Abstract
The ability of DJ-1 to modulate signal transduction has significant effects on how the cell regulates normal processes such as growth, senescence, apoptosis, and autophagy to adapt to changing environmental stimuli and stresses. Perturbations of DJ-1 levels or function can disrupt the equilibrium of homeostatic signaling networks and set off cascades that play a role in the pathogenesis of conditions such as cancer and Parkinson’s disease.
DJ-1 plays a major role in various pathways. It mediates cell survival and proliferation by activating the extracellular signal-regulated kinase (ERK1/2) pathway and the phosphatidylinositol-3-kinase (PI3K)/Akt pathway. It attenuates cell death signaling by inhibiting apoptosis signal-regulating kinase 1 (ASK1) activation as well as by inhibiting mitogen-activated protein kinase kinase kinase 1 (MEKK1/ MAP3K1) activation of downstream apoptotic cascades. It also modulates autophagy through the ERK, Akt, or the JNK/Beclin1 pathways. In addition, DJ-1 regulates the transcription of genes essential for male reproductive function, such as spermatogenesis, by relaying nuclear receptor androgen receptor (AR) signaling. In this chapter, we summarize the ways that DJ-1 regulates these pathways, focusing on how its role in signal transduction contributes to cellular homeostasis and the pathologic states that result from dysregulation.
Keywords: DJ-1, Signal transduction, Cell signaling, MAPK, ERK, MEK, Ras, Raf, PI3K, Akt, mTOR, MAPK, ASK1, Daxx, Trx1, JNK, p38, MEKK1, AR
8.1 Introduction
In the past decade, research into DJ-1, which has varied activities involved in cellular homeostasis, has revealed another major function: its ability to modulate signal transduction. Cell signaling pathways convey, amplify, and translate the information transmitted from the plasma membrane to the nucleus, regulating normal cellular processes to adapt to changing environmental conditions. Investigating the function of DJ-1 in cell signaling has been crucial in understanding its role in the pathogenesis of cancer and Parkinson’s disease (West et al. 2005; Devine et al. 2011). DJ-1 can tip the delicate balance between oncogenesis and cellular protection in either direction depending on cell type and extracellular stimuli. This equilibrium may be explained in the context of how DJ-1 influences the control processes that maintain important cellular signaling networks.
DJ-1, for example, can activate the extracellular signal-regulated kinase (ERK1/2) pathway and the phosphatidylinositol-3-kinase (PI3K)/Akt pathway to mediate cell survival and proliferation. It can attenuate cell death signaling by inhibiting apoptosis signal-regulating kinase 1 (ASK1) activation as well as mitogen-activated protein kinase kinase kinase 1 (MEKK1/MAP3K1) activation of downstream apoptotic cascades. It also appears to modulate autophagy through many signaling pathways, a process that can mediate either cell survival or cell death depending on the circumstances (Green and Llambi 2015). These pathways are regulated by DJ-1 in a multitude of ways. For instance, DJ-1 can bind directly to pathway effectors to modify their activity, and it can regulate a pathway indirectly by binding or modulating its co-activators or inhibitors.
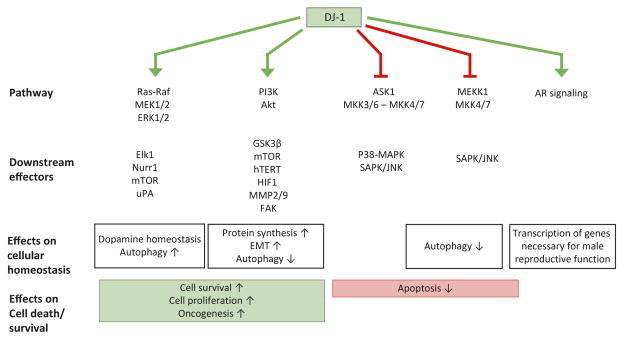
In this chapter, we focus on the different ways that DJ-1 can affect major cell signaling pathways in diverse cell types to play a crucial role in cellular transformation, death, and protection against a variety of stressors (Fig. 8.1).
Fig. 8.1. Major signaling pathways regulated by DJ-1.
DJ-1 activates the extracellular signal-regulated kinase (ERK1/2) pathway and the phosphatidylinositol-3-kinase (PI3K)/Akt pathway to mediate cell survival, proliferation, and autophagy. It can also activate androgen receptor (AR) signaling to induce transcription of genes necessary for male reproductive function. [Activation is indicated by pointed green arrows] DJ-1 can inhibit apoptosis signal-regulating kinase 1 (ASK1) activation as well as MEKK1 activation of downstream apoptotic cascades. [Inhibition is indicated by blunted red arrows]
EMT, epithelial-mesenchymal transition
8.2 DJ-1 Activates the ERK1/2 Pathway
The extracellular signal-regulated kinase (ERK1/2) pathway is a classic mitogen-activated protein kinase (MAPK) signaling cascade that regulates cell proliferation, growth, autophagy, and differentiation. The core pathway members include Ras (small GTP-binding protein; activator), Raf (serine/threonine kinase; MAPKKK), MEK1/2 (mitogen-activated protein kinase/ERK kinase; MAPKK), and ERK1/2 (MAPK) (McCubrey et al. 2007; Cargnello and Roux 2011). This pathway is activated by various stimuli, including growth factors, polypeptide hormones, neurotransmitters, chemokines, and phorbol esters, which bind or activate a variety of receptors and proteins such as receptor tyrosine kinases (RTKs), G-protein-coupled receptors (GPCRs), and protein kinase C (PKC) (Yoshioka 2004).
ERK1/2, also known as p44/42 MAPK, are serine-threonine kinases that are positively regulated by MEK1/2-mediated phosphorylation. MEK1/2 are MAPKK proteins with ERK1/2 as their only known physiological substrates and can be specifically inhibited by small molecule inhibitors such as U0126 or PD98059 (Chang et al. 2003). On the other hand, ERK is negatively regulated by a family of dual-specificity (Thr/Tyr) MAPK phosphatases (DUSPs/MKPs) (Jeffrey et al. 2007).
ERK phosphorylates several downstream transcription factors such as AP-1, c-Jun, and c-Myc (Plotnikov et al. 2011). ERK can also activate ribosome S6 kinase (RSK) and inhibitor kappa-B kinase (IKK), which can lead to the respective activation of transcription factors cAMP response element-binding (CREB) and nuclear factor immunoglobulin kappa-chain enhancer-B-cell (NF-kappa-B) (Chang et al. 2003; Burotto et al. 2014). MEK/ERKs have also been reported to participate in a non-canonical signaling pathway that interacts with the mammalian target of rapamycin complex 1 (mTORC1) to regulate autophagy via Beclin-1 modulation (Wang et al. 2009).
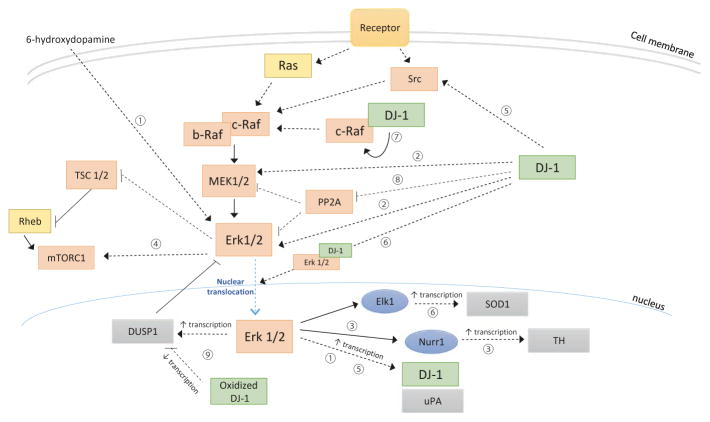
DJ-1 was initially discovered and identified in 1997 by Nagakubo et al. as a putative oncogene capable of transforming cells in cooperation with H-Ras, a GTPase that can activate c-Raf in the ERK pathway (Nagakubo et al. 1997). Since then, many studies have shown DJ-1 to activate ERK1/2. The activation of this MAPK pathway could contribute to, or explain, many of the roles that DJ-1 plays in protecting cells from oxidative injury, in regulating gene transcription, and in activating autophagy (Fig. 8.2).
Fig. 8.2. DJ-1 activates ERK1/2 signaling pathway.
1. 6-Hydroxydopamine-induced oxidative stress may upregulate DJ-1 through activation of ERK1/2 (Lev et al. 2009)
2. DJ-1, but not its L166P mutant, protects cells from oxidative injury by activating ERK1/2 and MEK1/2 (Gu et al. 2009)
3. DJ-1 mediates dopamine (DA) homeostasis through activation of ERK1/2 and the resulting nuclear translocation of the transcription factor Nurr1 (Lu et al. 2012; Lu et al. 2016)
4. DJ-1 activates the non-canonical MEK/ERK-mTOR pathway to activate autophagy (Gao et al. 2012; Krebiehl et al. 2010)
5. DJ-1 promotes oncogenic potential by activating the SRC/ERK/uPA axis (He et al. 2012)
6. DJ-1 interacts directly with ERK1/2 and may enhance the nuclear translocation of ERK1/2, where it can phosphorylate Elk1, leading to increased SOD expression (Wang et al. 2011)
7. DJ-1, but not its C106S mutant, can bind directly to and phosphorylate c-Raf, which can then activate MEK and ERK1/2 (Takahashi-Niki et al. 2015)
8. DJ-1, but not its L166P mutant, suppresses the expression of PP2A, an inhibitor of MEK1/2 and ERK1/2 family kinases (Gu et al. 2009)
9. DJ-1, but not its C106S mutant, may sequester p53 away from promoters in a DNA-binding affinity-dependent manner, resulting in downregulation of ERK1/2 inhibitor, DUSP1 (Kato et al. 2013)
[positive regulation is indicated by pointed arrows, and negative regulation is indicated by blunted arrows. Direct or known regulation is indicated by solid lines; indirect or unknown regulation is indicated by dotted lines]
8.2.1 Dopamine-Mediated Oxidative Stress May Upregulate DJ-1 Through Activation of ERK1/2
The involvement of DJ-1 with ERK was first reported in 2008, when it was demonstrated that ERK1/2 may upregulate DJ-1. As reactive oxygen species (ROS) generated from 6-hydroxydopamine (6-OHDA) could lead to upregulation of DJ-1 in SH-SY5Y human neuroblastoma cells (Lev et al. 2008), it was hypothesized that this augmentation may be mediated through the ERK pathway, which has been shown to play a role in forming a protective response against 6-OHDA stress (Lin et al. 2008). Phosphorylation/activation of ERK1/2 was seen to occur in both neuroblastoma cells treated with 6-OHDA and in the mouse striatum denervated with 6-OHDA. This ERK1/2 activation was shown in cellular models to precede the upregulation of DJ-1 mRNA, while inhibiting MEK1/2 by PD-98059 could attenuate ROS-induced upregulation of DJ-1 (Lev et al. 2009).
8.2.2 DJ-1 Protects Cells from Oxidative Injury by Activating ERK1/2 and MEK1/2
In contrast to studies suggesting that ERK pathway activation leads to upregulation of DJ-1, another experimental evidence has been reported suggesting that DJ-1 functions upstream of ERK1/2 phosphorylation (Gu et al. 2009; Letourneux et al. 2006; Kato et al. 2013; Wang et al. 2011). One such study found that overexpression of wild-type (WT) DJ-1 in COS-7 cells (African green monkey kidney fibroblast-like cell line) and MN9D cells (a fusion of mice embryonic ventral mesencephalic and neuroblastoma cells) upregulates ERK1/2 and MEK1/2 phosphorylation, whereas overexpression of a Parkinson’s disease-linked mutant form of DJ-1, L166P, does not enhance ERK1/2 or MEK1/2 phosphorylation (Gu et al. 2009). Additionally, DJ-1 overexpression in various models improves the viability of cells stressed with hydrogen peroxide (H2O2) compared to control (Sekito et al. 2006; Kahle et al. 2009). Under these conditions, inhibiting ERK1/2 activation by pre-treating cells with the MEK1/2 inhibitor U0126 abrogates the protective effect of DJ-1 overexpression against oxidative injury, suggesting that WT DJ-1 may provide neuroprotection through activation of the ERK pathway (Gu et al. 2009).
8.2.3 DJ-1 Mediates Dopamine (DA) Homeostasis Through Activation of ERK1/2 and the Resulting Nuclear Translocation of the Transcription Factor Nurr1
In addition to protection from oxidative injury, another function of DJ-1-mediated ERK1/2 signaling may be the regulation of tyrosine hydroxylase (TH) expression. In both in vivo and in vitro models, DJ-1 has been found to modulate the transcription factor Nurr1. Nurr1 plays a major role in DA homeostasis and can regulate the expression of TH and L-dopa decarboxylase (DDC), both of which are involved in DA synthesis, as well as the expression of vesicular monoamine transporter 2 (VMAT-2), which is necessary for the transport of DA from the cytosol into synaptic vesicles (Iwawaki et al. 2000; Hermanson et al. 2003; Ishikawa et al. 2009). MN9D cells that overexpress WT DJ-1 exhibit an increase in the nuclear translocation of Nurr1 as well as an increase in the mRNA levels of Nurr1 targets. However, cells that overexpress the Parkinson-associated pathogenic L166P mutant DJ-1 do not show such an increase. Knocking down DJ-1 expression using RNAi attenuates the activity of Nurr1 and downregulates the expression of its target proteins, which can be rescued by subsequent overexpression of WT DJ-1 (Lu et al. 2012).
