Abstract
Understanding factors contributing to individual differences in opioid addiction vulnerability is essential for developing more effective preventions and treatments. Sensation seeking has been implicated in addiction to several drugs of abuse, yet its relationship with individual differences in opioid addiction vulnerability has not been well established. The primary goal of this study was to evaluate the relationship between locomotor activity in a novel environment, a preclinical model of sensation-seeking, and individual differences in acquisition of i.v. morphine self-administration (SA) in rats. A secondary goal was to evaluate the relationship between activity and elasticity of demand (reinforcing efficacy) for morphine measured using a behavioral economic approach. Following an initial locomotor activity screen, animals were allowed to acquire morphine SA at a unit dose of 0.5 mg/kg/infusion in 4 hour/day sessions (Experiment 1) or 0.2 mg/kg/infusion in 2 hour/day sessions (Experiment 2) until infusion rates were stable. Unit price was subsequently manipulated via progressive reductions in unit dose (Experiment 1) or increases in response requirement per infusion (Experiment 2). Activity levels were not correlated with acquisition of morphine SA in either experiment. Morphine consumption was generally well described by an exponential demand function in both experiments (R2 values > 0.95 for rats as a group), but activity did not correlate with behavioral economic measures. Locomotor activity in a novel environment did not predict individual differences in acquisition of morphine SA. These data complement findings from some human studies and suggest that the role of sensation seeking in individual differences in opioid addiction vulnerability may be limited.
Keywords: Opioid, morphine, self-administration, individual differences, locomotor activity, sensation-seeking
1. Introduction
Opioid addiction poses a tremendous burden on public health (Center for Behavioral Health Statistics and Quality, 2013; Center for Behavioral Health Statistics and Quality, 2015). Although many people experiment with opioids, only a minority undergo the loss of control over drug use that defines addiction (American Psychiatric Association, 2013; Belin et al., 2016). Understanding the behavioral and neurobiological mechanisms contributing to individual differences in opioid addiction vulnerability is essential for developing more effective preventions and treatments.
Sensation-seeking, or the pursuit of novel and intense experiences and a willingness to take risks in order to attain such experiences (Zuckerman, 1994), has been implicated in vulnerability to addiction to a variety of drugs including stimulants (e.g. cocaine, amphetamine) and alcohol (Bardo et al., 2013; Belin et al., 2016; Hittner & Swickert, 2006; Piazza et al., 1989). However, the relationship between sensation-seeking and opioid addiction vulnerability has not been well established. Some studies have shown a positive relationship between sensation-seeking and opioid use in humans (Franques et al., 2003; Kosten et al., 1994; Vest et al., 2016), while others have shown either no relationship (Conrod et al., 2000; Marino et al., 2013; Nielsen et al., 2012) or a negative relationship (Ahn & Vassileva, 2016). The reasons for these discrepancies across studies are unclear, but may reflect differences in subject characteristics (e.g., age, sex, drug use history, and/or comorbidities), measure(s) of sensation-seeking, or other factors (Marino et al., 2013).
Animal models allow for greater experimental control than human studies, and could be useful for understanding the role of sensation-seeking in opioid addiction vulnerability. Spontaneous locomotor activity in a novel environment is a commonly used preclinical model of sensation-seeking (Blanchard et al., 2009; Pawlak et al., 2008; Piazza et al., 1989). Consistent with the relationship between sensation seeking and stimulant use in humans, higher activity reliably predicts greater self-administration (SA) of stimulants (e.g., cocaine, amphetamine), particularly in terms of acquisition (Belin et al., 2008; Belin et al., 2011; Belin & Deroche-Gamonet, 2012; Piazza et al., 1989; Piazza et al., 2000).
Only limited data are available regarding the relationship between spontaneous locomotor activity and individual vulnerability in i.v. opioid SA. In a comparison between several inbred rat strains, those strains with higher activity levels also exhibited greater acquisition of morphine SA under certain conditions (Ambrosio et al., 1995; see Discussion for further details). However, the relationship between locomotor activity and opioid SA in outbred rodents has not been evaluated. This represents an important research gap given that inbred and outbred rats are genetically distinct, and because findings on predictors of addiction vulnerability in inbred and outbred rat strains are not always concordant (Cadoni et al., 2015; Chaouloff et al., 1995; Dilleen et al., 2012; Meyer et al., 2010).
The primary goal of this study was to evaluate locomotor activity as a predictor of individual differences in the acquisition of morphine SA in outbred rats. Because activity did not predict acquisition of morphine SA under the conditions initially studied (0.5 mg/kg/infusion, 4 hr/day sessions), we evaluated the generality of this finding to a different model with a lower dose and shorter access period (0.2 mg/kg/infusion, 2 hr/day sessions). This approach was used because the relationship between activity and SA of other drugs (e.g. cocaine) can be more apparent when lower unit doses and/or shorter access periods are used (Belin et al., 2016; Kabbaj, 2006; Mantsch et al., 2001).
A secondary goal was to apply a behavioral economics framework to the analysis of individual differences in morphine SA. Behavioral economics involves evaluation of the extent to which consumption of a reinforcer (e.g., drug) is maintained following increases in its unit price, which in drug SA models is operationalized as the cost-benefit ratio of response requirement/unit dose (Bickel et al., 2000; Hursh, 1991; Hursh & Silberberg, 2008). Behavioral economics has been useful for studying individual differences in elasticity of demand (i.e., reinforcing efficacy) of numerous addictive drugs (e.g., cocaine) in both humans and animals (Diergaarde et al., 2008; Grebenstein et al., 2013, Hursh & Silberberg, 2008), but has not yet been applied to morphine SA in rodents. Therefore, we evaluated elasticity of demand in animals that acquired morphine SA in both experiments in order to 1) evaluate the precision and generalizability of a behavioral economic framework in the context of morphine SA, and 2) provide a preliminary evaluation of the relationship between locomotor activity and individual differences in behavioral economic measures.
