Abstract
Background
Diverticulosis and its complications are important healthcare problems in the United States and throughout the Western world. While mechanisms as to how diverticulosis occurs have partially been explored, few studies examined the relationship between colonic gases such as methane and diverticulosis in humans.
Aim
This study aimed to demonstrate prospective relationship between methanogenic Archaea and development of diverticulosis.
Methods
Subjects who consecutively underwent hydrogen breath test (HBT) at Rush University Medical Center (RUMC) between 2003 and 2010 were identified retrospectively through a database. Medical records were reviewed for presence of a colonoscopy report. Two hundred and sixty four subjects were identified who had both a breath methane level measurement and a colonoscopy result. Additional demographic and clinical data were obtained with chart review.
Results
Mean breath methane levels were higher in subjects with diverticulosis compared to those without diverticulosis (7.89 ppm vs. 4.94 ppm, p=0.04). Methane producers (defined as those with baseline fasting breath methane level >5 ppm) were more frequent among subjects with diverticulosis compared to those without diverticulosis (50.9% vs. 34%, p=0.0025). When adjusted for confounders, breath methane levels and age were the two independent predictors of diverticulosis on colonoscopy with logistic regression modeling.
Conclusions
Methanogenesis is associated with presence of diverticulosis. Further studies are needed to confirm our findings and prospectively evaluate a possible etiological role of methanogenesis and methanogenic archaea in diverticulosis.
Keywords: Breath methane level, diverticulosis, methanogenesis, methanogenic archaea
Introduction
Diverticulosis and diverticulitis are very common colonic problems encountered in clinical practice and are increasing over time. In 2008, diverticulosis and its complications cost the US healthcare system $3.066 billion [1]; constituting the 5th most important gastrointestinal cause of mortality and morbidity in the USA [1]. Due to a constant increase in the US elderly population percentage, the prevalence of diverticulosis and related problems and the total cost of diverticular disease are also expected to increase [2]. Tackling diverticular disease requires a better understanding of how and why diverticuli form.
The exact mechanisms for diverticuli formation are not known; however low fiber diet, constipation, connective tissue diseases and altered gut microbiome composition have all been postulated to play a role [3]. Diverticular disease is rare in countries with high fiber diet consumption such as Asian and African countries but it is widespread in Western countries where fiber consumption is relatively low [4]. The mechanism of how low fiber intake may lead to formation of diverticuli has been postulated to be as follows: Small-sized hard fecal matter forms with low-fiber and this causes robust segmental muscle contractions in the colon to propel it distally, leading to the outpouching of the colonic mucosa and submucosa to form diverticuli [3]. As such, fiber intake is often recommended for prevention of diverticulosis and associated constipation.
Recent studies also suggest that altered gut microbiome composition may contribute to the development of diverticulosis by production of toxic metabolites, alteration of homeostasis between gut microbiome and immune system with subsequent epithelial dysfunction, increased intraluminal pressure and delayed intestinal transit times [5]. Methanogenic archaea have been hypothesized to play a role in diverticulosis by releasing methane during fermentation of food products, which subsequently increases intraluminal pressure [3]. Furthermore, methane production in the gut has been shown to increase the amplitude of segmental muscle contractions and to slow the peristaltic velocity in dogs and guinea pigs [3]. This in turn can increase the intraluminal pressure especially in the presence of underlying constipation and low fiber intake. Increased methane levels in the breath have also been associated with constipation and constipation-predominant irritable bowel syndrome (IBS-C). As such, it is plausible that methanogenic archaea are key players in the development of diverticulosis.
High methanogen concentrations in the intestinal tract are associated with excretion of methane in breath that can be readily and easily measured in humans. The prevalence of methane producers in the general population has been reported to be 33.6% to 36.4% [6, 7]. Despite the ease of measurement of breath methane levels, no studies to date have established a link between breath methane detection and diverticulosis. Furthermore, no studies have characterized a dose-response relationship of breath methane levels and presence of diverticulosis. In this study, we wanted to see if there is an association between breath methane levels and presence of diverticulosis on colonoscopy.