As ERK1/2 has been reported to increase Nurr1 transcriptional activity (Nordzell et al. 2004), it was hypothesized that DJ-1 may mediate Nurr1 activation through the ERK1/2 pathway. WT DJ-1, as compared to its L166P mutant, phosphorylates ERK1/2, while blocking ERK1/2 activation using U0126 prevents DJ-1-mediated nuclear translocation of Nurr1 and the induction of Nurr1 target protein levels (Lu et al. 2016). Similarly, overexpression of WT DJ-1 but not its L166P mutant in the rat substantia nigra using a lentiviral vector increases ERK activation, Nurr1 nuclear translocation, and Nurr1 target protein levels (Lu et al. 2016).
8.2.4 DJ-1 Activates the Non-canonical MEK/ERK-mTOR Pathway to Activate Autophagy
DJ-1 appears to impact autophagy and neuronal cell survival through the ERK pathway. In a rat model made to overexpress DJ-1 by injection of adeno-associated viral vector, ERK activation in the substantia nigra is significantly greater compared with control animals injected with a vector expressing only green fluorescent protein. Additionally, overexpression of DJ-1 protects against rotenone-induced injury and enhances autophagy markers in this rat model as well as in MN9D cells (Gao et al. 2012). Rotenone is an inhibitor of mitochondrial complex I that can induce oxidative stress and apoptosis, inhibit proteasome activity, and cause dopaminergic neuronal death in rodents (Shamoto-Nagai et al. 2003). Further, blocking autophagy in MN9D cells by inhibiting either MEK1/2 with U0126 or phosphoinositol 3-kinase (PI3K) with 3-methyladenine (3MA) reverses autophagic activation by DJ-1 and abrogates DJ-1-mediated protection against rotenone (Gao et al. 2012). This implies that the neuroprotective effect of DJ-1 may be at least partly due to its effects on two signaling pathways that have been shown to regulate autophagy – the canonical Akt/ PI3K-mTOR pathway (Vasseur et al. 2009) and the non-canonical AMPK-MEK/ ERK-TSC-mTOR pathway (Wang et al. 2009).
This notion is partially supported by data from another study which shows that DJ-1 knockout (KO) mouse embryonal fibroblasts (MEFs) exhibit reduced basal autophagic degradation, impaired lysosomal activity, and accumulation of defective mitochondria (Krebiehl et al. 2010). These DJ-1 KO MEFs also show a reduction in phosphorylated ERK2 in the mitochondrial fraction, but no effect on cytosolic fractions, suggesting that DJ-1-mediated ERK2 phosphorylation may be controlling autophagic and lysosomal function (Krebiehl et al. 2010).
8.2.5 DJ-1 Promotes Oncogenic Potential by Activating the SRC/ERK/uPA Axis in Pancreatic Cancer
DJ-1 is associated with a vast number of tumor types, with its overexpression and secretion found frequently in conjunction with abnormal cell transformation and tumor progression (Cao et al. 2015). Activation of the ERK pathway also plays a prominent role in oncogenesis by driving inappropriate cell proliferation and survival (Caunt et al. 2015). An examination of the role of DJ-1 in tumor invasion and metastasis in human pancreatic cancer cell lines (BxPC-3 and SW1990) showed that knockdown of DJ-1 can lead to cytoskeleton disruption as well as diminished urokinase plasminogen activator (uPA) activity and expression. Active uPA converts plasminogen to active plasmin, which can break down the extracellular matrix around the cell or activate growth factors to promote cancer cell migration (Blasi and Carmeliet 2002). Knockdown of DJ-1 in pancreatic cancer cell lines also decreases ERK1/2 and SRC kinase phosphorylation, which regulates the ERK pathway (Chang et al. 2003; Eichhorn et al. 2007). This decrease in ERK1/2 and SRC phosphorylation can be reversed by restoring DJ-1 expression. Inhibiting ERK has the same effect on pancreatic cancer invasion potential and cell migration as knocking down DJ-1; both lead to decreased uPA expression and activity. This suggests that the effect of DJ-1 on pancreatic cancer invasion and migration may be dependent on the SRC/ERK pathway (He et al. 2012).
8.2.6 DJ-1 Interacts Genetically with Ras/ERK Signaling Components
DJ-1 appears to play a definitive role in the activation of ERK1/2 and downstream effectors, but there is a differing range of data as to exactly how DJ-1 may be participating in this signaling cascade. While one group has shown DJ-1 can directly interact with ERK1/2 (Wang et al. 2011), other groups have shown that DJ-1 modulates upstream factors in the MAPK cascade, either through direct interaction or by affecting protein expression (Gu et al. 2009; Kato et al. 2013; Takahashi-Niki et al. 2015).
Initial studies found that aged mice lacking both DJ-1 and the receptor tyrosine kinase (RTK) receptor Ret lose more dopaminergic neurons in the substantia nigra compared to mice lacking only Ret, suggesting a possible cooperation between DJ-1 and Ret (Aron et al. 2010). Ret is upstream of the ERK pathway and is necessary for the neuronal survival activity of glial neurotrophic factor (GDNF) (Kramer et al. 2007).
To study the interaction between DJ-1 and Ret further, a developing Drosophila eye model system was employed, which is very sensitive to dosage changes in RTK signaling and downstream MAPK pathways. Overexpressing constitutively active Ret in this model led to the development of adult eyes with reduced size and rough morphology. However, overexpression of constitutively active versions of Ret, Raf, ERK/rolled, or wild-type Akt1 did not affect endogenous DJ-1 levels. When flies expressing constitutively active Ret were crossed with flies expressing reduced DJ-1 levels (carrying DJ-1 microdeletions or DJ-1 loss-of-function alleles), the offspring exhibited normal eye phenotype, showing complete rescue of the eye defects. Conversely, when the flies expressing constitutively active Ret were crossed with flies overexpressing DJ-1, the offspring exhibited more severe eye defects. These findings indicate a genetic interaction between Ret and DJ-1 in controlling cell size and differentiation in the developing retinal photoreceptor neurons (Aron et al. 2010).
Similarly, DJ-1 interacts genetically with downstream RTK signaling component Ras and with ERK/rolled (rl), but not with PI3K/Akt. This suggests that DJ-1 does not modulate Akt activation in this model under normal circumstances, but it may synergize with Ret, Ras, and ERK during development, functioning either between Ras and ERK or in parallel to the Ras/ERK pathway to control cell differentiation and proliferation (Aron et al. 2010).
8.2.7 DJ-1 Interacts Directly with ERK1/2 and May Affect the Nuclear Translocation of ERK1/2 Rather Than the Direct Phosphorylation of ERK1/2
In a study investigating the effect of DJ-1 on increasing superoxide dismutase (SOD) expression and its subsequent ability to decrease ROS generation caused by either 1-methyl-4-phenylpyridinium (MPP +) or paraquat, the transcription factor Elk1 was found to bind to the SOD1 promoter to increase transcription. Additionally, Elk1 activation following the administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was found to be decreased in the substantia nigra of DJ-1 KO mice compared to WT mice (Wang et al. 2011). Elk1 is phosphorylated and activated by MAPK kinases such as ERK1/2 (Mut et al. 2012), suggesting that one of the mechanisms through which DJ-1 protects cells against ROS is via ERK1/2 – Elk1 activation leading to SOD induction.
However, in contrast to in vitro data showing that knocking down DJ-1 decreases ERK1/2 phosphorylation (Lu et al. 2012), ERK1/2 phosphorylation is unaffected in DJ-1 KO mice (Wang et al. 2011). This has led to the hypothesis that under oxidative insult, DJ-1 may act as a molecular chaperone, a function that is attributed to DJ-1 in previous studies (Shendelman et al. 2004), to affect the nuclear translocation of ERK1/2, rather than its phosphorylation. Evidence for direct interaction between DJ-1 and ERK2 has been presented in HEK293T (human embryonic kidney) cells as well as in mouse brain lysates using co-immunoprecipitation (Wang et al. 2011), although not yet replicated in human brain lysates (Meulener et al. 2005). Residues 1–100 of DJ-1 are necessary for this interaction, while mutation of its cysteine 106, which is necessary for its role as a redox sensor and peroxide scavenger (Taira et al. 2004a; Canet-Aviles et al. 2004; Martinat et al. 2004), does not affect its ability to interact with ERK1/2 (Wang et al. 2011). Additionally, the nuclear translocation of ERK1/2 is reduced in DJ-1 knockdown SH-SY5Y cells and in DJ-1 KO primary mouse neurons but is rescued by reconstituting DJ-1 expression (Wang et al. 2011). This suggests that DJ-1 may promote the translocation of ERK1/2 to the nucleus upon oxidative stress, allowing ERK1/2 to phosphorylate Elk1, leading to increased SOD expression, and allowing the cell to mount a defense response and suppress the production of superoxide induced by insults such as MPP+ or paraquat.
8.2.8 DJ-1 Can Bind Directly to and Phosphorylate c-Raf, Which Can Then Activate MEK and ERK1/2
When epidermal growth factor (EGF) binds to its receptor (EGFR), it can trigger the activation of Ras, which can then activate c-Raf, a serine/threonine kinase that can phosphorylate MEK, allowing it to activate ERK1/2 (McCubrey et al. 2007; Cargnello and Roux 2011). Pulldown assays using GST-DJ-1 mixed with 35S-labeled c-Raf, and co-immunoprecipitation assays in HeLa (human cervical epithelial adenocarcinoma) and HEK293T cells transfected with FLAG-c-Raf and DJ-1-HA, have shown that DJ-1 binds directly to the kinase domain of c-Raf, the MAPKKK in the ERK signaling pathway (Takahashi-Niki et al. 2015). This interaction is enhanced when cells are treated with EGF (Takahashi-Niki et al. 2015), which augments c-Raf activation.
Phosphorylation of c-Raf at several residues regulates its activity. This kinase is normally inactive and phosphorylated at serines 43, 259, and 621. However, when Ser-259 and Ser-621 are dephosphorylated by protein phosphatase 2A (PP2A), and when Ser-338 is phosphorylated following EGF stimulation, c-Raf becomes activated (Dhillon et al. 2002; Abraham et al. 2000; Oehrl et al. 2003). When DJ-1 knockdown NIH3T3 (mouse embryonic fibroblast) and HeLa cells or DJ-1 knockout (KO) mouse primary fibroblasts are treated with EGF, the loss of DJ-1 is associated with decreased levels of phospho-Ser-338 c-Raf compared to control. However, loss of DJ-1 does not impact the levels of phospho-Ser-259 c-Raf. The reintroduction of DJ-1 into cells derived from DJ-1 KO mice rescues the level of phospho-Ser- 338 c-Raf to control levels (Takahashi-Niki et al. 2015). Additionally, when a constitutively active c-Raf fragment (consisting of the kinase domain that binds DJ-1) is incubated with purified DJ-1 and ATP, the fragment exhibits increased autophosphorylation activity in a DJ-1-dose-dependent manner (Takahashi-Niki et al. 2015). This suggests that by binding to c-Raf, DJ-1 stimulates the kinase activity and autophosphorylation of c-Raf at Ser-338, which aids in the activation of the protein and the ERK pathway.
Interestingly, expression of C106S mutant DJ-1 into DJ-1 null cells reportedly does not rescue the level of phospho-Ser-338 c-Raf to control levels, and the binding activity of C106S mutant DJ-1 to c-Raf in pulldown assays is weaker than that of wild-type DJ-1 (Takahashi-Niki et al. 2015). The cysteine 106 (Cys-106) residue is highly sensitive to oxidative stress and is essential for the role of DJ-1 as a cytoprotective redox sensor (Taira et al. 2004a, b; Canet-Aviles et al. 2004; Martinat et al. 2004). The level of Cys-106 oxidation dictates the level of DJ-1 activation, and when Cys-106 is excessively oxidized, it renders DJ-1 inactive (Wilson 2011). To investigate whether oxidized forms of DJ-1 are necessary to activate c-Raf, the level of DJ-1 oxidized at Cys-106 was examined in cells treated with EGF, H2O2, or both. As expected, H2O2 treatment increases highly oxidized forms of DJ-1 at Cys-106 (SO2H and SO3H forms), but does not increase the level of phospho-Ser-338 c-Raf, indicating that oxidized forms of DJ-1 do not aid in the autophosphorylation of c-Raf. Also, EGF treatment, which increases the level of phospho-Ser-338, does not induce DJ-1 oxidation, and subsequent H2O2 treatment in EGF-treated cells decreases the level of phospho-Ser-338 c-Raf (Takahashi-Niki et al. 2015). This suggests that C106 is important for the interaction of DJ-1 with c-Raf and for subsequent c-Raf activation and that the oxidation of Cys-106 to SO2H and SO3H forms is not necessary for this interaction.
8.2.9 DJ-1 Suppresses the Expression of PP2A, An Inhibitor of MEK1/2 and ERK1/2 Family Kinases
Members of the ERK1/2 pathway are regulated by a variety of kinases and phosphatases, one of which is protein phosphatase 2A (PP2A). PP2A is a serine/threonine phosphatase, a holoenzyme whose well-conserved catalytic (C) subunit is tightly regulated by a regulatory (B) subunit and held together by a scaffolding (A) subunit (Janssens and Goris 2001). PP2A can negatively regulate the ERK pathway by dephosphorylating MEK1/2 and ERK1/2 kinases (Letourneux et al. 2006; Zhou et al. 2002; Alessi et al. 1995; Sonoda et al. 1997; Silverstein et al. 2002) and also by dephosphorylating and inhibiting cellular SRC kinase (c-SRC), an activator of the ERK pathway (Eichhorn et al. 2007).