2. Materials and Methods
2.1. Animals
Male adult Sprague Dawley rats (Envigo, Indianapolis, IN) weighing 276-300 g at arrival were used. All rats were individually housed in a temperature- and humidity-controlled colony room with unlimited access to water under a reversed 12-h light/dark cycle (lights off at 10:00 hr). All behavioral testing occurred during the dark (active) phase. Beginning one week following arrival, food was restricted to 18 g/day to facilitate operant performance, avoid detrimental health effects of long-term ad libitum feeding, and limit catheter migration. Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 2011 National Research Council’s Guide for the Care and Use of Laboratory Animals and the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.
2.2. Apparatus
2.2.1. Locomotor Activity
Locomotor activity was monitored in 43 × 43 cm open field activity chambers (Med Associates, Inc., St. Albans, VT). Each chamber had two 16-beam photocell arrays placed 5 cm and one array 18 cm above the chamber floor to monitor horizontal and vertical activity, respectively. Chambers were placed inside sound-attenuating cubicles equipped with exhaust fans that provided masking noise and ambient lighting. Open-field activity software (Med Associates) was used for operating the apparatus and recording data.
2.2.2. Morphine Self-Administration
Self-administration (SA) sessions were conducted using 16 standard operant conditioning chambers (model ENV-007, Med Associates, Inc). Each chamber contained two response levers, a white stimulus light located 2 cm above each lever, and a house light that provided ambient illumination. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. An infusion pump (model PHM-100-15, Med Associates) placed outside each cubicle delivered infusions at a rate of 100 μl/kg per second. MED-PC IV software (Med Associates) was used for operating the apparatus and recording data.
2.3. Drugs
Morphine sulfate (NIH National Institute on Drug Abuse Drug Supply Program, Bethesda, MD) was dissolved in sterile saline and heparin (30 units/ml) was added to maintain catheter patency. Morphine doses are expressed as the weight of the salt.
2.4 Surgical Procedures
Each rat was implanted with a chronic indwelling catheter into the right jugular vein under isoflurane (1%-3%) anesthesia, using general surgical procedures described in detail elsewhere (Harris et al, 2008; LeSage et al, 2002). The catheter was externalized between the scapulae and attached to A vascular-access harness (VAH95AB, Instech Laboratories, Plymouth Meeting, PA) that allowed connection to a fluid swivel via a tether for morphine administration. Animals were allowed to recover for one week after surgery, during which time they received daily i.v. infusions of heparinized saline, ceftriaxone antibiotic (5.25 mg, first three days only), and s.c. injections of buprenorphine (0.05 mg/kg; first two days only) for analgesia. Infusions of methohexital (0.1 ml, 10 mg/ml, i.v.) were administered to check patency post-session on Fridays throughout all protocols. If a catheter became occluded (indicated by a failure of the animal to exhibit anesthesia within 3-5 sec after methohexital infusion), another catheter was implanted into the ipsilateral femoral vein. Failure of this second catheter resulted in removal of the animal from the study.
2.6. Experimental Protocols
2.6.1. Experiment 1
Six days after arrival, rats (N = 16) were monitored for locomotor activity in a novel open field for 2 hours. At least 24 hours later, rats were catheterized as described above. After a 7-10-day recovery period, rats were allowed to acquire i.v. morphine SA during daily 4 hr sessions conducted Mon-Fri. During each session, responding on the left (“active”) response lever resulted in an i.v. infusion of morphine sulfate at a unit dose (0.5 mg/kg/infusion) that maintains robust SA and that has previously been used to evaluate other determinants (e.g., pain sensitivity) of individual differences in morphine SA (Park et al., 2012; Nishida et al., 2016). Each infusion was accompanied by offset of the house light and the onset of a white cue light above the active response lever. Following a 5-second timeout period, the cue light above the active lever was extinguished to signal availability of the next infusion. Responses on the other lever in the operant chamber (the “inactive” lever) were recorded but had no programmed consequences. On the first day of acquisition (always a Friday), food powder was placed on the active lever to facilitate contact with the lever. Data from this session were not included in the data analysis. Beginning the following Monday, rats were tested under an FR 1 schedule for at least 10 sessions and until acquisition criteria were met (≥ 5 infusions per session, ≤20% coefficient of variation, and ≥ 2:1 response ratio on the active to inactive lever) across 3 sessions, at which point the FR was increased to FR 2 for at least 5 sessions and then to FR 3. We used a FR 3 schedule prior to unit dose manipulation in order to be consistent with previous studies evaluating elasticity of demand for other drugs (e.g, nicotine) in our lab (Grebenstein et al., 2013; Grebenstein et al., 2015; Raleigh et al., 2014). When infusion rates were stable under the FR 3 schedule (same stability criteria as above), unit price was manipulated by progressively reducing the morphine unit dose according to the following progression: 0.3, 0.1, 0.05, 0.025, 0 mg/kg. Each unit dose was tested for 5 sessions. Four rats exhibited self-mutilation and were tested under an accelerated schedule in order to expedite the protocol. These animals were tested in 1-3 sessions per unit dose at either 1 (n = 3) or 2 (n = 1) of the 6 unit doses. All of these animals completed the standard 5 sessions per unit dose for the remaining unit doses. Data for these 4 animals did not impact our overall conclusions and are included in the analyses below.
2.6.2. Experiment 2
Rats (N = 22) were tested for locomotor activity as described in Experiment 1, catheterized, and allowed to acquire i.v. morphine SA at a unit dose of 0.2 mg/kg/infusion (n = 16) or 0 mg/kg/infusion (saline) (n = 6) in 2 hour/day sessions under a FR 1 schedule for at least 10 sessions. A longer timeout period following each infusion (30 sec rather than 5 sec) was used in an attempt to avoid the self-mutilation observed in Experiment 1. No mutilation was observed at any point in this experiment. In animals exhibiting stable SA at FR 1 (same criteria as above), the FR was increased every 5 sessions according to the following progression: FR 2, 3, 6, 12, 24, etc., until infusion rates declined by at least 90% compared to FR1. Unit price was manipulated via increases in FR requirement rather than decreases in unit dose for two reasons. First, based on data from Experiment 1, it was unclear whether a dose-reduction protocol using a 0.2 mg/kg/infusion training dose would provide a sufficient number and range of unit prices for a behavioral economic analysis (see Fig 1D). This approach also allowed us to evaluate the precision of the exponential demand function for describing morphine consumption when unit price was manipulated in this manner. In theory, increasing unit price via dose reduction or FR escalation should produce functionally equivalent effects on consumption (Bickel et al., 1995; Greenwald & Hursh, 2006).
