Abstract
PURA syndrome is a recently described developmental encephalopathy presenting with neonatal hypotonia, feeding difficulties, global developmental delay, severe intellectual disability, and frequent apnea and epilepsy. We describe 18 new individuals with heterozygous sequence variations in PURA. A neuromotor disorder starting with neonatal hyptonia, but ultimately allowing delayed progression to walking, was present in nearly all individuals. Congenital apnea was present in 56% during infancy, but all cases in this cohort resolved during the first year of life. Feeding difficulties were frequently reported, with gastrostomy tube placement required in 28%. Epilepsy was present in 50% of the subjects, including infantile spasms and Lennox-Gastaut syndrome. Skeletal complications were found in 39%. Disorders of gastrointestinal motility and nystagmus were also recurrent features. Autism was diagnosed in one individual, potentially expanding the neurodevelopmental phenotype associated with this syndrome. However we did not find additional PURA sequence variations in a cohort of 120 subjects with autism. We also present the first neuropathologic studies of PURA syndrome, and describe chronic inflammatory changes around the arterioles within the deep white matter. We did not find significant correlations between mutational class and severity, nor between location of the sequence variation in PUR repeat domains. Further studies are required in larger cohorts of subjects with PURA syndrome to clarify these genotype-phenotype associations.
Keywords: PURA, deletion 5q31.3, intellectual disability, epilepsy, congenital apnea
Introduction
PURA syndrome (OMIM #616158) is a recently described disorder characterized by early hypotonia and global developmental delay, with most affected individuals having disability in expressive language development. Many individuals, but not all, have epilepsy and in some cases the seizures may be intractable. Persons affected with PURA haploinsufficiency were first identified with deletions on 5q31.3 [Brown et al., 2013]. Diagnostic whole exome sequencing allowed the subsequent identification of pathogenic sequence variations in the gene [Hunt et al., 2014; Lalani et al., 2014; Tanaka et al., 2015]. While there was early suggestion that sequence variations disrupting the PUR repeat II and PUR repeat III regions may correlate with clinical severity, [Hunt et al., 2014] there is much regarding the phenotypic spectrum associated with PURA syndrome that is not yet understood.
We report 18 new individuals with de novo pathogenic sequence variations in PURA, the largest clinical series described to date. We detail the developmental history, and elaborate on the neurodevelopmental phenotype to include a less severely affected individual with a diagnosis of autism. We also detail the nature and incidence of medical complications, and describe the first neuropathological examination of an individual with PURA syndrome. Finally, we make several recommendations for the medical management of individuals with this disorder.
Methods
Subject ascertainment
All subjects with PURA syndrome were consented through the Genetic Studies of Developmental Brain Disorders. Subjects with autism spectrum disorders were consented through the Autism Taste & Smell or Autism Family Study protocols. The protocols were approved by the University of Rochester Medical Center Research Subjects Review Board.
Whole exome sequencing
Subject DB13-043 and both parents had research whole exome sequencing performed on saliva-derived DNA, using the Agilent Sure-Select 50 Mb whole exome capture kit. Paired end 100 bp reads were generated on an Illumina HiSeq2500 sequencer at the University of Rochester Genomics Research Center. Sequence was aligned to hg19 with BWA v.0.6.2 and analyzed with Picard v.1.84, SAMtools v.0.1.18, and GATK v.2.3-9. Annotation of variants was performed with Annovar, and de-novo, autosomal recessive, and X-linked variants were identified and common variants in dbSNP 137 excluded with SOLVE-Brain v.1.0.1. Common population variants were identified in the NHLBI Exome Variant Server and the Exome Aggregation Consortium data sets. Ensembl’s Variant Effect Predictor provided SIFT and PolyPhen predictions of pathogenicity. Candidate sequence variants were confirmed with Sanger methods. All other subjects had clinical whole exome sequencing performed as part of routine care.
PURA Sanger Sequencing
Subjects (n=120) with autism underwent sequencing of saliva-derived DNA for the single exon of PURA (NM_005859) using Sanger methods. Primer sequences are given in Supplemental Table 1.
Clinical data acquisition
Clinical phenotyping
Birth history, medical history, developmental history, and family history were obtained through standardized phone interviews and/or standardized questionnaires with parents of affected individuals. Medical records, including developmental assessments, were also reviewed. Routine clinical brain magnetic resonance imaging (MRI) studies were reviewed in 12 of the 18 individuals with PURA syndrome.
Autism assessments
Individuals with autism were diagnosed based upon the Autism Diagnostic Observation Schedule (ADOS), and the Autism Diagnostic Interview, Revised (ADI-R).
Neuropathologic studies
Gross and microscopic examination of the formalin-fixed brain of subject DB16-016 was performed using standard methods.
Statistical analyses
All statistical analyses were performed using R version 3.2.0 (cran-project.org). For genotype-phenotype correlations, we established a clinical severity index comprised of the outcomes epilepsy, apnea, G-tube placement, skeletal abnormalities, and nonambulation. A higher index score indicated greater severity (values range 0–5). We assigned a value of “severe outcome” to those scoring ≥3 on the severity index, and then tested for correlations with genotype.