Overexpression of WT DJ-1 in MN9D and COS-7 cells reduces PP2A levels, while the overexpression of L166P mutant DJ-1 has negligible effect on PP2A (Gu et al. 2009). Additionally, overexpression of neither WT DJ-1 nor L166P mutant had an effect on protein kinase A (PKA) levels (Gu et al. 2009), a protein that has been shown to activate the ERK pathway (Gu et al. 2009; Zanassi et al. 2001). This suggests that DJ-1 may positively regulate ERK1/2 signaling by decreasing the expression of PP2A.
It should be noted, however, that PP2A has also been shown to activate the ERK pathway through its interactions with c-Raf and Ras (Abraham et al. 2000; Adams et al. 2005), and the effect of DJ-1 on the ERK pathway through PP2A regulation may be context-dependent (Junttila et al. 2008).
8.2.10 DJ-1 May Sequester p53 Away from Promoters in a DNA-Binding Affinity-Dependent Manner, Resulting in the Downregulation of ERK1/2 Inhibitor, DUSP1
p53 is a crucial tumor-suppressor protein and a transcription factor that is activated by cellular stress to induce transcription of genes involved in DNA repair, apoptosis, cell cycle arrest, or autophagy (Vousden and Prives 2009; Menendez et al. 2009). p53 is closely associated with DJ-1, with experimental evidence showing that DJ-1 may repress p53 transcriptional activity to prevent apoptosis (Fan et al. 2008), that p53 may inhibit DJ-1 activation through phosphorylation (Rahman-Roblick et al. 2008), and that p53 may decrease DJ-1 protein levels through a posttranscriptional route (Vasseur et al. 2012). In a study using co-immunoprecipitation experiments, p53 was shown to bind strongly to WT DJ-1 in an oxidative stress-dependent manner, with their interaction enhanced by H2O2 treatment (Kato et al. 2013). On the other hand, C106S mutant DJ-1 failed to co-immunoprecipitate with p53 under oxidative stress, suggesting that the Cys-106 residue is necessary for this interaction. Further examination of their interaction using pulldown assays of tagged DJ-1 with p53 deletion mutants showed that DJ-1 binds p53 at its DNA-binding domain, suggesting that it may interfere with the transcriptional activity of p53 (Kato et al. 2013). As expected, this interaction also impacts the transcriptional expression of p53 target genes such as DUSP1, which is a mitogen-activated protein kinase phosphatase that can dephosphorylate ERK and regulate apoptosis by inhibiting downstream effectors of the ERK pathway (Jeffrey et al. 2007). In mouse primary fibroblasts, DUSP1 mRNA and protein expression is increased under oxidative stress, and this induction is even greater in fibroblasts obtained from DJ-1 KO mice, suggesting that DJ-1 may modulate DUSP1 levels (Kato et al. 2013). These findings provide support to the notion that under conditions of oxidative stress, DJ-1 forms a complex with p53, sequestering it away from the DUSP1 promoter. Decreased DUSP1 transcription would prevent the dephosphorylation of ERK, allowing for the promotion of cell survival.
8.3 DJ-1 Activates the PI3K/Akt Pathway
The PI3K/Akt pathway is another classic signaling cascade that regulates cell growth, proliferation, and survival by affecting a multitude of complementary downstream pathways. The cascade is initiated when various stimuli induce the lipid kinase phoaphatidylinositol-3-kinase (PI3K) to generate phosphatidylinositol-3,4,5- triphosphate (PIP3) from phosphatidylinositol-4,5 bisphosphate (PIP2). This allows for the translocation of the serine/threonine kinase Akt (also known as protein kinase B/PKB) to the plasma membrane. At the membrane, Akt is phosphorylated at residue Thr-308 by phosphoinositide-dependent kinase 1 (PDK1) to induce partial activation. Subsequent phosphorylation of Akt at residue Ser-473 by mTORC2 or members of the PI3K-related kinase (PIKK) family induces full enzymatic Akt activity. Phosphatase and tensin homologue (PTEN) negatively regulates the PI3K/Akt pathway by dephosphorylating PIP3 back to PIP2. And Akt is dephosphorylated by protein phosphatase 2A (PP2A) and the PH-domain leucine-rich-repeat-containing protein phosphatases (PHLPP1/2) (Maehama and Dixon 1998; Andjelkovic et al. 1996; Brognard et al. 2007; Gao et al. 2005).
One of the major downstream effects of Akt is the mammalian target of rapamycin complex 1 (mTORC1) which promotes the translation of proteins necessary for cell growth and protein synthesis. Akt can directly phosphorylate and activate mTOR and also inhibit mTOR inhibitor proteins proline-rich Akt substrate of 40 kDa (PRAS40) and tuberin (TSC2). Phosphorylation of mTORC1 activates the mTORC1/S6 kinase axis and its downstream effectors such as eukaryote translation initiation factor 4E-binding protein 1(4E-BP1) and 40S ribosomal protein S6 (RPS6) (Manning and Cantley 2007).
Another important target of Akt is glycogen synthase kinase 3 (GSK-3), which is inhibited by Akt (Cross et al. 1995). This results in cell cycle progression through the inhibition of GSK3-mediated phosphorylation and degradation of cyclin D and cyclin E as well as the transcription factors c-Jun and c-Myc. Inhibition of GSK3 can also promote glycogen metabolism, regulate wnt signaling, and affect the formation of neurofibrillary tangles in Alzheimer’s disease. Akt also positively regulates cell proliferation through inhibition of cyclin-dependent kinase inhibitors p21 (CDKN1A/CIP1/WAF1) and p27 (CDKN1B/KIP1).
Akt can mediate cell survival by inhibiting pro-apoptotic proteins such as Bcl-2-associated death promoter (Bad) or by inhibiting forkhead transcription factors (FoxO1/3a) from generating pro-apoptotic proteins such as Bim and cytokine Fas ligand (FasL) (Zhang et al. 2011). Akt also phosphorylates and activates the E3 ubiquitin ligase murine double minute 2 (MDM2), which triggers p53 degradation. This prevents the transcription of pro-apoptotic BH3-only proteins such as Puma and Noxa (Mayo and Donner 2002).
Additionally, there is significant cross talk between Akt and other major signaling pathways, often under specific conditions. Under stimuli such as tumor necrosis factor (TNFα) or platelet-derived growth factor (PDGF), Akt activates NF-kB/p65 signaling by phosphorylating pathway activators – IκB kinase α (IκKα). In certain cell types, or under stresses such as ischemia, Akt has also been reported to block ERK signaling through direct phosphorylation and inhibition of c-Raf (Zhou et al. 2015).
DJ-1 has been shown to activate the PI3k/Akt pathway, a process that may mediate its transforming effects during oncogenesis, as well as its cytoprotective effects against oxidative and nitrosative stress as discussed below (Fig. 8.3) (Kim et al. 2005; Yang et al. 2005).
Fig. 8.3. DJ-1 activates PI3K/AKT1 signaling pathway.
1. DJ-1 increases phosphorylated Akt, and activates the PI3k/Akt pathway, which contributes to oncogenesis and neuronal survival (Kim et al. 2005; Yang et al. 2005; Aleyasin et al. 2010; Zhang et al. 2016)
2. Under hypoxic conditions, DJ-1 mediates the activation of HIF1 that regulates the expression of many genes needed for cells to adapt to hypoxic conditions. DJ-1 does this by increasing mTORC activity through PI3k/Akt pathway modulation, decreasing p53 activation, and increasing AMPK activation of mTORC (Vasseur et al. 2009), as well as by inhibiting HIF-VHL interaction through direct binding with VHL (Parsanejad et al. 2014)
3. There may be significant cross talk and feedback regulation between p53, DJ-1, and the Akt pathway. p53 may inhibit DJ-1 activation through phosphorylation (Rahman-Roblick et al. 2008) and may also decrease DJ-1 protein levels through a posttranscriptional route (Vasseur et al. 2012). p53 negatively regulates the IGF-1/PI3k/Akt pathway by increasing the transcription of target genes such as IGF-BP3 or PTEN (Feng 2010), and is also conversely regulated by Akt, which activates the E3 ubiquitin ligase MDM2, a trigger for p53 degradation (Mayo and Donner 2002). Oxidative stress may induce DJ-1-mediated phosphorylation/activation of p53 (Vasseur et al. 2012), a process that may be mediated by active Akt (Zhan et al. 2010). Active p53 may then respond appropriately to stress by activating transcriptional programs (Kruiswijk et al. 2015). In a negative feedback loop, p53 can also decrease DJ-1 levels and attenuate Akt activation, preventing abnormal cellular transformation (Vasseur et al. 2012)
4. PD pathogenic mutants (L166P, D146A) of DJ-1 lose the ability to inhibit GSK-3β through the regulation of the Akt pathway and may contribute to increased tau phosphorylation and PD pathogenesis (Wang et al. 2013)
5. By activating the PI3K/Akt pathway, DJ-1 may increase the activity of downstream transcription factor c-myc to modulate the expression of human telomerase reverse transcriptase (hTERT), which is implicated in cellular differentiation and neoplastic transformation (Sitaram et al. 2009)
6. DJ-1 may play a role in modulating recruitment of Akt to the membrane in an ROS-dependent manner (Aleyasin et al. 2010)
7. DJ-1 binds mRNAs of PI3K/Akt pathway (Akt1, IGF2) and may release them to be translated under oxidative stress (van der Brug et al. 2008)
8. DJ-1 may decrease ceramide-induced autophagy and cell death through its effects on the PI3k/ AKT pathway (Jaramillo-Gomez et al. 2015)
9. DJ-1 may bind PTEN directly in a manner dependent on the redox status of its Cys-106 residue (Kim et al. 2009). And under nitrosative stress, DJ-1 may directly bind and inhibit PTEN activity via transnitrosylation
[positive regulation is indicated by pointed arrows, and negative regulation is indicated by blunted arrows. Direct or known regulation is indicated by solid lines; indirect or unknown regulation is indicated by dotted lines]
8.3.1 DJ-1 Negatively Regulates PTEN to Activate the PI3K/ Akt Pathway Mediating Oncogenic and Cytoprotective Properties of DJ-1
A study using Drosophila genetic screen for gain-of-function mutants was the first to report in 2005 that DJ-1 may antagonize PTEN function and activate the PI3K cell survival pathway to induce oncogenesis (Kim et al. 2005). The negative regulation of PTEN by DJ-1 has since been corroborated in various other model systems (Yang et al. 2005; Klawitter et al. 2013; Sitaram et al. 2009; Yao et al. 2011; Fang et al. 2010; Davidson et al. 2008; Liu et al. 2015). DJ-1 overexpression in PTEN overexpressing NIH-3T3 fibroblasts and in PTEN+/− mouse embryonic fibroblasts (MEFs) rescues cell survival under apoptotic stress, suggesting that DJ-1 may protect cells from apoptosis in a PTEN-dependent manner. In COS-7 cells, A597 cells, NIH-3T3, and PTEN+/− MEFs, DJ-1 increases phosphorylation of Akt and its downstream effectors in a PI3K-dependent manner, whereas knockdown of DJ-1 decreases the phosphorylation of Akt. In PTEN−/− MEFs, however, DJ-1 knockdown does not affect Akt phosphorylation, suggesting that PTEN is necessary for DJ-1 effects on Akt phosphorylation and that DJ-1 may suppress PTEN functionality to aid in PI3K/Akt pathway activation (Kim et al. 2005).
The connection between DJ-1, PTEN, and human carcinogenesis has also been examined in carcinomas (Kim et al. 2005). In primary breast cancer tissue samples, mutations in PTEN are not a major factor in the development of sporadic breast cancers (Feilotter et al. 1999; Freihoff et al. 1999), and alterations in PTEN levels in primary ductal adenocarcinomas are thought to be primarily epigenetic (Perren et al. 1999; Shi et al. 2003). Examination of the expression of DJ-1, phospho-Akt, and PTEN in serial histologic sections of breast cancer from 73 patients with lymph node-negative disease suggested an inverse relationship between PTEN and DJ-1 expression and a positive relationship between DJ-1 and phosphorylated Akt (Kim et al. 2005). Further, an examination of DJ-1 and phosphorylated Akt levels in primary lung cancer samples from 40 patients showed positive correlation between DJ-1 and phosphorylated Akt levels (Kim et al. 2005). Taken together, this suggests that DJ-1 may affect oncogenesis through epigenetic modulation of PTEN functionality in multiple cancer types, allowing for Akt to become hyperphosphorylated.
This assertion was further supported by a report showing that inhibition of DJ-1A in Drosophila leads to impaired PI3K/Akt signaling (Yang et al. 2005). DJ-1A RNAi induces phenotypes such as photoreceptor loss in the eye, dopaminergic neuron reduction in aging brains, and hypersensitivity to oxidative stress. When wild-type (WT) PTEN or a dominant-negative form of the PI3K catalytic subunit is co-expressed with DJ-1A RNAi transgene, the pathologic eye phenotype is exacerbated. Conversely, co-expression of the WT form of the PI3K catalytic subunit or Akt overexpressing transgene with DJ-1A RNAi suppresses the dysfunctional phenotype (Yang et al. 2005). This suggests that the dysfunctional phenotypes induced by DJ-1 knockdown are mediated through the PI3K/Akt pathway. Finally, phosphorylated Akt levels are significantly reduced in DJ-1A RNAi expressing fly head extracts, suggesting that DJ-1A is necessary for maintaining normal phosphorylation of Akt and the activation of PI3K/Akt signaling in the fly brain that may be needed to mediate dopaminergic neuron survival and responses to oxidative stress (Yang et al. 2005; Aleyasin et al. 2010). The protective effect of DJ-1 against oxidative stress is also seen in cardiomyocytes from mice (Billia et al. 2013; Dongworth et al. 2014; Mukherjee et al. 2011) and humans (Klawitter et al. 2013). DJ-1 is upregulated in cardiac tissue samples taken from patients with chronic ischemia dilated cardiomyopathy (Klawitter et al. 2013), a condition which has been linked to endothelial dysfunction and ROS (Tentolouris et al. 2004; Sorescu et al. 2002). Increased levels of DJ-1 in these tissues are associated with decreased PTEN protein expression and increased Akt phosphorylation (Klawitter et al. 2013), which may serve as a compensatory mechanism to protect from ischemic injury.