Figure 1.
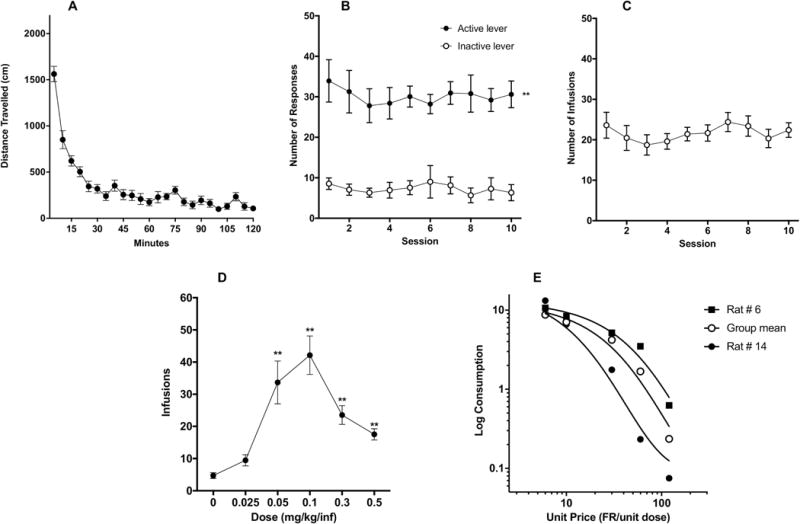
(A) Mean (± SEM) distance traveled in 5-minute blocks during locomotor testing. Mean (± SEM) number of response on the active and inactive levers (B) and infusions per session (C) during acquisition in Experiment 1. (D) Mean (± SEM) number of infusions at each morphine SA unit dose during demand testing. (E) Exponential demand curve describing morphine consumption as a function of unit price for rats as a group. Demand curves for individual rats with relatively low (rat #6) and high (rat #14) elasticity of demand are also shown.
2.7. Statistical Analyses
All statistical analyses and graphing were performed either in GraphPad Prism 7 or R (ver. 3.2.3). Locomotor activity was measured as total distance traveled (in cm) during the 2-hour activity test and was analyzed using one-way ANOVA. Secondary measures of activity included ambulatory count (horizontal photobeam breaks), stereotypic count (repetitive horizontal photobeam breaks) and vertical count (vertical photobeam breaks). In general, lever presses or infusions during morphine SA were analyzed using ANOVA followed by Sidak’s or Dunnet’s multiple comparison tests (see Results for more details). The primary measure of morphine SA acquisition was the mean number of infusions per session during the first 10 days of acquisition. Secondary measures of acquisition were 1) mean number of infusions during the first 10 minutes of each session during the first 10 days of acquisition, a period that can be especially sensitive for detecting predictors of morphine SA (Nishida et al., 2016), and 2) number of days to achieve acquisition criteria. Pearson’s correlation was used to examine relationships between locomotor activity and measures of morphine SA acquisition and demand (see below). To confirm that collapsing locomotor data across the entire 2 hour locomotor session did not obscure a relationship between early-session activity and morphine SA, these correlational analyses were repeated using locomotor activity during only the first 30 minutes of the session. Finally, in an additional analysis, measures of morphine SA were compared between subgroups of rats designated as high or low responders based on whether their 2 hour activity level was above or below the median of the sample (see Dellu et al., 1996; Piazza et al., 1989; Piazza et al., 2000). In both experiments, these comparisons yielded the same conclusions as the correlational analyses and are not reported.
To determine elasticity of demand (reinforcing efficacy) during unit dose reduction (Experiment 1) or FR escalation (Experiment 2), exponential demand curve analyses were conducted using the following equation:
In this model, Q is the quantity consumed. The independent variable, C, is the cost of morphine based on the unit price (FR/unit dose). The free parameters, Q0 and α are estimated from the best-fit function and refer to the maximum level of consumption at zero price (i.e., level or “intensity” of demand) and the rate of change in consumption with increases in unit price, respectively. The range of the exponential function, k is a constant specifying the range of consumption in log units. The k value is held constant across all data sets being compared in each experiment (set to 2.3 in Experiment 1 and 1.8 in Experiment 2), because changes in k impact the value of α. The α parameter is considered a measure of reinforcing efficacy, such that drugs that produce rapidly declining (elastic) demand curves have higher α values and lower reinforcing efficacy than demand curves with slower declining (inelastic) demand curves. Therefore, α served as the index of elasticity of the demand for, or the reinforcing efficacy of morphine. Other demand measures of interest included: Q0, the level or intensity of demand as described above; Omax, the maximal response output; and Pmax, the unit price at which maximal response output occurred. Demand functions were generated using a template for GraphPad Prism software provided by the Institutes for Behavior Resources, Inc. (Baltimore, MD) on their website.
3. Results
3.1. Experiment 1
3.1.1. Locomotor Activity
One-way ANOVA of locomotor activity in rats that later completed at least 10 days of morphine self-administration (SA) indicated a main effect of time (F(6.939, 104.1) = 43.35, p < 0.0001). Activity levels were highest during the first 30 minutes of the 2-hour session (Fig 1A), consistent with previous literature (e.g., Piazza et al., 1989).
3.1.2. Acquisition
All data were removed for one rat that pulled out its catheter following 8 acquisition sessions and died during i.v. catheter re-implantation. A two-factor ANOVA on data for the remaining 15 animals indicated a significant main effect of lever (F(1, 14) = 119.2, p<0.0001), but no main effect of session or interaction during the first 10 acquisition sessions (Fig 1B). Sidak’s multiple comparisons test further showed significant differences in responding on the active versus inactive levers during all 10 sessions (t = 5.24 – 6.93, p<0.0001). Infusion rate did not differ significantly across sessions (Fig 1C).
3.1.3. Elasticity of Demand
One rat did not complete dose-response testing due to multiple catheter occlusions. Data for this animal have been removed. One-way ANOVA on data for the remaining animals revealed a significant effect of dose on number of infusions (F (1.786, 23.21) = 19.91, p<0.0001). Post-hoc Dunnett’s multiple comparisons test showed that infusions at 0.05, 0.1, 0.3, 0.5 mg/kg/infusion were all significantly higher than at 0 mg/kg/infusion (q = 4.41 – 6.35, p < 0.0001-0.003), while there was only a marginally significant difference between infusions at 0.025 versus 0 mg/kg/infusion (q = 2.69, p= 0.0686, see Fig 1D).
