs 2 & 3) was analyzed using Wilcoxon rank sum test with continuity correction (two-sided). The numbers of patients with worst mucositis grade 0, 1, 2, 3 were 44, 54, 16, and 4, respectively for the AUC evaluation (N=118 total). The numbers of patients with worst mucositis grade 0, 1, 2, 3 were 43, 52, 16, and 4, respectively for the log IC50 evaluation (N=115 total).
Table 5.
Summary statistics of AUCinf, Cmax, and ex vivo IC50 values
| Mucositis grade 0 |
Mucositis grade 1 |
Mucositis grade 2 |
Mucositis grade 3 |
Overall | ||
|---|---|---|---|---|---|---|
|
| ||||||
| AUCinf (mg•hr/L) | 2 hour cohort | |||||
| n | 21 | 27 | 7 | 2 | 57 | |
| mean (sd) | 12.8 (3.7) | 14.5 (4.7) | 16.0 (4.5) | 19.4 (9.7) | 14.2 (4.6) | |
| median (range) | 12.2 (6.7–20.0) | 13.9 (5.6–27.3) | 16.2 (7.0–20.3) | 19.4 (12.6–26.3) | 13.9 (5.6–27.3) | |
|
| ||||||
| 6 hour cohort | ||||||
| n | 23 | 27 | 9 | 2 | 61 | |
| mean (sd) | 14.4 (3.6) | 13.8 (3.1) | 15.5 (2.1) | 17.9 (0.4) | 14.4 (3.2) | |
| median (range) | 14.9 (6.8–21.7) | 14.3 (6.9–19.2) | 14.7 (13.7–20.2) | 17.9 (17.7–18.2) | 14.7 (6.8–21.7) | |
|
| ||||||
| Cmax (µg/mL) | 2 hour cohort | |||||
| n | 21 | 27 | 7 | 2 | 57 | |
| mean (sd) | 8.09 (1.67) | 8.85 (1.76) | 8.63 (2.16) | 10.53 (1.52) | 8.60 (1.80) | |
| median (range) | 8.16 (5.40–11.22) | 8.64 (4.66–11.94) | 9.30 (5.25–11.25) | 10.53 (9.45–11.60) | 8.52 (4.66–11.94) | |
|
| ||||||
| 6 hour cohort | ||||||
| n | 23 | 27 | 9 | 2 | 61 | |
| mean (sd) | 8.39 (1.47) | 8.21 (1.69) | 9.38 (1.13) | 10.21 (0.29) | 8.51 (1.57) | |
| median (range) | 8.19 (6.25–12.16) | 8.22 (5.15–11.29) | 8.75 (7.81–11.15) | 10.21 (10.00–10.41) | 8.54 (5.15–12.16) | |
|
| ||||||
| log(IC50)# | 2 hour cohort | |||||
| n | 21 | 25 | 7 | 2 | 55 | |
| mean (sd) | 1.32 (0.35) | 1.18 (0.29) | 1.13 (0.43) | 0.93 (0.32) | 1.22 (0.34) | |
| median (range) | 1.31 (0.50–1.89) | 1.18 (0.54–1.74) | 1.34 (0.52–1.55) | 0.93 (0.70–1.16) | 1.24 (0.50–1.89) | |
|
| ||||||
| 6 hour cohort | ||||||
| n | 22 | 27 | 9 | 2 | 60 | |
| mean (sd) | 1.18 (0.42) | 1.27 (0.32) | 1.06 (0.34) | 1.16 (0.27) | 1.20 (0.36) | |
| median (range) | 1.32 (0.46–1.81) | 1.27 (0.56–1.70) | 0.96 (0.61–1.52) | 1.16 (0.97–1.35) | 1.27 (0.46–1.81) | |
AUCinf, area under the concentration-time curve calculated from time 0 to infinity ; Cmax, maximum predicted concentration.
The ex vivo IC50 values were log (base 10) transformed.