8.3.2 The Effect of DJ-1 on the PI3K/Akt Pathway Is Implicated in Multiple Disease States
The role of DJ-1 in the PI3k/Akt pathway is implicated in many disease models. For example, DJ-1 may affect the phosphorylation of tau, a protein that when hyperphosphorylated is found as a main component of neurofibrillary tangles in Alzheimer’s disease and progressive supranuclear palsy (Avila et al. 2004). Overexpression of the Parkinson’s disease-linked L166P mutant or D149A DJ-1 in cells induces less phosphorylation of Akt compared to wild-type DJ-1, leading to increased activity of GSK-3β and increased tau phosphorylation (Wang et al. 2013). This suggests that abnormal function of mutant DJ-1 in Akt activation may play a role in tauopathies.
In human clear cell renal cell carcinoma (ccRCC), DJ-1 may regulate the PTEN/ PI3K/Akt pathway to modulate hTERT levels and contribute to disease progression (Sitaram et al. 2009). Human telomerase reverse transcriptase (hTERT) is the catalytic subunit of telomerase, a ribonucleoprotein reverse transcriptase, which has been implicated in cellular differentiation and neoplastic transformation (Kim et al. 1994; Takakura et al. 1999). hTERT gene may be regulated by the transcription factor c-myc which is a downstream target of the PI3K/Akt pathway (Wu et al. 1999; Asano et al. 2004). In human ccRCC tissue samples, DJ-1 mRNA levels are positively correlated with c-myc and hTERT mRNA levels (Sitaram et al. 2009). Follow-up experiments in human kidney carcinoma A498 cells showed that knockdown of DJ-1 reduces c-myc and hTERT RNA expression and also decreases protein levels of p-PTEN and p-Akt, as well as that of Akt’s downstream effectors, p-GSK-3β and c-Myc (Sitaram et al. 2009). As such, the effect of DJ-1 on the activation of PI3k/Akt pathway appears to have far-reaching consequences on downstream effectors that can contribute to disease states.
Indeed, the effect of DJ-1 on the PI3k/Akt pathway may also be involved in several pathologic states including renal tubular epithelial-mesenchymal transition (EMT), which can lead to renal interstitial fibrosis and end-stage renal failure (Yao et al. 2011), in the peritoneal metastasis of gastric carcinoma by modulating levels of matrix metallopeptidases MMP-2 and MMP-9 (Zhu et al. 2014), in the migration and invasion of human glioma SWO-38 cells by affecting focal adhesion kinase (FAK) phosphorylation (Fang et al. 2010), in the cell survival and aggressiveness of ovarian carcinoma (Davidson et al. 2008), in the progression of uterine cervical neoplasia (Choi et al. 2015), in the tumorigenesis of medulloblastomas (Lin et al. 2014), and in the malignant properties of the hepatocellular carcinoma (HCC) cell line, HepG2 (Liu et al. 2015).
8.3.3 DJ-1 Mediates Stress-Induced Cellular Responses Through PI3K/Akt Pathway, Possibly Through PTEN-Independent Mechanisms
Under cellular stress, many of the protective effects of DJ-1 appear to be mediated through the PI3k/Akt pathway. For example, under hypoxic stress, DJ-1 may regulate transcription factor hypoxia-inducible factor-1 (HIF1) through the PI3K/Akt/ mTOR survival pathway as well as the metabolic sensor AMPK to protect cells against hypoxia-induced cell death (Vasseur et al. 2009). HIF1 regulates the expression of many genes known to be affected by Akt and may operate in parallel with AMPK to create a concerted response to hypoxia (Laderoute et al. 2006). mTOR activity has been reported to stabilize HIF1 protein (Zhong et al. 2000), while excess p53 may promote HIF1 degradation (Ravi et al. 2000). Under hypoxia, loss of DJ-1 was shown to significantly reduce mTOR activity (as measured by phosphorylation of p70-S6K and 4E-BP1) and increase p53 induction (as measured by p53 phosphorylation) in human osteosarcoma U2OS cells and in MEFs derived from DJ-1 KO mice. Congruently, DJ-1 is required for full HIF1 induction and activation under hypoxic stress, as DJ-1 knockdown in U2OS cells and MEFs exhibits decreased HIF1 protein levels and HIF1 target gene expression compared to control (Vasseur et al. 2009). DJ-1 has also been shown to interact with von Hippel-Lindau (VHL) protein, an E3 ubiquitin ligase, which can form a complex with HIF1 to induce its degradation (Parsanejad et al. 2014). DJ-1 inhibits HIF-VHL interaction and protects cells from hypoxia-induced apoptosis. Additionally, loss of DJ-1 alters AMPK activity and expression, where DJ-1 knockdown cells exhibit total decreased AMPK levels as well as decreased phosphorylation of AMPK under hypoxic stress (Vasseur et al. 2009). Taken together, these findings suggest that the ability of DJ-1 to modulate AMPK activity and regulate the PI3k/Akt/mTOR pathway or VHL-HIF1 interaction to increase HIF1 transcriptional activity may be crucial in coordinating the induction of genes necessary to adapt to hypoxia.
DJ-1 may also regulate Akt through its association with p53. p53 negatively regulates the IGF-1/PI3k/Akt pathway by increasing the transcription of target genes such as IGF-BP3 or PTEN (Feng 2010), and is also conversely regulated by Akt, which activates the MDM2, a trigger for p53 degradation (Mayo and Donner 2002). Accordingly, a study investigating the link between DJ-1, p53, and cell transformation showed that when p53 is deleted, DJ-1 is able to exert oncogenic effects through the phosphorylation and activation of Akt (Vasseur et al. 2012). In wild-type MEFs with normal p53 levels, DJ-1 knockdown results in inhibition of Akt phosphorylation. And in p53 null MEFs, which exhibit a higher level of Akt phosphorylation, DJ-1 is required for full activation of Akt. Interestingly, under oxidative stress, DJ-1 is necessary for the full phosphorylation and activation of p53 at serine 15 (Vasseur et al. 2012), which may be mediated by active Akt protein in certain cellular contexts (Vasseur et al. 2012; Zhan et al. 2010). Thus, there may be cross talk and feedback regulation between p53, DJ-1, and the Akt pathway, whereby oxidative stress induces DJ-1-mediated phosphorylation of p53, which may be mediated by active Akt. Active p53 may then be able to respond appropriately to stress by activating transcriptional programs (Kruiswijk et al. 2015), and also by consequently decreasing DJ-1 expression in a negative feedback loop, leading to attenuated Akt activation and preventing abnormal cellular transformation.
Under oxidative stress, DJ-1 may regulate Akt signaling by affecting Akt localization, and this may be crucial for neuronal protection in the context of Parkinson’s disease (PD). Upon hydrogen peroxide (H2O2) treatment, primary neuronal cultures from DJ-1 KO mice show a reduction in Akt phosphorylation (Aleyasin et al. 2010). Similarly, dopaminergic neurons in the substantia nigra from DJ-1 KO mice exhibit reduced Akt phosphorylation in response to in vivo administration of MPTP (Aleyasin et al. 2010). Further, when lymphoblasts isolated from PD patients harboring the Parkinson’s disease pathogenic mutation L166P are treated with H2O2, Akt phosphorylation is found to be reduced compared to that of healthy control lymphoblasts (Aleyasin et al. 2010). Suppression of Akt phosphorylation using a pharmacological inhibitor of AKT, LY294002 (LY), diminishes the neuroprotective function of DJ-1 in primary mouse neurons. This suggests that DJ-1 exerts some of the aforementioned protective effects through the Akt pathway (Aleyasin et al. 2010). Yet, exogenous WT Akt is unable to protect DJ-1-deficient neurons from oxidative stress both in vivo and in vitro (Aleyasin et al. 2010). On the other hand, myristoylated Akt (Myr-Akt), a membrane-anchored, constitutively active form of Akt, is able to protect DJ-1-deficient neurons (Aleyasin et al. 2010). Early studies have shown that Akt localization to the membrane occurs prior to its phosphorylation and activation (James et al. 1996; Franke et al. 1997) and that membrane-bound Myr-Akt is sufficient to provide cytoprotection (Ries et al. 2006). As loss of DJ-1 reduces Akt phosphorylation, and only membrane-anchored constitutively active form of Akt can provide protection to neurons lacking DJ-1, these findings suggest that DJ-1 may be an upstream activator of WT Akt. And consistent with this notion, DJ-1 has been shown to be necessary for Akt to translocate from the cytoplasmic compartment to membranous fractions following H2O2 treatment (Aleyasin et al. 2010). This suggests that DJ-1 may play a role in modulating recruitment of Akt to the membrane in an ROS-dependent manner, further supporting the notion that DJ-1 is necessary for Akt pathway activation and Akt-mediated neuroprotection from ROS.
It is also worthwhile to note that DJ-1 has been shown to associate with mRNA that encode members of the PTEN/PI3k/Akt pathway, raising the possibility that DJ-1 may also regulate this pathway posttranscriptionally. mRNA interacting with DJ-1 includes Akt1, IGF2, JUND, RPS6KB2, PPP2R2C, BCL2L1, RASL10B, MAPK8IP1, and EIF3EPI1 (van der Brug et al. 2008).
8.3.4 DJ-1 Inhibits Autophagy Through Activation of the PI3K/AKT Pathway
While the exact role of DJ-1 in regulating autophagy is under debate, it is clear that it can affect autophagy in a variety of models (Vasseur et al. 2009; McCoy and Cookson 2014). Previously, it was shown that DJ-1 activates autophagy in a neuronal model through the activation of the non-canonical MEK-ERK-mTOR pathway (Gao et al. 2012). In contrast, DJ-1 has been reported to activate autophagy through the PI3K/AKT pathway, independent of downstream mTOR effects.
The effect of DJ-1 on autophagy has also been studied in relation to C2-ceramide, a neurotoxic lipid that is associated with early inhibition of the PI3K/Akt pathway. Overexpression of DJ-1 in CAD cells (mouse catecholaminergic neuronal tumor cells) reportedly prevents C2-ceramide-induced inhibition of the PI3K/Akt pathway by keeping PTEN phosphorylated (inhibited). DJ-1 overexpression also decreases C2-ceramide-induced autophagy in a manner independent of changes in mTOR, exhibiting decreased autophagosome formation and autophagic flux. This suggests that DJ-1 may decrease ceramide-induced autophagy and cell death through its effects on the PI3k/AKT pathway (Jaramillo-Gomez et al. 2015).
8.3.5 DJ-1 May Inhibit PTEN Through Direct Binding and Transnitrosylation
Evidence has been reported suggesting that DJ-1 can interact with PTEN directly (Kim et al. 2009; Choi et al. 2014). Both wild-type and C106S mutant DJ-1 bind PTEN to inhibit its phosphatase activity in NIH3T3 cells, but the non-oxidizable C106S mutant binds and inhibits PTEN activity to a greater extent than WT DJ-1 does (Kim et al. 2009). Early in oxidative stress, WT DJ-1 strongly inhibits PTEN activity. But this is not sustained as stress continues and highly oxidized forms of DJ-1 at Cys-106 (SO2H and SO3H forms) accumulate. On the other hand, C106S mutant DJ-1, which cannot be oxidized at the Cys-106, strongly inhibits PTEN activity in a sustained manner (Kim et al. 2009). MALDI-TOF/TOF-MS (matrix-assisted laser desorption/ionization/time-of-flight mass spectrometry) analysis of the oxidation state of Cys-106 has revealed that in order for DJ-1 to inhibit PTEN activity and increase phosphorylation of Akt, over 50% of the DJ-1 population needs to be in the reduced/non-oxidized (SH) form (Kim et al. 2009). This suggests that under acute oxidative stress, the ability of DJ-1 to inhibit PTEN and consequently increase protective PI3k/Akt signaling is initially increased. However, with prolonged oxidation, when highly oxidized forms of DJ-1 make up more than 50% of total DJ-1, DJ-1 loses its ability to inhibit PTEN, indicating that DJ-1’s modulation of the PI3k/Akt pathway is dependent on its redox status.