Morphine consumption during demand testing was well-described by an exponential demand function, with R2 values typically ≥ 0.85 for individual animals and R2 = 0.97 for rats as a group (Table 1, Figure 1E). There was a considerable degree of individual variability in α values (i.e., elasticity of demand), with some rats showing a rapid decline in morphine consumption following increases in unit price (i.e., reductions in unit dose) (e.g., rat #14 in Table 1 and Fig 1E) and others maintaining significant consumption despite the increases in unit price (e.g., rat #6 in Table 1 and Fig 1E).
Table 1.
Exponential demand curve parameters for individual subjects
| Subject | α | Q0 | Pmax | Omax | R2 |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| 1 | 0.00046 | 23.0 | 22.8 | 168.5 | 0.89 |
| 2 | 0.00057 | 17.0 | 24.9 | 136.0 | 0.91 |
| 3 | 0.00078 | 14.0 | 22.1 | 99.4 | 0.83 |
| 4 | 0.00060 | 9.9 | 40.6 | 129.2 | 0.95 |
| 5 | 0.00070 | 14.0 | 24.6 | 110.8 | 0.85 |
| 6 | 0.00048 | 13.0 | 38.6 | 161.5 | 0.96 |
| 7 | 0.00079 | 9.9 | 30.8 | 98.1 | 0.98 |
| 8 | 0.00290 | 1.7 | 48.9 | 26.7 | 0.81 |
| 9 | 0.00078 | 15.0 | 20.6 | 99.4 | 0.98 |
| 10 | 0.00100 | 12.0 | 20.1 | 77.5 | 0.96 |
| 11 | 0.00320 | 2.6 | 29.0 | 24.2 | 0.89 |
| 12 | 0.00092 | 13.0 | 20.2 | 84.3 | 0.96 |
| 13 | 0.00100 | 17.0 | 14.2 | 77.5 | 0.92 |
| 14 | 0.00130 | 19.0 | 9.8 | 59.6 | 0.97 |
| Mean | 0.00111 | 12.94 | 26.23 | 96.62 | 0.92 |
| SEM | 0.00229 | 1.54 | 2.83 | 11.62 | 0.02 |
| Experiment 2 | |||||
| 1 | 0.00160 | 6.8 | 29.7 | 64.3 | 0.65 |
| 2 | 0.00650 | 12.0 | 4.1 | 15.8 | 0.83 |
| 3 | 0.00150 | 4.7 | 45.8 | 68.6 | 0.85 |
| 4 | 0.00098 | 6.2 | 53.1 | 105.0 | 0.92 |
| 5 | 0.00240 | 4.8 | 28.0 | 42.9 | 0.77 |
| 6 | 0.00140 | 8.4 | 27.4 | 73.5 | 0.92 |
| 7 | 0.00058 | 11.0 | 50.6 | 177.4 | 0.91 |
| 8 | 0.00076 | 3.5 | 121.3 | 135.4 | 0.85 |
| Mean | 0.00197 | 7.18 | 45 | 85.36 | 0.84 |
| SEM | 0.00068 | 1.08 | 12.27 | 18.36 | 0.03 |
Note. The parameter k is set to 2.3 log units for experiment 1 and 1.8 log units for experiment 2.
3.1.4. Correlations
Pearson’s correlation indicated that total distance traveled during the 2-hour locomotor test was not correlated with average daily infusion rate during acquisition of morphine self-administration (SA) (r = 0.05, p = 0.86), average infusions during the first 10 minutes of each session during acquisition (r = −0.21, p = 0.46) (Figure 2A-B), or days to acquire (r = 0.23, p = 0.41) (Figure 2C; n = 15). Moreover, distance traveled during locomotor testing did not predict elasticity of demand (reinforcing efficacy) (r = −0.11, p = 0.70) (Figure 2D), intensity of demand (maximum consumption at zero price) (r = 0.10, p = 0.73) (Figure 2E), Pmax (r = 0.14, p = 0.63), or Omax (r = 0.18, p = 0.55) (data not shown graphically; n = 14). Consistent with the above analyses, activity during the first 30 minutes of the 2 hour locomotor test was not correlated with average daily infusion rate during acquisition (r = 0.31, p = 0.26) or any other measure of morphine SA (data not shown). Secondary activity measures including ambulatory count (mean ± SEM = 2027.7 ± 181.1), stereotypic count (12403.3 ± 702.1), and vertical count (208.7 ± 21.5) were not significantly correlated with average daily infusion during acquisition or other measures of morphine SA (all p ≥ 0.33) (data not shown).
Figure 2.
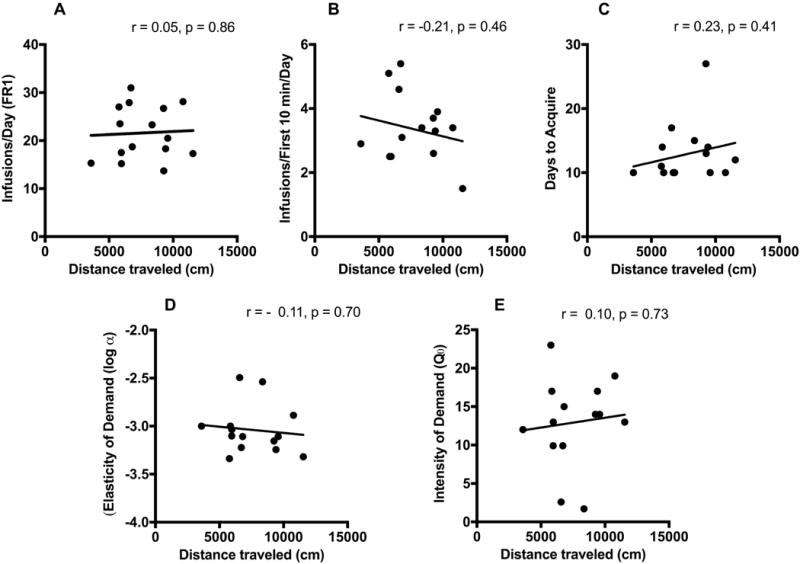
Relationship between distance traveled during the 2 hour locomotor activity test and (A) mean daily infusions during the first 10 sessions of acquisition, (B) mean daily infusions during the first 10 minutes of each session during the first 10 acquisition sessions, (C) number of days to acquire, (D) elasticity of demand (log α) and (E) intensity of demand (Q0).
