Abstract
Alcohol acts as a sedative that interacts with several neurotransmitter systems important in the regulation of sleep. Acute administration of large amounts of alcohol prior to sleep leads to decreased sleep onset latency and changes in sleep architecture early in the night, when blood alcohol levels are high, with subsequent disrupted, poor quality sleep later in the night. Alcohol abuse and dependence are associated with chronic sleep disturbance, lower slow wave sleep, and more rapid eye movement sleep than normal, that last long into periods of abstinence and may play a role in relapse. The chapter outlines the evidence for acute and chronic alcohol effects on sleep architecture and sleep EEG, evidence for tolerance with repeated administration, and possible underlying neurochemical mechanisms for alcohol’s effects on sleep. Also discussed are sex differences as well as effects of alcohol on sleep homeostasis and circadian regulation. Evidence for the role of sleep disruption as a risk factor for developing alcohol dependence is discussed in the context of research conducted in adolescents. The utility of sleep evoked potentials in the assessment of the effects of alcoholism on sleep and the brain and in abstinence-mediated recovery is also outlined. The chapter concludes with a series of questions that need to be answered to determine the role of sleep and sleep disturbance in the development and maintenance of problem drinking and the potential beneficial effects of the treatment of sleep disorders for maintenance of abstinence in alcoholism.
Keywords: alcoholism, acute alcohol K-complex, REM, slow wave sleep
1.0 Introduction
Sleep is a fundamental, necessary and complex behavior in humans. The mammalian nervous system demonstrates two distinct states of sleep, rapid eye movement (REM) and non-rapid eye movement (NREM) sleep, that involve different patterns of neurological activation and neurotransmitter release, and presumably different functions. Many of the neurotransmitters known to be involved in wake-sleep regulation are also affected by alcohol, and thus not surprisingly, alcohol affects sleep in a number of ways. As with other drugs, alcohol demonstrates tolerance with repeated administration and dependence, and this tolerance is accompanied by adaptation of neurotransmitter systems, either by modulation of their release or by modifying the sensitivity of their response mechanisms. Withdrawal of alcohol in dependent individuals can be associated with neurological manifestations of the consequent neurochemical imbalance. Over time, recovery can take place to restore a normal balance of inhibitory and excitatory systems; however, it is possible that some changes induced by alcohol may be resistant to restoration. In addition to the direct pharmacological effect of alcohol on sleep-regulatory systems, the long-term consequences of alcohol abuse and dependence on brain macrostructure and microstructure, may also themselves lead to changes in sleep regulation or changes in electroencephalographic (EEG) manifestations of sleep. The influence of alcohol on sleep thus needs to be evaluated in multiple contexts. This chapter reports the evidence for the acute effects of alcohol on sleep in humans and in animal models, the effects of short-term repeated administration of alcohol on sleep, and the observations of the long-term consequences of alcohol dependence on sleep.
Human sleep is defined on the basis of changes in EEG activity, augmented by measurement of eye movements and postural muscle tone to aid in the differentiation of REM sleep from wakefulness. The movement from wakefulness to deep sleep largely maps onto an EEG frequency continuum with very high frequency gamma oscillations (~40 Hz and higher) and desynchronized beta activity (~20–30 Hz) associated with active wakefulness, giving way to synchronized alpha (~10Hz) in relaxed wakefulness, theta activity (4–7 Hz) in light Stage 1 NREM sleep (now called N1 sleep, Iber et al., 2007).
Stage 2 NREM sleep (N2) is characterized by phasic sleep spindles (synchronized sigma activity: 12 – 16Hz) and K-complexes, high amplitude delta waves, and constitutes between 45–55 % of the night. Stages 3 and 4 NREM sleep (N3 or slow wave sleep, SWS) are characterized by slow delta activity (0.3 to 2 Hz), constituting about 18 % of the night. REM sleep, constituting 20–25 % of the night, demonstrates desynchronized activity in theta and beta ranges reflective of the partial reactivation of brain stem mechanisms that are fully active in wakefulness and deactivated in NREM sleep. NREM and REM sleep alternate across the night in approximately 90-minute cycles. As the sleep episode progresses, Stage 2 sleep accounts for most of NREM sleep, and the amount of REM sleep increases. According to the two-process model of sleep (Borbely 1982), the timing, depth, and duration of sleep are controlled by the interaction between circadian (Process C) and homeostatic (Process S) processes. Process S reflects the buildup of sleep pressure during wake, which dissipates during sleep. EEG delta wave activity in NREM sleep is considered a marker of Process S and is highest in the first part of the sleep period when sleep pressure is highest. Process C reflects circadian regulation of sleep timing. While the overarching purpose of sleep remains unknown, evidence suggests that it serves several functions, including conservation of brain energy, facilitation of certain forms of learning and memory, and support of cognitive capacity including pruning and maintenance of synaptic connectivity (Tononi and Cirelli 2006). Insufficient sleep or insomnia (difficulty initiating or maintaining sleep or non-restorative sleep) are associated with negative consequences to immune function, impaired cardiovascular and cerebrovascular health, cognitive impairment, and a change in emotional reactivity. Insomnia is a risk factor for the development of psychiatric disorders, including depression, anxiety, and alcohol abuse (Breslau et al. 1996). Insomnia complaints may also interfere with the recovery process and contribute to relapse among patients in recovery from alcohol dependence (Brower 2001; Arnedt, Conroy, and Brower 2007). Therefore, it has, been suggested that treating insomnia may aid recovery in alcoholics and prevent or reduce the likelihood of relapse (Brower 2001; Arnedt, Conroy, and Brower 2007)
2.0 Acute effects of alcohol on sleep
Sleep occurs over a sustained period, typically lasting approximately 8 hours in humans. In the absence of continued dosing, alcohol consumed prior to the onset of sleep, therefore, will not be at a constant level throughout the sleep period. Depending on the timing of sleep onset relative to consumption, blood alcohol levels may continue to rise for some time during sleep, but inevitably they will start to fall as a function of metabolism, the time course of which is unaltered by sleep itself (Rundell et al. 1972). Sleep, therefore, could be expected to be affected differently during the initial period of high alcohol levels from the subsequent elimination phase. The presence of alcohol metabolites such as aldehyde need to be considered in terms of their own possible influence on sleep mechanisms as do secondary effects of alcohol, such as diuresis.
Effects of an acute pre-bedtime dose of alcohol on sleep have been extensively studied although methodology has varied greatly between studies in terms of dose and timing of alcohol administration, age and gender of subjects, and sample size. Alcohol initially acts as a sedative. Commonly reported phenomena include shortened sleep onset latency (MacLean and Cairns 1982; Roehrs et al. 1999; Williams, MacLean, and Cairns 1983; Stone 1980; Scrima et al. 1982) and increased SWS in the first half of the night (Williams, MacLean, and Cairns 1983; Van Reen, Jenni, and Carskadon 2006; Sagawa et al. 2011; Rundell et al. 1972; Chan et al. 2013; MacLean and Cairns 1982; Prinz et al. 1980; Feige et al. 2006; Arnedt, Rohsenow, et al. 2011). REM sleep is suppressed, with a longer latency to REM sleep and decreased REM sleep in the first half of the night (Williams, MacLean, and Cairns 1983; Van Reen, Jenni, and Carskadon 2006; Sagawa et al. 2011; Rundell et al. 1972; Chan et al. 2013) or across the whole night (Van Reen, Jenni, and Carskadon 2006; Williams, MacLean, and Cairns 1983; Roehrs et al. 1999; Rundell et al. 1972; Roehrs, Yoon, and Roth 1991; Arnedt, Rohsenow, et al. 2011). In the second half of the night, sleep is disrupted, with increased wakefulness and/or stage 1 sleep. This pattern of initial sleep augmentation followed by a period of poor quality sleep can lead to a downward spiral, with insomnia being self-treated with alcohol to produce a rapid sleep onset, subsequent poor sleep then leading to daytime sleepiness that is self-treated with caffeine, which exacerbates insomnia, requiring more alcohol to fall asleep, etc. It is estimated that alcohol is used by more than one in ten individuals as a hypnotic agent to self-medicate sleep problems (Arnedt, 2007).
2.1 Acute effects of alcohol on sleep: repeated administration
While there is reasonable consensus on the effects of acute alcohol administration on a single night’s sleep in non-alcohol-dependent individuals, less is known regarding the effects of repeated administration over multiple nights, a situation that better represents the normal experience for non-dependent drinkers in the community. Studies of the effects of repeated alcohol administration over multiple nights are rare and suffer from small sample sizes. To our knowledge, only five such studies have been published with a total of 19 men and 5 women evaluated in experiments that vary in the dose of alcohol administered, the timing of the alcohol relative to sleep, and the number of nights of consecutive usage.
Yules, Freedman, and Chandler (1966) studied three young non-alcohol dependent, men over 5 nights of drinking, with 1g/Kg ethanol administered 15 minutes before bedtime. Yules, Lippman and Freedman (1967) studied four young men over three or five nights of drinking with 1 g/Kg ethanol administered 4 hours before bedtime. Unfortunately, these studies predate the acceptance of a standardized sleep scoring system, and the data are difficult to interpret (e.g. stage 1 NREM and REM sleep are combined), however their data are consistent with REM suppression and enhancement of slow wave sleep in the first part of the night.
Rundell et al. (1972) studied seven young men over three nights of drinking with alcohol administered over an hour, ending 30 minutes before bed, with blood alcohol concentrations at bedtime between 0.05 and 0.095 mg percent. Data are presented from a baseline night, three drinking nights and the mean of two recovery nights. Prinz et al. (1980) studied five young men over nine nights of drinking (seven of them at home) with a 0.8g/Kg dose (0.08 Breath Alcohol Concentration (BAC) on the laboratory nights) consumed over the hour before bedtime. Data are reported from a baseline night; the first and ninth alcohol nights and a recovery night. Feige et al. (2006) studied five young men and five young women over three nights of drinking. Alcohol was consumed before bed to obtain BAC of 0.03 or 1.0% in two different conditions. Data are presented from a baseline night, three drinking nights and two recovery nights. The results for the first half of the night from these studies are summarized in Figure 1.
Figure 1.