Under mild nitrosative stress, DJ-1 directly binds and may inhibit PTEN activity via transnitrosylation (Choi et al. 2014). In HEK cells stably expressing neuronal nitric oxide synthase (nNOS) that can be made to generate cellular nitric oxide (NO) using calcium ionophore A23187 treatment, both DJ-1 and PTEN are nitrosylated at cysteine residues when endogenous NO production is induced: DJ-1 at its Cys106 residue forming SNO-DJ-1 and PTEN at Cys83 residue forming SNO-PTEN (Choi et al. 2014). As the transfer of an NO group from one protein thiol to another according to their respective Nernstian redox potentials is a common mechanism in mammalian systems (Kornberg et al. 2010; Nakamura and Lipton 2013), it was hypothesized that DJ-1 may interact with and S-nitrosylated PTEN. In in vitro pulldown assays using GST-tagged DJ-1 and PTEN, as well as in co-immunoprecipitation experiments in HEK293A cells using antibodies directed against endogenous PTEN or DJ-1, it was found that PTEN and DJ-1 form a complex in cells (Choi et al. 2014). When purified recombinant SNO-DJ-1 is incubated with PTEN, SNO-DJ-1 but not unmodified DJ-1 is able to act as an NO donor to PTEN. In the converse experiment using SNO-PTEN and DJ-1, nitrosylated PTEN is unable to transfer its NO group to DJ-1 (Choi et al. 2014). This transnitrosylation from DJ-1 to PTEN is abrogated in DJ-1 knockdown SH-SY5Y cells, while DJ-1 overexpression increases the level of SNO-PTEN. Additionally, while the co-transfection of WT DJ-1 protects SH-SY5Y cells from cell death induced by exogenous PTEN overexpression, co-transfection of the system with either non-nitrosylatable Cys-106 DJ-1 mutant or non-nitrosylatable C83A PTEN mutant cannot confer the same cytoprotection (Choi et al. 2014). This suggests that the neuroprotective activity of DJ-1 may require at least in part the transnitrosylation and inhibition of PTEN. Notably, in human PD brains, SNO-PTEN levels have been found to be significantly elevated and SNO-DJ-1 slightly decreased compared to controls, suggesting that DJ-1 may transnitrosylate PTEN in PD brains to detoxify neurotoxic levels of NO until this adaptive protective system is overwhelmed. Dysfunctional DJ-1 may lose this protective transnitrosylation activity due to mutations or through oxidation of its Cys106 residue (Choi et al. 2014).
8.4 DJ-1 Inhibits the ASK1 Pathway
Apoptosis signal-regulating kinase 1 (ASK1), also known as mitogen-activated protein kinase kinase kinase 5 (MAP3K5), is a MAPKKK that plays a key role in stress-induced apoptosis as well as cell survival and differentiation. Downstream of ASK1 are its MAPKKs, mitogen-activated protein kinase kinases ( MKK4/MKK7/ SEK1 and MKK3/MKK6), which in turn activate the MAPK proteins, c-Jun N-terminal kinases (JNKs), and p38 mitogen-activated protein kinases. This results in activation of the mitochondrial cell death pathway to induce apoptosis. These separate pathways are also referred to as the MKK4/MKK7-JNK pathway and MKK3/MKK6-p38 pathway (Cargnello and Roux 2011; Lawler et al. 1998; Keshet and Seger 2010; Tobiume et al. 2001).
Under normal conditions, ASK1 is oligomerized through its C-terminal coiled-coil domain but rendered inactive by various inhibitors such as thioredoxin (Trx) (Saitoh et al. 1998) and 14-3-3 proteins (Goldman et al. 2004). Reduced thioredoxin (Trx) binds ASK1 at its N-terminal coiled-coil domain, and 14-3-3 protein binds ASK1 at its phosphorylated Ser967 residue (Saitoh et al. 1998; Goldman et al. 2004; Yoon et al. 2009). These inhibitors regulate ASK1 activation in a redox sensitive manner and compete with ASK1 activators – such as TNF-alpha receptor-associated factors (TRAFs) or death-domain-associated protein 6 (Daxx).
Under cellular stresses such as oxidative stress, ultraviolet (UV) light, endoplasmic reticulum stress, tumor necrosis factor (TNF), and withdrawal of growth factor or serum, phosphorylation of ASK1 at Ser-967 is lost. Daxx then helps relieve inhibitory intramolecular interactions between the N- and C- termini of the ASK1 kinase, and TRAF2 and TRAF6 are recruited to ASK1 to form a larger molecular mass complex dubbed the ASK1 signalosome. ASK1 is then able to form homo-oligomeric interactions through both its C-terminal and N-terminal coiled-coil domains, undergo autophosphorylation at threonine 845, and become fully activated (Matsuzawa et al. 2005; Chang et al. 1998; Nishitoh et al. 1998; Ichijo et al. 1997; Leisner et al. 2016).
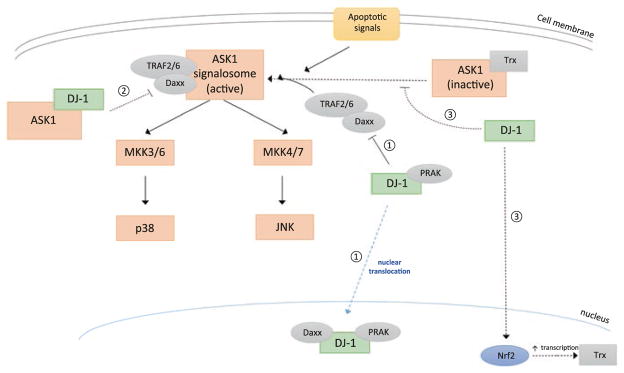
DJ-1 has been shown to inhibit ASK1 activation through a variety of mechanisms: (1) through sequestration or inhibition of the ASK1 activator, Daxx; (2) through direct binding with ASK1; by (3) increasing the expression of the ASK1 inhibitor, Trx1; and by (4) stabilizing Trx1-ASK1 interaction. This ultimately enhances cell survival by inhibiting cell death signaling and the mitochondrial apoptotic cascade (Fig. 8.4) (Junn et al. 2005; Waak et al. 2009; Hwang et al. 2013; Tang et al. 2014; Mo et al. 2010; Karunakaran et al. 2007).
Fig. 8.4. DJ-1 inhibits the ASK1 pathway.
1. DJ-1 inhibits ASK1 activity through nuclear sequestration of Daxx, an ASK1 activator and a part of the active ASK1 signalosome (Junn et al. 2005; Hwang et al. 2013; Karunakaran et al. 2007). Nuclear localization of DJ-1 may occur through its interaction with PRAK/MK5 which contains a nuclear localization sequence (NLS) (Tang et al. 2014)
2. DJ-1 may regulate ASK1 activity through direct binding (Waak et al. 2009; Mo et al. 2010; Cao et al. 2014)
3. DJ-1 inhibits ASK1 activation by preventing the dissociation of Trx1 from ASK1 and also by increasing the transcription of Trx1 (Im et al. 2010; Im et al. 2012)
[positive regulation is indicated by pointed arrows, and negative regulation is indicated by blunted arrows. Direct or known regulation is indicated by solid lines; indirect or unknown regulation is indicated by dotted lines]
8.4.1 DJ-1 Inhibits Daxx/ASK1 Activity Through Nuclear Sequestration of Daxx
The death protein Daxx has been identified as a DJ-1-interacting protein through yeast two-hybrid screen and subsequent immunoprecipitation experiments (Junn et al. 2005). In SH-SY5Y and COS-7 cells, overexpression of Daxx and ASK1 increases ASK1 activation (detected by in vitro kinase assay) and cell death. Co-expression of wild-type (WT) DJ-1 in this system is able to repress ASK1 activation and reduce cell death, whereas the Parkinson’s disease-associated pathogenic L166P DJ-1 mutant cannot protect cells from the effects of Daxx and ASK1 overexpression (Junn et al. 2005). In an unstressed cell, Daxx is localized mainly in the nucleus, while ASK1 is localized in the cytoplasm. Under conditions of cell death signaling, Daxx translocates to the cytoplasm where it can interact with ASK1 to activate apoptosis (Chang et al. 1998; Ko et al. 2001). Fluorescent immunocytochemical experiments showed that WT DJ-1 blocks the translocation of Daxx to the cytoplasm by binding Daxx and sequestering it in the nucleus, whereas L166P mutant DJ-1 fails to do the same. This trend is magnified when cells are treated with H2O2 (Junn et al. 2005). M26I mutant DJ-1 is also unable to suppress the export of Daxx from the nucleus to the cytoplasm (Waak et al. 2009), indicating that WT DJ-1 but not its pathogenic mutants can protect cells against oxidative stress-induced Daxx/ASK1 cell death signaling.
The stress of MPTP administration in mice also results in the activation of ASK1 signaling as well as the translocation of Daxx from the nucleus to cytosol (Karunakaran et al. 2007; Lee et al. 2012). As expected in ASK1 KO mice, MPTP-induced motor impairments are not as pronounced, and striatonigral dopaminergic neurons are relatively preserved compared to wild-type littermates (Lee et al. 2012). MPTP administration also lowers DJ-1 levels in the ventral midbrain of the animals, particularly in the nuclear fraction, which is consistent with the increase in the nuclear export of Daxx and its association with and activation of ASK1 in the cytosol (Karunakaran et al. 2007). Together, this indicates that ASK1 is a crucial effector of MPTP and oxidative stress-induced toxicity and apoptosis in the brain and that the cytoprotective function of DJ-1 may be mediated in part by its ability to regulate Daxx and ASK1 signaling.
DJ-1 may also bind to p38 regulated/activated kinase (PRAK/MK5) under cellular stress to help localize DJ-1 into the nucleus, allowing it to sequester Daxx in the nucleus and prevent cell death (Tang et al. 2014). PRAK is a downstream effector of the ASK1-MKK3/MKK6-p38 pathway and can be activated by p38 MAPK (Cargnello and Roux 2011). PRAK, which contains a putative nuclear localization sequence (NLS) and a nuclear export sequence (NES), is localized primarily in the cytoplasm under normal conditions (New et al. 2003). DJ-1 has been identified as a PRAK interactor in a yeast two-hybrid screen and found to bind directly to PRAK in immunoprecipitation assays and fluorescence resonance energy transfer (FRET) assays (Tang et al. 2014). DJ-1 also co-localizes with PRAK in the nuclei of NIH3T3 cells under oxidative stress. Following H2O2 treatment, PRAK increases phosphorylation of DJ-1 and its nuclear localization, as well as the nuclear sequestration of Daxx. Conversely, cells lacking PRAK exhibit impaired nuclear localization of DJ-1 and Daxx as well as increased cell death under oxidative stress (Tang et al. 2014). As DJ-1 lacks both a NLS and a NES, PRAK may be the crucial partner that assists DJ-1 in regulating the cellular localization of Daxx and ASK1 signaling.
Finally, DJ-1 may not only modulate Daxx localization but also its expression through the PI3k/Akt/dFOXO axis. In Drosophila, DJ-1β loss-of-function mutants are acutely sensitive to oxidative stress conditions and exhibit neuronal death after H2O2 treatment (Meulener et al. 2005; Menzies et al. 2005). As Daxx has been shown in mammalian systems to induce apoptosis by activating the JNK/FOXO cell death signaling pathway (Chang et al. 1998), one study examined the relationship of DJ-1β with the Drosophila homologue of Daxx, Daxx-like protein (DLP) (Hwang et al. 2013). DLP, seen to be elevated by H2O2 and UV exposure in wild-type Drosophila, has been shown to render flies more sensitive to oxidative stress when overexpressed in neurons (Hwang et al. 2013). In DJ-1β mutant flies, DLP expression as well as the translocation of DLP from the nucleus to the cytoplasm is increased, whereas overexpression of WT DJ-1β reduces the level of endogenous DLP. Additionally, DLP deficiency rescues the phenotypes of DJ-1β Drosophila mutants (Hwang et al. 2013). These findings suggest that DJ-1β may regulate the activity of DLP at least in part by limiting its expression and cytosolic localization. As DLP promoter harbors a consensus forkhead box subgroup O (FoxO) response element (FRE), it was also investigated whether DJ-1β could downregulate DLP expression transcriptionally under oxidative stress. Indeed, in DJ-1β mutants, the transcriptional activity of dFOXO is increased, inducing DLP transcription and apoptosis (Hwang et al. 2013). Interestingly, DLP overexpression also activates the JNK/dFOXO axis in Drosophila, meaning that DLP activation may further increase DLP expression in a feed-forward loop of DLP-JNK-dFOXO (Hwang et al. 2013). However, the PI3k/Akt pathway, which is activated by DJ-1, inhibits dFOXO (Greer and Brunet 2005). Thus, DJ-1β may play a complex role in the regulation of DLP, where it cannot only modulate the cellular localization of the protein, but also inhibit the activation and overexpression of DLP and the subsequent propagation of apoptotic signaling through modulating the PI3k/Akt/dFOXO axis.
8.4.2 DJ-1 Inhibits ASK1 Activity Through Direct Interaction
In addition to DJ-1 binding to Daxx leading to ASK1 regulation (Junn et al. 2005), evidence has also been reported that DJ-1 may regulate ASK1 activity through direct binding as well (Waak et al. 2009; Mo et al. 2010; Cao et al. 2014). Following overexpression of both tagged DJ-1 and ASK1, the two proteins were shown to co-immunoprecipitate. By binding to ASK1, DJ-1 may disrupt ASK1 homo-oligomerization and activation, thus inhibiting H2O2-induced ASK1 activation of MKK3 and p38 (Mo et al. 2010). Although DJ-1 is able to inhibit ASK1 activity, it has little effect on the enzyme activity of either MKK3 or p38 (Mo et al. 2010), indicating that DJ-1 targets primarily ASK1 in the ASK1/MKK2/p38 cascade.
While one study observed DJ-1/ASK1 co-immunoprecipitation both in the presence and absence of oxidative stress (Mo et al. 2010), others have reported that oxidative stress and the resulting oxidized DJ-1 forms are necessary for DJ-1/ASK1 interaction (Waak et al. 2009; Cao et al. 2014). In support of the latter point, substitution of Cys-106 residue of DJ-1 to non-oxidizable alanine reportedly abrogates DJ-1/ASK1 interaction, whereas mutations of peripheral conserved cysteine residues, such as C53A and C46A, still allow the association of DJ-1 with ASK1. This suggests that oxidation of DJ-1 at Cys-106 may be crucial for its binding to ASK1 (Waak et al. 2009).