3.2. Experiment 2
3.2.1. Locomotor Activity
As in Experiment 1, one-way ANOVA of locomotor activity indicated a main effect of time (F(7.058, 105.9) = 69.27, p < 0.0001), with most activity occurring during the first 30 minutes of the 2-hour session (Fig 3A).
Figure 3.
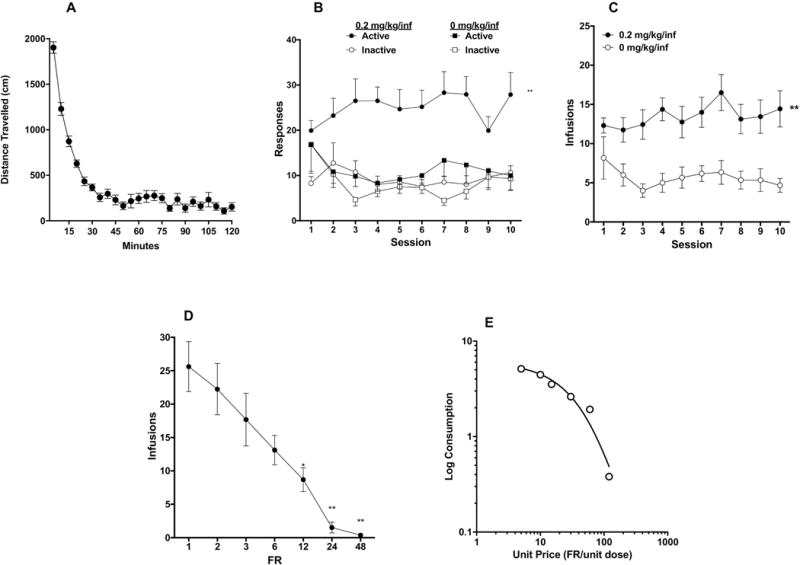
(A) Mean (± SEM) distance traveled in 5-minute blocks during locomotor testing. Mean (± SEM) response on the active and inactive levers (B) and total infusions per session (C) during the first 10 sessions of acquisition in rats responding for morphine (0.2 mg/kg/inf) or saline (0 mg/kg/inf) rats in Experiment 2. (D) Mean (± SEM) number of infusions at FR during demand testing. (E) Exponential demand curve describing morphine consumption as a function of unit price for rats as a group.
3.2.2. Acquisition
A three-factor ANOVA (lever x drug x session) indicated significant main effects of lever (active vs inactive) (F(1, 20) = 10.70, p=0.004) and drug (morphine vs. saline) (F(1, 20) = 10.25, p=0.004), and a significant lever x drug interaction (F(1, 20) = 5.14, p=0.035). There was no main effect of session or other interactions. A two-factor ANOVA on data for the morphine group indicated a significant effect of lever (F (1, 15) = 22.16, p=0.0003), but no effect of session or interaction (Fig 3B). Sidak’s multiple comparisons test further showed significant differences in responding on the active versus inactive levers during all 10 sessions (t = 3.33 – 6.46, p<0.01). Analysis of active and inactive lever data for rats in the saline group indicated no effect of lever, session, or interaction.
Analysis of infusion rates indicated a significant effect of drug (F (1, 20) = 11.36, p=0.0030), but no effect or session or interaction (Fig 3C).
3.2.3. Elasticity of Demand
None of the animals in the saline group were tested for demand because none of them achieved SA acquisition criteria. Eight of the 16 animals in the morphine group did not complete this phase due to failure to acquire, loss of catheter patency, or other problem. One-way repeated measures ANOVA on data from the 8 remaining animals revealed significant effect of FR on number of morphine infusions (F (1.941, 15.53) = 21.29, p<0.0001). Post-hoc Dunnett’s multiple comparisons test showed that infusions at FR 12, 24 and 48 were all significantly lower than at FR 1 (q = 3.94 – 6.62, p = 0.02-0.0007). Infusions at FR 6 only marginally differed from infusions at FR 1 (q = 3.00, p= 0.07) (Fig 3D).
Morphine consumption during demand testing was generally well-described by an exponential demand function (R2 = 0.96 for rats as a group, Fig 3E), with considerable individual variability in α values (Table 1).
3.2.4. Correlations
Distance traveled during the 2-hour locomotor test was not correlated with average daily infusion rate during acquisition of morphine SA (r = 0.06, p = 0.82, n = 16), average infusions during the first 10 minutes of each session (r = −0.16, p = 0.56, n = 16) (Figure 4A-B), or days to acquire (r = −0.11, p = 0.73, n = 13) (Figure 4C). Moreover, distance traveled did not predict elasticity of demand (r = −0.21, p = 0.62) (Figure 4D), intensity of demand (r = −0.13, p = 0.77) (Figure 4E), Pmax (r = 0.30, p = 0.47), or Omax (r = 0.20, p = 0.64) (data not shown graphically; n = 8 for all behavioral economic measures). Activity during the first 30 minutes of the 2 hour locomotor session also was not correlated with average daily infusion rate during acquisition (r = 0.002, p = 0.99) or any other measures of morphine SA (data not shown). Other measures including ambulatory count (mean ± SEM = 2640.9 ± 177.5), stereotypic count (14046.4 ± 755.6) and vertical count (228.3 ± 15.7) were not correlated with average daily infusion during acquisition or other measures of morphine SA (all p ≥ 0.25) (data not shown).
Figure 4.