Results
We report a total of 18 new individuals with PURA syndrome, 10 males and 8 females. The mean age at the time of study was 7.4 years, with the youngest individual 11 months and the oldest 27 years. Prior to identification of PURA mutations, subjects had a range of previous diagnoses, including autism, cerebral palsy, “Rett-like disorder”, pervasive developmental disorder, and “likely mitochondrial disease”.
Whole exome sequencing
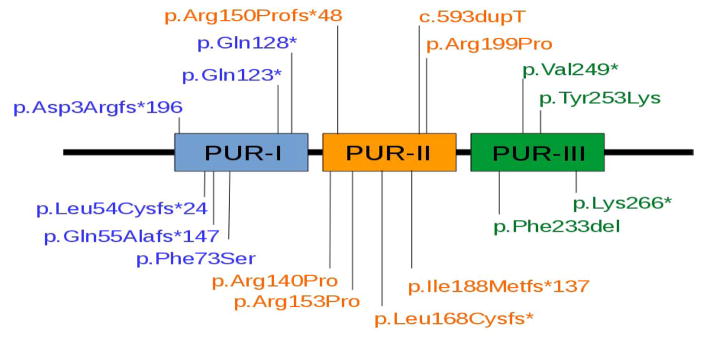
A de novo c.596G>C (p.Arg199Pro) sequence variation in PURA in subject DB13-043 was identified using research whole exome sequencing at the University of Rochester Medical Center. This PURA mutation was previously described, [Lalani et al., 2014] confirming the pathogenicity of this sequence variation. The remainder of the subjects reported here had their sequence variations in PURA identified using clinical whole exome sequencing, illustrating the value of this diagnostic technology [Yang et al., 2014]. The genotype results of the subjects reported in this study are detailed in Table 1. We observed 5 missense, 4 nonsense, 7 frameshift indel, and 2 nonframeshift indel mutations. Subject DB15-021 had both a missense and a nonsense mutation in PURA, but for the purposes of genotype-phenotype correlations the nonsense mutation was regarded as causative. Six mutations were located in the PUR-I domain, seven in the PUR-II domain, and five in the PUR-III domain. The locations of the sequence variations within the PUR domains of PURA are illustrated in Figure 1.
Table 1.
Demographic, genetic, developmental, epilepsy, and MRI findings in 18 individuals with PURA syndrome.
| ID | Sex/Age at study | PURA mutation /amino acid substitution | Developmental history | Epilepsy | Brain MRI | Other neurologic history |
|---|---|---|---|---|---|---|
| DB13-043 | F/5y | c.596G>C p.Arg199Pro | GDD, infantile hypotonia, ambulatory | Yes | Thin white matter, increased XAX | Nystagmus |
| DB15-021 | M/14y | c.683A>G, Asp228Ser c.796A>T p.Lys266* | GDD, infantile hypotonia, ambulatory | Yes, Lennox-Gastaut syndrome | Enlarged lateral ventricles, cyst right massa intermedia | Nystagmus |
| DB15-023 | M/1.5y | c.593dupT | GDD, neonatal hypotonia, not yet sitting up on own | No | ND | Nystagmus |
| DB15-027 | M/4y | c.697_699del p.Phe233del |
Hypotonia, GDD, ambulatory | No | Thin white matter, increased posterior fossa XAX | Cortical visual impairment |
| DB15-030 | M/12y | c.419G>C / p.Arg140Pro | Infantile hypotonia, autism diagnosis at age 11 years, ambulatory | Yes, epilepsy onset at 5 years. Currently treated with levetiracetam. | Normal | Ataxic gait |
| DB15-033 | F/1.5y | c.502del / p.Leu168Cysfs* | Hypotonia, slow feeding at birth | Yes, infantile spasms at 11 months, responded to vigabatrin. Currently on lamotrigine, no further seizures | Thin corpus callosum, immature white matter, right perihippocampal cyst | Nystagmus |
| DB15-035 | F/3y | c.458G>C / p.Arg153Pro | Neonatal hypotonia, ambulatory with frequent falls | No | Underdevelopment of white matter, mildly dilated lateral ventricles | |
| DB15-037 | M/13.5y | c.264delC / p.Ile188Metfs*137 | GDD, infantile hypotonia, ambulatory at age 4 yrs, but more trouble walking over past 2 years | Yes. Generalized tonic-clonic seizures, on levetiracetam, topiramate | Normal | |
| DB15-045 | M/7y | c.218T>C / p.Phe73Ser | GDD, infantile hypotonia, ambulatory at age 4 yrs | No | ND | Exaggerated startle response since newborn |
| DB16-002 | F/15y | c.382C>T / p.Gln128* | GDD, infantile hypotonia, ambulatory | No | Mild cerebellar tonsillar ectopia | Strabismus, exaggerated startle response |
| DB16-003 | F/2.5y | c.745delG / p.Val249* | GDD, infantile hypotonia, not yet ambulatory | No | Mildly dilated lateral ventricles | Nystagmus |
| DB16-009 | M/11 mo | c.759T>G,p.Tyr253Lys | Props in sitting position, infantile hypotonia | No | Normal | |
| DB16-012 | M/8y | c.159_182dup p.Gln55Alafs*147 | GDD, infantile hypotonia, ambulatory at age 4 yrs. Falls frequently. No speech. | Yes, neonatal seizures, then complex partial seizures onset at 6 years. Currently treated with levetiracetam. | ND | Exaggerated startle response since infancy. Some anxiety. |
| DB16-016 | F/27y | c.7_11delGACCG / p.Asp3Argfs*196 | GDD, infantile hypotonia, was independently ambulatory but decline in ability to walk and interact since onset of epilepsy | Yes, onset at age 16 years, multiple seizure types: complex partial seizures, drop seizures, generalized tonic-clonic seizures | Normal | Exaggerated startle |
| DB16-017 | F/4y | chr5:139494213-139494221 GCGCGAGAA > G / p.Arg150Profs*48 | GDD, infantile hypotonia. Ambulatory. | Yes, infantile spasms at 1 year of age. Treated with vigabatrin, then ACTH. | ND | |
| DB16-032 | F/13m | c.697_699delTTC / p.Phe233del | GDD, neonatal hypotonia | Yes, seizures during first 2 weeks after birth | ND | |
| DB16-035 | M/1.8y | c.367C>T / p.Gln123* | GDD, neonatal hypotonia | No | Low central white matter volume | Bilateral axonal motor neuropathy |
| DB16-043 | M/10.6y | c.159delG / p.Leu54Cysfs*24 | GDD, infantile hypotonia, no speech, began walking with support at age 7y | Spells without electrographic correlate | ND | Exaggerated startle response since infancy |
Abbreviations: GDD = global developmental delay; XAX = extra-axial fluid; PDD = pervasive developmental disability; ACTH = adrenocorticotropic hormone; ND = no data
Figure 1.