Size-exclusion chromatography has also shown that oxidized DJ-1 is incorporated into native ASK1 complexes. These complexes are dissolved upon reducing SDS-PAGE, implying that DJ-1 is incorporated into ASK1 signalosome by mixed disulfide formation that is dependent on the central Cys-106 residue (Waak et al. 2009). This mixed disulfide formation is similar to the interaction between thioredoxin 1 (Trx1) and ASK1 at its N-terminal Trx1 binding site. And, overexpression of DJ-1 or Trx1 is able to repress ASK1 homo-oligomerization (Mo et al. 2010), suggesting that DJ-1 acts similarly to Trx1 in suppressing ASK1 activation.
Interestingly, WT DJ-1 is unable to bind ASK1 that lacks this N-terminal Trx1 binding site, but M26I mutant DJ-1 is able to constitutively bind both WT ASK1 and the N-terminal deleted ASK1, presumably at a dysfunctional site (Waak et al. 2009). Additionally, while L166P mutant DJ-1 also associates with ASK1, the interaction is much weaker than that of wild-type DJ-1 (Mo et al. 2010). Unlike WT DJ-1, its C106A, L166P, and M26I mutants fail to provide cytoprotection against oxidative stress (Wilson 2011; Malgieri and Eliezer 2008). This shows that proper interaction of WT DJ-1 with ASK1, presumably at the N-terminal Trx1 binding site, is necessary to provide protection. Parkinson-associated pathogenic mutant DJ-1, which differs in protein stability and lacks normal functionality compared to WT DJ-1, may be unable to properly interact with ASK1, thus contributing to pathogenic consequences.
8.4.3 DJ-1 Inhibits ASK1 Activation by Preventing the Dissociation of Trx1 from ASK1 and by Upregulating Trx1 Expression
An additional mechanism by which DJ-1 may inhibit ASK1 signaling is through affecting the inhibitory complex of Trx1-ASK1 (Im et al. 2010). In unstressed HEK293T cells, Trx1 co-immunoprecipitates with ASK1 equally in the presence or absence of exogenous DJ-1. However, under oxidative stress, the interaction between ASK1 and Trx1 decreases dramatically. This dissociation of Trx1 from ASK1 upon oxidative stress is prevented by co-expression of WT DJ-1, whereas co-expression of L166P or C106S mutant DJ-1 fails to do the same. Additionally, in DJ-1 KO mouse brain homogenates, the Trx1-ASK1 complex is found to dissociate more readily under oxidative challenge compared to brains from WT mice, suggesting that DJ-1 plays an important role in the maintenance of the Trx1-ASK1 inhibitory complex (Im et al. 2010).
A follow-up study reported that WT DJ-1, but not its L166P or M26I mutants, can also upregulate Trx1 mRNA and protein expression by upregulating the levels of the transcription factor Nrf2 and stimulating its translocation into the nucleus (Im et al. 2012). This enhances Nrf2 recruitment to the antioxidant response element (ARE) of the Trx1 gene promoter, increasing the level of Trx1 within the cell and blocking ASK1 activation (Im et al. 2012). Interestingly, Trx1 has also been shown to bind PTEN to activate the PI3K/Akt pathway (Meuillet et al. 2004). Knocking down Trx1 impairs the ability of DJ-1 to induce AKT phosphorylation and activation (Im et al. 2012), suggesting that DJ-1-mediated modulation of Trx1 may impact both the ASK1 pathway and the PI3K/Akt pathway, dually regulating major cell death and cell survival signaling pathways.
8.5 DJ-1 in Other Signaling Pathways
8.5.1 DJ-1 Can Interact with MEKK1 to Suppress MEKK1-MKK4-JNK1 Cell Death Signaling
Apoptotic signaling via MKK4-JNK can be activated not only by ASK1 but also by other MAPKKKs such as MAP-ERK kinase kinase 1 (MEKK1), MEKK4, TAK1, and MLKs (Whitmarsh and Davis 1998). It has been demonstrated that wild-type (WT) DJ-1, but not its L166P mutant form, can interact physically with MEKK1 to inhibit its activity, protecting cells from UV-induced MEKK1-MKK4-JNK1 apoptotic signaling (Mo et al. 2008). By binding to MEKK1, DJ-1 also sequesters the kinase in the cytoplasm, preventing it from translocating to the nucleus and regulating gene expression through its downstream transcription factor effectors such as NF-kB and c-Jun. On the other hand, L166P mutant DJ-1 enhances the nuclear accumulation of MEKK1. DJ-1 does not appear to inhibit other MAPKKKs such as MLK3 and TAK1 (Mo et al. 2008).
8.5.2 DJ-1 Suppresses the JNK/Beclin 1 Pathway to Regulate Autophagy
In addition to its effects on autophagy through the ERK and AKT pathway, DJ-1 can inhibit autophagy in a cancer cell model through the JNK/Beclin1 pathway (Ren et al. 2010). In H1299 cells, a p53 null lung cancer cell line, overexpression of DJ-1 decreases autophagy, whereas knocking down of DJ-1 increases autophagy. DJ-1 knockdown also activates JNK1/2 in H1299 cells without affecting ERK or p38 activation and increases Beclin1 expression (Ren et al. 2010). JNK1/2 phosphorylation, Beclin1 upregulation, and autophagy activation that occur with DJ-1 knockdown are abrogated in the presence of the JNK-specific inhibitor, SP600125, suggesting that regulation of autophagy by DJ-1 is JNK dependent. Under conditions of starvation, DJ-1 knockdown in H1299 cells also exhibits increased autophagy as well as increased cell death (Ren et al. 2010). Thus, in a cancer model, the upregulation of DJ-1 may enhance oncogenesis by inhibiting autophagy in a JNK-dependent manner. This would lead to downregulation of the tumor-suppressor Beclin1 as well as accumulation of p62, which has been shown to contribute to tumorigenesis (Moscat and Diaz-Meco 2009; Mathew et al. 2009).
8.5.3 DJ-1 Positively Regulates Androgen Receptor Signaling
The nuclear receptor androgen receptor (AR) relays androgen signaling from the cell surface to the nucleus and activates the transcription of genes essential for male reproductive function such as spermatogenesis (O’Hara and Smith 2015). DJ-1 has been shown to be necessary for normal AR function and to positively regulate AR signaling in a variety of ways (Taira et al. 2004b). DJ-1 directly binds to the protein PIASxa/ARIP3, an inhibitor of AR, and prevents PIASxa/ARIP3 from forming a complex with AR (Takahashi et al. 2001). In addition, DJ-1 binds to AR to stimulate its transcriptional activity in hormonally treated prostate cancer cells, potentially contributing to cancer progression and androgen independence (Pitkanen-Arsiola et al. 2006; Tillman et al. 2007).
DJ-1 also binds and sequesters a novel DJ-1-binding protein (DJBP), which was identified by yeast two-hybrid screen (Niki et al. 2003). DJBP can bind to the DNA-binding domain of AR and repress its transcriptional activity through recruitment of a histone deacetylase (HDAC) co-repressor complex. Normally, when hormone receptors bind their putative ligand, they assume a configuration that leads to transcriptional activation (Xu et al. 1999). However, an HDAC-co-repressor complex may change the active form of AR into an inactive form. DJ-1 can partially restore AR function by abrogating the DJBP-HDAC complex.
Interestingly, Daxx has been shown to sumoylate and repress the DNA binding activity of AR, leading to inhibition of its transcriptional function (Shih et al. 2007). Daxx also binds DJ-1 in the nucleus (Junn et al. 2005). Additionally, sumoylation of DJ-1 at lysine residue K130 has been found to be crucial for its normal activity (Shinbo et al. 2005). The latter finding raises the possibility that Daxx might play a part in the sumoylation of DJ-1 and that DJ-1 binding to Daxx may impact the ability of Daxx to repress AR through a competitive mechanism as well. However, any direct link between Daxx and sumoylated DJ-1 as well as a link between sumoylated DJ-1 and AR remain to be investigated.
8.5.4 Mutant DJ-1 Interacts with TTRAP and TRAF6, Affecting Cell Death Signaling, Protein Aggregation, and rRNA Biogenesis
Several pathogenic missense mutations in DJ-1, such as L166P, M26I, and E64D, have been linked to recessively inherited PD (Bonifati et al. 2003; Bonifati et al. 2004; Abou-Sleiman et al. 2003). L166P and M26I mutations affect DJ-1 stability (although with mixed results for M26I), and its ability to form homodimers, and result in reduced cellular DJ-1 levels (Moore et al. 2003; Takahashi-Niki et al. 2004; Milkovic et al. 2015). E64D mutant DJ-1 may retain the ability to form homodimers, but this mutation may potentiate DJ-1 aggresome formation (Repici et al. 2013).
As there is still no unifying consensus on how these various mutants abrogate normal DJ-1 function, one study sought to investigate whether these mutants may be involved in a pathological “gain-of-function” protein-protein interaction (Zucchelli et al. 2009). To this end, a yeast two-hybrid screen of a human fetal brain cDNA library was employed to identify proteins that interact with both wild-type and mutant DJ-1. The TNFR-associated factor (TRAF) and tumor necrosis factor (TNF) receptor-associated protein (TTRAP/EAPII) was one such protein identified as a novel DJ-1 interactor (Zucchelli et al. 2009). TTRAP is a member of the non-canonical TGFβ-TRAF6-TAK1 apoptotic pathway and was originally isolated for its ability to bind TNF receptor and TRAFs and consequently inhibit NF-kβ activation (Pype et al. 2000; Varady et al. 2011).
TTRAP is found in the nucleus of SH-SY5Y cells and throughout the adult mouse brain, with strong expression in the dentate gyrus of the hippocampus. In the adult mouse mesencephalon, TTRAP is expressed both in non-dopaminergic and dopaminergic neurons of the substantia nigra. Interestingly, TTRAP solubility and subcellular localization are modified by proteasomal impairment. Treatment with the proteasomal inhibitor MG132 shifts TTRAP from the soluble fraction into the insoluble fraction, as TTRAP moves from the nucleus to the cytoplasm to form a single large juxta-nuclear aggresome-like structure. In co-immunoprecipitation experiments, TTRAP binding is stronger to M26I and L166P mutant DJ-1 than to WT DJ-1. When proteasomal function is impaired with MG132 treatment, the binding of all DJ-1 isoforms to TTRAP is enhanced. TTRAP overexpression protects SH-SY5Y cells from MG132-induced apoptosis, reducing JNK phosphorylation and poly-(ADP-ribose) polymerase (PARP) activation. However, co-transfection of M26I or L166P mutant DJ-1 with TTRAP results in a “gain-of-function” phenomenon, in which TTRAP assumes a novel signaling property that not only abrogates its protective effects against proteasomal impairment, but induces the activation of JNK- and p38 MAPK-mediated apoptosis. Cells that express only M26I or L166P DJ-1 without TTRAP do not activate the JNK- and p38 MAPK-mediated apoptotic pathway (Zucchelli et al. 2009). Thus L166P and M26I mutant DJ-1 may be involved in a pathological interaction with TTRAP, in a process that can render cells more sensitive to proteasomal stress and activate apoptotic pathways.
In a follow-up investigation, TRAF6, which interacts with TTRAP, was also shown to interact with L166P mutant but not with WT DJ-1 (Zucchelli et al. 2010). TRAF6 is an E3 ubiquitin ligase that is involved in activating the non-canonical TGFβ-TRAF6-TAK1 apoptotic pathway and can help relay neuronal cell signaling from the neurotrophin receptor p75 and TrkA (Geetha et al. 2005). It is also part of the active ASK1 signalosome, which can induce apoptosis (Fujino et al. 2007). Notably, TRAF6 promotes an atypical mode of polyubiquitination of DJ-1, stimulating the accumulation of mutant DJ-1 into insoluble aggregates (Zucchelli et al. 2010). Normally, polyubiquitin chains form through covalent conjugations using any one of seven lysine residues present in the ubiquitin protein (K6, K11, K27, K29, K33, K48, and K63), each contributing distinct but sometimes intersecting roles. For example, to target a protein for degradation by the proteasome, polyubiquitin chains are mainly formed through linkages of the K48 residue (Chen and Sun 2009). K11 linkages appear to function in endoplasmic reticulum-associated degradation (Xu et al. 2009). TRAF6 promotes K63-specific chain assembly that contributes to the non-canonical TGFβ-TRAF6-TAK1 apoptotic pathway, ultimately leading to NF-kβ activation (Chen 2005). While TRAF6 does not bind or affect the ubiquitination of WT DJ-1, it does bind and enhance the ubiquitination of L166P mutant DJ-1. Furthermore, instead of using the typical K63-specific chain assembly, TRAF6 promotes atypical polyubiquitination of L166P DJ-1 by using K6, K27, K29, and K33 isotype linkages. And rather than triggering degradation, these atypical polyubiquitin chains on L166P DJ-1 mediate its accumulation into insoluble aggregates (Zucchelli et al. 2010). In addition, an examination of postmortem brains from sporadic PD patients in a subsequent study revealed that TTRAP is associated with cytoplasmic Lewy bodies and localized to the nucleolus of surviving dopamine neurons. In a cell model, L166P mutant but not WT DJ-1 promotes the accumulation of TTRAP into insoluble cytoplasmic aggresomes, which in turn impair rRNA biogenesis by inhibiting TTRAP localization into nucleolar cavities (Vilotti et al. 2012).
Thus L166P mutant DJ-1 appears to gain dominant-negative functions over WT DJ-1 through its binding interactions with TTRAP and TRAF6. Under proteasomal impairment, L166P DJ-1 binds strongly to TTRAP, which localizes as an aggregate around the nucleus, and triggers p38-/JNK-mediated apoptosis (Zucchelli et al. 2009). In addition, L166P DJ-1 can bind TRAF6, an E3 ubiquitin ligase, and become poly-ubiquitinated in an atypical fashion. The poly-ubiquitinated L166P DJ-1 is able to form insoluble aggregates and sequester TTRAP in the cytoplasm, preventing TTRAP from localizing to the nucleolus and negatively affecting rRNA biogenesis (Zucchelli et al. 2010; Vilotti et al. 2012).