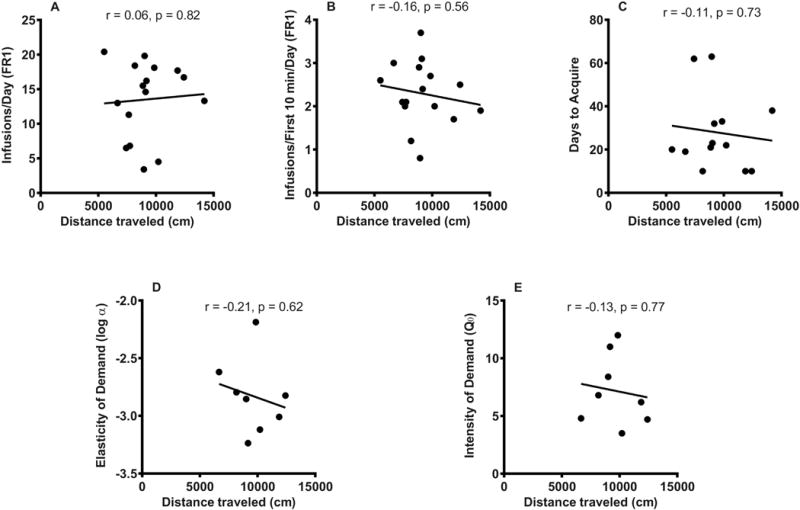
Relationship between distance traveled during the 2 hour locomotor activity test and (A) mean daily infusions during the first 10 sessions of acquisition, (B) mean daily infusions during the first 10 minutes of each session during the first 10 acquisition sessions, (C) number of days to acquire; (D) elasticity of demand (log α), and (E) intensity of demand (Q0) in the morphine SA group. There are fewer data points for (C-E) than in (A) and (B) due to attrition (see text).
4. Discussion
The main finding of this study is that spontaneous locomotor activity did not predict acquisition of morphine self-administration (SA) in rats using two distinct morphine SA protocols. To the extent that activity in an open field is relevant to sensation-seeking, our data are consistent with some human studies indicating a lack of positive relationship between sensation seeking and opioid addiction vulnerability (Ahn & Vassileva, 2016; Marino et al., 2013; Nielsen et al., 2012). For example, a recent analysis showed that high sensation-seeking and impulsivity are predictors of greater vulnerability to amphetamine addiction but not heroin addiction (Ahn & Vassileva, 2016). In contrast, other human studies indicate that sensation-seeking is positively associated with opioid addiction vulnerability (Cheng et al., 2015; Franques et al., 2003; Kosten et al., 1994). Taken together, these data suggest that the relationship between sensation-seeking and opioid addiction vulnerability may be complex and may not be observable under all conditions.
Our data with outbred rats contrast with a previous report that inbred rat strains with higher rates of spontaneous locomotor activity also exhibited greater acquisition of morphine SA (Ambrosio et al., 1995). This discrepancy may reflect genetic differences between outbred and inbred rat strains (Zhou et al., 2008) and/or our use of a within-strain rather than between-strain comparison. In addition, the significant between-strain correlation reported in Ambrosio et al. (1995) was only observed when activity was measured after catheterization. This relationship was lost when activity was measured before catheterization (i.e., catheterization changed the rank order of inbred strains in terms of locomotor activity), which is the approach used in this study and most others evaluating activity/drug SA relationships. These factors, as well as other methodological differences across studies (e.g., unit dose), could account for the difference.
Based on their findings that spontaneous locomotor activity predicted acquisition of both food and cocaine SA, Mitchell and colleagues suggested that locomotor activity predicts a general learning capability for operant lever pressing rather than drug use propensity per se (Mitchell et al., 2005). Our findings that locomotor activity did not predict acquisition of morphine SA contrasts with this prediction, and suggests that the relationship between activity and self-administration may be more complex.
Our current extension of behavioral economics to morphine SA further supports the precision, generalizability, and utility of this analytical approach. An exponential demand function described morphine consumption well, consistent with findings using drugs and other reinforcers in animals and humans (Grebenstein et al., 2013, Hursh, 1991, Hursh & Silberberg, 2008). Importantly, considerable individual differences were observed in α in both studies (Table 1), supporting the use of this approach to measure individual differences in addiction vulnerability. Evaluation of other factors (e.g., opioid withdrawal sensitivity) as determinants of individual differences in demand for morphine is warranted.
The primary goal of this study was to understand the relationship between locomotor activity and morphine SA rather than direct comparison between the determinants of individual differences in morphine versus stimulant addiction. Nonetheless, our data contrast with findings indicating that activity reliably predicts acquisition of SA and reinforcing efficacy of stimulants (e.g., cocaine, amphetamine, nicotine) (Belin et al., 2011, Belin et al., 2016, Suto et al., 2001; Turner et al., 2008; Piazza et al., 2000). They also complement studies that other predictors of stimulant SA (e.g., impulsivity) do not predict individual differences in opioid SA (Ahn & Vassileva, 2016; Dilleen et al., 2012; McNamara et al., 2010). The cause of these potential differences across drug classes is unclear, but may include differences in their neurobiological effects (e.g. drug-induced synaptic and structural plasticity) or different needs for self-medication (Badiani et al., 2011; Markou et al., 1998). That is, due to the different psychoactive effects of opioids versus stimulants (e.g., anxiolytic effects versus arousal), individuals with varying psychological characteristics (e.g., degree of sensation-seeking) might prefer one drug class over the other (Khantzian, 1985; Markou et al., 1998).
Alternatively, the lack of concordance between the current findings and those with other drugs may reflect methodological factors unique to this study (rat strain, equipment, etc.) rather than our use of an opioid instead of other drugs. However, the rat strain, equipment, and general procedure used here were almost identical to that used in several studies reporting positive relationships between activity and acquisition of SA of non-opioids (e.g., Belin et al., 2008; Smith et al., 2015; Suto et al., 2001). Furthermore, the ability of activity to predict acquisition of SA of other drugs has remained robust despite numerous methodological differences across studies and laboratories including variations in rat strain, method of activity assessment (e.g., circular runway versus open field), operant response (lever press versus nose-poke), etc. Indeed, locomotor activity has been described as the most reliable predictor of acquisition of drug SA in the preclinical individual differences literature (Bardo et al., 2013; Blanchard et al., 2009). Nonetheless, inclusion of a positive control group tested under the same conditions but responding for a stimulant (e.g., cocaine) in future studies is needed to confirm that our findings reflect a true difference in predictors of SA across drugs. Further evaluating the relationship between activity and opioid SA using other unit doses, durations of access, types of opioids, etc., is also needed to confirm the generality of our findings. Understanding the ability of other putative measures of sensation-seeking (e.g., preference for a novel environment) (Belin et al., 2011; Cain et al., 2005) to predict individual differences in opioid SA is also of interest.