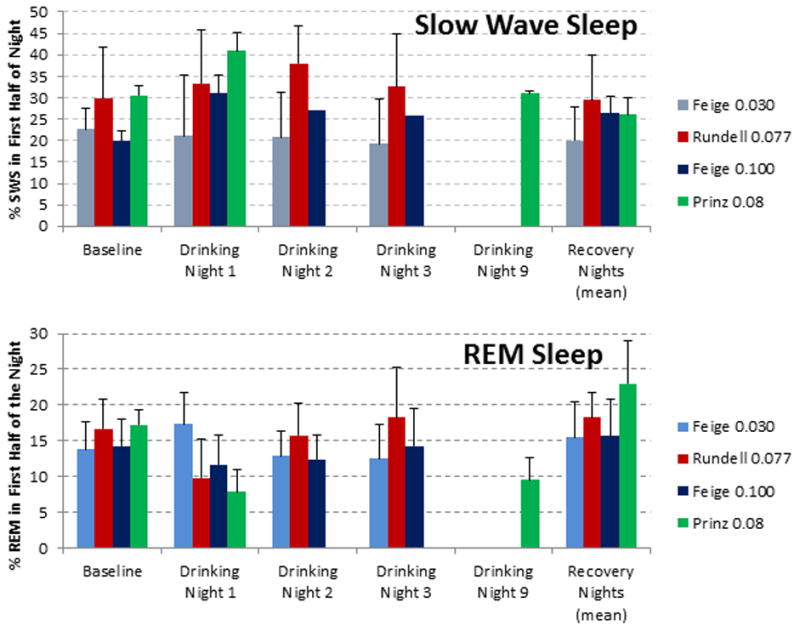
The percentage of (A) slow wave sleep (SWS) and (B) rapid eye movement (REM) sleep in the first half of the night across multiple nights of drinking. Data are drawn from (Feige et al. 2006; Prinz et al. 1980; Rundell et al. 1972).
SWS was significantly increased over baseline on the first drinking night in the Prinz et al. (1980) and Feige et al. (2006) (0.10% BAC dose) studies but not in the Feige et al. (2006) (0.03% BAC dose) or Rundell et al. (1972) studies. Prinz et al. (1980) show SWS as recovered to baseline on night nine; however, Feige et al. (2006) showed significant increases in SWS for the first half of the night on all three drinking nights, so if tolerance to the SWS induction effect occurs with repeated nightly use, it would appear to take some time to develop.
The percentage of REM sleep in the first half of the night was not decreased on the first drinking night at either the 0.03 or 0.10% BAC doses in the Feige et al. (2006) study. However, REM density (a measure of the number of eye movements per unit time) was significantly reduced on all three drinking nights for the 0.10% BAC relative to baseline, and recovered to baseline with no evidence of rebound on the first recovery night (Feige et al., 2006). Prinz et al. (1980) reported that REM sleep in the first half of the first drinking night (7.0 ± 3.1%) and of the ninth drinking night (9.5± 3.18%) was lower than baseline (17.26 ± 2.20%), although the difference was not statistically reliable in this small sample. Rundell et al. (1972) reported a decrease in REM sleep on the first drinking night in their study, but values on the second and third drinking nights were not different to baseline. While these studies support others showing a suppressing effect of REM sleep by a single dose of alcohol, more studies are needed to determine whether the effect persists after multiple drinking nights.
Given the clear importance of tolerance to sleep effects as a mechanism for the development of abusive drinking (Conroy and Brower 2011), the lack of consistent results from the small number of studies and of subjects evaluated provides a clear argument for more work to be done to investigate the effects of repeated administration of alcohol on sleep.
2.2 Acute alcohol: sleep EEG data
Computerized analysis of the sleep EEG may reveal subtle effects of alcohol that may vary according to brain region and that are not evident from manual scoring of the polysomnogram (PSG). Few investigations have examined the acute effect of alcohol on EEG power spectra (Dijk et al. 1992; Rundell et al. 1972; Landolt et al. 1996; Van Reen, Jenni, and Carskadon 2006). Rundell et al. (1972) conducted an automated period analysis of EEG in the first half of the night in young men following administration of 0.9g/Kg alcohol and reported decreases in beta along with increases in alphoid (1st derivative of the zero crossing count). Dijk et al. (1992) observed increases in delta power in a single central derivation in young men following administration of 0.6g/Kg alcohol, consistent with increased SWS and decreases in sigma power only in the first two hours of the sleep period (Dijk et al. 1992). Landolt et al. (Landolt et al. 1996) reported elevated delta and theta power during SWS in frontal, central, parietal, and occipital derivations in older men, with the increase in delta appearing to be most prominent in the frontal and central derivations following moderate alcohol consumption (0.55 g/kg). Effects were only seen in the first sleep cycle, with significantly reduced delta power with alcohol in subsequent cycles. This cycle effect is complicated, however, by the fact that alcohol was administered 6 hours before sleep. Van Reen and colleagues (2006) reported that0.49 g/kg of alcohol was associated with increased alpha activity in anterior derivations (Fz/Cz) with no difference in posterior derivations (Pz/Oz) during NREM sleep compared to baseline in young women, suggesting that the effect of alcohol on the sleep EEG differs according to region. Given that these studies differed in alcohol dose (0.49 – 0.90 g/Kg body weight), timing (35 minutes to 6 hours prior to sleep), analysis methods, and results, it is difficult to determine if there is a consistent effect of alcohol on power spectra on the basis of these studies.
3.0 Sleep in alcoholism
Studies consistently show a high comorbidity of insomnia and alcoholism (reviewed in (Arnedt, Conroy, and Brower 2007)). Based on studies of clinical populations, between 36 – 91% of patients report insomnia either while drinking or within several weeks of stopping (Brower and Perron 2010). Many alcoholics develop poor sleep habits and irregular sleep-wake schedules when drinking, which may persist after quitting. Napping and maintaining an irregular sleep-wake schedule are associated with greater wakefulness and poorer sleep quality at night (Currie et al., 2003).
Laboratory based polysomnographic studies of abstinent alcoholics typically show a pattern of sleep disturbance with increased wakefulness consistent with self-reports of persistent sleep disturbance common in this population. Sleep efficiency is a simple index of the proportion of the time in bed spent asleep and thus a polysomnographic marker of general sleep quality. It is generally decreased in recently detoxified alcoholics (periods of sobriety, ranging from 16–46 days) (Drummond et al. 1998; Gillin et al. 1990; Irwin et al. 2000; Gann et al. 2001; Snyder and Karacan 1985), but seems to show recovery, with most studies examining alcoholics after longer periods of abstinence not showing differences between alcoholics and controls (Adamson and Burdick 1973; Williams and Rundell 1981; Drummond et al. 1998; Colrain, Turlington, and Baker 2009b).
Laboratory studies also reveal decreased SWS (Gillin et al. 1990; Drummond et al. 1998; Thompson et al. 1995; Feige et al. 2007; Gann et al. 2001; Allen and Wagman 1975; Allen, Wagman, and Funderburk 1977; Wagman and Allen 1975; Othmer et al. 1982; Rundell, Williams, and Lester 1975; Le Bon et al. 1997; Irwin et al. 2002; Irwin et al. 2000; Colrain, Turlington, and Baker 2009b) as a highly consistent finding (Ehlers 2000; Benca et al. 1992) in alcoholics relative to controls. There is also evidence of increased REM sleep pressure (Gillin et al. 1990; Drummond et al. 1998; Thompson et al. 1995; Gann et al. 2001; Feige et al. 2007; Colrain, Turlington, and Baker 2009b). It is reasonable to expect increased REM pressure in actively drinking or recently detoxified alcoholics, given that REM sleep is suppressed with high doses of alcohol (Aldrich 1998). This form of REM rebound cannot explain the increased REM in those who have been abstinent for a long time, relative to controls. It is possible that increased REM sleep may represent a predisposition to altered sleep rather than a consequence of alcohol abuse; although REM is not elevated in adolescents with a positive family history of alcoholism (Tarokh et al. 2012). Another possibility is that alcohol abuse leads to long-lasting neurochemical changes in the brain stem. Possible mechanisms will be discussed below in section 6. Figure 2 (adapted from (Colrain, Turlington, and Baker 2009b) gives an example of the proportions of wakefulness (pre-sleep and throughout the night), and different sleep stages in alcoholic and control men and women.
Figure 2.
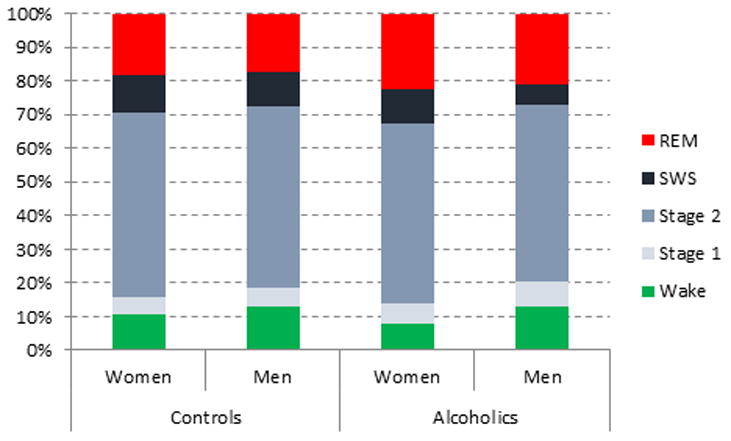
The percentage of the night spent in different sleep stages (Rechtschaffen and Kales 1968) in men and women with alcohol dependence and sex-matched control. Data are dawn from (Colrain, Turlington, and Baker 2009b).
3.1 Alcoholism: Sleep EEG Data
Spectral analysis of sleep EEG data has been conducted in a smaller number of studies. Consistent with the reports of reduced SWS, Irwin et al. (2000) reported significantly lower NREM delta power (0.75–4 Hz) across the entire night in alcoholics relative to controls, particularly in the first NREM period. Total power in the measured spectrum was also lower, with a trend for lower theta power in the first NREM period in alcoholics. The delta power result was confirmed in the baseline data in (Irwin et al. 2002), although it appeared to be stronger in African American than in European American alcoholics. On the other hand, analysis of placebo condition sleep EEG data from a study investigating REM sleep induction tests using galanthamine hydrobromide did not show differences between alcoholics and controls in delta or theta frequencies during NREM or REM sleep (Feige et al. 2007). Our own data from multiple EEG sites (Colrain, Turlington, and Baker 2009b) revealed that relative to controls alcoholics had significantly lower slow wave activity (SWA) in NREM sleep across the whole night and in the first NREM period in the slow (<1 Hz) band as well as in each of the 1 to <2, 2 to <3, and 3 to <4 Hz delta EEG bands. The slower end of theta activity (up to 6 Hz) displayed a similar pattern to delta frequencies. The effects were more prominent in frontal than posterior scalp derivations in alcoholic men and women. Interestingly, the delta effects were not seen in REM sleep but have been reported by others during wakefulness (Bauer 2001; Saletu-Zyhlarz et al. 2004). These lower levels of delta power during NREM sleep could reflect underlying gray and white matter volume deficits and compromised connectivity in the brain that characterize chronic alcoholics, as described later.