8.6 Conclusion
In a mere 10 years, DJ-1 has emerged as a significant player in many major signaling pathways, including ERK, Akt/PI3k, and ASK1, with distinct effects on different cancers and neuronal models. However, a bulk of the studies utilize in vitro reductionist methods, studying how cultured cells respond to various stresses and stimuli. Much of the data remains to be confirmed in in vivo models, where complex cross talk between signaling pathways and resulting biological compensation may attenuate or strengthen effects by DJ-1.
Nevertheless, many studies have shown that DJ-1 undoubtedly impacts the signaling processes that maintain cellular homeostasis, as well as the careful balance between uncontrolled oncogenesis and premature cell death. This indicates the importance of pursuing the role of DJ-1 in regulating signal transduction and of elucidating ways to target it for therapeutic intervention in cancer and neurodegeneration.
References
- Abou-Sleiman PM, et al. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol. 2003;54(3):283–286. doi: 10.1002/ana.10675. [DOI] [PubMed] [Google Scholar]
- Abraham D, et al. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J Biol Chem. 2000;275(29):22300–22304. doi: 10.1074/jbc.M003259200. [DOI] [PubMed] [Google Scholar]
- Adams DG, et al. Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/ threonine phosphatase 2A holoenzymes. J Biol Chem. 2005;280(52):42644–42654. doi: 10.1074/jbc.M502464200. [DOI] [PubMed] [Google Scholar]
- Alessi DR, et al. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Current Biology. 1995;5(3):283–295. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- Aleyasin H, et al. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci U S A. 2010;107(7):3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic M, et al. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci U S A. 1996;93(12):5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron L, et al. Pro-survival role for Parkinson’s associated gene DJ-1 revealed in trophically impaired dopaminergic neurons. PLoS Biol. 2010;8(4):e1000349. doi: 10.1371/journal.pbio.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, et al. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23(53):8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- Avila J, et al. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84(2):361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- Billia F, et al. Parkinson-susceptibility gene DJ-1/PARK7 protects the murine heart from oxidative damage in vivo. Proc Natl Acad Sci U S A. 2013;110(15):6085–6090. doi: 10.1073/pnas.1303444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3(12):932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Oostra BA, Heutink P. Linking DJ-1 to neurodegeneration offers novel insights for understanding the pathogenesis of Parkinson’s disease. J Mol Med (Berl) 2004;82(3):163–174. doi: 10.1007/s00109-003-0512-1. [DOI] [PubMed] [Google Scholar]
- Brognard J, et al. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25(6):917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Burotto M, et al. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120(22):3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet-Aviles RM, et al. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101(24):9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, et al. The oxidation states of DJ-1 dictate the cell fate in response to oxidative stress triggered by 4-HPR: autophagy or apoptosis? Antioxid Redox Signal. 2014;21(10):1443–1459. doi: 10.1089/ars.2013.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, et al. DJ-1 as a human oncogene and potential therapeutic target. Biochem Pharmacol. 2015;93(3):241–250. doi: 10.1016/j.bcp.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caunt CJ, et al. MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat Rev Cancer. 2015;15(10):577–592. doi: 10.1038/nrc4000. [DOI] [PubMed] [Google Scholar]
- Chang HY, et al. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281(5384):1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- Chang F, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17(7):1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7(8):758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33(3):275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Choi MS, et al. Transnitrosylation from DJ-1 to PTEN attenuates neuronal cell death in parkinson’s disease models. J Neurosci. 2014;34(45):15123–15131. doi: 10.1523/JNEUROSCI.4751-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SK, et al. Overexpression of PI3K-p110alpha in the progression of uterine cervical neoplasia and its correlation with pAkt and DJ-1. Eur J Gynaecol Oncol. 2015;36(4):389–393. [PubMed] [Google Scholar]
- Cross DA, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Davidson B, et al. Expression and clinical role of DJ-1, a negative regulator of PTEN, in ovarian carcinoma. Hum Pathol. 2008;39(1):87–95. doi: 10.1016/j.humpath.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Devine MJ, Plun-Favreau H, Wood NW. Parkinson’s disease and cancer: two wars, one front. Nat Rev Cancer. 2011;11(11):812–823. doi: 10.1038/nrc3150. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, et al. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol. 2002;22(10):3237–3246. doi: 10.1128/MCB.22.10.3237-3246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongworth RK, et al. DJ-1 protects against cell death following acute cardiac ischemia-reperfusion injury. Cell Death Dis. 2014;5:e1082. doi: 10.1038/cddis.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn PJ, et al. A RNA interference screen identifies the protein phosphatase 2A subunit PR55gamma as a stress-sensitive inhibitor of c-SRC. PLoS Genet. 2007;3(12):e218. doi: 10.1371/journal.pgen.0030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, et al. DJ-1 decreases Bax expression through repressing p53 transcriptional activity. J Biol Chem. 2008;283(7):4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- Fang M, et al. Role of DJ-1-induced PTEN down-regulation in migration and invasion of human glioma cells. Chin J Cancer. 2010;29(12):988–994. doi: 10.5732/cjc.010.10307. [DOI] [PubMed] [Google Scholar]
- Feilotter HE, et al. Analysis of the 10q23 chromosomal region and the PTEN gene in human sporadic breast carcinoma. Br J Cancer. 1999;79(5–6):718–723. doi: 10.1038/sj.bjc.6690115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z. p53 Regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment. Cold Spring Harb Perspect Biol. 2010;2(2):a001057. doi: 10.1101/cshperspect.a001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, et al. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4- bisphosphate. Science. 1997;275(5300):665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Freihoff D, et al. Exclusion of a major role for the PTEN tumour-suppressor gene in breast carcinomas. Br J Cancer. 1999;79(5–6):754–758. doi: 10.1038/sj.bjc.6690121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino G, et al. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27(23):8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18(1):13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Gao H, et al. DJ-1 protects dopaminergic neurons against rotenone-induced apoptosis by enhancing ERK-dependent mitophagy. J Mol Biol. 2012;423(2):232–248. doi: 10.1016/j.jmb.2012.06.034. [DOI] [PubMed] [Google Scholar]
- Geetha T, Jiang J, Wooten MW. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell. 2005;20(2):301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Goldman EH, Chen L, Fu H. Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J Biol Chem. 2004;279(11):10442–10449. doi: 10.1074/jbc.M311129200. [DOI] [PubMed] [Google Scholar]
- Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7(12):a006080. doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24(50):7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Gu L, et al. Involvement of ERK1/2 signaling pathway in DJ-1-induced neuroprotection against oxidative stress. Biochem Biophys Res Commun. 2009;383(4):469–474. doi: 10.1016/j.bbrc.2009.04.037. [DOI] [PubMed] [Google Scholar]
- He X, et al. DJ-1 promotes invasion and metastasis of pancreatic cancer cells by activating SRC/ERK/uPA. Carcinogenesis. 2012;33(3):555–562. doi: 10.1093/carcin/bgs002. [DOI] [PubMed] [Google Scholar]
- Hermanson E, et al. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells. Exp Cell Res. 2003;288(2):324–334. doi: 10.1016/s0014-4827(03)00216-7. [DOI] [PubMed] [Google Scholar]
- Hwang S, et al. Drosophila DJ-1 decreases neural sensitivity to stress by negatively regulating Daxx-like protein through dFOXO. PLoS Genet. 2013;9(4):e1003412. doi: 10.1371/journal.pgen.1003412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo H, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275(5296):90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Im JY, et al. DJ-1 protects against oxidative damage by regulating the thioredoxin/ASK1 complex. Neurosci Res. 2010;67(3):203–208. doi: 10.1016/j.neures.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im JY, et al. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Genet. 2012;21(13):3013–3024. doi: 10.1093/hmg/dds131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, et al. Oxidative status of DJ-1-dependent activation of dopamine synthesis through interaction of tyrosine hydroxylase and 4-dihydroxy-L-phenylalanine (L-DOPA) decarboxylase with DJ-1. J Biol Chem. 2009;284(42):28832–28844. doi: 10.1074/jbc.M109.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwawaki T, Kohno K, Kobayashi K. Identification of a potential nurr1 response element that activates the tyrosine hydroxylase gene promoter in cultured cells. Biochem Biophys Res Commun. 2000;274(3):590–595. doi: 10.1006/bbrc.2000.3204. [DOI] [PubMed] [Google Scholar]
- James SR, et al. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996;315(3):709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353(3):417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo-Gomez J, et al. Overexpression of DJ-1 protects against C2-ceramide-induced neuronal death through activation of the PI3K/AKT pathway and inhibition of autophagy. Neurosci Lett. 2015;603:71–76. doi: 10.1016/j.neulet.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Jeffrey KL, et al. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007;6(5):391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- Junn E, et al. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci U S A. 2005;102(27):9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22(4):954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med. 2009;47(10):1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Karunakaran S, et al. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: protection by alpha-lipoic acid. FASEB J. 2007;21(9):2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- Kato I, et al. Oxidized DJ-1 inhibits p53 by sequestering p53 from promoters in a DNA-binding affinity-dependent manner. Mol Cell Biol. 2013;33(2):340–359. doi: 10.1128/MCB.01350-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet Y, Seger R. The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol. 2010;661:3–38. doi: 10.1007/978-1-60761-795-2_1. [DOI] [PubMed] [Google Scholar]
- Kim NW, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kim RH, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7(3):263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kim YC, et al. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int J Oncol. 2009;35(6):1331–1341. [PubMed] [Google Scholar]
- Klawitter J, et al. Association of DJ-1/PTEN/AKT- and ASK1/p38-mediated cell signalling with ischaemic cardiomyopathy. Cardiovasc Res. 2013;97(1):66–76. doi: 10.1093/cvr/cvs302. [DOI] [PubMed] [Google Scholar]
- Ko YG, et al. Apoptosis signal-regulating kinase 1 controls the proapoptotic function of death-associated protein (Daxx) in the cytoplasm. J Biol Chem. 2001;276(42):39103–39106. doi: 10.1074/jbc.M105928200. [DOI] [PubMed] [Google Scholar]
- Kornberg MD, et al. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12(11):1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ER, et al. Absence of ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5(3):e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebiehl G, et al. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1. PLoS One. 2010;5(2):e9367. doi: 10.1371/journal.pone.0009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruiswijk F, Labuschagne CF, Vousden KH. p53 In survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16(7):393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26(14):5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S, et al. Synergistic activation of SAPK1/JNK1 by two MAP kinase kinases in vitro. Curr Biol. 1998;8(25):1387–1391. doi: 10.1016/s0960-9822(98)00019-0. [DOI] [PubMed] [Google Scholar]
- Lee KW, et al. Apoptosis signal-regulating kinase 1 mediates MPTP toxicity and regulates glial activation. PLoS One. 2012;7(1):e29935. doi: 10.1371/journal.pone.0029935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisner TM, et al. CIB1: a small protein with big ambitions. FASEB J. 2016;30(8):2640–2650. doi: 10.1096/fj.201500073R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneux C, Rocher G, Porteu F. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J. 2006;25(4):727–738. doi: 10.1038/sj.emboj.7600980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev N, et al. Oxidative insults induce DJ-1 upregulation and redistribution: implications for neuroprotection. Neurotoxicology. 2008;29(3):397–405. doi: 10.1016/j.neuro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Lev N, et al. DJ-1 protects against dopamine toxicity. J Neural Transm (Vienna) 2009;116(2):151–160. doi: 10.1007/s00702-008-0134-4. [DOI] [PubMed] [Google Scholar]
- Lin E, et al. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J Neurosci Res. 2008;86(1):108–117. doi: 10.1002/jnr.21478. [DOI] [PubMed] [Google Scholar]
- Lin J, et al. DJ-1 is activated in medulloblastoma and is associated with cell proliferation and differentiation. World J Surg Oncol. 2014;12:373. doi: 10.1186/1477-7819-12-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, et al. DJ-1 knockdown inhibits growth and xenograft induced tumor generation of human hepatocellular carcinoma cells. Oncol Rep. 2015;33(1):201–206. doi: 10.3892/or.2014.3594. [DOI] [PubMed] [Google Scholar]