There are several limitations to our study. First, group sizes were relatively small, which may have limited our ability to detect a significant relationship between activity and morphine SA. However, previous studies have detected correlations between locomotor activity and acquisition of stimulant SA using similar or even smaller group sizes (e.g., Mitchell, Cunningham & Mark, 2005; Smith et al., 2015). Given the absence of even a trend for activity to predict acquisition measures in either experiment (see Fig 2 and 4), it is unlikely that the use of larger group sizes would have changed our conclusions regarding these relationships. An additional limitation is that some rats in Experiment 1 exhibited self-mutilation during dose-response testing, which required testing some rats for a shorter number of days at 1-2 of the 6 unit doses. However, removal of data for these animals did not change our conclusions. Importantly, this issue was not present during acquisition, which was our primary outcome.
While not a primary goal of this study, our use of open-field activity testing provided the opportunity to examine the relationship between time in the periphery versus center of the activity chamber (i.e., thigmotaxis), a measure of anxiety-like behavior (Cohen et al., 2009; Prut & Belzung, 2003; Treit & Fundytus, 1988), and individual differences in morphine SA. Anxiety has been linked to opioid addiction vulnerability in humans (Lejuez et al., 2008; Martins et al., 2012; Norton, 2001), and anxiety-like behavior in the elevated plus maze predicted individual differences in cocaine self-administration in rodents (Dilleen et al., 2012; Pelloux et al., 2009; Walker et al., 2009). We found that thigmotaxis did not predict individual differences in any measure of morphine SA, which is consistent with findings indicating that anxiety-like behavior in the elevated plus maze did not predict individual differences in heroin self-administration (Dilleen et al., 2012).
In conclusion, our findings indicate that locomotor activity in a novel environment did not predict individual differences in morphine SA in rats. These data complement findings from some human studies and suggest that the role of sensation-seeking in individual differences in opioid addiction vulnerability may be limited.
Highlights.
We evaluated locomotor activity as a predictor of morphine self-administration.
Behavioral economics was used to measure the reinforcing efficacy of morphine.
Morphine consumption was well described by an exponential demand function.
Activity did not predict acquisition of morphine self-administration or demand.
Acknowledgments
Supported by NIDA R21 DA037728 (Gewirtz/Harris, Co-PIs), the Minneapolis Medical Research Foundation (MMRF) Translational Addiction Research Program (Harris PI), an MMRF Career Development Award for PhD Investigators (Harris PI), and NIDA training grant T32 DA007097 (Swain, Y; Molitor T, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn WY, Vassileva J. Machine-learning identifies substance-specific behavioral markers for opiate and stimulant dependence. Drug Alcohol Depend. 2016;161:247–257. doi: 10.1016/j.drugalcdep.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio E, Goldberg S, Elmer G. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behavioural pharmacology. 1995;6(3):229–237. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association; Washington, D.C.: 2013. [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nature Reviews Neuroscience. 2011;12(11):685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65(1):255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin‐Rauscent A, Everitt BJ, Dalley JW. In search of predictive endophenotypes in addiction: insights from preclinical research. Genes, Brain and Behavior. 2016;15(1):74–88. doi: 10.1111/gbb.12265. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36(3):569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med. 2012;2(11) doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320(5881):1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology (Berl) 2000;153(1):44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology (Berl) 1995;118(3):250–259. doi: 10.1007/BF02245952. [DOI] [PubMed] [Google Scholar]
- Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: Further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33(7):1145–1154. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Simola N, Espa E, Fenu S, Di Chiara G. Strain dependence of adolescent Cannabis influence on heroin reward and mesolimbic dopamine transmission in adult Lewis and Fischer 344 rats. Addict Biol. 2015;20(1):132–142. doi: 10.1111/adb.12085. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13(4):367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. The DAWN report: highlights of the 2011 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. National survey on drug use and health: detailed tables. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. [Google Scholar]
- Chaouloff F, Kulikov A, Sarrieau A, Castanon N, Mormède P. Male Fischer 344 and Lewis rats display differences in locomotor reactivity, but not in anxiety-related behaviours: relationship with the hippocampal serotonergic system. Brain Res. 1995;693(1-2):169–178. doi: 10.1016/0006-8993(95)00733-7. [DOI] [PubMed] [Google Scholar]
- Cheng GL, Liu YP, Chan CC, So KF, Zeng H, Lee TM. Neurobiological underpinnings of sensation seeking trait in heroin abusers. Eur Neuropsychopharmacol. 2015;25(11):1968–1980. doi: 10.1016/j.euroneuro.2015.07.023. [DOI] [PubMed] [Google Scholar]
- Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar OM, Ettenberg A. Anxiolytic effects of nicotine in a rodent test of approach– avoidance conflict. Psychopharmacology. 2009;204(3):541–549. doi: 10.1007/s00213-009-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Stewart SH, Dongier M. Validation of a system of classifying female substance abusers on the basis of personality and motivational risk factors for substance abuse. Psychol of Addict Behav. 2000;14(3):243–256. doi: 10.1037//0893-164x.14.3.243. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza P, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34(3):136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63(3):301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ, Dalley JW, Belin D. High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology (Berl) 2012;222(1):89–97. doi: 10.1007/s00213-011-2626-4. [DOI] [PubMed] [Google Scholar]