Differences in activity in the fast frequency bands (beta and gamma) during sleep between alcoholics and controls are less consistent. Feige et al. (2007) reported elevated beta activity in REM and gamma activity in stage 2 NREM sleep, but only in data from the adaptation nights, with no differences for subsequent placebo nights from their drug study. Irwin et al. (2002) reported a trend for elevated beta activity in alcoholics across the entire night at baseline that became a significant difference during a recovery night following a night of partial sleep deprivation. Colrain et al. (2009b) did not see any differences between alcoholics and controls in high frequency EEG activity during sleep. Because these analyses are performed on stable sleep epochs, results suggest that once sleep is attained, it is not necessarily characterized by elevated fast frequency activity. By contrast, primary insomniacs have greater beta power during NREM sleep than normal sleepers, thought to reflect higher levels of cortical arousal (Riemann et al. 2010). Topographic differences in EEG spectral power during sleep evaluated in alcoholics compared with controls revealed that slow frequency activity was maximal over frontal scalp regions in both alcoholics and control subjects (Colrain, Turlington, and Baker 2009b). This is consistent with previous studies of normal subjects (Finelli, Borbely, and Achermann 2001), positron emission tomography data (Dang-Vu et al. 2005), and high density EEG array (Massimini et al. 2004) studies, which indicate that the frontal cortex is preferentially involved in the generation of SWA during sleep. Differences in slow frequency between alcoholics and controls were also more marked over the frontal scalp with alcoholics showing lower delta EEG power (Figure 3). This topographic pattern is consistent with the known frontal susceptibility to alcoholism-related alterations in brain structure and function (Zahr et al. 2013; Oscar-Berman et al. 2013).
Figure 3.
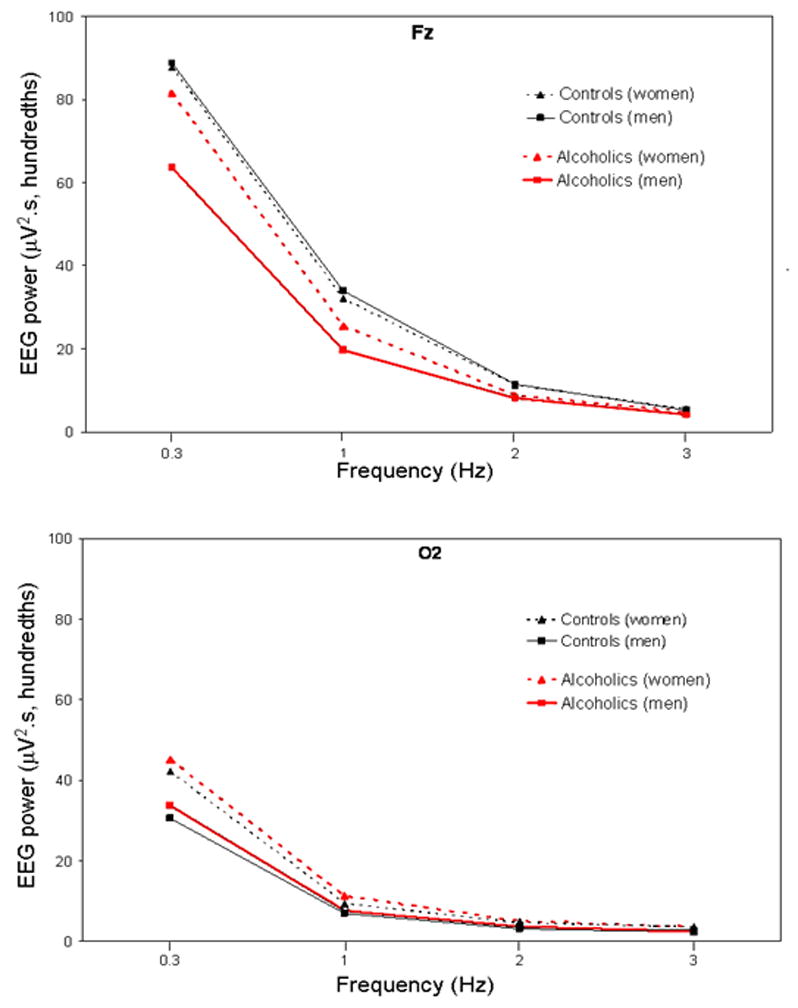
Figure 1: All-night NREM sleep slow wave activity (; 0.3 – <4 Hz) at Fz (upper panel) and O2 (lower panel) for 34 alcoholics (21 men) and 41 controls (18 men). Values are averaged within the 0.3–1 Hz bin, and within each 1-Hz frequency bin thereafter. Bins are identified by their lower boundary. Data are dawn from (Colrain, Turlington, and Baker 2009b).
3.2 Sex effects in the impact of alcohol and alcoholism on sleep
Few studies have investigated the potential of sex differences in acute alcohol intake or chronic alcoholism on sleep. There are sex differences in drinking patterns and pharmacokinetics of alcohol. Women tend to have less body water and more body fat than men and therefore tend to have higher blood alcohol levels than men after consuming the same amount of alcohol due to alcohol’s hydrophilic properties (Ely et al. 1999). Alcohol consumption and the response to alcohol in women may also be impacted by the hormonal and/or mood fluctuations associated with the menstrual cycle. There are reports of greater alcohol consumption in women with severe premenstrual syndrome, although not necessarily limited to the premenstrual phase when symptoms are prominent, and there are also reports of increased alcohol consumption premenstrually in a portion of alcoholic women (Kouri and Halbreich 1998). The interaction between menstrual cycle phase, alcohol consumption and premenstrual symptoms is further complicated by a family history of alcoholism (Evans and Levin 2011). Historically, Alcoholism has been more prevalent among men than women; however, the gender gap is decreasing with regard to both alcohol consumption and alcohol use disorders, at least in the United States (Keyes, Grant, Hasin et al., 2008). Women are more vulnerable to the development of alcohol-related disease such as liver cirrhosis (Walter et al. 2003) and alcohol-dependent women have worse quality of life scores than alcohol-dependent men (Peters, Millward, and Foster 2003). With regard to sleep, there is limited evidence for sex differences in the impact of alcohol. Interestingly, despite the commonly reported sex difference in prevalence rates of insomnia in community samples, with the risk for developing insomnia being 1.41 times greater in women (Manber, Baker, and Gress 2006), no sex differences were identified in the frequency of insomnia in men and women undergoing treatment for alcohol dependence (Brower, Aldrich, Robinson et al, 2001).
In a study of 93 healthy adults (59 women), Arnedt et al. (2011) found that drinking to reach peak breath alcohol concentrations of 0.1 g% before bedtime resulted in more disrupted sleep (decreased sleep efficiency and increased wakefulness) but similar impact on sleep architecture (% SWS and % REM sleep) in healthy women compared with men. Women also reported higher ratings of sleepiness after consuming alcohol than did men. No sex differences in the effects of alcohol on sleep were seen in the group of older adolescents studied by Chan et al. (Chan et al. 2013). In a study of 42 recovering alcoholics (15 women) and 42 controls (23 women), we found that women had a better sleep efficiency and more delta activity during NREM sleep than men, regardless of diagnosis (Colrain, Turlington, and Baker 2009a). Both male and female alcoholics had less delta activity during NREM sleep than sex-matched controls and although the sex by diagnosis interaction term was not significant, alcoholic men appeared to show a more prominent reduction in delta activity during NREM sleep compared with male controls than female alcoholics did compared with female controls. Further, estimated lifetime alcohol consumption predicted percentage of SWS in alcoholic men but not alcoholic women (Colrain, Turlington, and Baker 2009a). Estimated lifetime alcohol consumption was higher in alcoholic men than women, and the women had longer periods of sobriety prior to testing on average. Studies that include larger groups of male and female alcoholics are needed to further evaluate sex differences in the impact of alcohol dependence on sleep.
4.0 Alcohol dependence and sleep in adolescence
As described above, insomnia is a risk factor for the development of alcohol dependence in adults. Recent studies suggest that sleep problems may developmentally precede onset of alcohol use and alcohol problems in adolescents (reviewed in (Shibley, Malcolm, and Veatch 2008)). There are substantial changes in sleep behavior across adolescence, with a shift to later bedtimes, a shift in chronotype towards eveningness, with a preference for evening activities, and a shorter sleep duration (Reviewed in (Colrain and Baker 2011)). There are also changes in sleep architecture, the most notable of which is a dramatic decrease in the amount of SWS during the teenage years (Carskadon 1982). Changes in adolescent sleep and sleep behavior may be driven by social and environmental factors as well as by physiological changes in circadian and homeostatic sleep regulation. The loss of SWS and its associated sleep protective delta activity may relate to the high prevalence of sleep problems in adolescents; the lifetime prevalence of DSM-IV diagnosed insomnia in adolescents (aged 13–16 years) is estimated as 10.7 % (Johnson et al. 2006). Adolescents reporting sleep problems also report more mood disturbances, inattention and memory problems, conduct disorders, and increased drug and alcohol use (Shibley, Malcolm, and Veatch 2008). Based on survey data, adolescents who use alcohol are more likely to report sleep problems than those who abstain even after adjusting for internalizing and externalizing problems (Johnson and Breslau 2001). Short (< 6 h) sleep (Roberts, Roberts, and Duong 2009) and poor sleep quality (Manni et al. 1997) have both significantly predicted the development of alcohol use disorders. Perceived tiredness and poor sleep habits accounted for up to 26% of the variance in psychoactive substance use in adolescents (Tynjala, Kannas, and Levalahti 1997). A greater tendency for either circadian phase delay (Pieters et al. 2010) or evening chronotype (Negriff et al. 2011) is also predictive of alcohol use in adolescents. One of the few prospective studies found that chronic insomnia predicted alcohol use in adolescents aged 11–17 years, 12 months after an initial assessment (Roberts, Roberts, and Duong 2008). Also, mothers’ ratings of early childhood sleep disturbances significantly predicted an early onset of alcohol, marijuana, and illicit drug use in adolescence (Wong et al. 2004). These findings have led to the recommendation that clinicians should treat sleep disturbances in adolescents to prevent or delay the adverse effects of addiction (Shibley, Malcolm, and Veatch 2008). Longitudinal studies that track the development of insomnia symptoms along with changes in alcohol use across adolescence are critical to establish definitively the impact of sleep behavior on subsequent alcohol abuse.
5.0 Sleep homeostasis and circadian problems with alcohol abuse
While several studies have evaluated sleep in alcoholics under baseline conditions, in the absence of a sleep challenge, few studies have evaluated sleep homeostasis in abstinent alcoholics. Irwin et al. (Irwin et al. 2002) studied 46 primary alcoholics in a partial sleep deprivation paradigm in which sleep was studied at baseline, on a night in which sleep was restricted to between 3:00 and 6:30 AM and on a recovery night. In both Caucasian and African American alcoholics there was no evidence of homeostatic recovery in either the amount of SWS or in delta EEG power. Two studies from the Michigan group, one in 10 alcoholics (Brower et al. 2011) and the other in 48 alcoholics (Armitage et al. 2012), used a mild sleep challenge of delaying sleep by 3 hours to assess homeostatic recovery. Both papers reported a blunted response in the alcoholics relative to controls, with the smaller study showing alcoholics to have a slower decay of slow wave activity over the night than a group diagnosed with major depression (Brower et al. 2011).