Methods
This study was conducted in compliance with ethical guidelines and was approved by Rush University Institutional Review Board. Two hundred and sixty four human subjects who underwent hydrogen breath testing (HBT) at Rush University Medical Center (RUMC) between 2003 and 2010 were identified retrospectively using the Rush University Gastrointestinal HBT database. Subjects were not allowed to use antibiotics 2 weeks prior to HBT analysis. HBT was performed after a 12 hour fast, and subjects were asked to abstain from smoking and strenuous activity in the morning of the study and until its completion. HBT subjects received either 25 grams of lactose (Quintron Inc, Milwaukee, WI, USA) or 20 grams of lactulose (Inalco S.p.A., Milano, Italy, packaged by Cumberland Pharmaceuticals Inc., Nashville, TN, USA) for their breath test. Two fasting baseline breath samples were obtained; subsequent breath samples after sugar administration were taken every 15 minutes up to 150 minutes, and data was reported as parts per million (ppm). End expiratory breath samples were taken to ensure alveolar gas sampling and CO2 levels were also analyzed to ensure quality of the breath sample. Breath samples were then analyzed using a Quintron gas chromatograph (Quintron Instrument Company, Milwaukee, Wisconsin, USA) for presence of carbon dioxide, hydrogen, and methane. HBT results were plotted graphically and interpreted by one of the authors (Ali Keshavarzian) who is an experienced clinician. Methane producers were defined as having baseline fasting breath methane level > 5 ppm because ambient air concentrations of methane are expected to be much lower than 5ppm [8] and the detection limit of methane by the gas chromatography is at 1ppm [9]. Diverticulosis was defined as having one or more diverticuli at the colonoscopy performed closest to date of the breath test. Patient demographics and other clinical data and colonoscopy results were obtained with chart review. Statistical analysis was completed with two-sided Chi-Square or Fisher exact tests for categorical data and t-tests for continuous variables using SPSS v. 17.0 (SPSS Inc., Chicago, IL). A p-value less than 0.05 was considered statistically significant.
Results
There were two baseline breath methane measurements available for each patient. Two baseline breath methane levels as well as follow up breath methane levels were highly correlated (n = 406, Pearson Corr. Coefficient > 0.87 for all), therefore the first baseline breath methane level was used for further analyses. Out of the 406 studies noted in the database, 264 subjects had a documented colonoscopy in the medical record demonstrating either presence or absence of diverticulosis. Data on these 264 subjects were used for the analysis.
Characteristics of study subjects
Mean age for the entire study group was 51.92 years. The study group consisted of 193 females (73.1%) and 71 males (26.9%); and 183/264 (69.3%) subjects were white. BMI at the time of the breath test was recorded for only 262 subjects, and of that 30.5% (80/262) were obese with a BMI> 30. Our investigation of social habits showed that 12.1% of the subjects were smokers, 42.4% used alcohol, and 0.0075 % used illicit drugs.
Demographic characteristics in subjects with and without diverticulosis
Table 1 summarizes the differences in subject characteristics between those with diverticulosis vs. without diverticulosis. There was a significant difference in mean age between the subjects with diverticulosis vs. without diverticulosis (60.58 vs. 46.73 years respectively, p<0.001). Females were 63.6% among the subjects with diverticulosis vs. 78.8% among the subjects without diverticulosis, indicating a gender difference between the two study groups (p=0.013). There was no significant difference in race or ethnicity between the two groups (p=0.080). Social habits between the two groups were also not significantly different.
Table 1.