Location of pathogenic sequence variations in domains of the PURA protein reported in this series.
Neonatal hypotonia leading to consistent neuromotor phenotype
Our subjects nearly universally had neonatal onset hypotonia, often with difficulties tolerating oral feeding in the newborn period resulting in prolonged hospitalizations. Gross motor development in general was delayed, but of the subjects older than 5 years of age at the time of study (n=9) all were ambulatory. Gait was notable in many subjects for increased tone in the lower extremities, ataxia in others, and a diagnosis of “cerebral palsy” was frequently applied. In other published cohorts, motor development was severely impaired in some individuals, with many never attaining independent ambulation [Hunt et al., 2014; Lalani, et al., 2014; Tanaka et al., 2015].
Common language impairment, intellectual disability, and autism spectrum disorder (ASD)
Nearly all subjects in this series had absent verbal language, consistent with previous reports in PURA syndrome [Hunt et al., 2014; Lalani et al., 2014; Tanaka et al., 2015]. One exception was subject DB15-030, a 15 year old boy with a missense PURA p.Arg140Pro sequence variation and higher functioning language skills. This subject received a clinical diagnosis of autism at age 11 years. This individual was ambulatory and had seizures controlled on a single medication, suggesting that some missense PURA sequence variations may be associated with a milder phenotype. Due to this diagnosis of autism, we investigated a cohort of 120 individuals with ASD for variations in PURA. As we did not find any pathogenic sequence variations, it is possible the association of autism with sequence variation in PURA is only coincidental.
Variability of epilepsy
Nine (50%) out of 18 individuals in this series had a diagnosis of epilepsy, with some having recognizable electroclinical syndromes such as infantile spasms (2 subjects) and Lennox-Gastaut syndrome (1 subjects). Several individuals reported previously also had diagnoses of Lennox-Gastaut syndrome or other intractable seizure types [Lalani et al., 2014]. Two new subjects in this report (DB15-033 and DB16-017) had infantile spasms. Subject DB15-033 had onset of spasms at 11 months which responded to vigabatrin. This individual had a p.Leu168CysFS* mutation in the PUR-II domain of PURA. Subject DB16-017 had onset of spasms at 1 year, which initially did not respond to vigabatrin but spasms subsequently stopped and the EEG normalized after two doses of adrenocorticotropic hormone. This individual had a p.Arg150Profs*48 frameshift mutation in the PUR-II domain of PURA. The previously reported individual in the literature with infantile spasms had a p.Phe233del mutation in the PUR-III domain [Hunt et al., 2014].
Two individuals in our series had epilepsy presenting later in childhood, and one individual (DB16-012) had neonatal seizures that remitted, with recurrence of epilepsy at age 6 years. Subject DB16-016 did not have seizure onset until 16 years of age, but her epilepsy became intractable, requiring several anti-seizure medications for treatment and was associated with loss of cognitive and motor skills. Five (28%) individuals in our series had exaggerated startle response to stimuli, but without epileptiform correlate on EEG. This was a finding reported in several previously published individuals, and appears to be a neuromotor behavior recurrent in PURA syndrome. Nystagmus was present in 5 (28%) of our subjects, was horizontal and was associated with poor visual attentiveness. Details of neurodevelopment, epilepsy, and other neurologic symptoms are provided in Table 1.