Despite strong evidence for disruption of circadian rhythms in rodent chronic drinking models (Spanagel et al. 2005), few studies have evaluated circadian rhythms in chronic alcoholics. Most studies have been conducted in small groups and in the acute detoxification phase of withdrawal. Two studies have shown no melatonin rhythm in the first few days following drinking cessation (Schmitz et al. 1996; Fonzi et al. 1994) with restoration of normal rhythmicity after 2 weeks (Schmitz et al. 1996; Fonzi et al. 1994) and a third (Mukai et al. 1998) reported a delayed rhythm only in alcoholics who were suffering from delirium tremens. Kuhlwiein et al. (Kuhlwein, Hauger, and Irwin 2003) found a delay in the rise time of nocturnal melatonin as well as a delay in the peak, associated with a delay in sleep onset in African American alcoholics studied two weeks post withdrawal. African American subjects were selected as they have previously shown more pronounced sleep disturbance (Irwin et al. 2002).
The apparently delayed melatonin rhythms are in contrast to the single study showing evidence of an advanced body temperature rhythm early in withdrawal (Kodama et al. 1988), although this was more pronounced in alcoholics with comorbid depression. The temperature rhythm had normalized by three weeks in most patients.
Cortisol rhythms show no evidence for disruption early in withdrawal or two to four weeks post drinking in two studies (Mukai et al. 1998; Fonzi et al. 1994). However, those with delirium tremens did have altered rhythms (Mukai et al. 1998; Fonzi et al. 1994). Kuhlwein, Hauger and Irwin (2003) reported lower cortisol early in the night and higher levels later in the night in their African American alcoholics after two weeks.
Two remaining studies focused on REM sleep showed advanced timing of REM sleep which did not normalize for several months (Imatoh et al. 1986) and abnormal 5HIAA (serotonin metabolite) rhythms in those with delirium tremens or clouded sensorium but not alcoholics with only autonomic dysregulation symptoms (Sano et al. 1993).
The studies outlined above provide limited support for evidence of blunted sleep homeostasis that may persist long-term into periods of abstinence and possible circadian rhythm problems in the acute withdrawal period, especially in those suffering severe withdrawal symptom such as delerium tremens. Abnormalities in the timing of REM sleep would appear to last longer into the abstinence period. The role of circadian misalignment in disturbed brain reward function, and its role in the development of alcohol use disorders is the subject of a recent review by Hasler and Clark (2013).
6.0 Evoked Potentials during sleep
Sleep event-related potentials (ERPs) provide an opportunity to evaluate the brain’s ability to generate slow frequency EEG activity during sleep as a function of acute changes in neurochemical state following consumption of alcohol or as a consequence of micro- and macrostructural changes in the brain caused by long-term alcohol dependence. The presentation of auditory or respiratory stimuli during sleep leads to a reliable series of modality independent (Colrain, Webster, and Hirst 1999) evoked potential responses: a P2 component at around 200 ms, and N350, N550 and P900 components. The major contributor to the sleep ERP response is the evoked K-complex (Halasz 2005; Colrain 2005), with the N350, N550 and P900 responses all substantially larger when K-complexes are included in the average (Figure 4), although there is evidence that the N350 can be substantial in the absence of K-complexes if evoked vertex sharp waves are present in the average (Colrain et al. 2000).
Figure 4.
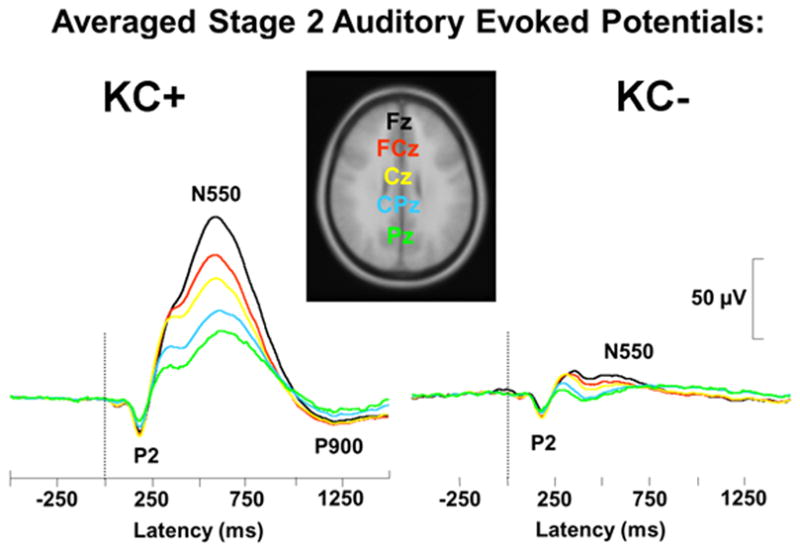
Examples of averaged evoked responses from stage 2 NREM sleep. The left panel (KC+) shows the result of averaging responses that included K-complexes. The right panel (KC-) show the result of averaging responses not including K-complexes. Waveforms are presented from Fz, FCz, Cz, CPz and Pz.
The K-complex reflects a single instance of high amplitude delta wave during sleep and probably reflects a sleep protective process (Czisch et al. 2009; Colrain 2005; De Gennaro, Ferrara, and Bertini 2000). Data linking the K-complex to delta generation is supported by both animal (Amzica and Steriade 1997, 1998b, 2002) and human (De Gennaro, Ferrara, and Bertini 2000; Nicholas et al. 2006) experiments. Further, the scalp distribution of the prominent negative (N550) component of the averaged evoked K-complex (Colrain 2005) shows the same bilaterally symmetrical, frontal predominant distribution seen in spontaneous delta activity in SWS (Finelli, Borbely, and Achermann 2001). Converging evidence suggests that the K-complex is a cortically generated EEG phenomenon because it is associated with a voltage polarity inversion between superficial (negative voltage) and deeper cortical layers (positive voltage) observed in animal studies (Amzica and Steriade 1998a) and intracranial recordings in human epileptic patients (Wennberg and Lozano 2003). This polarity inversion would appear not to support hypotheses of a subcortical generator (Sassin and Johnson 1968; Dang-Vu et al. 2008; Czisch et al. 2009). The large amplitude of the K-complex (and the N550) is typically an order of magnitude greater than even relatively large event related potential components such as the P300 seen during wakefulness. This implies a need for large numbers of healthy neurons to be involved in its generation. These neurons need to be simultaneously active and the synchronization of their firing is contingent on healthy white matter tracts. It is therefore not surprising that the amplitude of the prominent negative component of the averaged evoked K-complex (N550) declines with normal aging in healthy men and women (Crowley, Trinder, and Colrain 2002; Crowley, Trinder, and Colrain 2004; Colrain et al. 2010). This decrease parallels age-related decreases in gray matter volume (Pfefferbaum et al. 1994). We have thus hypothesized that the K-complex may also be a sensitive marker of the brain degradation seen in chronic alcoholism (Colrain et al. 2010).
6.1 Sleep evoked responses in alcoholism
Two studies have evaluated sleep evoked responses in abstinent long-term alcoholics. Nicholas et al. (2002) studied 7 abstinent long-term alcoholic men meeting DSM – IV criteria for alcohol dependence and 8 normal control men. Alcoholics were less likely to generate a K-complex in response to a tone than matched controls. The alcoholic group also showed a significantly smaller amplitude N550 component at a frontal site compared with controls; however, the latency of the component did not differ between the groups. The P2, N350 and P900 components measured at Cz showed no group differences for amplitude or latency.
In a larger study, Colrain et al. (2009) studied 42 abstinent long-term alcoholics (27 men) and 42 controls (19 men). As in the previous study (Nicholas et al. 2002), alcoholics were significantly less likely to produce K-complexes than controls. P2 amplitude was, however, smaller in alcoholics than controls with the difference being largest at Cz, where the component was maximal, but smaller at other sites (see Figure 5). P2 latency was longer in alcoholics. There were no sex differences or interactions between diagnosis and sex for K-complex incidence, P2 amplitude or P2 latency. Frontal (but not posterior) N550 and P900 amplitudes were smaller in alcoholics than controls and smaller in men than women, but the sex difference was not related to diagnosis. Latencies of N550 and P900 did not differ as a function of diagnosis or sex.
Figure 5.
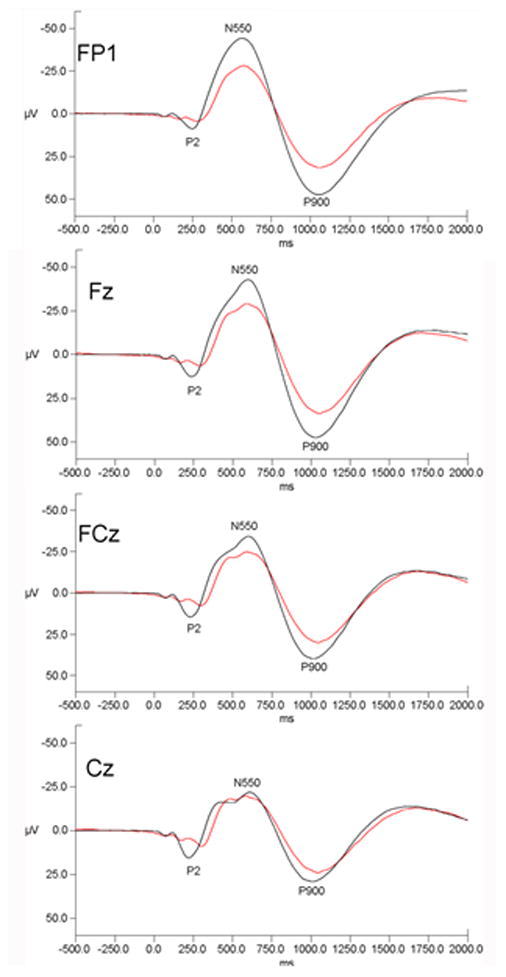
Grand mean evoked potential waveforms for alcoholics (red lines) and control subjects (black lines) for the FP1, Fz, FCz and Cz electrode sites. Data are presented with negative voltages up the Y axis. Data are drawn from (Colrain et al. 2009).
Chronic alcoholism is thus associated with both an impaired ability to produce evoked delta frequency responses (lower K-complex incidence) and smaller magnitudes of the responses when evoked (smaller N550 and P900 amplitudes).
6.2 Links between sleep EEG effects and altered brain structure in alcoholism
In vivo MRI data show that alterations in both cortical gray matter and white matter volumes are associated with chronic, excessive alcohol consumption with the prefrontal cortex being the supratentorial area most affected (Pfefferbaum and Sullivan 2013). These data are supported by evidence for reduced postmortem neuronal count in frontal cortex in alcoholics (Harper and Kril 1989; Harper, Kril, and Daly 1987; Sutherland, Sheedy, and Kril 2013) (see Chapter xx in this volume for review). A reduced number of neurons in alcoholics could result in reduced delta EEG amplitude, as fewer neural columns are available for synchronized burst firing. Chronic heavy alcohol use also disrupts white matter microstructure as manifest by abnormally low fractional anisotropy (FA), both in localized regions of white matter, such as the corpus callosum and centrum semiovale, and more broadly throughout the cortex(Pfefferbaum and Sullivan 2013; Pfefferbaum, Adalsteinsson, and Sullivan 2006; Pfefferbaum et al. 2009; Pfefferbaum et al. 2010). Compromised white matter integrity could conceivably reduce the ability of the brain to coordinate synchronized burst firing across large cortical areas.