Baseline demographic characteristics of subjects with diverticulosis and subjects without diverticulosis
| Subjects with diverticulosis (n=99) | Subjects without diverticulosis (n=165) | P values | |
|---|---|---|---|
| Gender | 0.013 | ||
| Female | 63 (63.6%) | 130 (78.8%) | |
| Male | 36 (36.4%) | 35 (21.2%) | |
| Mean age | 60.58 | 46.73 | 0.000 |
| Ethnicity | 0.080 | ||
| Hispanic | 8 (8.08%) | 12 (7.2%) | |
| Non-Hispanic | 91 (91.92%) | 153 (92.8%) | |
| Race | 0.080 | ||
| American Indian/Alaska Native | 0 (0%) | 0 (0%) | |
| Asian | 1 (1.01%) | 3 (1.8%) | |
| African American | 18 (18.1%) | 28 (16.9%) | |
| Pacific Islander/Native Hawaiian | 2 (2.02%) | 0 (0%) | |
| White | 70 (70.7%) | 113 (68.4%) | |
| Others | 8 (8.08%) | 21 (12.7%) | |
| Smoking | 13 (13.1%) | 19 (11.5%) | 0.391 |
| Mean Body Mass Index | 28.24 | 26.78 | 0.155 |
| Alcohol use | 47 (47.4%) | 65 (39.3%) | 0.218 |
| Illicit drug use | 0 (0%) | 2 (1.2%) | 0.633 |
Clinical characteristics in subjects with or without diverticulosis
The differences in comorbidities between subjects with diverticulosis vs. without diverticulosis are presented in Table 2. Subjects with diverticulosis had a higher prevalence of hypertension (41.4% with diverticulosis vs. 25.4% without diverticulosis, p=0.008), and they were more likely to have rheumatologic diseases (38.3% with diverticulosis vs. 22.4% without diverticulosis, p=0.006), compared to subjects without diverticulosis. Subjects with diverticulosis had lower prevalence of celiac disease compared to subjects without diverticulosis (1% vs. 6.06%, p=0.046).
Table 2.
Co-morbidities of subjects with diverticulosis and subjects without diverticulosis
| Co-morbidities | Subjects with diverticulosis (n=99) | Subjects without diverticulosis (n=165) | P values |
|---|---|---|---|
| Hypertension | 41 (41.4%) | 42 (25.4%) | 0.008 |
| Rheumatologie diseases | 38 (38.3%) | 37 (22.4%) | 0.006 |
| Heart disease | 34 (34.3%) | 47 (28.4) | 0.333 |
| Gastroesophageal reflux disease | 31 (31.1%) | 41 (24.8%) | 0.266 |
| Laparotomy / Laparoscopy | 28 (28.2%) | 43 (26.06%) | 0.737 |
| Psychiatric disease | 28 (28.2%) | 36 (21.8%) | 0.246 |
| Diabetes Mellitus | 21 (21.2%) | 24 (14.5%) | 0.184 |
| Neurologic disease | 21 (21.2%) | 30 (18.1%) | 0.562 |
| Cholecystectomy | 18 (18.1%) | 26 (15.7%) | 0.640 |
| Malignancy | 17 (17.1%) | 20 (12.1%) | 0.261 |
| Thyroid disorder | 17 (17.1%) | 23 (13.9%) | 0.504 |
| Hysterectomy | 17 (17.1%) | 32 (19.3%) | 0.620 |
| Lactose intolerance | 12 (12.1%) | 22 (13.3%) | 0.762 |
| Peptic ulcer | 11 (11.1%) | 12 (7.2%) | 0.291 |
| Kidney disease | 10 (10.1%) | 21 (12.7%) | 0.510 |
| Gall bladder disease | 10 (10.1%) | 25 (15.1%) | 0.234 |
| Appendectomy | 7 (7.07%) | 14 (8.48%) | 0.661 |
| Hernia surgery | 7 (7.07%) | 9 (5.4%) | 0.612 |
| Gastroparesis | 7 (7.07%) | 6 (3.06%) | 0.216 |
| Liver disease | 4 (4.04%) | 9 (5.4%) | 0.501 |
| Inflammatory Bowel Disease | 2 (2.02%) | 7 (4.2%) | 0.331 |
| Pancreas disease | 2 (2.02%) | 9 (5.4%) | 0.174 |
| Transplantation | 2 (2.02%) | 4 (2.4%) | 0.820 |
| Nissen fundoplication | 2 (2.02%) | 3 (1.8%) | 0.918 |
| Celiac disease | 1 (1%) | 10 (6.06%) | 0.046 |
The differences in medication usage between subjects with diverticulosis vs. without diverticulosis are presented in Table 3. Subjects with diverticulosis had a higher prevalence of antihypertensive medication use compared to subjects without diverticulosis (46.4% vs. 25.6%, p=0.001); which is consistent with the increased prevalence of hypertension in our dataset. We did not find any significant differences in the usage of other medications including those that pertain to gastrointestinal system such as stool softeners, narcotics, NSAIDs, prokinetics, antacids and proton pump inhibitors.