MRI findings consistent with a generalized disorder of white matter maturation
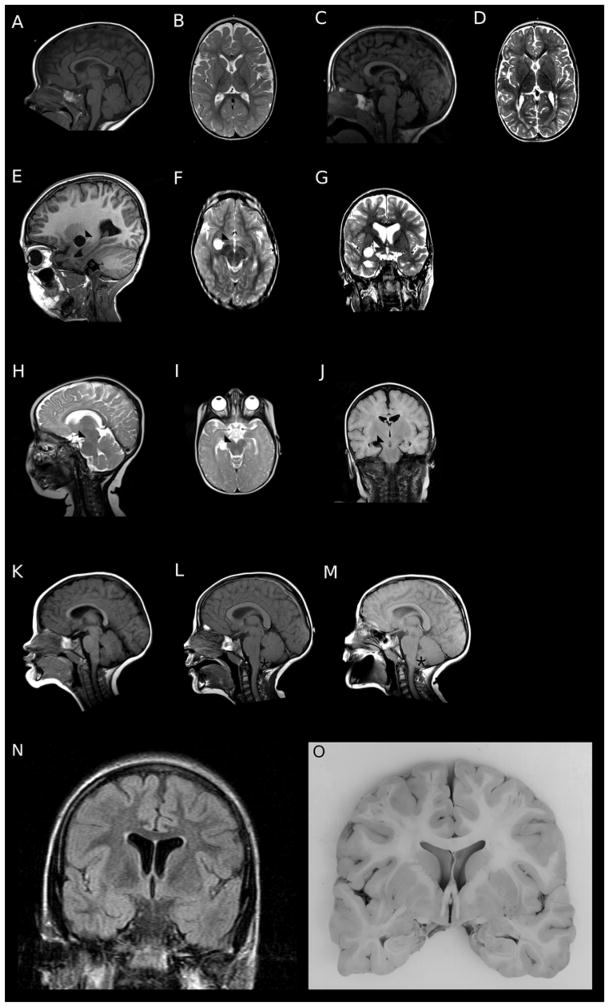
Brain MRIs from 12 of 18 subjects were available for review. No malformations of cortical or subcortical development were observed, consistent with previously published findings. In general, most subjects had reduced volume of the cerebral white matter, with mildly increased extra-axial fluid spaces and mild to moderately dilated lateral ventricles. Two subjects had prominent subarachnoid cysts, and one had progressive cerebellar tonsilar ectopia. The neuroimaging findings are illustrated in Figure 3.
Figure 3.
Representative brain MRI findings in individuals with PURA syndrome. Subject DB13-043, a 5 year old girl with hypotonia and epilepsy, had thinning of the corpus callosum (A) and of the subcortical white matter with increased extra-axial fluid spaces (B). Subject DB15-027, a 4 year old boy with hypotonia and cortical visual impairment, had mildly increased extra-axial fluid in the posterior fossa (C) and thinning of the cerebral white matter (D). Subjects DB15-021 (E–G) and DB15-033 (H–J) had subarachnoid cysts (arrowhead). Subject DB16-002 had thickening of the corpus callosum and cerebellar tonsilar ectopia (asterix) develop over scans at ages 2 years (K), 6 years (L), and 9 years (M). Coronal T2 FLAIR image from subject DB16-016 at age 20 years showing mild dilation of the lateral ventricles and increased signal in the subcortical white matter (N). Gross brain specimen of subject DB16-016 confirming ventricular and white matter findings seen on neuroimaging (O).
Congenital apnea resolving in the first year of life
Congenital apnea was a frequent finding in our cohort, with 10 (56%) individuals with congenital apnea complicating their neonatal course. In most cases, the neonatal central apnea improved by the first year of life. There were no reports of other breathing dysrythmias later in life, and there was no report of pulmonary complications. Several patients developed obstructive apnea later in life. Subject DB16-016 died after a prolonged respiratory illness, but the mortality was attributed more to generalized neurologic decline, and not to a specific disorder of breathing regulation.
Frequent skeletal complications
Skeletal complications occurred in seven (39%) of the individuals in our cohort, with three subjects developing scoliosis, two with hip dysplasia or hip dislocation, and two with osteopenia or osteoporosis. As these complications tended to be diagnosed as individuals grew older, it is possible the true prevalence is higher as ten of our subjects were five years old or younger.
Frequent abnormalities of gastric motility
Six (33%) individuals had gastroesophageal reflux disease, with four requiring fundoplication. Five (28%) individuals had gastric feeding tubes placed, and these were usually placed early in infancy as a consequence of poor neonatal oral feeding. Nine (50%) individuals had constipation, which usually responded to oral medications.
Single incidences of other malformations occurred in our cohort, including one case of cardiac ventricular septal defect not requiring surgery, two occurrences of patent foramen ovale. Other nonrecurrent medical complications included hypothyroidism (n=1), eosinophilic esophagitis (n=1), renal stones (n=1), anemia (n=2), neutropenia (n=1), and short stature (n=1). The occurrences of anemia, neutropenia, and renal stones may have been complications of anti-seizure medications. The medical co-morbidities observed in our cohort with PURA syndrome are summarized in Table 2.
Table 2.