An indirect test of the neuronal loss hypothesis of K-complex amplitude deficit in chronic alcoholism was conducted using gray matter volumes from structural MRI data acquired from the subjects in Colrain et al. (2009). Statistical models were constructed to determine the extent to which cortical and subcortical volumes could predict evoked potential component amplitudes in sleeping alcoholics and controls. Stepwise multiple regression entering age, intracranial volume, diagnosis, lobar gray matter volumes and subcortical tissue volumes to predict N550 amplitude at Fz produced different models in men and women (Colrain et al. 2011). For men, sensorimotor gray matter volume made a significant independent contribution to N550 amplitude with the amount of variance explained significantly improving with the addition of diagnostic group. These data support the hypothesis that diminished gray matter volume in chronic alcoholism contributes to an impaired ability to generate large amplitude slow waves, although not all the variance could be explained by loss of volume. Poor connectivity (i.e., deficits in white matter integrity) likely also contributes, although relations between evoked potential amplitude and diffusion tensor imaging (DTI) measures of white matter integrity are yet to be tested. Interestingly, in women, while age and temporal gray matter volume provided the best model, the addition of diagnosis did not improve the model.
7.0 Possible neurochemical mechanisms of the acute and chronic alcohol effects on sleep EEG
Sleep is associated with a complex set of interactions between different neurotransmitter systems (see (Espana and Scammell 2011; Fuller, Gooley, and Saper 2006) for reviews). NREM sleep is a period in which cholinergic and noradrenergic brainstem arousal mechanisms are dramatically reduced. The acute effects of alcohol on sleep EEG can arguably be explained by alcohol’s GABA agonist properties. The subsequent withdrawal of tonic input to the reticular nucleus of thalamus allows the release of GABA and inhibition of thalmo-cortical circuits (Steriade 1999). The hyperpolarizing effect of GABA causes the opening of low threshold calcium ion channels and a pattern of synchronized burst firing that manifests as sleep spindles in the sleep EEG. Further release of GABA causes greater levels of hyperpolarization and the production of delta EEG waveforms (Steriade, McCormick, and Sejnowski 1993).
The movement between NREM and REM sleep involves a complex interaction between REM-on and REM-off neuronal groups in the brainstem. The REM-on groups largely consist of cholinergic cells in the lateral dorsal tegmentum (LDT) and the pedunculo pontine tegemental (PPT) nuclei. REM-off cells involve the serotonergic dorsal raphe nucleus and noradrenergic locus ceruleus. The model originally developed by McCarley and Hobson (1975) proposed a set of reciprocal interactions between the two groups of neurons whereby REM-on neurons are influenced by a self-excitatory loop but also have an excitatory link to REM-off neurons. Once a threshold level of activation is reached in the REM-off cells, they become dominant. These have an inhibitory action on REM-on cells but also a self-inhibitory feedback loop that progressively decreases their activity. Eventually, activity drops below a threshold point and REM-on cells regain dominance. Recent work has identified an important role for GABAergic interneurons that act to facilitate the REM-off process (McCarley 2011). It is, therefore, plausible, that alcohol could influence this REM-off process through its effects on GABA, leading to the suppression of REM sleep in the short-term.
7.1 Neurochemistry of acute alcohol effects
Animal data indicate that administration of GABAergic antagonists lead to increased REM (Sanford et al. 2003; Xi, Morales, and Chase 2001, 1999). Alcohol leads to presynaptic release of GABA in the brainstem and spinal cord (Kelm, Criswell, and Breese 2011) and thus, it is reasonable to hypothesize that this sequence plays a role in alcohol’s suppression of REM sleep in the context of high doses of alcohol.
The increase in delta activity is also consistent with alcohol’s GABA agonist properties. GABA mediated hyperpolarization of cortical and thalamo-cortical neurons is thought to underlie the calcium channel mediated burst firing that results in EEG delta activity (Steriade 1999). While alcohol does not lead to presynaptic GABA release in the thalamus or cortex the way it does in some other brain regions (Kelm, Criswell, and Breese 2011), it does enhance the function of GABAA receptors. Further, there is evidence for acute ethanol modulation of metobatropic glutamate receptor (mGluR) mediated slow currents (Su, Sun, and Shen 2010) that are thought to underlie the slow oscillation in thalamo-cortical cells underlying delta generation (Hughes et al. 2002).
Sleep spindles (associated with sigma frequency power) are produced when thalamo-cortical cells become hyperpolarized resulting in low threshold spikes and the generation of spindle frequency activity in thalamic reticular cells (Steriade 1993). Further hyperpolarization leads to cessation of spindle activity and the development of delta activity (Steriade 1993). Alcohol preferentially facilitates tonic GABAergic neurotransmission (Wei, Faria, and Mody 2004), which is more likely to likely to lead to delta than sigma production. The observed decrease in sigma power, following alcohol consumption in Dijk (1992), is in contrast to the spindle and sigma facilitation seen following administration of benozdiazepines (Johnson, Hanson, and Bickford 1976). In addition, no studies have found altered sigma power in alcoholics. These findings are consistent with recent data showing that alcohol facilitates benzodiazepine insensitive GABAA receptor subtypes (Krystal 2006) and older data showing inverse effects of benzodiazepines on sigma and delta activity (Johnson et al. 1979).
7.2 Neurochemistry of alcoholism effects
The sleep EEG effects in those with long-term alcohol dependence are the opposite to those following acute alcohol administration. One possible mechanism is long-term alteration in responsiveness of GABA mechanisms. There is evidence of allosteric modification of GABA receptors (Kang, Spigelman, and Olsen 1998; Follesa et al. 2006) and reduced GABAA receptor function (Valenzuela and Harris 1997; Mihic and Harris 1995) in rodent models of alcohol dependence. Thus down regulation of brainstem GABAergic systems following development of alcohol dependence would lead to diminished activity in REM-off systems (see Figure 6) leading to an increased propensity for REM. This hypothesis has not been directly tested, and it should be noted that other factors may play a role in the increased REM seen in long-term abstinent alcoholics. For example, administration of the tumor necrosis factor α (TNF-α) antagonist etanercept led to normalization of REM sleep in 18 abstinent alcoholics (Irwin et al. 2009).
Figure 6.
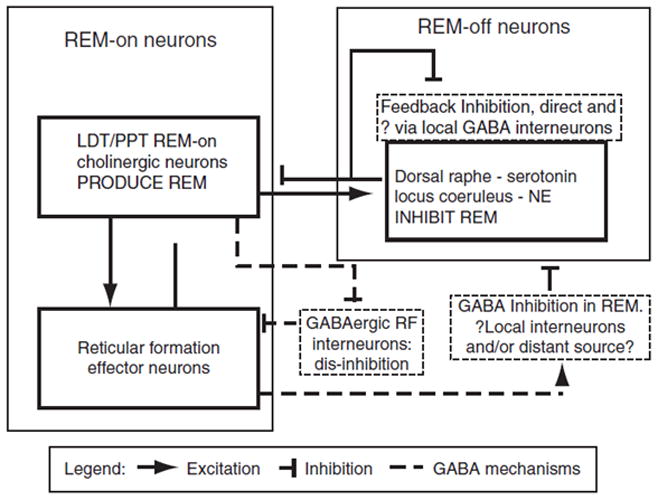
A structural model of rapid eye movement (REM) sleep control highlighting the role of GABAergic interneurons (McCarley 2011). LDT, laterodorsal tegmental nucleus; PPT, pedunculopontine tegmental nucleus; RF, reticular formation; GABA, gamma-aminobutyric acid; NE, norepinephrine.
In addition to loss of gray matter volume and reduced connectivity, down regulation of GABA systems could also partially explain the decrease in both delta power and the amplitude of evoked delta responses in abstinent alcoholics. However, again, there are other possible mechanisms that may also contribute to these effects. For example, there is evidence of altered amplitude of T-type Calcium current following chronic alcohol consumption in non-human primates (Welsh et al. 2011; Carden et al. 2006), evidence of blocked T-type Calcium currents in reticular thalamocortical neurons in vitro (Joksovic et al. 2005) and evidence for chronic ethanol modulation of mRNA of mGluR1 (Simonyi et al. 1996), all of which could lead to attenuated delta generation.
8.0 Familial predisposition for alcoholism effects on sleep?
As has been discovered in awake EEG and evoked potentials, it is also possible that sleep EEG effects in alcoholism may partially reflect a genetic predisposition to alcoholism. The studies addressing this possibility in sleep have used a comparison of those with positive and negative alcoholism family histories rather than genetic analysis. Arendt et al. (Arnedt, Rohsenow, et al. 2011) did not find any differences in the acute alcohol effects on sleep in family history positive or negative men and women. Tarokh et al. (2012) study of sleep EEG in family history positive and negative youth provides evidence that a familial predisposition is not associated with greater REM sleep, suggesting that increased REM sleep is more likely to be a consequence of alcohol abuse per se. Comparison of the child and adolescent cohorts in Tarokh et al. (2012) reveals a tendency for family history positive children to have lower levels of NREM delta, with more consistent differences emerging in the adolescent cohort. However, Tarokh et al. (2012) did not show family history differences in sleep homeostasis using NREM delta power as the dependent measure; nor did Colrain et al. (2011) find a family history effect for NREM evoked delta amplitude.
While there have been no studies evaluating genotype influences on sleep EEG, the data from wake EEG studies are clearly relevant, especially the known association between the GABA gene GABRA2 and beta oscillations (Porjesz et al. 2002) and between the cholinergic gene CHRM2 and delta oscillations (Porjesz and Rangaswamy 2007; Rangaswamy and Porjesz 2013). It should be noted however that EEG during wakefulness that is measured in association with cognitive or behavioral tasks, reflects the activity in task specific pathways and networks, whereas sleep EEG is measuring a baseline state. Family history linkages with wake but not sleep EEG phenomena may thus reflect specificity in the predisposition to altered circuitry associated with the awake behaviors, rather than with EEG generation per se. There are data showing relations between genes associated with circadian rhythm regulation and alcoholism-related insomnia. Brower et al. (2012) reported that a specific PER3 genotype (PER3 (4/4)) conferred a small but significantly increased risk of insomnia over and above drinking frequency, mental and physical health status and childhood abuse in a large sample of alcohol dependent patients. PER3 (4/4) is associated with evening circadian preference and lower homeostatic sleep drive (Brower et al. 2012).