- Franques P, Auriacombe M, Piquemal E, Verger M, Brisseau-Gimenez S, Grabot D, Tignol J. Sensation seeking as a common factor in opioid dependent subjects and high risk sport practicing subjects. A cross sectional study. Drug Alcohol Depend. 2003;69(2):121–126. doi: 10.1016/s0376-8716(02)00309-5. [DOI] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013;114–115:70–81. doi: 10.1016/j.pbb.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein PE, Burroughs D, Roiko SA, Pentel PR, LeSage MG. Predictors of the nicotine reinforcement threshold, compensation, and elasticity of demand in a rodent model of nicotine reduction policy. Drug Alcohol Depend. 2015;151:181–193. doi: 10.1016/j.drugalcdep.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Hursh SR. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of unit price and pre-session drug supply. Drug Alcohol Depend. 2006;85(1):35–48. doi: 10.1016/j.drugalcdep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Harris AC, Burroughs D, Pentel PR, LeSage MG. Compensatory nicotine self administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharmacol. 2008;16(1):86–97. doi: 10.1037/1064-1297.16.1.86. [DOI] [PubMed] [Google Scholar]
- Hittner JB, Swickert R. Sensation seeking and alcohol use: A meta-analytic review. Addict behav. 2006;31(8):1383–1401. doi: 10.1016/j.addbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56(2):377–393. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115(1):186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets. 2006;5(5):513–520. doi: 10.2174/187152706778559318. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The cocaine crisis. Springer; Boston, MA: 1987. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence; pp. 65–74. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ball SA, Rounsaville BJ. A sibling study of sensation seeking and opiate addiction. J Nerv Ment Dis. 1994;182(5):284–289. doi: 10.1097/00005053-199405000-00006. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Zvolensky MJ, Daughters SB, Bornovalova MA, Paulson A, Tull MT, Otto MW. Anxiety sensitivity: A unique predictor of dropout among inner-city heroin and crack/cocaine users in residential substance use treatment. Behav Res Ther. 2008;46(7):811–818. doi: 10.1016/j.brat.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemenager T, Richter A, Reinhard I, Gelbke J, Beckmann B, Heinrich M, Kniest A, Mann K, Hermann D. Impaired decision making in opiate addiction correlates with anxiety and self-directedness but not substance use parameters. J Addict Med. 2011;5(3):203–213. doi: 10.1097/ADM.0b013e31820b3e3d. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72(1-2):279–289. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Staley M, Muelken P, Smethells JR, Stepanov I, Vogel RI, Pentel PR, Harris AC. Abuse liability assessment of an e-cigarette refill liquid using intracranial self-stimulation and self-administration models in rats. Drug Alcohol Depend. 2016;168:76–88. doi: 10.1016/j.drugalcdep.2016.08.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157(1):31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- Marino EN, Rosen KD, Gutierrez A, Eckmann M, Ramamurthy S, Potter JS. Impulsivity but not sensation seeking is associated with opioid analgesic misuse risk in patients with chronic pain. Addict Behav. 2013;38(5):2154–2157. doi: 10.1016/j.addbeh.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18(3):135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Martins SS, Fenton MC, Keyes KM, Blanco C, Zhu H, Storr CL. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42(6):1261–1272. doi: 10.1017/S0033291711002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D. Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology. 2010;212(4):453–464. doi: 10.1007/s00213-010-1974-9. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Rahman S, Charnigo RJ, Dwoskin LP, Crabbe JC, Bardo MT. Genetics of novelty seeking, amphetamine self-administration and reinstatement using inbred rats. Genes Brain Behav. 2010;9(7):790–798. doi: 10.1111/j.1601-183X.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav Neurosci. 2005;119(2):464–472. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Ho A, Bahl A, Varma P, Kellogg S, Borg L, Kreek MJ. Former heroin addicts with or without a history of cocaine dependence are more impulsive than controls. Drug Alcohol Depend. 2012;124(1):113–120. doi: 10.1016/j.drugalcdep.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida KS, Park TY, Lee BH, Ursano RJ, Choi KH. Individual differences in initial morphine sensitivity as a predictor for the development of opiate addiction in rats. Behav Brain Res. 2016;313:315–323. doi: 10.1016/j.bbr.2016.07.038. [DOI] [PubMed] [Google Scholar]
- Norton G. Substance use/abuse and anxiety sensitivity: What are the relationships? Addict Behav. 2001;26(6):935–946. doi: 10.1016/s0306-4603(01)00244-1. [DOI] [PubMed] [Google Scholar]
- Park TY, Nishida KS, Wilson CM, Jaiswal S, Scott J, Hoy AR, Selwyn RG, Dardzinski BJ, Choi KH. Effects of isoflurane anesthesia and intravenous morphine self-administration on regional glucose metabolism ([(18) F]FDG-PET) of male Sprague-Dawley rats. Eur J Neurosci. 2017;45(7):922–931. doi: 10.1111/ejn.13542. [DOI] [PubMed] [Google Scholar]
- Pawlak CR, Ho YJ, Schwarting RK. Animal models of human psychopathology based on individual differences in novelty-seeking and anxiety. Neurosci Biobehav Rev. 2008;32(8):1544–1568. doi: 10.1016/j.neubiorev.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. Anxiety increases the place conditioning induced by cocaine in rats. Behav Brain Res. 2009;197(2):311–316. doi: 10.1016/j.bbr.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20(11):4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J of Pharmacol. 2003;463(1):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Raleigh MD, Pentel PR, LeSage MG. Pharmacokinetic correlates of the effects of a heroin vaccine on heroin self-administration in rats. PLoS One. 2014;9(12):e115696. doi: 10.1371/journal.pone.0115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Schaff MB, Rupprecht LE, Schassburger RL, Buffalari DM, Murphy SE, Sved AF, Donny EC. Effects of MAO inhibition and a combination of minor alkaloids, beta-carbolines, and acetaldehyde on nicotine self-administration in adult male rats. Drug Alcohol Depend. 2015;155:243–252. doi: 10.1016/j.drugalcdep.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158(2):175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31(4):959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Turner CA, Flagel SB, Clinton SM, Akil H, Watson SJ. Cocaine interacts with the novelty-seeking trait to modulate FGFR1 gene expression in the rat. Neurosci Lett. 2008;446(2-3):105–107. doi: 10.1016/j.neulet.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest N, Reynolds CJ, Tragesser SL. Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addict Behav. 2016;60:184–190. doi: 10.1016/j.addbeh.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Walker QD, Schramm-Sapyta NL, Caster JM, Waller ST, Brooks MP, Kuhn CM. Novelty induced locomotion is positively associated with cocaine ingestion in adolescent rats; anxiety is correlated in adults. Pharmacol Biochem Behav. 2009;91(3):398–408. doi: 10.1016/j.pbb.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153(4):1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge University Press; 1994. [Google Scholar]