Medical co-morbidities in 18 individuals with PURA syndrome.
| ID | Breathing/Respiratory | Feeding / GI | Skeletal | Other medical history |
|---|---|---|---|---|
| DB13-043 | Neonatal apnea, later sleep apnea | Constipation, GERD, Nissen fundoplication | None | None |
| DB15-021 | None | Constipation | Scoliosis s/p spinal fusion, bilateral femoral osteotomies | None |
| DB15-023 | Tracheostomy, history of apnea as neonate | Constipation, GERD, Nissen fundoplication, G-tube | None | None |
| DB15-027 | None | Poor neonatal feeding | None | None |
| DB15-030 | None | GERD, Nissen fundoplication, G-tube | Low bone density | Hypothyroidism. Eosinophilic esophagitis. Sleep difficulty. Renal stones while on topiramate. Anemia. |
| DB15-033 | None | Slow feeding at birth | None | None |
| DB15-035 | Apnea during RSV infection. Breath-holding spells when younger. | Constipation | Hip dysplasia, mild osteopenia | None |
| DB15-037 | Neonatal apnea, aspiration pneumonia | GERD, Nissen fundoplication, G-tube (as infant, removed at 1 year), constipation | None | Patent foramen ovale |
| DB15-045 | Asthma | Poor neonatal feeding, constipation | Scoliosis | None |
| DB16-002 | Congenital apnea | Poor neonatal feeding, required G-tube at 6 weeks, removed at 7 months of age | Left arm fracture after fall | None |
| DB16-003 | None | Poor neonatal feeding. Now G-tube fed. | None | None |
| DB16-009 | CPAP at birth, then hi-flow 02 for 6 weeks. On 02 at home for 3 weeks. | Poor neonatal feeding, had nasogastric tube until 4 weeks. Constipation. | None | None |
| DB16-012 | None | Poor neonatal feeding, required nasogastric tube first 4 weeks of life | None | None |
| DB16-016 | Sleep apnea | Poor neonatal feeding | Scoliosis, spinal fusion surgery | Neutropenia, anemia |
| DB16-017 | Recurrent apnea first year of life | Poor neonatal feeding | None | None |
| DB16-032 | Central and mixed apneas | G-tube fed since 5 weeks of life, but now with improving oral feeding; Constipation | None | Muscular ventricular septal defect |
| DB16-035 | Central apnea first 8 months of life | GERD, silent aspiration, G-tube fed for 8 months in infancy, constipation | None | PFO |
| DB16-043 | Apneas with bradycardia began at 7 days of life, stopped at 3 months | Poor neonatal feeding, required nasogastric tube. GERD. | Hip surgery at 8y | Short stature. Low body temperature as newborn. |
Abbreviations: GERD = gastroesophageal reflex disease; CPAP = continuous positive airway pressure; RSV = respiratory syncytial virus
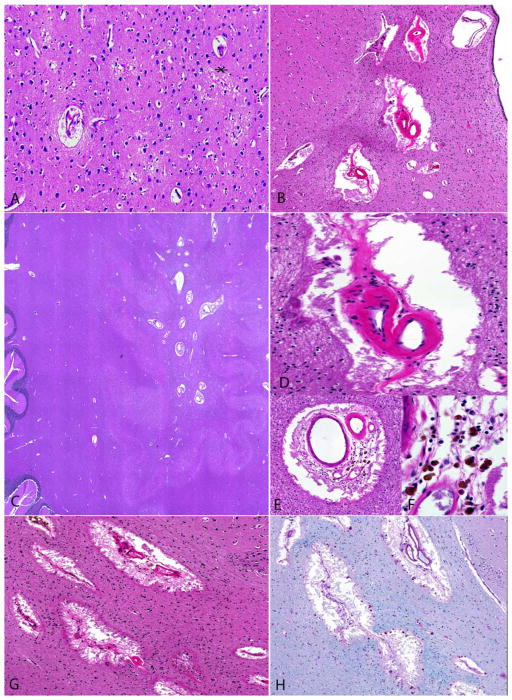
Neuropathologic examination identifies chronic inflammatory changes in the arterioles of the deep white matter
Subject DB16-016 died at the age of 27 years after a prolonged respiratory illness. Gross examination found a normal brain weight, no major malformations, and confirmed mild ventricular enlargement seen on prior neuroimaging (Figure 3N–O). Microscopic examination identified perivascular changes in the arterioles of the deep white matter consistent with chronic inflammation (Figure 4). Trichrome, Congo Red, and Periodic Acid-Schiff stains were normal (Supplementary Figure 1).
Figure 4.
Histologic examination of brain of subject DB16-016 revealed mild vacuolization and reactive gliosis within neuropil of the cerebral cortical (asterix; A). Subcortical deep white matter vessels were notably thickened and hyalinized, with prominent involvement of arterioles in the periventricular regions (B) and cerebellar hemispheres (C). The deep white matter vessels revealed variably high sclerotic indices (D to F), with occasional perivascular lymphocytes, hemosiderin-laden macrophages (E–F) and mild gliosis. Associated white matter rarefaction and perivascular space widening were present (G). The vascular and perivascular features were highlighted on luxol fast blue myelin stain (H).
Recurrent PURA mutations demonstrate clinical variability
We observed several mutations in PURA that were recurrent in our series, and/or were reported previously in the literature. Two individuals in this report (subjects DB15-027 and DB16-032) had the p.Phe233del sequence variation. Subject DB16-032 had seizure onset during the first two weeks of life and central apnea, but the other did not have epilepsy or a history of apnea. Interestingly, a previously reported patient with the p.Phe233del mutation had infantile spasms with progressive epilepsy and central apnea [Hunt et al., 2014]. Another previously reported patient with this sequence variation did not have any seizures or apnea at 6 months of age [Tanaka et al., 2015]. Subject DB15-027 had cortical visual impairment, and both previously reported patients with this sequence variation were noted to have eye abnormalities as well [Hunt et al., 2014; Tanaka et al., 2015].