Table 3.
Medication use among subjects with diverticulosis and subjects without diverticulosis
| Medications | Subjects with diverticulosis | Subjects without diverticulosis | P values |
|---|---|---|---|
| Proton pump inhibitors | 59/99 (58.5%) | 78/164 (47.5%) | 0.083 |
| Antihypertensive medications | 46/99 (46.4%) | 42/164 (25.6%) | 0.001 |
| Psychiatric medications | 34/99 (34.3%) | 44/164 (26.8%) | 0.196 |
| Vitamin supplements | 32/99 (32.3%) | 44/164 (26.8%) | 0.341 |
| Cardiovascular medications | 31/99 (31.3%) | 38/164 (23.1%) | 0.146 |
| NSAIDs | 30/99 (30.3%) | 35/164 (21.3%) | 0.103 |
| Anticholinergics | 24/99 (24.2%) | 33/164 (20.1%) | 0.432 |
| Previous use of antibiotics | 24/99 (24.2%) | 32/164 (19.5%) | 0.364 |
| Stool softeners | 22/99 (22.2%) | 22/164 (13.4%) | 0.064 |
| Pulmonary and allergy | 21/99 (21.2%) | 33/164 (20.1%) | 0.832 |
| Diabetes medications | 19/99 (19.1%) | 18/164 (10.9%) | 0.063 |
| Other neurological medications | 17/99 (17.1%) | 26/164 (15.8%) | 0.779 |
| Thyroid medications | 15/99 (15.1%) | 20/164 (12.1%) | 0.494 |
| IBS medications | 14/99 (14.1%) | 28/164 (17.07%) | 0.529 |
| Immunosuppresants | 12/99 (12.1%) | 15/164 (9.01%) | 0.441 |
| Corticosteroids | 9/99 (9.09%) | 17/164 (10.3%) | 0.737 |
| H2Blockers | 7/83 (8.4%) | 18/127 (14.1%) | 0.209 |
| Narcotics | 8/98 (8.1%) | 12/164 (7.3%) | 0.803 |
| Other antacids | 6/99 (6.06%) | 14/164 (8.5%) | 0.463 |
| Prokinetics | 5/99 (5.05%) | 14/164 (8.5%) | 0.290 |
| Iron supplements | 4/99 (4.04%) | 10/164 (6.09%) | 0.472 |
| Anticonvulsants | 4/99 (4.04%) | 4/164 (2.43%) | 0.464 |
NSAIDs, non-steroidal anti-inflammatory drugs; IBS, irritable bowel syndrome.