Further, increasing AUCinf and Cmax was also observed to be associated with worse mucositis grade (median (min – max): AUCinf mg•hr/L: mucositis grade 0–1: 14.1 (5.6 – 27.3); mucositis grade 2–3: 16.1 (7.0 – 26.3); Cmax µg/mL: mucositis grade 0–1: 8.27 (4.66 – 12.16); mucositis grade 2–3: 9.70 (5.25 – 11.60)). Figure 2 and Table 5 describe these associations by mucositis grade. The risk of more severe mucositis (grade 2 or 3) was increased by 21% for every 100 mg•min/L, equivalent to 1.67 mg•hr/L, increase in AUCinf, adjusted for age (age adjusted relative risk for 100 mg•min/L increase: 1.21, 95% CI: 1.04, 1.41, p=0.015). Similarly, as Cmax increased so did the risk of more severe mucositis (age adjusted relative risk for 1 µg/mL increase: 1.26, 95% CI: 0.99, 1.60, p=0.058). Finally, while both AUCinf and Cmax were observed to be strongly related to the duration of neutropenia (3.2% increase in duration (95% CI: 2.2%, 5.3%, p=0.003) for every 100 mg•min/L increase in AUCinf; 4.8% increase in duration (95% CI: 0.8%, 9.0%, p=0.019) for each 1 µg/mL increase in Cmax, adjusted for age, sex, race and treatment group), only AUCinf was associated with days hospitalized (0.23 day increase in days hospitalized (95% CI: 0.01, 0.45, p=0.043) for every 100 mg•min/L increase in AUCinf, adjusted for age, sex, race and treatment group).
Neither AUCinf or Cmax were strongly associated with time to progression or to overall survival. For our analysis, follow up for progression and death ended in November 2016, with 53 of 118 patients observed with progression and 28 deaths for median follow up time of 2.9 years. Adjusted for age, gender, race and treatment group, the risk of progression decreased marginally as Cmax increased (adjusted HR: 0.84, 95% CI: 0.67, 1.05, p=0.130). No association was observed between progression and AUC (adjusted HR for 100 mg•min/L increase: 0.97, 95% CI: 0.85, 1.10, p=0.601). A modest association was observed between overall survival and Cmax (adjusted for age, race and gender HR: 0.80, 95% CI: 0.59, 1.08, p=0.139) but not with AUCinf (adjusted HR for 100 mg•min/L increase: 0.95, 95% CI: 0.81, 1.14, p=0.632).
DISCUSSION
The primary purpose of this trial was to investigate whether a shorter duration of cryotherapy was non-inferior to the standard 6-hour regimen in MM patients. Results from this study indicated that 2 hours of cryotherapy was similar (non-inferior) to 6 hours with respect to grade 3/4 mucositis, length of hospitalization, or development of positive blood cultures; findings that support a 2-hour cryotherapy regimen as the new standard of care (20). As expected, we found a significant association between drug exposure (AUC) and grade of mucositis. This is consistent with results reported in a recent publication by Nath et al. who observed high melphalan AUC is a risk factor for severe mucositis grade (21). Additionally, serum creatinine and body weight, which were relevant to the final covariates in our PK model, have also been reported as risk factors for the development of oral mucositis (22).
We utilized patient samples and relevant clinical data to establish a PK model based on data from 118 patients. Results from our study were consistent with previously identified covariates of creatinine clearance, fat free mass, and hematocrit as important factors that explain significant portions of inter-patient variability (13). In our PK model, we observed that fat free mass explains a significant portion of variability in estimated inter-compartmental clearance and central volume among patients, and it enhanced model fitting better (likelihood ratio test, p < 0.001) than other alternative body size descriptors, e.g. body surface area (p < 0.01) or total body weight (p < 0.05). Similarly, several indicators of renal clearance, including calculated creatinine clearance based on serum creatinine using different equations (Cockcroft and Gault or CKD-EPI) and measured creatinine clearance from 24-hour urine data, were tested to describe the inter-patient variability of melphalan CL. The calculated creatinine clearance using the Cockcroft-Gault equation with total body weight (ΔOFV=−56.49 compared to the base model) was ultimately chosen based on statistical significance and model fitting results.