We identified subject DB13-043 with the missense p.Arg199Pro mutation, which had been reported previously in the literature [Lalani et al., 2014]. While both individuals had intractable epilepsy, the previously reported patient was diagnosed with Lennox-Gastaut syndrome. Both subjects had nystagmus and neither had any skeletal abnormalities.
Genotype-phenotype correlations
We established a clinical severity index comprised of the outcomes epilepsy, apnea, G-tube placement, skeletal abnormalities, and nonambulation after age 5 years. A higher index score indicated greater severity. We then tested for correlations with genotype. When we grouped likely haploinsufficient variations (i.e. nonsense and frameshift indels) and compared to variations that perhaps might not lead to haploinsufficiency (i.e. missense and nonframeshift indels), we did not find significant differences in clinical severity. There was also no significant difference in clinical severity between PUR-I domain sequence variations (mean severity score 1.8), PUR-II domain variations (mean severity score 2), and PUR-III variations (mean severity score 1.4) (Supplementary Figure 2).
Discussion
PURA syndrome is the latest developmental encephalopathy to emerge where symptoms are attributed to pathogenic sequence variations in a transcription factor expressed during early brain development. Other examples include FOXG1 syndrome [Ariani et al., 2008], MEF2C syndrome [Le Meur et al., 2010], Mowat-Wilson syndrome [Mowat et al., 2003], and Pitt-Hopkins syndrome [Amiel et al., 2007]. Like those disorders, early hypotonia, severe language impairment, variable epilepsy severity, and abnormal patterns of movement are common features. We did not find consistent dysmorphologic features in our cohort, but rather observed that the facies in infancy in PURA syndrome is notable for sequelae of hypotonia which improved as the child matured.
Global developmental delay and intellectual disability with an absence of verbal language development are also common themes in developmental encephalopathies caused by haploinsufficiency of transcription factors important during early brain development. However there can be considerable variability, as evidenced by the individual reported here with a missense PURA mutation with verbal language development and a diagnosis of autism. All other patients in our cohort had severe intellectual disability. This observation has been made as well in FOXG1 syndrome, where most affected individuals have severe cognitive impairment, precluding a diagnosis of autism, but only recently have more mildly affected individuals been identified who received a prior diagnosis of autism [McMahon et al., 2015]. We were therefore interested in identifying PURA mutations in our cohort of individuals with autism spectrum disorders. While we were not successful, the presence of an individual in our PURA syndrome cohort with a diagnosis of autism suggests that the neurodevelopmental phenotype associated with this syndrome may be broader than was first suspected. A larger sample size may be needed to test the true prevalence of PURA mutations among individuals diagnosed with autism spectrum disorders.
We observed variability in epilepsy type and severity in our cohort, with half of our subjects having a diagnosis of epilepsy. Some individuals had a diagnosis of electroclinical syndromes (infantile spasms and Lennox-Gastaut syndrome), while others developed seizures later in childhood. In some cases, the seizures were intractable, even though their onset was in the second decade of life. We did not appreciate an overall correlation between sequence variation type or location and epilepsy, but this observation must be treated with caution since the number of patients overall described with PURA syndrome is still small. The variability of both epilepsy type and severity however is typical of this class of developmental brain disorders due to sequence variations in transcription factors.
Brain MRI findings in our cohort were consistent with those previously described [Hunt et al., 2014; Lalani et al., 2014; Tanaka et al., 2015], and included frequent dysmaturation of the cortical white matter. Two of our subjects had arachnoid cysts, and one had the onset of cerebellar tonsillar ectopia. However, we do not hold any of those findings to be clinically significant, and certainly not specific to PURA syndrome.
Congenital and central apnea was present in 55% of our subjects in this cohort. Neonatal apnea was also described previously in infants with PURA syndrome [Hunt et al., 2014; Lalani et al., 2014; Tanaka et al., 2015], indicating this may be a helpful diagnostic feature if present in an infant with severe hypotonia and feeding difficulties. Abnormalities in breathing rhythm are also found in MECP2 syndrome [Ramirez et al., 2013] as well as Pitt-Hopkins syndrome [Zweier et al., 2008], suggesting that the molecular function of PURA may be similarly involved in automatic regulation of breathing by the brainstem. In most of the subjects described in this report, apnea occurred in the neonatal period, could persist throughout infancy, but generally improved over time. We found no correlation between sequence variation type or location and the occurrence of apnea in our subjects.
Skeletal complications were also present in more than a third of the individuals in this series, and ranged from osteoporosis to hip dysplasia and, most frequently, scoliosis. While osteopenia was reported in several individuals treated with anticonvulsant medications, a known complication of prolonged anti-seizure medication [Beerhorst et al., 2013], our data suggest scoliosis is a significant risk for patients with PURA syndrome. This may be a secondary consequence of the severe hypotonia in the early years of the syndrome. Early screening for scoliosis is therefore a reasonable recommendation for children with PURA syndrome.
Disorders of gastrointestinal motility were a frequent complication in this series. Gastroesophageal reflux disease (GERD) were severe enough in some individuals to require fundoplication. Many individuals also had significant difficulty with oral feeding due to hypotonia, and required gastrostomy tube placement. Constipation in most cases required stool softeners and/or motility agents. These motility problems may be related to the hypotonia seen in this syndrome, although this may be caused by primary neurologic dysregulation as well.