Methane production and diverticulosis on colonoscopy
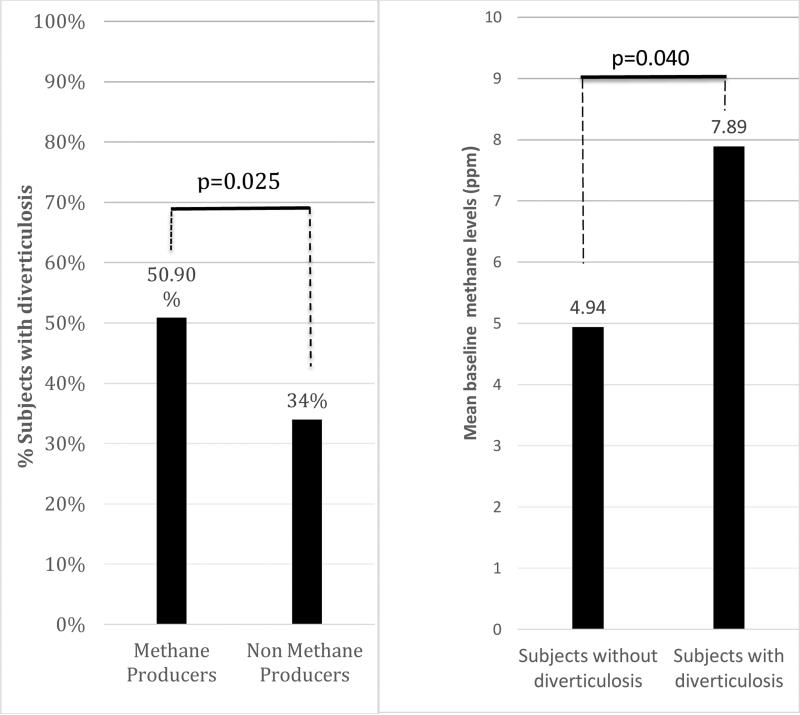
Subjects who were methane producers (MPs) (see definition in methods) had higher prevalence of diverticulosis compared to those subjects who were not methane producers (Non-MPs) (50.9% vs. 34%, p=0.025) (Figure 1.(a)). The mean breath methane level was higher in subjects with diverticulosis compared to subjects without diverticulosis (p=0.040) (Figure 1.(b)).
Figure 1.
(a) Comparisons of percentages of subjects with diverticulosis among methane producers vs. non-methane producers. (b) Comparisons of mean baseline methane levels (ppm) among subjects without diverticulosis vs. subjects with diverticulosis.
There was no significant difference in age (p=0.869), gender (p=0.558) and co-morbidities such as hypertension (p=0.928), celiac disease (p=0.35), rheumatological disease (p=0.704) in subjects who were MPs compared to those who were not Non-MPs (Table 4).
Table-4.
Comorbidities of methane producers and non-methane producers
| Comorbidities | Methane Producers | Non Methane Producers | P value |
|---|---|---|---|
| Hypertension | 17/53 (32.07%) | 66/210 (31.4%) | 0.928 |
| Celiac Disease | 1/53 (1.8%) | 10/210 (4.7%) | 0.350 |
| Rheumatologic Diseases | 14/53 (26.4%) | 61/210 (29.04%) | 0.704 |
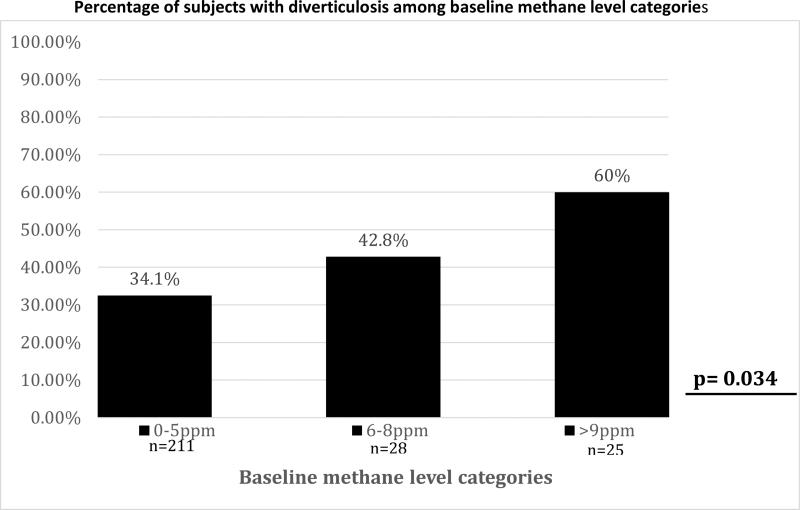
To determine if there is any association between increasing baseline methane levels and percentage of subjects with diverticulosis on colonoscopy, we divided our sample set into 3 groups: Group 1 had baseline methane levels between 0-5 ppm (Non-MPs); group 2 had baseline methane levels between 6-8 ppm; and group 3 had baseline methane levels over 9 ppm. Our results showed that the percentage of subjects with diverticulosis increased with rising baseline methane levels (p=0.034) (Figure 2). We performed a logistic regression analysis to take into account the baseline differences in subjects with and without diverticulosis to see if there is an independent effect of baseline breath methane levels. When age, gender, history of hypertension, celiac disease and rheumatologic disease were used to predict presence of diverticulosis together with baseline breath methane levels, only age (exp (B) =1.08, p=0.001) and baseline breath methane levels (exp (B) =1.031, p=0.025) were found to be independent predictors of diverticulosis.