Our study also prospectively evaluated for the first time the contribution of a SNP in SLC7A5 (rs4240803) to melphalan PK, and this revealed a significant effect of SLC7A5 on drug distribution within the peripheral compartment. The SLC7A5 gene is primarily responsible for the uptake of melphalan into cells (23), and it was identified as a significant variable affecting PK. Drug transporter pharmacogenetics is known to impact drug disposition (24), and the rs4240803 polymorphism in SLC7A5 had previously been shown to be associated with increased use of TPN following HDM (18). Until our trial however, this SLC7A5 polymorphism had not been identified as a predictor of melphalan PK. While a previous study by Kuhne et al. (25) evaluated several polymorphisms in SLC7A5, SLC7A8, and SLC3A2 (the gene encoding the 4F2hc protein, which is the common heavy chain associated protein for both LAT-1 and LAT-2 transporters), this study did not evaluate rs4240803 nor did it identify any significant associations between melphalan pharmacokinetics and any of the polymorphisms evaluated within the 64 patients enrolled. The rs4240803 polymorphism is in the first intron of SLC7A5, and its function is not currently known. Giglia et al. (18) point out this intron is within a region near a putative transcriptional enhancer, and it could therefore impact mRNA expression levels (26). Our observations suggest SLC7A5 may influence melphalan distribution within peripheral compartments, which is consistent with other findings for drug transporter pharmacogenetics (27). Incorporating this covariate into our PK model further enhanced the ability of the model to predict melphalan plasma concentrations.
Fat free mass, creatinine clearance, and hematocrit explain 17.5% of inter-patient variability of melphalan CL compared to the structural PK model (unexplained inter-patient variability decreased from 33.8% to 27.9%), which is nearly identical to the findings previously published by Nath et al. in a 100-patient dataset (33.6% to 26.7%) (13). Overall, results from our study serve as a validation for the previously published model (13). While we discovered that inclusion of SLC7A5 as a covariate on V2 did further improve the model fitting, inter-patient variability was not decreased by addition of this covariate. Furthermore, with data from 118 patients, we observed good model stability and precision with narrow 95% CIs of bootstrap iterations compared to the parameter estimates from the final model (Table 4). In the VPC results, plasma concentrations were slightly over-predicted at early time points, presumably due to under-predicted drug distribution caused by sparse data, extended infusion time (28 – 59 minutes), or highly variable protein-binding fraction of melphalan in some patients (Figure 1). Based on the previous study and the data presented herein, this PK model will be a useful tool for predicting patients’ melphalan PK and melphalan exposure-related outcomes, though more work will need to be done to better predict melphalan concentrations at the early time points.
The relationship observed between ex vivo IC50 and mucositis suggests that IC50 may be a potential biomarker for melphalan toxicities. Induction of apoptosis upon ex vivo melphalan exposure in PBMCs from MM patients was previously correlated to their in vivo response to melphalan treatment (14). This suggests that ex vivo IC50 might be a useful indicator for the sensitivity level of plasma cells in multiple myeloma patients to melphalan exposure. In this respect, while the PK results are important for clinicians to consider, melphalan-induced cell death is critical to understanding variability in outcomes from melphalan therapy. These results suggest more specific PD biomarkers may enable us to further improve the melphalan dosing regimen.