The neuropathologic examination of the brain of a single individual with PURA syndrome revealed unexpected inflammatory changes in the arteriolar walls of the deep white matter, suggestive of chronic perivascular disease. This individual had a longstanding history of progressive cognitive decline over the preceding decade, associated with worsening epilepsy. It is possible these perivascular findings are associated with the increasing neurologic impairment seen. As these observations are from a single subject only, it is not possible to be certain these changes are a feature of PURA syndrome individuals.
Although sequence variations in the PUR-II and PUR-III regions may correlate with clinical severity [Hunt et al., 2014], we were not able to confirm this correlation. We would like to emphasize the relatively small size of our series and with the description of further PURA syndrome patients a trend may become clearer in future studies.
In summary, we further expand the clinical phenotype in the largest series of individuals with PURA syndrome published to date. PURA syndrome has several core features, and this diagnosis should be considered in any individual with severe neonatal hypotonia, feeding difficulties, congenital apnea, global developmental delay with significant language impairment, as well as frequent occurrence of epilepsy and skeletal abnormalities. Many patients have hypotonic facies, particularly in infancy and early childhood. Data from the one individual who has undergone neuropathologic examination suggests a tendency for chronic vascular inflammation, although we emphasize this is an isolated observation. These data allow us to make recommendations for the clinical care of individuals with PURA syndrome, summarized in Table 3. The rapid characterization of this disorder, largely through clinically available whole exome sequencing, demonstrates the diagnostic utility of massively parallel sequencing approaches in medical genetics. Further studies to better understand the phenotypes associated with PURA syndrome are indicated.
Table 3.
Recommendations for management of individuals with PURA syndrome.
| Complication (Incidence) | Recommendations for management |
|---|---|
| Hypotonia with motor disorder (All) | Although hypotonia may be severe, some individuals may attain ambulation after the age of 5 years, suggesting efficacy for physical therapy. |
| Perinatal apnea (55%) | Close monitoring and support of respiration, usually in NICU setting. Usually resolves by 12 months of age. Long-term ventilatory support uncommon. |
| Epilepsy (50%) | Confirmation that observed spells are seizures. Routine EEG may be insufficient, may require long-term video monitoring. Non-epileptic spells, including exaggerated startle response, have also been reported. No data available on efficacy of specific anti-seizure medications. |
| Skeletal complications (39%) | Imaging for scoliosis, hip dysplasia, or other complications, as appropriate. Screening of vitamin D, calcium, and phosphate levels as appropriate. Baseline measurement and periodic monitoring appropriate if anti-seizure medicines are prescribed. |
| Gastric motility disorder (GERD 33%; constipation 50%) | GERD may be severe enough to require fundoplication. Many individuals also have difficulties with oral feeding due to hypotonia, and may require gastrostomy tube placement. Constipation may require stool softeners and/or motility agents. |
| Nystagmus (28%) | Referral to pediatric ophthalmologist for assessment and management. |
Supplementary Material
Supplemental Table 1. Primers used for PCR amplification and Sanger sequencing of PURA.
Supplemental Figure 1. Special stains performed on periventricular samples from the brain of subject DB16-016: (a) Trichrome; (b) Congo Red and (C) Periodic Acid-Schiff (PAS). These stains did not identify specific abnormalties.
(A) There was no significant difference in clinical severity between PUR-I domain sequence variations (mean severity score 1.8), (B) PUR-II domain variations (mean severity score 2), and (C) PUR-III variations (mean severity score 1.4).
Figure 2.
Representative facial photographs of individuals with PURA syndrome. Facial photographs of subjects with PURA syndrome. (A) DB13-043; (B) DB15-021; (C) DB15-023; (D) DB15-027; (E) DB15-030; (F) DB15-033; (G) DB15-035; (H) DB15-037; (I) DB16-002; (J) DB16-009; (K) DB16-043. Facial images of subject DB16-016 across her lifespan at age 4 months (L), 4.5 years (M), 18 years (N), and 27 years (O) demonstrate early facial hypotonia in infancy that resolved with maturity. Other individuals (A,C,G) had similar hypotonic facies. Some had downslanting palpebral fissures (A,B) but overall there were no consistent dysmorphic facial features across this cohort of individuals with PURA syndrome.
Acknowledgments
We wish to acknowledge the PURA Syndrome foundation, as well as the families of our research subjects. Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) under award numbers K08NS078054 (to A.R.P.) and K08NS089830 (to RIM), and the National Institute for Deafness and Communications Disorders R01DC009439 (to LB). We would also like to acknowledge the University of Rochester Genomics Research Center for sequencing support, and the University of Rochester Center for Integrated Research Computing for providing high-performance computing resources.
Footnotes
Disclosures
The authors have no conflicts of interest to declare.