9.0 A role for sleep in treatment, recovery, and relapse
Given that sleep problems persist during alcohol recovery, it raises the possibility that treatment of sleep problems in alcoholic patients could aid recovery and decrease relapse rates (Brower 2001). Self-reported sleep problems as well as sleep continuity and REM sleep measures derived from the PSG are significant predictors of relapse (Reviewed in (Arnedt, Conroy, and Brower 2007)). Periodic leg movements, seen in recently detoxified alcoholics are also more common in those who go on to relapse (Gann et al. 2002). A recent pilot study showed that cognitive behavioral treatment for insomnia (CBTI) in alcohol dependent patients was more effective than placebo in improving subjective sleep and in reducing daytime fatigue (Arnedt, Conroy, et al. 2011). However, there were no differences in relapse rates between those treated with placebo and those treated with CBTI in this small sample (n= 17). In a larger controlled trial of CBTi in recovering alcoholic men and women, Currie and colleagues (2004) reported that patients treated with CBTi had improved subjective sleep quality, reduced sleep onset latency, and fewer awakenings compared to pre-treatment whereas wait-listed controls showed no significant changes in sleep. Improvements in the CBTi group were maintained 3 and 6 months after treatment. There was no difference in relapse rates between those treated with CBTi and waitlist controls.
We have found intriguing evidence that the amplitude of the N550 component of the K-complex may act as a marker of brain recovery with abstinence. After correction for age and estimated lifetime alcohol consumption, time sober demonstrated significant correlations with increased N550 and P900 amplitudes in recovering alcoholics (Colrain et al. 2009). Additional data were collected from 15 alcohol dependent subjects (12 men) who had been studied previously (Colrain et al. 2009). N550 and P900 amplitude were significantly larger at frontal sites with the additional 12 months of abstinence (Colrain, Padilla, and Baker 2012) (see Figure 7). These data are suggestive of the N550 and P900 reflecting functional improvement associated with recovery in gray matter volume and white matter integrity (Cardenas et al. 2007). However, this apparent marker of brain recovery should not be interpreted as a recovery of sleep. Sleep problems persist even after several months of abstinence (Arnedt, Conroy, and Brower 2007).
Figure 7.

Grand mean evoked potential waveforms for alcoholics at initial assessment(redlines) and at 12 month follow-up (blue lines) Fz, FCz, Cz, CPz and Pz. Data are presented with negative voltages up the Y axis. Data are drawn from (Colrain, Padilla, and Baker 2012).
10.0 Conclusion
Alcohol has a profound impact on sleep, with effects dependent on acute versus chronic use and dependence. While alcohol is initially sedating, this effect disappears after a few hours, resulting in a fragmented and disturbed sleep in the second half of the night. Sustained use of alcohol in chronic alcoholism is associated with major sleep problems. Ongoing sleep and circadian disruption are features of alcohol drinking binges. For those who are homeless, there are almost insurmountable obstacles to obtaining appropriately restorative sleep. When abstinent, sleep issues persist, with insomnia and vivid dreams being common complaints, which can be a factor leading to relapse.
Animal (typically rodent) models of acute or chronic alcohol administration on sleep have provided valuable insights into the neurochemical effects of alcohol on brain structures and systems that play a role in sleep regulation, however, many questions remain unanswered. Further studies are needed in humans and animal models to establish whether there are genetic predispositions to sleep differences or susceptibilities to alcohol, whether the REM increase seen in alcoholics is a reflection of an irreversible change in the brainstem, and whether there are periods of vulnerability to the onslaught of alcohol on sleep regulatory systems, such as during adolescence. It will also be valuable for future studies of adolescents and family history positive individuals to explore further what aspects of altered brain structure and sleep EEG pre-date the onset of alcohol abuse compared to changes that occur as a result of the impact of alcohol on the brain. It is critically important from a clinical perspective to determine whether preventing sleep disorders may help prevent the development of alcohol use disorders in high-risk individuals and which treatments are most effective in alcoholics, both in terms of improving sleep quality and supporting continued abstinence. At this time when poly-substance dependence is common, it also is becoming increasingly relevant to investigate the interactive effects of substances of abuse on sleep behavior and regulation.
Abbreviations
- EEG
Electroencephalogram
- REM
Rapid Eye Movement
- NREM
Non-Rapid Eye Movement
- Hz
Hertz
- SWS
Slow Wave Sleep
- BAC
Breath Alcohol Concentration
- PSG
Polysomnogram
- SWA
Slow Wave Activity
- ERPs
Event-Related Potentials
- FA
Fractional Anisotropy
- DTI
Diffusion Tensor Imaging
- GABA
gamma-Aminobutyric acid
- LDT
Lateral Dorsal Tegmentum
- PPT
Pedunculo Pontine Tegmentum
- CBTI
Cognitive Behavioral Treatment for Insomnia
- RF
Reticular Formation
- NE
Norepinephrine
References
- Adamson J, Burdick JA. Sleep of dry alcoholics. Arch Gen Psychiatry. 1973;28(1):146–9. doi: 10.1001/archpsyc.1973.01750310116019. [DOI] [PubMed] [Google Scholar]
- Aldrich M. National Institute on Alcohol Abuse and Alcoholism Research Monograph 33 Alcohol Problems and Aging. U.S. Department of Health and Human Services; 1998. Effects of alcohol on sleep; pp. 281–300. [Google Scholar]
- Allen RP, Wagman AM. Do sleep patterns relate to the desire for alcohol? In: Grosss Milton M., editor. Alcohol Intoxication and Withdrawal. New York: Plenum Press; 1975. pp. 495–508. [DOI] [PubMed] [Google Scholar]
- Allen RP, Wagman AM, Funderburk FR. Slow wave sleep changes: alcohol tolerance and treatment implications. In: Gross Milton M., editor. Alcohol Intoxication and Withdrawal IIIA. New York: Plenum Press; 1977. pp. 629–640. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. The K-complex: its slow (<1-Hz) rhythmicity and relation to delta waves. Neurology. 1997;49(4):952–9. doi: 10.1212/wnl.49.4.952. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998a;82(3):671–86. doi: 10.1016/s0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998b;107(2):69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. The functional significance of K-complexes. Sleep Medicine Reviews. 2002;6(2):139–49. doi: 10.1053/smrv.2001.0181. [DOI] [PubMed] [Google Scholar]
- Armitage R, Hoffmann R, Conroy DA, Arnedt JT, Brower KJ. Effects of a 3-hour sleep delay on sleep homeostasis in alcohol dependent adults. Sleep. 2012;35(2):273–8. doi: 10.5665/sleep.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227–33. doi: 10.1016/j.brat.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. J Addict Dis. 2007;26(4):41–54. doi: 10.1300/J069v26n04_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedt JT, Rohsenow DJ, Almeida AB, Hunt SK, Gokhale M, Gottlieb DJ, Howland J. Sleep following alcohol intoxication in healthy, young adults: effects of sex and family history of alcoholism. Alcohol Clin Exp Res. 2011;35(5):870–8. doi: 10.1111/j.1530-0277.2010.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology. 2001;25(3):332–40. doi: 10.1016/S0893-133X(01)00236-6. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39(6):411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25(2):110–25. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Hoffmann R, Conroy DA, Arnedt JT, Armitage R. Sleep homeostasis in alcohol-dependent, depressed and healthy control men. Eur Arch Psychiatry Clin Neurosci. 2011;261(8):559–66. doi: 10.1007/s00406-011-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Perron BE. Prevalence and correlates of withdrawal-related insomnia among adults with alcohol dependence: results from a national survey. Am J Addict. 2010;19(3):238–44. doi: 10.1111/j.1521-0391.2010.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Wojnar M, Sliwerska E, Armitage R, Burmeister M. PER3 polymorphism and insomnia severity in alcohol dependence. Sleep. 2012;35(4):571–7. doi: 10.5665/sleep.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden WB, Alexander GM, Friedman DP, Daunais JB, Grant KA, Mu J, Godwin DW. Chronic ethanol drinking reduces native T-type calcium current in the thalamus of nonhuman primates. Brain Res. 2006;1089(1):92–100. doi: 10.1016/j.brainres.2006.02.135. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34(3):879–87. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA. The Second Decade. In: Guilleminault C, editor. Sleeping and waking disorders: indications and techniques. Menlo Park: Addison Wesley; 1982. pp. 99–125. [Google Scholar]
- Chan JKM, Trinder J, Colrain IM, Nicholas CL. The Acute Effects of Alcohol on Sleep Architecture in Late Adolescence. Alcoholism: Clinical and Experimental Research. 2013 doi: 10.1111/acer.12141. no. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Baker FC. Changes in Sleep as a Function of Adolescent Development. Neuropsychol Rev. 2011 doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Crowley KE, Nicholas CL, Afifi L, Baker FC, Padilla M, Turlington SR, Trinder J. Sleep evoked delta frequency responses show a linear decline in amplitude across the adult lifespan. Neurobiology of Aging. 2010;31(5):874–883. doi: 10.1016/j.neurobiolaging.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Sullivan EV, Rohlfing T, Baker FC, Nicholas CL, Padilla ML, Chanraud S, Pitel AL, Pfefferbaum A. Independent contributions of cortical gray matter, aging, sex and alcoholism to K-complex amplitude evoked during sleep. Sleep. 2011;34(6):787–95. doi: 10.5665/SLEEP.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Turlington S, Baker FC. Impact of alcoholism on sleep architecture and EEG power spectra in men and women. Sleep. 2009a;32(10):1341–52. doi: 10.1093/sleep/32.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response? J Sleep Res. 1999;8(4):273–280. doi: 10.1046/j.1365-2869.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Webster KE, Hirst G, Campbell KB. The roles of vertex sharp waves and K-complexes in the generation of N300 in auditory and respiratory-related evoked potentials during early stage 2 NREM sleep. Sleep. 2000;23(1):97–106. [PubMed] [Google Scholar]