The recent study by Nath et al. (21) demonstrated that higher melphalan exposure is associated with more severe grade of mucositis, although higher exposure was also associated with longer overall survival. Pharmacokinetic results from our study (AUC range 5.6 – 27.3 mg•hr/L, median 14.4 mg•hr/L) were similar to those reported in the Nath study (4.9 – 24.6 mg•hr/L, median 12.8 mg•hr/L). Time to progression and overall survival are modestly associated with Cmax in our study. The observation that increased melphalan AUC may lead to improved progression-free and overall survival in the Nath study highlights a potential advantage to being able to predict melphalan PK and exposure in individual patients. However, it is still not clear how this information can be used to optimize dosing to achieve maximum efficacy (i.e. prolong progression-free and overall survival) while maintaining a reasonable margin of safety. Mucositis can be a severe and painful toxicity, but other melphalan-induced toxicities, such as prolonged severe neutropenia, can be life-threatening. To highlight this point, Nath et al. indicated “Eighty-two percent of patients experienced febrile neutropenia. One patient, with a melphalan AUC of 18.4 mg l–1 h, died due to bacterial pneumonia on day 100 post-transplant…” (21). In our study, the duration of severe neutropenia (ANC < 500 cells per µL) ranged from 2.9 to 13.2 days with a median of 5.7 days. As the risk of infection increases with the duration of severe neutropenia, one strategy for dose optimization may be to maximize melphalan AUC while minimizing the duration of severe neutropenia. As melphalan exposure was associated with duration of severe neutropenia in our study, we are currently pursuing such a strategy.
The primary aim of this study was to compare the effectiveness of 2-hour vs. 6-hour cryotherapy with respect to mucositis and patient tolerance of HDM. The results indicated that 2 hours of cryotherapy was at least as effective at reducing severe oral mucositis as 6 hours. Correlative analyses, including the development of a population PK model and identification of significant covariates that explain inter-patient variability, have provided us tools for predicting melphalan exposure prior to treatment via analysis of relevant patient factors including creatinine clearance, fat free mass, hematocrit, and SLC7A5 rs4240803 genotype. Further work to improve and link this model to outcomes from therapy is underway for personalizing melphalan dosing to minimize adverse outcomes without sacrificing efficacy from HSCT therapy in MM patients.
METHODS
Clinical study design
This single institution, randomized, controlled non-inferiority trial was approved by The Ohio State University Cancer Institutional Review Board, and written informed consent was obtained from all enrolled patients (NCT01653106). Adult patients with multiple myeloma as defined by the International Myeloma Working Group (IWMG) diagnostic criteria admitted for autologous stem cell transplantation were eligible for enrollment (28). Patients were enrolled and randomized in equal numbers to receive either 2 or 6 hours of cryotherapy, with the randomization scheme blocked and stratified by: hemoglobin (< 11 g/dL or ≥ 11 g/dL), fat free mass (30 – 50 kg, 50 – 70 kg, or > 70 kg), and measured creatinine clearance (< 30 mL/min, 30 – 60 mL/min, or > 60 mL/min).
We hypothesized that 2 hours of cryotherapy would be non-inferior to 6 hours of cryotherapy with a non-inferiority margin of 15% with respect to the rate of severe (grade 3 or worse) mucositis. Previous work had observed that approximately 15% of patients on 3 hours of cryotherapy developed grade 3/4 mucositis. Our null hypothesis was that if the true severe mucositis rate for 6 hours of cryotherapy is 15% then the corresponding true severe mucositis rate for 2 hours of cryotherapy is 30% or greater. With a sample size of 146 evaluable patients (73 per arm), we would have at least 80% power for a one-sided test of these hypotheses with a significance level of 0.05.
The primary study endpoint of non-inferiority in severe mucositis and related secondary endpoints (any mucositis and grade 2 or worse mucositis) were evaluated through the difference and associated 90% confidence intervals in these rates between randomized treatment groups (all patients received therapy as assigned). Non-inferiority was considered to be established if the upper limit of the 90% CI was below the non-inferiority margin of 15%, based on a large sample difference in proportions confidence interval. Differences between treatment groups in the incidence of bacteremia were estimated by the two-sample test of proportions and by a two-sample t-test for length of hospital stay, at the two-sided 5% level. Associations between mucositis grade and bacteremia or length of hospital stay were estimated through logistic or linear regression models, adjusted for age and treatment group. All reported p-values and confidence intervals were two-sided and reported at the nominal level unadjusted for multiple comparisons; all analyses were performed using Stata 13.0 (29).