Figure 2.
Increase in the percentage of subjects with diverticulosis in three successive groups which were clustered according to the baseline breath methane levels (ppm).
Relationship between constipation, methane production and diverticulosis
Because methane has been linked to constipation, we have examined the relationship between constipation and diverticulosis and methane production. Those patients reporting constipation were more likely to be MPs compared to Non-MPs (27.3% vs.16%, p=0.027), however, the frequency of constipation was similar in those with diverticulosis vs. those without (36.4% vs. 38%, p=0.901), suggesting constipation is not the primary determinant for the presence of diverticulosis in our dataset.
Discussion
Our study demonstrates an association between breath methane levels and diverticulosis on colonoscopy for the first time, in the largest number of patients reported to date. Those with diverticulosis were more likely to be methane producers and mean breath methane levels were higher in subjects with diverticulosis in our dataset. These findings suggest methanogens could be playing a role in the development of diverticulosis independent of the effects of age and other clinical factors. Our study now constitutes ground for exploring further mechanisms of diverticuli formation in relation to the gut microbiota.
In this dataset, we noted an expected relationship between age and diverticulosis. As age increased, the prevalence of diverticulosis rose. Among subjects with diverticulosis, we also noted a higher prevalence of rheumatologic diseases. The latter association is in line with earlier studies [10] that found associations between inflammatory connective tissue disorders and diverticulosis. Similarly, subjects with diverticulosis were more likely to have hypertension and use anti-hypertensive medications compared to those without diverticulosis. This may also be a reflection of the higher age among subjects with diverticulosis, and the increase of blood pressure with age.
Methane has been shown to decrease peristaltic velocity, increase contraction amplitude and intraluminal pressure in the colon of pigs [11]. These in-vivo preclinical findings are in parallel with elevated methane levels in subjects with diverticulosis in our study. One plausible mechanism by which methanogenesis could be related to diverticulosis is via methane altering colonic motility and thereby causing constipation, which has been previously reported [12-14]. In fact, constipation and low fiber diet (resulting in constipation) have also long been implicated as potential risk factors for diverticulosis, although the data in this regard is not definitive or strong [15-16]. In our dataset, we confirmed a relationship with breath methane levels with clinical report of constipation, however, constipation was not related to diverticulosis. This finding suggests that the relationship between methane and diverticulosis is more complex than clinical constipation alone. It is plausible that constipation and methanogenesis might work synergistically to lead to increased intraluminal pressure, which in turn may contribute to diverticula formation. It is also plausible that methanogenesis by itself (or as a surrogate marker for other factors that lead to methanogenesis) could have an independent association with diverticulosis. Lastly, the lack of a relationship between constipation and diverticulosis in our dataset could be because of the subjective nature of this symptom, which not only encompasses slow transit in the colon, but could also be reported by patients who experience a variety of bowel issues such as straining as a result of pelvic floor dysfunction or hard stool consistency, which may or may not be related to diverticulosis. To date, only two prior studies investigated the relationship between diverticulosis and methanogenesis in limited number of patients [3, 17]. The first study [17] primarily looked at a relationship between fecal abundance of methanogenic archaea by stool culture in enema fluid obtained before a flexible sigmoidoscopy in 57 subjects with diverticulosis. While there was no difference between the presence of methanogens in controls and diverticulosis patients, higher