References
- Amiel J, Rio M, de Pontual L, Redon R, Malan V, Boddaert N, Plouin P, Carter NP, Lyonnet S, Munnich A, Colleaux L. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am J Hum Genet. 2007;80:988–993. doi: 10.1086/515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariani F, Hayek G, Rondinella D, Artuso R, Mencarelli MA, Spanhol-Rosseto A, Pollazzon M, Buoni S, Spiga O, Ricciardi S, Meloni I, Longo I, Mari F, Broccoli V, Zappella M, Renieri A. FOXG1 is responsible for the congenital variant of Rett syndrome. Am J Hum Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerhorst K, van der Kruijs SJM, Verschuure P, Tan IYF, Aldenkamp AP. Bone disease during chronic antiepileptic drug therapy: general versus specific risk factors. J Neurol Sci. 2013;331:19–25. doi: 10.1016/j.jns.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Brown N, Burgess T, Forbes R, McGillivray G, Kornberg A, Mandelstam S, Stark Z. 5q31.3 Microdeletion syndrome: clinical and molecular characterization of two further cases. Am J Med Genet A. 2013;161:2604–2608. doi: 10.1002/ajmg.a.36108. [DOI] [PubMed] [Google Scholar]
- Hunt D, Leventer RJ, Simons C, Taft R, Swoboda KJ, Gawne-Cain M, Magee AC, Turnpenny PD, Baralle D DDD study. Whole exome sequencing in family trios reveals de novo mutations in PURA as a cause of severe neurodevelopmental delay and learning disability. J Med Genet. 2014;51:806–813. doi: 10.1136/jmedgenet-2014-102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani SR, Zhang J, Schaaf CP, Brown CW, Magoulas P, Tsai ACH, El-Gharbawy A, Wierenga KJ, Bartholomew D, Fong CT, Barbaro-Dieber T, Kukolich MK, Burrage LC, Austin E, Keller K, Pastore M, Fernandez F, Lotze T, Wilfong A, Purcarin G, Zhu W, Craigen WJ, McGuire M, Jain M, Cooney E, Azamian M, Bainbridge MN, Muzny DM, Boerwinkle E, Person RE, Niu Z, Eng CM, Lupski JR, Gibbs RA, Beaudet AL, Yang Y, Want MC, Xia F. Mutations in PURA cause profound neonatal hypotonia, seizures, and encephalopathy in 5q31.3 microdeletion syndrome. Am J Hum Genet. 2014;95:579–583. doi: 10.1016/j.ajhg.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur N, Holder-Espinasse M, Jaillard S, Goldenberg A, Joriot S, Amati-Bonneau P, Guichet A, Barth M, Charollais A, Journel H, Auvin S, Boucher C, Kerckaert JP, David V, Manouvrier-Hanu S, Saugier-Veber P, Frebourg T, Dubourg C, Andrieux J, Bonneau D. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J Med Genet. 2010;47:22–29. doi: 10.1136/jmg.2009.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon KQ, Papandreou A, Ma M, Barry BJ, Mirzaa GM, Dobyns WB, Scott RH, Trump N, Kurian MA, Paciorkowski AR. Familial recurrences of FOXG1-related disorder: Evidence for mosaicism. Am J Med Genet A. 2015 doi: 10.1002/ajmg.a.37353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat DR, Wilson MJ, Goossens M. Mowat-Wilson syndrome. J Med Genet. 2003;40:305–310. doi: 10.1136/jmg.40.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Ward CS, Neul JL. Breathing challenges in Rett syndrome: lessons learned from humans and animal models. Respir Physiol Neurobiol. 2013;189:280–287. doi: 10.1016/j.resp.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka AJ, Bai R, Cho MT, Anyane-Yeboa K, Ahimaz P, Wilson AL, Kendall F, Hay B, Moss T, Nardini M, Bauer M, Retterer K, Juusola J, Chung WK. De novo mutations in PURA are associated with hypotonia and developmental delay. Cold Spring Harb Mol Case Stud. 2015;1:a000356. doi: 10.1101/mcs.a000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Muzny DM, Xia F, Niu Z, Person R, Ding Y, Ward P, Braxton A, Wang M, Buhay C, Veeraraghavan N, Hawes A, Chiang T, Leduc M, Beuten J, Zhang J, He W, Scull J, Willis A, Landsverk M, Craigen WJ, Bekheirnia MR, Stray-Pedersen A, Liu P, Wen S, Alcaraz W, Cui H, Walkiewicz M, Reid J, Bainbridge M, Patel A, Boerwinkle E, Beaudet AL, Lupski JR, Plon SE, Gibbs RA, Eng CM. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier C, Sticht H, Bijlsma EK, Clayton-Smith J, Boonen SE, Fryer A, Greally MT, Hoffmann L, den Hollander NS, Jongmans M, Kant SG, King MD, Lunch SA, McKee S, Midro AT, Park SM, Ricotti V, Tarantino E, Wessels M, Peippo M, Rauch A. Further delineation of Pitt-Hopkins syndrome: phenotypic and genotypic description of 16 novel patients. J Med Genet. 2008;45:738–744. doi: 10.1136/jmg.2008.060129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Primers used for PCR amplification and Sanger sequencing of PURA.
Supplemental Figure 1. Special stains performed on periventricular samples from the brain of subject DB16-016: (a) Trichrome; (b) Congo Red and (C) Periodic Acid-Schiff (PAS). These stains did not identify specific abnormalties.
(A) There was no significant difference in clinical severity between PUR-I domain sequence variations (mean severity score 1.8), (B) PUR-II domain variations (mean severity score 2), and (C) PUR-III variations (mean severity score 1.4).