Treatment
Cryotherapy was initiated 15 minutes prior to the start of the melphalan infusion and continued for either 2 or 6 hours depending on treatment arm. Over the duration of the cryotherapy regimen, bone marrow transplant specific nursing staff documented patient adherence and total number of cups of ice consumed. Patients refrained from other oral intake during cryotherapy and were instructed to melt approximately 1 ounce of shaved ice (flavoring permitted) in their mouth with immediate replenishment. A single dose of melphalan was infused over 30 minutes on day −2 (two days prior to transplant) in all patients and included 200 mg/m2 except those patients > 75 years, body mass index (BMI) < 19, and/or with significant renal or cardiac dysfunction, who were treated with 140 mg/m2. All patients received 12 mg dexamethasone on day −2, 8 mg on day −1, and 8 mg either once or twice daily on days 0 and +1. Stem cell support with G-CSF was started either on day +1 or +7 based on institutional standard of care protocol at the time. Patients were followed for adverse events and patient reported outcomes.
Safety and tolerability assessments
Baseline demographics were obtained at the time of enrollment and included age, race, gender, height, weight, measured creatinine clearance, hemogram, myeloma disease status, albumin, total bilirubin, total serum protein, and c-reactive protein. Other data was collected daily and included presence or absence of bacteremia and/or neutropenic fever, TPN use, and diarrhea. Patients were assessed for oral mucositis per the World Health Organization (WHO) criteria (Supplementary Table 2).
Blood sample collection and pharmacokinetic studies
Blood samples were collected from 119 patients enrolled for PK analysis. Similar to a sampling scheme recommended previously for melphalan (13), venous blood samples were collected in heparin tubes prior to melphalan administration and then 5, 30, 45, 60, 180, and 360 minutes after completion of melphalan infusion (a subset of patients also had samples collected at the end of the infusion and at 15 minutes after infusion). Blood sample analyses and PK modeling methods are described in Supplementary Methods.
PBMC collection for PD biomarker studies
Approximately 24 mL samples of venous blood were obtained in green top (heparin) tubes within 2 hours prior to the infusion of melphalan. Samples were collected, mixed, placed on ice and transported to The Ohio State University Leukemia Tissue Bank where Ficoll-Hypaque was used to isolate PBMCs prior to storage at −70°C. While every attempt was made to obtain PBMCs from all patients who provided plasma PK samples, adequate quantities of viable PBMCs to conduct ex vivo and genotyping studies, as described below, were available from only a subset of the patients. PD biomarker experiments are described in Supplementary Methods.
Relationship among PK, PD biomarker, and clinical outcomes
The relationship between ex vivo IC50, AUCinf, and Cmax values with worst mucositis grade (0 & 1 vs 2 & 3) was initially assessed by two-sided Wilcoxon rank sum tests with continuity correlation (R, version 3.1.1). The adjusted (for age, race, gender, and treatment group) relative risk of worse mucositis grade and IC50, AUCinf, and Cmax was made via modified Poisson regression (30). The ex vivo IC50 values of PBMCs were subjected to log (base 10) transformation prior to statistical analyses. Associations between each PK, PD marker and duration of neutropenia or hospital stay was assessed via linear regression, with duration of neutropenia natural log transformed to account for the strong skewness in distribution. The risk of progression or the risk of death associated with level of AUCinf or Cmax was made via Cox proportional hazards models, with baseline hazard stratification on age group. The time to progression was measured as the time from transplant until the date of progression, or progression time was censored on the date a patient was last known to be progression free. Overall survival was calculated as the time from transplant to death or the date last known to be alive.
Supplementary Material
STUDY HIGHLIGHTS.
-What is the current knowledge on the topic?
Current practice utilizes fixed doses of high dose melphalan for HSCT conditioning, but the factors underlying the high variability in patient outcomes, including adverse effects, remain unknown.
-What question does this study address?
This randomized trial aimed to identify an efficacious and tolerable cryotherapy regimen. Additionally, correlative analyses aimed to identify pharmacokinetic/pharmacodynamic factors that account for inter-patient variability with respect to patient outcomes.