colony numbers of methanogens were observed in the group with diverticulosis on flexible sigmoidoscopy compared to controls [17]. Limitations of this study included lack of an entire colon exam to exclude any other colonic pathology, use of culture technology which has limitations in assessing methanogens, an extraordinarily high number of subjects (71%) with presence of methanogens, and lack of assessment of potential confounders including age. While our findings are in accordance with those of this latter study, they also go further to suggest: 1) that detectable breath methane may be a marker of the high relative abundance of methanogens in the colon; and 2) that higher levels of breath methane are associated with increasing prevalence of diverticulosis, irrespective of confounders such as age and co-morbid conditions. A second investigation did not find any association between breath methane levels in 30 subjects with right-sided diverticulosis vs. those without diverticulosis [3]. We did not explore the differences between right sided vs. left sided diverticulosis in our dataset. Considering the retrospective nature of our study and the fact that a significant portion of clinical colonoscopy reports may not specify the exact distribution of diverticuli, looking for differences in right and left side diverticuli in terms of methanogenesis requires a prospective design. The percentage of MPs in our study was 20.15% which is very close to 23% observed in a recent comprehensive assessment of the gut microbiome in healthy twins [18]. This is lower than some of the other previously reported values of 33.6%-36.4% [7, 8] and is likely due to a lower cut-off value of 2.8-3 ppm used in these studies to classify a subject as a methane-producer, compared to ours. We have elected to use the cut-off of 5 ppm to classify a subject as a methane producer, considering potential variability in the assay, in addition to ambient concentrations of methane that can be up to 2 ppm or higher, used in the previous studies.
One limitation of our study was that our analysis was done on patients who presented to a tertiary gastroenterology clinic with symptoms, not the general population. Another limitation of our study is its retrospective design, which did not allow for evaluation of any associations between the number or extent of diverticuli on colonoscopy and methanogenesis, which could be looked for in prospective studies that collect more detailed information on these parameters at the time of colonoscopy. Diverticulosis especially when complicated with gastrointestinal bleeds and diverticulitis can become an important cause of morbidity and mortality, especially in high-risk groups such as the elderly. Further studies using breath methane levels as a non-invasive tool can enable examining additional relationships between complications of diverticulosis and methanogenesis.
The strength of our study was its use of an easy, affordable and clinically accessible assessment of methanogens (breath methane measurement). In addition, a comprehensive look at demographics including patient social habits, co-morbid conditions and medications provided us the opportunity to evaluate possible confounding factors. In addition, our study is the largest investigation up to date that looked into the relationship between methanogenesis and diverticulosis in humans.
In summary, methanogenesis and diverticulosis are associated independent of the effects of age. Further studies are necessary to prospectively confirm our findings and explore changes in the microbiota of patients with diverticulosis with and without complications.
Acknowledgements
We would like to acknowledge Meltem Yalcin, Mark T. DeMeo, Michael D. Brown, Keith Bruninga, Sohrab Mobarhan, Garth Swanson, John Losurdo, Neha Mathur and Disha Mahendra for their support.
Footnotes
Disclosure of Conflicts of Interest
There is no conflict of interest that arises from financial relationships between the authors of this article (Cemal Yazici, Deniz Cagil Arslan, Rana Abraham, Kelly Cushing, Ali Keshavarzian and Ece A. Mutlu) and any commercial or proprietary entity that produces healthcare-related products and/or services related to the content of the article.