- Lu L, et al. DJ-1 upregulates tyrosine hydroxylase gene expression by activating its transcriptional factor Nurr1 via the ERK1/2 pathway. Int J Biochem Cell Biol. 2012;44(1):65–71. doi: 10.1016/j.biocel.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Lu L, et al. DJ-1/PARK7, but not its L166P mutant linked to autosomal recessive parkinsonism, modulates the transcriptional activity of the orphan nuclear receptor Nurr1 In vitro and in vivo. Mol Neurobiol. 2016;53:7363. doi: 10.1007/s12035-016-9772-y. [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(22):13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Malgieri G, Eliezer D. Structural effects of Parkinson’s disease linked DJ-1 mutations. Protein Sci. 2008;17(5):855–868. doi: 10.1110/ps.073411608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinat C, et al. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ESderived cell model of primary parkinsonism. PLoS Biol. 2004;2(11):e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137(6):1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6(6):587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27(9):462–467. doi: 10.1016/s0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Cookson MR. DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy. 2014;7(5):531–532. doi: 10.4161/auto.7.5.14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9(10):724–737. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Yenisetti SC, Min KT. Roles of drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15(17):1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Meuillet EJ, et al. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN’s lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN’s tumor suppressor activity. Arch Biochem Biophys. 2004;429(2):123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Meulener M, et al. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol. 2005;15(17):1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Milkovic NM, et al. Transient sampling of aggregation-prone conformations causes pathogenic instability of a parkinsonian mutant of DJ-1 at physiological temperature. Protein Sci. 2015;24(10):1671–1685. doi: 10.1002/pro.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JS, et al. DJ-1 modulates UV-induced oxidative stress signaling through the suppression of MEKK1 and cell death. Cell Death Differ. 2008;15(6):1030–1041. doi: 10.1038/cdd.2008.26. [DOI] [PubMed] [Google Scholar]
- Mo JS, et al. DJ-1 modulates the p38 mitogen-activated protein kinase pathway through physical interaction with apoptosis signal-regulating kinase 1. J Cell Biochem. 2010;110(1):229–237. doi: 10.1002/jcb.22530. [DOI] [PubMed] [Google Scholar]
- Moore DJ, et al. A missense mutation (L166P) in DJ-1, linked to familial Parkinson’s disease, confers reduced protein stability and impairs homo-oligomerization. J Neurochem. 2003;87(6):1558–1567. doi: 10.1111/j.1471-4159.2003.02265.x. [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137(6):1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee UA, et al. 37 A novel role for DJ-1 in cardioprotection. Heart. 2011;97(24):e8. [Google Scholar]
- Mut M, et al. Both mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinases (ERK) 1/2 and phosphatidylinositide-3-OH kinase (PI3K)/Akt pathways regulate activation of E-twenty-six (ETS)-like transcription factor 1 (Elk-1) in U138 glioblastoma cells. Int J Biochem Cell Biol. 2012;44(2):302–310. doi: 10.1016/j.biocel.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Nagakubo D, et al. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231(2):509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA. Emerging role of protein-protein transnitrosylation in cell signaling pathways. Antioxid Redox Signal. 2013;18(3):239–249. doi: 10.1089/ars.2012.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New L, Jiang Y, Han J. Regulation of PRAK subcellular location by p38 MAP kinases. Mol Biol Cell. 2003;14(6):2603–2616. doi: 10.1091/mbc.E02-08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki T, et al. DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol Cancer Res. 2003;1(4):247–261. [PubMed] [Google Scholar]
- Nishitoh H, et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2(3):389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- Nordzell M, et al. Defining an N-terminal activation domain of the orphan nuclear receptor Nurr1. Biochem Biophys Res Commun. 2004;313(1):205–211. doi: 10.1016/j.bbrc.2003.11.079. [DOI] [PubMed] [Google Scholar]
- O’Hara L, Smith LB. Androgen receptor roles in spermatogenesis and infertility. Best Pract Res Clin Endocrinol Metab. 2015;29(4):595–605. doi: 10.1016/j.beem.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Oehrl W, Rubio I, Wetzker R. Serine 338 phosphorylation is dispensable for activation of c-Raf1. J Biol Chem. 2003;278(20):17819–17826. doi: 10.1074/jbc.M209951200. [DOI] [PubMed] [Google Scholar]
- Parsanejad M, et al. Regulation of the VHL/HIF-1 pathway by DJ-1. J Neurosci. 2014;34(23):8043–8050. doi: 10.1523/JNEUROSCI.1244-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren A, et al. Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol. 1999;155(4):1253–1260. doi: 10.1016/S0002-9440(10)65227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen-Arsiola T, et al. Androgen and anti-androgen treatment modulates androgen receptor activity and DJ-1 stability. Prostate. 2006;66(11):1177–1193. doi: 10.1002/pros.20450. [DOI] [PubMed] [Google Scholar]
- Plotnikov A, et al. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813(9):1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Pype S, et al. TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated factors (TRAFs), and that inhibits nuclear factor-kappa B activation. J Biol Chem. 2000;275(24):18586–18593. doi: 10.1074/jbc.M000531200. [DOI] [PubMed] [Google Scholar]
- Rahman-Roblick R, et al. Proteomic identification of p53-dependent protein phosphorylation. Oncogene. 2008;27(35):4854–4859. doi: 10.1038/onc.2008.124. [DOI] [PubMed] [Google Scholar]
- Ravi R, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14(1):34–44. [PMC free article] [PubMed] [Google Scholar]
- Ren H, et al. DJ-1, a cancer and Parkinson’s disease associated protein, regulates autophagy through JNK pathway in cancer cells. Cancer Lett. 2010;297(1):101–108. doi: 10.1016/j.canlet.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Repici M, et al. Parkinson’s Disease-associated mutations in DJ-1 modulate its dimerization in living cells. J Mol Med (Berl) 2013;91(5):599–611. doi: 10.1007/s00109-012-0976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V, et al. Oncoprotein Akt/PKB induces trophic effects in murine models of Parkinson’s disease. Proc Natl Acad Sci U S A. 2006;103(49):18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17(9):2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekito A, et al. DJ-1 interacts with HIPK1 and affects H2O2-induced cell death. Free Radic Res. 2006;40(2):155–165. doi: 10.1080/10715760500456847. [DOI] [PubMed] [Google Scholar]
- Shamoto-Nagai M, et al. An inhibitor of mitochondrial complex I, rotenone, inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells. J Neurosci Res. 2003;74(4):589–597. doi: 10.1002/jnr.10777. [DOI] [PubMed] [Google Scholar]
- Shendelman S, et al. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2(11):e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, et al. Dysregulated PTEN-PKB and negative receptor status in human breast cancer. Int J Cancer. 2003;104(2):195–203. doi: 10.1002/ijc.10909. [DOI] [PubMed] [Google Scholar]
- Shih HM, et al. Daxx mediates SUMO-dependent transcriptional control and subnuclear compartmentalization. Biochem Soc Trans. 2007;35(Pt 6):1397–1400. doi: 10.1042/BST0351397. [DOI] [PubMed] [Google Scholar]
- Shinbo Y, et al. Proper SUMO-1 conjugation is essential to DJ-1 to exert its full activities. Cell Death Differ. 2005;13(1):96–108. doi: 10.1038/sj.cdd.4401704. [DOI] [PubMed] [Google Scholar]
- Silverstein AM, et al. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci. 2002;99(7):4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram RT, et al. The PTEN regulator DJ-1 is associated with hTERT expression in clear cell renal cell carcinoma. Int J Cancer. 2009;125(4):783–790. doi: 10.1002/ijc.24335. [DOI] [PubMed] [Google Scholar]
- Sonoda Y, et al. Stimulation of Interleukin-8 production by Okadaic acid and vanadate in a human Promyelocyte cell line, an HL-60 subline: possible role of mitogen-activated protein kinase on the Okadaic acid-induced nf-κb activation. J Biol Chem. 1997;272(24):15366–15372. doi: 10.1074/jbc.272.24.15366. [DOI] [PubMed] [Google Scholar]
- Sorescu D, et al. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105(12):1429. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- Taira T, et al. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004a;5(2):213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T, Iguchi-Ariga SM, Ariga H. Co-localization with DJ-1 is essential for the androgen receptor to exert its transcription activity that has been impaired by androgen antagonists. Biol Pharm Bull. 2004b;27(4):574–577. doi: 10.1248/bpb.27.574. [DOI] [PubMed] [Google Scholar]
- Takahashi K, et al. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor. J Biol Chem. 2001;276(40):37556–37563. doi: 10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- Takahashi-Niki K, et al. Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson’s disease patients. Biochem Biophys Res Commun. 2004;320(2):389–397. doi: 10.1016/j.bbrc.2004.05.187. [DOI] [PubMed] [Google Scholar]
- Takahashi-Niki K, et al. Epidermal growth factor-dependent activation of the extracellular signal-regulated kinase pathway by DJ-1 protein through its direct binding to c-Raf protein. J Biol Chem. 2015;290(29):17838–17847. doi: 10.1074/jbc.M115.666271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura M, et al. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59(3):551–557. [PubMed] [Google Scholar]
- Tang J, et al. PRAK interacts with DJ-1 and prevents oxidative stress-induced cell death. Oxidative Med Cell Longev. 2014;2014:735618. doi: 10.1155/2014/735618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tentolouris C, et al. Endothelial function and proinflammatory cytokines in patients with ischemic heart disease and dilated cardiomyopathy. Int J Cardiol. 2004;94(2):301–305. doi: 10.1016/j.ijcard.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Tillman JE, et al. DJ-1 binds androgen receptor directly and mediates its activity in hormonally treated prostate cancer cells. Cancer Res. 2007;67(10):4630–4637. doi: 10.1158/0008-5472.CAN-06-4556. [DOI] [PubMed] [Google Scholar]
- Tobiume K, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2(3):222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Brug MP, et al. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci U S A. 2008;105(29):10244–10249. doi: 10.1073/pnas.0708518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady G, Sarkadi B, Fatyol K. TTRAP is a novel component of the non-canonical TRAF6-TAK1 TGF-beta signaling pathway. PLoS One. 2011;6(9):e25548. doi: 10.1371/journal.pone.0025548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur S, et al. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci U S A. 2009;106(4):1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur S, et al. Consequences of DJ-1 upregulation following p53 loss and cell transformation. Oncogene. 2012;31(5):664–670. doi: 10.1038/onc.2011.268. [DOI] [PubMed] [Google Scholar]
- Vilotti S, et al. Parkinson’s disease DJ-1 L166P alters rRNA biogenesis by exclusion of TTRAP from the nucleolus and sequestration into cytoplasmic aggregates via TRAF6. PLoS One. 2012;7(4):e35051. doi: 10.1371/journal.pone.0035051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Waak J, et al. Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J Biol Chem. 2009;284(21):14245–14257. doi: 10.1074/jbc.M806902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J Biol Chem. 2009;284(32):21412–21424. doi: 10.1074/jbc.M109.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, et al. DJ-1 modulates the expression of cu/Zn-superoxide dismutase-1 through the Erk1/2-Elk1 pathway in neuroprotection. Ann Neurol. 2011;70(4):591–599. doi: 10.1002/ana.22514. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Parkinson’s Disease-associated DJ-1 mutations increase abnormal phosphorylation of tau protein through Akt/GSK-3beta pathways. J Mol Neurosci. 2013;51(3):911–918. doi: 10.1007/s12031-013-0099-0. [DOI] [PubMed] [Google Scholar]
- West AB, Dawson VL, Dawson TM. To die or grow: Parkinson’s disease and cancer. Trends Neurosci. 2005;28(7):348–352. doi: 10.1016/j.tins.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem Sci. 1998;23(12):481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 2011;15(1):111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KJ, et al. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21(2):220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9(2):140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- Xu P, et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137(1):133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Inactivation of drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102(38):13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, et al. Upregulated DJ-1 promotes renal tubular EMT by suppressing cytoplasmic PTEN expression and Akt activation. J Huazhong Univ Sci Technolog Med Sci. 2011;31(4):469–475. doi: 10.1007/s11596-011-0475-3. [DOI] [PubMed] [Google Scholar]
- Yoon KW, et al. CIB1 Functions as a ca(2+)-sensitive modulator of stress-induced signaling by targeting ASK1. Proc Natl Acad Sci U S A. 2009;106(41):17389–17394. doi: 10.1073/pnas.0812259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K. Scaffold proteins in mammalian MAP kinase cascades. J Biochem. 2004;135(6):657–661. doi: 10.1093/jb/mvh079. [DOI] [PubMed] [Google Scholar]
- Zanassi P, et al. cAMP-dependent protein kinase induces cAMP-response element-binding protein phosphorylation via an intracellular calcium release/ERK-dependent pathway in striatal neurons. J Biol Chem. 2001;276(15):11487–11495. doi: 10.1074/jbc.M007631200. [DOI] [PubMed] [Google Scholar]
- Zhan H, et al. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J Biol Chem. 2010;285(38):29662–29670. doi: 10.1074/jbc.M110.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta (BBA) Mol Cell Res. 2011;1813(11):1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Protective effects of DJ-1 medicated Akt phosphorylation on mitochondrial function are promoted by da-Bu-yin-wan in 1-methyl-4-phenylpyridinium-treated human neuroblastoma SH-SY5Y cells. J Ethnopharmacol. 2016;187:83–93. doi: 10.1016/j.jep.2016.04.029. [DOI] [PubMed] [Google Scholar]
- Zhong H, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60(6):1541–1545. [PubMed] [Google Scholar]
- Zhou B, et al. The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J Biol Chem. 2002;277(35):31818–31825. doi: 10.1074/jbc.M203969200. [DOI] [PubMed] [Google Scholar]
- Zhou J, et al. Crosstalk between MAPK/ERK and PI3K/AKT signal pathways during brain ischemia/reperfusion. ASN Neuro. 2015;7(5):175909141560246. doi: 10.1177/1759091415602463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZM, et al. DJ-1 is involved in the peritoneal metastasis of gastric cancer through activation of the Akt signaling pathway. Oncol Rep. 2014;31(3):1489–1497. doi: 10.3892/or.2013.2961. [DOI] [PubMed] [Google Scholar]
- Zucchelli S, et al. Aggresome-forming TTRAP mediates pro-apoptotic properties of Parkinson’s disease-associated DJ-1 missense mutations. Cell Death Differ. 2009;16(3):428–438. doi: 10.1038/cdd.2008.169. [DOI] [PubMed] [Google Scholar]
- Zucchelli S, et al. TRAF6 promotes atypical ubiquitination of mutant DJ-1 and alpha-synuclein and is localized to Lewy bodies in sporadic Parkinson’s disease brains. Hum Mol Genet. 2010;19(19):3759–3770. doi: 10.1093/hmg/ddq290. [DOI] [PubMed] [Google Scholar]