-What this study adds to our knowledge
We show that 2 hours of cryotherapy is equally effective to 6 hours. Population pharmacokinetic modeling and investigation of pharmacodynamic endpoints verified fat free mass, hematocrit, and creatinine clearance as significant covariates; furthermore, the SLC7A5 genotype was identified as a significant covariate and improved model fitting.
-How this might change clinical pharmacology or translational science
This study provides randomized clinical trial results supporting a shorter and well tolerated cryotherapy regimen that is at least as efficacious as the standard. Combining the presented data and pharmacokinetic model with other significant covariates that influence patient outcomes could lead to construction of a dosing algorithm that optimizes patient safety and clinical response.
Acknowledgments
This research was supported by Multiple Myeloma Opportunities for Research and Education (MMORE) [CCH, MAP, YKC], a Pelotonia IDEA award (46050-502048) [MAP, CCH, MP], the Ohio State University Comprehensive Cancer Center Core Grant (P30 CA016058) [EH, MAP], and an Eli-Lilly fellowship [YKC]. The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST/DISCLOSURES
None of the authors have a relevant conflict of interest to report. The authors confirm that neither the submitted manuscript nor any similar manuscript, in whole or in part, other than an abstract, is under consideration, in press, or published elsewhere.
AUTHOR CONTRIBUTIONS
M.A.P., Y.K.C., D.W.S., M.L., J.L., J.W., E.M.H., Y.G., D.M.B., Y.A.E., A.E.R., S.M.D., M.J.P., and C.C.H. wrote the manuscript; M.A.P., M.J.P., and C.C.H. designed the research; M.A.P., Y.K.C., D.W.S., M.L., J.L., J.W., Y.G., K.T., N.W., D.M.B., Y.A.E., A.E.R., S.M.D., M.J.P., and C.C.H. performed the research; M.A.P., Y.K.C., D.W.S., J.L., J.W., and E.M.H. analyzed the data.
References
- 1.Attal M, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N. Engl. J. Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Gay F, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–1629. doi: 10.1016/S1470-2045(15)00389-7. [DOI] [PubMed] [Google Scholar]
- 4.Siegel DS, et al. Outcome with lenalidomide plus dexamethasone followed by early autologous stem cell transplantation in the ECOG E4A03 randomized clinical trial. Blood. 2010;116:22–23. doi: 10.1038/bcj.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parmar SR, et al. Comparison of 1-day vs 2-day dosing of high-dose melphalan followed by autologous hematopoietic cell transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2014;49:761–766. doi: 10.1038/bmt.2014.56. [DOI] [PubMed] [Google Scholar]
- 6.Sonis ST, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J. Clin. Oncol. 2001;19:2201–2205. doi: 10.1200/JCO.2001.19.8.2201. [DOI] [PubMed] [Google Scholar]
- 7.Blijlevens N, et al. In a high-dose melphalan setting, palifermin compared with placebo had no effect on oral mucositis or related patient's burden. Bone Marrow Transplant. 2013;48:966–971. doi: 10.1038/bmt.2012.257. [DOI] [PubMed] [Google Scholar]
- 8.Lilleby K, et al. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37:1031–1035. doi: 10.1038/sj.bmt.1705384. [DOI] [PubMed] [Google Scholar]
- 9.Nooka AK, et al. Pharmacoeconomic analysis of palifermin to prevent mucositis among patients undergoing autologous hematopoietic stem cell transplantation. Biol. Blood. Marrow Transplant. 2014;20:852–857. doi: 10.1016/j.bbmt.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvador P, Azusano C, Wang L, Howell D. A pilot randomized controlled trial of an oral care intervention to reduce mucositis severity in stem cell transplant patients. J. Pain. Symptom Manage. 2012;44:64–73. doi: 10.1016/j.jpainsymman.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Toro JJ, et al. A prospective, randomized clinical trial of cryotherapy vs. supersaturated calcium phosphate rinses vs. saline rinses for the prevention of oral mucositis in patients with multiple myeloma (MM) receiving high-dose melphalan (HDM) and autotransplantation. Biol. Blood. Marrow Transplant. 2014;20:S204–205. [Google Scholar]