References
- 1.Vikram BR, Walter EL. The Burden of diverticular disease on patients and healthcare Systems. Gastroenterology & Hepatology (N Y) 2013;9(1):21–27. [PMC free article] [PubMed] [Google Scholar]
- 2.Schoetz DJ., Jr Diverticular disease of the colon: a century old problem. Diseases of the Colon & Rectum. 1999;42(6):703–709. doi: 10.1007/BF02236921. [DOI] [PubMed] [Google Scholar]
- 3.Jang SI, Kim JH, Youn YH, Park H, Lee SI, Conklin JL. Relationship between intestinal gas and the development of right colonic diverticula. Journal of Neurogastroenterology and Motility. 2010;16(4):418–23. doi: 10.5056/jnm.2010.16.4.418. doi: 10.5056/jnm.2010.16.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Inca R, Pomerri F, Vettorato MG, Dal Pont E, Di Leo V, Ferronato A, Medici V, Sturniolo GC. Interaction between rifaximin and dietary fibre in patients with diverticular disease. Alimentary Pharmacology & Therapeutics. 2007;25(7):771–9. doi: 10.1111/j.1365-2036.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- 5.Daniels L, Philipszoon LE, Boermeester MA. A hypothesis: important role for gut microbiota in the etiopathogenesis of diverticular disease. Diseases of Colon & Rectum. 2014;57(4):539–43. doi: 10.1097/DCR.0000000000000078. doi: 10.1097/DCR.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 6.Levitt MD, Furne JK, Kuskowski M, Ruddy J. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clinical Gastroenterology and Hepatology. 2006;4(2):123–9. doi: 10.1016/j.cgh.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Bond JH, Jr., Engel RR, Levitt MD. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. Journal of Experimental Medicine. 1971;133(3):572–88. doi: 10.1084/jem.133.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leiby A, Mehta D, Gopalareddy V, Jackson-Walker S, Horvath K. Bacterial overgrowth and methane production in children with encopresis. Journal of Pediatrics. 2010;156(5):766–70. 770, e1. doi: 10.1016/j.jpeds.2009.10.043. doi: 10.1016/j.jpeds.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 9.Florin TH, Woods HJ. Inhibition of methanogenesis by human bile. Gut. 1995;37(3):418–21. doi: 10.1136/gut.37.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myasoedova E, Matteson EL, Talley NJ, Crowson CS. Increased incidence and impact of upper and lower gastrointestinal events in patients with rheumatoid arthritis in Olmsted County, Minnesota: a longitudinal population-based study. The Journal of Rheumatology. 2012;39(7):1355–1362. doi: 10.3899/jrheum.111311. doi: 10.3899/jrheum.111311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahng J, Jung IS, Choi EJ, Conklin JL, Park H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Journal of Neurogastroenterology and Motility. 2012;24(2):185–90. e92. doi: 10.1111/j.1365-2982.2011.01819.x. doi: 10.1111/j.1365-2982.2011.01819.x. [DOI] [PubMed] [Google Scholar]
- 12.Attaluri A, Jackson M, Valestin J, Rao SSC. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010;105:1407–1411. doi: 10.1038/ajg.2009.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoshal UC, Srivastava D, Verma A, Misra A. Slow transit constipation associated with excess methane production and its improvement following rifaximin therapy: a case report. J Neurogastroenterol Motil. Apr. 2011;17(2):185–8. doi: 10.5056/jnm.2011.17.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil. Jan. 2014;20(1):31–40. doi: 10.5056/jnm.2014.20.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strate LL. Diverticulosis and dietary fiber: Rethinking the relationship. Gastroenterology. 2012 Feb;142(2):205–7. doi: 10.1053/j.gastro.2011.12.019. doi: 10.1053/j.gastro.2011.12.019. Epub 2011 Dec 17. [DOI] [PubMed] [Google Scholar]
- 16.Braunschmid T, Stift A, Mittlbock M, Lord A, Weiser FA, Riss S. Constipation is not associated with diverticular disease – Analysis of 976 patients. Int J Surg. 2015 Jul;19:42–5. doi: 10.1016/j.ijsu.2015.04.045. doi: 10.1016/j.ijsu.2015.04.045. Epub 2015 May 15. [DOI] [PubMed] [Google Scholar]
- 17.Weaver GA, Krause JA, Miller TL, Wolin MJ. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986;27(6):698–704. doi: 10.1136/gut.27.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen EE, Lozupone CA, Rey FE, Wu M, Guruge JL, Narra A, Goodfellow J, Zaneveld JR, McDonald DT, Goodrich JA, Heath AC, Knight R, Gordon JI. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proceedings of National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4599–606. doi: 10.1073/pnas.1000071108. doi: 10.1073/pnas.1000071108. [DOI] [PMC free article] [PubMed] [Google Scholar]