- Colrain IM. The K-complex: A seven-decade history. Sleep. 2005;28(2):255–273. doi: 10.1093/sleep/28.2.255. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Crowley KE, Nicholas CL, Padilla ML, Baker FC. The impact of alcoholism on sleep evoked Delta frequency responses. Biological Psychiatry. 2009;66(2):177–84. doi: 10.1016/j.biopsych.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Padilla ML, Baker FC. Partial recovery of alcohol dependence-related deficits in sleep evoked potentials following twelve months of abstinence. Frontiers in Neurology. 2012 doi: 10.3389/fneur.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Turlington SR, Baker FC. The impact of alcoholism on sleep Architecture and EEG power Spectra in Men and women. Sleep. 2009b;32(10):1341–52. doi: 10.1093/sleep/32.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DA, Brower KJ. Alcohol, toxins, and medications as a cause of sleep dysfunction. Handb Clin Neurol. 2011;98:587–612. doi: 10.1016/B978-0-444-52006-7.00038-1. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Trinder J, Colrain IM. An examination of evoked K-complex amplitude and frequency of occurrence in the elderly. J Sleep Res. 2002;11(2):129–40. doi: 10.1046/j.1365-2869.2002.00293.x. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Trinder J, Colrain IM. Evoked K-Complex Generation: The Impact of Sleep Spindles and Age. Clinical Neurophysiology. 2004;115(2):471–476. doi: 10.1016/j.clinph.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Currie SR, Clark S, Rimac S, Malhotra S. Comprehensive assessment of insomnia in recovering alcoholics using daily sleep diaries and ambulatory monitoring. Alcohol Clin Exp Res. 2003 Aug;27(8):1262–9. doi: 10.1097/01.ALC.0000081622.03973.57. [DOI] [PubMed] [Google Scholar]
- Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004 Sep;99(9):1121–32. doi: 10.1111/j.1360-0443.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- Czisch M, Wehrle R, Stiegler A, Peters H, Andrade K, Holsboer F, Samann PG. Acoustic oddball during NREM sleep: a combined EEG/fMRI study. PLoS One. 2009;4(8):e6749. doi: 10.1371/journal.pone.0006749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, Desseilles M, Laureys S, Degueldre C, Perrin F, Phillips C, Maquet P, Peigneux P. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage. 2005;28(1):14–21. doi: 10.1016/j.neuroimage.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, Carrier J, Moonen G, Balteau E, Degueldre C, Luxen A, Phillips C, Maquet P. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A. 2008;105(39):15160–5. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Bertini M. The spontaneous K-complex during stage 2 sleep: is it the ‘forerunner’ of delta waves? Neuroscience Letters. 2000;291:41–43. doi: 10.1016/s0304-3940(00)01366-5. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Brunner DP, Aeschbach D, Tobler I, Borbely AA. The effects of ethanol on human sleep EEG power spectra differ from those of benzodiazepine receptor agonists. Neuropsychopharmacology. 1992;7(3):225–32. [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22(8):1796–802. [PubMed] [Google Scholar]
- Ehlers Cindy L. Alcohol and Sleep. In: Noronha Antonio, Eckardt Michael, Warren Kenneth., editors. Review of NIAA’s Neuroscience and Behavioral Research Portfolio. Bethesda, MD: U.S. Department of Health and Human Services; 2000. pp. 417–436. [Google Scholar]
- Ely M, Hardy R, Longford NT, Wadsworth ME. Gender differences in the relationship between alcohol consumption and drink problems are largely accounted for by body water. Alcohol Alcohol. 1999;34(6):894–902. doi: 10.1093/alcalc/34.6.894. [DOI] [PubMed] [Google Scholar]
- Espana RA, Scammell TE. Sleep neurobiology from a clinical perspective. Sleep. 2011;34(7):845–58. doi: 10.5665/SLEEP.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Levin FR. Response to alcohol in women: role of the menstrual cycle and a family history of alcoholism. Drug Alcohol Depend. 2011 Mar 1;114(1):18–30. doi: 10.1016/j.drugalcdep.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B, Gann H, Brueck R, Hornyak M, Litsch S, Hohagen F, Riemann D. Effects of alcohol on polysomnographically recorded sleep in healthy subjects. Alcohol Clin Exp Res. 2006;30(9):1527–37. doi: 10.1111/j.1530-0277.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- Feige B, Scaal S, Hornyak M, Gann H, Riemann D. Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res. 2007;31(1):19–27. doi: 10.1111/j.1530-0277.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Finelli LA, Borbely AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. European Journal of Neuroscience. 2001;13(12):2282–90. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology (Berl) 2006;186(3):267–80. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Fonzi S, Solinas GP, Costelli P, Parodi C, Murialdo G, Bo P, Albergati A, Montalbetti L, Savoldi F, Polleri A. Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia. 1994;21(1–2):109–12. [PubMed] [Google Scholar]
- Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21(6):482–93. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- Gann H, Feige B, Fasihi S, van Calker D, Voderholzer U, Riemann D. Periodic limb movements during sleep in alcohol dependent patients. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):124–9. doi: 10.1007/s00406-002-0371-8. [DOI] [PubMed] [Google Scholar]
- Gann H, Feige B, Hohagen F, van Calker D, Geiss D, Dieter R. Sleep and the cholinergic rapid eye movement sleep induction test in patients with primary alcohol dependence. Biol Psychiatry. 2001;50(5):383–90. doi: 10.1016/s0006-3223(01)01172-6. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Kripke DF, Schuckit M. EEG sleep studies in “pure” primary alcoholism during subacute withdrawal: relationships to normal controls, age, and other clinical variables. Biological Psychiatry. 1990;27:477–488. doi: 10.1016/0006-3223(90)90439-9. [DOI] [PubMed] [Google Scholar]
- Halasz P. K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment. Sleep Med Rev. 2005;9(5):391–412. doi: 10.1016/j.smrv.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril JJ. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. Journal of Neurological Science. 1989;92:81–89. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Daly JM. Are we drinking our neurones away? British Medical Journal. 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33(6):947–58. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine 2007 [Google Scholar]
- Imatoh N, Nakazawa Y, Ohshima H, Ishibashi M, Yokoyama T. Circadian rhythm of REM sleep of chronic alcoholics during alcohol withdrawal. Drug Alcohol Depend. 1986;18(1):77–85. doi: 10.1016/0376-8716(86)90116-x. [DOI] [PubMed] [Google Scholar]
- Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51(8):632–41. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- Irwin M, Miller C, Gillin JC, Demodena A, Ehlers CL. Polysomnographic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcohol Clin Exp Res. 2000;24(9):1376–84. [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66(2):191–5. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Breslau N. Sleep problems and substance use in adolescence. Drug Alcohol Depend. 2001;64(1):1–7. doi: 10.1016/s0376-8716(00)00222-2. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roth T, Schultz L, Breslau N. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117(2):e247–56. doi: 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- Johnson LC, Hanson K, Bickford RG. Effect of flurazepam on sleep spindles and K-complexes. Electroencephalogr Clin Neurophysiol. 1976;40(1):67–77. doi: 10.1016/0013-4694(76)90180-2. [DOI] [PubMed] [Google Scholar]
- Johnson LC, Seales DM, Naitoh P, Church MW, Sinclair M. The effects of flurazepam hydrochloride on brain electrical activity during sleep. Electroencephalogr Clin Neurophysiol. 1979;47(3):309–21. doi: 10.1016/0013-4694(79)90282-7. [DOI] [PubMed] [Google Scholar]
- Joksovic PM, Brimelow BC, Murbartian J, Perez-Reyes E, Todorovic SM. Contrasting anesthetic sensitivities of T-type Ca2+ channels of reticular thalamic neurons and recombinant Ca(v)3.3 channels. Br J Pharmacol. 2005;144(1):59–70. doi: 10.1038/sj.bjp.0706020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, Spigelman I, Olsen RW. Alteration in the sensitivity of GABA(A) receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol Clin Exp Res. 1998;22(9):2165–73. [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev. 2011;65(2):113–23. doi: 10.1016/j.brainresrev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM1, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008 Jan 11;93(1–2):21–9. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama H, Nakazawa Y, Kotorii T, Nonaka K, Inanaga K, Ohshima M, Tokoyama T. Biorhythm of core temperature in depressive and non-depressive alcoholics. Drug Alcohol Depend. 1988;21(1):1–6. doi: 10.1016/0376-8716(88)90002-6. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Halbreich U. Effects of alcohol and other drugs in women of reproductive age: hormonal interactions. Drugs Today (Barc) 1998 Oct;3410:837–43. doi: 10.1358/dot.1998.34.10.487470. [DOI] [PubMed] [Google Scholar]
- Kuhlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry. 2003;54(12):1437–43. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Roth C, Dijk DJ, Borbely AA. Late-afternoon ethanol intake affects nocturnal sleep and the sleep EEG in middle-aged men. J Clin Psychopharmacol. 1996;16(6):428–36. doi: 10.1097/00004714-199612000-00004. [DOI] [PubMed] [Google Scholar]
- Le Bon O, Verbanck P, Hoffmann G, Murphy JR, Staner L, De Groote D, Mampunza S, Den Dulk A, Vacher C, Kornreich Ch, Pelc I. Sleep in detoxified alcoholics: Impairment of most standard sleep parameters and increased risk for sleep apnea, but not for myoclonias- A controlled study. Journal of Studies on Alcohol. 1997;58:30–36. doi: 10.15288/jsa.1997.58.30. [DOI] [PubMed] [Google Scholar]
- MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young men. J Stud Alcohol. 1982;43(5):434–44. doi: 10.15288/jsa.1982.43.434. [DOI] [PubMed] [Google Scholar]
- Manber R, Baker FC, Gress JL. Sex differences in sleep and sleep disorders: a focus on women’s sleep. Int J Sleep Disorders. 2006;1:7–15. [Google Scholar]
- Manni R, Ratti MT, Marchioni E, Castelnovo G, Murelli R, Sartori I, Galimberti CA, Tartara A. Poor sleep in adolescents: a study of 869 17-year-old Italian secondary school students. J Sleep Res. 1997;6(1):44–9. doi: 10.1046/j.1365-2869.1997.00025.x. [DOI] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. Journal of Neuroscience. 2004;24(31):6862–70. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM sleep. Handb Clin Neurol. 2011;98:151–71. doi: 10.1016/B978-0-444-52006-7.00010-1. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189(4196):58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Harris RA. Alcohol actions at the GABAA receptor/chloride channels complex. In: Deitrich RA, Erwin G, editors. Pharmacological Effects of Ethanol on the Nervous System. Boca Raton, FL: CRC Press; 1995. pp. 51–71. [Google Scholar]
- Mukai M, Uchimura N, Hirano T, Ohshima H, Ohshima M, Nakamura J. Circadian rhythms of hormone concentrations in alcohol withdrawal. Psychiatry Clin Neurosci. 1998;52(2):238–40. doi: 10.1111/j.1440-1819.1998.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Negriff S, Dorn LD, Pabst SR, Susman EJ. Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Res. 2011;185(3):408–13. doi: 10.1016/j.psychres.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CL, Trinder J, Crowley KE, Colrain IM. The impact of slow wave sleep proximity on evoked K-complex generation. Neuroscience Letters. 2006;404(1–2):127–31. doi: 10.1016/j.neulet.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Nicholas CL, Sullivan EV, Pfefferbaum A, Trinder J, Colrain IM. The effects of alcoholism on auditory evoked potentials during sleep. Journal of Sleep Research. 2002;11(3):247–53. doi: 10.1046/j.1365-2869.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Mosher-Ruiz S, Luhar R, Gravitz ZR. Profile of Spared, Impaired, and Recovered Neuropsychological Processes. In: Pfefferbaum A, Sullivan EV, editors. Handbook of Clinical Neurology: Alcohol and the Nervous System. 2013. [Google Scholar]