- 12.Mori T, Aisa Y, Yamazaki R, Mihara A, Ikeda Y, Okamoto S. Cryotherapy for the prevention of high-dose melphalan-induced oral mucositis. Bone Marrow Transplant. 2006;38:637–638. doi: 10.1038/sj.bmt.1705494. [DOI] [PubMed] [Google Scholar]
- 13.Nath CE, et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br. J. Clin. Pharmacol. 2010;69:484–497. doi: 10.1111/j.1365-2125.2010.03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gkotzamanidou M, Sfikakis PP, Kyrtopoulos SA, Bamia C, Dimopoulos MA, Souliotis VL. Chromatin structure, transcriptional activity and DNA repair efficiency affect the outcome of chemotherapy in multiple myeloma. Br. J. Cancer. 2014;111:1293–1304. doi: 10.1038/bjc.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gkotzamanidou M, et al. Progressive changes in chromatin structure and DNA damage response signals in bone marrow and peripheral blood during myelomagenesis. Leukemia. 2014;28:1113–1121. doi: 10.1038/leu.2013.284. [DOI] [PubMed] [Google Scholar]
- 16.Yanagida O, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg GJ, Lam HY, Begleiter A. Active carrier-mediated transport of melphalan by two separate amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J. Biol. Chem. 1979;254:1057–1064. [PubMed] [Google Scholar]
- 18.Giglia JL, et al. A single nucleotide polymorphism in SLC7A5 is associated with gastrointestinal toxicity after high-dose melphalan and autologous stem cell transplantation for multiple myeloma. Biol. Blood. Marrow Transplant. 2014;20:1014–1020. doi: 10.1016/j.bbmt.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikhael JR, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin. Proc. 2013;88:360–376. doi: 10.1016/j.mayocp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Sborov DW, et al. 2-hour cryotherapy effectively reduces severe mucositis associated with high-dose melphalan followed by stem cell rescue: results from a randomized trial. Blood. 2014;124:3960. [Google Scholar]
- 21.Nath CE, et al. High melphalan exposure is associated with improved overall survival in myeloma patients receiving high dose melphalan and autologous transplantation. Br. J. Clin. Pharmacol. 2016;82:149–159. doi: 10.1111/bcp.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grazziutti ML, et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant. 2006;38:501–506. doi: 10.1038/sj.bmt.1705471. [DOI] [PubMed] [Google Scholar]
- 23.Kuhne A, Tzvetkov MV, Hagos Y, Lage H, Burckhardt G, Brockmoller J. Influx and efflux transport as determinants of melphalan cytotoxicity: Resistance to melphalan in MDR1 overexpressing tumor cell lines. Biochem. Pharmacol. 2009;78:45–53. doi: 10.1016/j.bcp.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Undevia SD, Gomez-Abuin G, Ratain MJ. Pharmacokinetic variability of anticancer agents. Nat. Rev. Cancer. 2005;5:447–458. doi: 10.1038/nrc1629. [DOI] [PubMed] [Google Scholar]
- 25.Kuhne A, et al. Genetic polymorphisms in the amino acid transporters LAT1 and LAT2 in relation to the pharmacokinetics and side effects of melphalan. Pharmacogenet. Genomics. 2007;17:505–517. doi: 10.1097/FPC.0b013e3280ea77cd. [DOI] [PubMed] [Google Scholar]
- 26.Cooper DN. Functional intronic polymorphisms: Buried treasure awaiting discovery within our genes. Hum. Genomics. 2010;4:284–288. doi: 10.1186/1479-7364-4-5-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grover A, Benet LZ. Effects of drug transporters on volume of distribution. AAPS J. 2009;11:250–261. doi: 10.1208/s12248-009-9102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durie BG, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 29.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 30.Zou G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.