- Othmer O, Dauhaday WH, Goodin DW, Levine WR, Malarkey WB, Freemon F, Halikas JA. Sleep and growth hormone secretion in adults. Journal of Clinical Psychiatry. 1982;43(10):411–414. [PubMed] [Google Scholar]
- Peters TJ, Millward LM, Foster J. Quality of life in alcohol misuse: comparison of men and women. Arch Womens Ment Health. 2003 Nov;6(4):239–43. doi: 10.1007/s00737-003-0012-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological Psychiatry. 2006;59(4):364–372. doi: 10.1016/j.biopsych.2005.06.025. doi:16125148. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, Sullivan EV. Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholics men and women: Selective relations to dissociable functions. Alcoholism: Clinical and Experimental Research. 2010;34(7):1201–1211. doi: 10.1111/j.1530-0277.2010.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biological Psychiatry. 2009;65(8):680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. DTI and MRI. In: Pfefferbaum A, Sullivan EV, editors. Handbook of Clinical Neurology: Alcohol and the Nervous System. Elsevier; 2013. [Google Scholar]
- Pieters S, Van Der Vorst H, Burk WJ, Wiers RW, Engels RC. Puberty-dependent sleep regulation and alcohol use in early adolescents. Alcohol Clin Exp Res. 2010;34(9):1512–8. doi: 10.1111/j.1530-0277.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99(6):3729–33. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. ScientificWorldJournal. 2007;7:131–41. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz PN, Roehrs TA, Vitaliano PP, Linnoila M, Weitzman ED. Effect of alcohol on sleep and nighttime plasma growth hormone and cortisol concentrations. J Clin Endocrinol Metab. 1980;51(4):759–64. doi: 10.1210/jcem-51-4-759. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Understanding Alcohol Use Disorders with Neuroelectrophysiology. In: Pfefferbaum A, Sullivan EV, editors. Handbook of Clinical Neurology: Alcohol and the Nervous System. Elsevier; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardised Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Washington D.C: U.S. Government Printing Office; 1968. [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Duong HT. Sleepless in adolescence: prospective data on sleep deprivation, health and functioning. Journal of adolescence. 2009;32(5):1045–57. doi: 10.1016/j.adolescence.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Duong HT. Chronic insomnia and its negative consequences for health and functioning of adolescents: a 12-month prospective study. J Adolesc Health. 2008;42:294–302. doi: 10.1016/j.jadohealth.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Papineau K, Rosenthal L, Roth T. Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood. Neuropsychopharmacology. 1999;20(3):279–86. doi: 10.1016/S0893-133X(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Yoon J, Roth T. Nocturnal and next-day effects of ethanol and basal level of sleepiness. Hum Psychopharmacol. 1991;6:307–312. [Google Scholar]
- Rundell OH, Lester BK, Griffiths WJ, Williams HL. Alcohol and sleep in young adults. Psychopharmacologia. 1972;26(3):201–18. doi: 10.1007/BF00422697. [DOI] [PubMed] [Google Scholar]
- Rundell OH, Williams HL, Lester BK. Sleep in alcoholic patients: Longitudinal findings. In: Gross Milton M., editor. Alcohol Intoxication and Withdrawal. New York: Plenum Press; 1975. pp. 389–402. [DOI] [PubMed] [Google Scholar]
- Sagawa Y, Kondo H, Matsubuchi N, Takemura T, Kanayama H, Kaneko Y, Kanbayashi T, Hishikawa Y, Shimizu T. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35(11):2093–100. doi: 10.1111/j.1530-0277.2011.01558.x. [DOI] [PubMed] [Google Scholar]
- Saletu-Zyhlarz GM, Arnold O, Anderer P, Oberndorfer S, Walter H, Lesch OM, Boning J, Saletu B. Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol Alcohol. 2004;39(3):233–40. doi: 10.1093/alcalc/agh041. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90(2):938–45. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- Sano H, Suzuki Y, Yazaki R, Tamefusa K, Ohara K, Yokoyama T, Miyasato K. Circadian variation in plasma 5-hydroxyindoleacetic acid level during and after alcohol withdrawal: phase advances in alcoholic patients compared with normal subjects. Acta Psychiatr Scand. 1993;87(4):291–6. doi: 10.1111/j.1600-0447.1993.tb03374.x. [DOI] [PubMed] [Google Scholar]
- Sassin JF, Johnson LC. Body motility during sleep and its relation to the K-complex. Experimental Neurology. 1968;22(1):133–144. doi: 10.1016/0014-4886(68)90025-3. [DOI] [PubMed] [Google Scholar]
- Schmitz MM, Sepandj A, Pichler PM, Rudas S. Disrupted melatonin-secretion during alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20(6):983–95. doi: 10.1016/0278-5846(96)00078-4. [DOI] [PubMed] [Google Scholar]
- Scrima L, Broudy M, Nay KN, Cohn MA. Increased severity of obstructive sleep apnea after bedtime alcohol ingestion: diagnostic potential and proposed mechanism of action. Sleep. 1982;5(4):318–28. doi: 10.1093/sleep/5.4.318. [DOI] [PubMed] [Google Scholar]
- Shibley HL, Malcolm RJ, Veatch LM. Adolescents with insomnia and substance abuse: consequences and comorbidities. J Psychiatr Pract. 2008;14(3):146–53. doi: 10.1097/01.pra.0000320113.30811.46. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Zhang JP, Sun AY, Sun GY. Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons. Neuroreport. 1996;7(13):2115–8. doi: 10.1097/00001756-199609020-00010. [DOI] [PubMed] [Google Scholar]
- Snyder S, Karacan I. Sleep patterns of sober chronic alcoholics. Neuropsychobiology. 1985;13(1–2):97–100. doi: 10.1159/000118169. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body’s biological clock. Alcohol Clin Exp Res. 2005;29(8):1550–7. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Steriade M. Cellular Substrates of Brain Rhythms. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Baltimore: Williams and Wilkins; 1993. pp. 27–62. [Google Scholar]
- Steriade M. Cellular Substrates of Brain Rhythms. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Baltimore: Williams and Wilkins; 1999. pp. 28–75. [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Stone BM. Sleep and low doses of alcohol. Electroencephalogr Clin Neurophysiol. 1980;48(6):706–9. doi: 10.1016/0013-4694(80)90427-7. [DOI] [PubMed] [Google Scholar]
- Su LD, Sun CL, Shen Y. Ethanol acutely modulates mGluR1-dependent long-term depression in cerebellum. Alcohol Clin Exp Res. 2010;34(7):1140–5. doi: 10.1111/j.1530-0277.2010.01190.x. [DOI] [PubMed] [Google Scholar]
- Sutherland GT, Sheedy D, Kril JJ. Neuropathology of Alcoholism. In: Pfefferbaum A, Sullivan EV, editors. Handbook of Clinical Neurology: Alcohol and the Nervous System. Elsevier; 2013. [DOI] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, Achermann P. Dissipation of sleep pressure is stable across adolescence. Neuroscience. 2012;216:167–77. doi: 10.1016/j.neuroscience.2012.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Van Reen E, Acebo C, LeBourgeois M, Seifer R, Fallone G, Carskadon MA. Adolescence and parental history of alcoholism: insights from the sleep EEG. Alcohol Clin Exp Res. 2012;36(9):1530–41. doi: 10.1111/j.1530-0277.2012.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Gillin JC, Golshan S, Irwin M. Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short-term abstinence. Biological Psychiatry. 1995;38:831–836. doi: 10.1016/0006-3223(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tynjala J, Kannas L, Levalahti E. Perceived tiredness among adolescents and its association with sleep habits and use of psychoactive substances. J Sleep Res. 1997;6(3):189–98. doi: 10.1046/j.1365-2869.1997.00048.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Harris RA. Alcohol Neurobiology. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse: A comprehensive Textbook. Baltimore, MD: Williams & wilkins; 1997. pp. 119–142. [Google Scholar]
- Van Reen E, Jenni OG, Carskadon MA. Effects of alcohol on sleep and the sleep electroencephalogram in healthy young women. Alcohol Clin Exp Res. 2006;30(6):974–81. doi: 10.1111/j.1530-0277.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- Wagman AMI, Allen RP. Effects of alcohol ingestion and abstinence on slow wave sleep of alcoholics. In: Gross Milton M., editor. Alcohol Intoxication and Withdrawal. New York: Plenum Press; 1975. pp. 453–466. [DOI] [PubMed] [Google Scholar]
- Walter H, Gutierrez K, Ramskogler K, Hertling I, Dvorak A, Lesch OM. Gender-specific differences in alcoholism: implications for treatment. Arch Womens Ment Health. 2003;6(4):253–8. doi: 10.1007/s00737-003-0014-8. [DOI] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24(38):8379–82. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, V, Han Z, Rossi DJ, Mohr C, Odagiri M, Daunais JB, Grant KA. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc Natl Acad Sci U S A. 2011;108(25):10314–9. doi: 10.1073/pnas.1017079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg RA, Lozano AM. Intracranial volume conduction of cortical spikes and sleep potentials recorded with deep brain stimulating electrodes. Clinical Neurophysiology. 2003;114(8):1403–18. doi: 10.1016/s1388-2457(03)00152-4. [DOI] [PubMed] [Google Scholar]
- Williams DL, MacLean AW, Cairns J. Dose-response effects of ethanol on the sleep of young women. J Stud Alcohol. 1983;44(3):515–23. doi: 10.15288/jsa.1983.44.515. [DOI] [PubMed] [Google Scholar]
- Williams HL, Rundell OH., Jr Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol Clin Exp Res. 1981;5(2):318–25. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res. 2004;28(4):578–87. doi: 10.1097/01.alc.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- Xi MC, Morales FR, Chase MH. A GABAergic pontine reticular system is involved in the control of wakefulness and sleep. Sleep Res Online. 1999;2(2):43–8. [PubMed] [Google Scholar]
- Xi MC, Morales FR, Chase MH. Induction of wakefulness and inhibition of active (REM) sleep by GABAergic processes in the nucleus pontis oralis. Arch Ital Biol. 2001;139(1–2):125–45. [PubMed] [Google Scholar]
- Yules RB, Freedman DX, Chandler KA. The effect of ethyl alcohol on man’s electroencephalographic sleep cycle. Electroencephalogr Clin Neurophysiol. 1966;20(2):109–11. doi: 10.1016/0013-4694(66)90153-2. [DOI] [PubMed] [Google Scholar]
- Yules RB, Lippman ME, Freedman DX. Alcohol administration prior to sleep. The effect on EEG sleep stages. Arch Gen Psychiatry. 1967;16(1):94–7. doi: 10.1001/archpsyc.1967.01730190096012. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Pitel AL, Chanraud S, Sullivan EV. Contributions of Studies on Alcohol Use Disorders to Understanding Cerebellar Function. In: Pfefferbaum A, Sullivan EV, editors. Handbook of Clinical Neurology: Alcohol and the Nervous System. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


