Abstract
Objective
While pre-exposure prophylaxis with oral tenofovir (TFV) disoproxil fumarate/emtricitabine reduces HIV acquisition rates, poor adherence to and acceptability of daily vaginal gels has led to development of vaginal film formulations to improve adherence and, potentially, enable episodic use.
Study Design
In this two-arm, cross-over study of a fast-dissolving tenofovir film (40 mg) compared to a previously studied semisolid tenofovir 1% gel (40 mg), 10 healthy women received a single vaginal dose of each study product. Clinical, pharmacokinetic, and antiviral assessments were performed over one week post-dose.
Results
Nine of 10 participants experienced mild to moderate adverse effects, similar between products, with no severe adverse events or events attributed to study products. TFV concentrations after film dosing exceeded concentrations after gel dosing in plasma between 8 and 24 hours (p≤0.02). TFV concentrations in cervicovaginal fluid and both TFV and TFV diphosphate concentrations in cervical tissue homogenates were higher following film dosing (all p values < 0.04). The differences ranged from median (interquartile range) 2.9-fold (1.1, 9.0; midvaginal cervicovaginal fluid) to 4.4-fold (2.9, 7.7; plasma). Neither film nor gel demonstrated reduced cervical tissue biopsy infectivity after ex vivo HIV challenge.
Conclusion
Single dose tenofovir film demonstrated consistently higher concentrations in plasma and cervicovaginal samples when compared to gel during the first day following dosing. Single dose cervical tissue TFV-DP concentrations at 5 hours exceeded steady-state concentrations previously reported with daily oral Truvada® dosing. Tenofovir film may provide an alternative to tenofovir oral and gel formulations. Clinical efficacy remains to be tested.
Keywords: HIV, Pre-exposure prophylaxis, Pharmacokinetics, Pharmacodynamics, Tenofovir, Vaginal gel, Vaginal film
INTRODUCTION
Human immunodeficiency virus (HIV) infection remains a global health problem with sexual intercourse being the most common mode of transmission. Pre-exposure prophylaxis (PrEP) with tenofovir (TFV) containing regimens has proven effective in randomized controlled trials using daily oral tenofovir/emtricitabine. While effectiveness is best when product adherence is high 1–5, poor adherence may result in no protection, especially in women6–8. Responding to the negative PrEP impact of poor adherence, alternatives to daily oral dosing have been pursued including sustained release products that provide long-term protection with infrequent dosing and topical products potentially suitable for either episodic or sustained use9–11. Topical PrEP efficacy has been demonstrated with vaginal gel and ring formulations of tenofovir and dapivirine, though these have been only modestly effective2,7,8,12–14. It is hoped that, similar to contraceptive product development where multiple formulation options lead to increased adherence and efficacy across the population, alternative PrEP formulations will boost overall adherence15.
On-demand microbicide products may be preferred for persons at risk of HIV infection who desire PrEP, but struggle with daily oral dosing, prefer to avoid the potential for long-lasting toxicity from the systemic exposure of injectable formulations, or who only have occasional episodic HIV exposure risks not necessarily requiring long-term formulations. One such option in development is fast-dissolving vaginal film formulations of dapivirine and TFV, which, in gel and intravaginal ring formulations, have proven PrEP efficacy2,7,8,12,13,16. Listerine® breath mint strips are one example of fast-dissolving film formulations on the market. Among topical formulations, film may overcome limitations of the vaginal gel which include messiness; frequent leakage from the vagina after application; and product storage and transport complications due to the bulkiness of the applicator 17. By contrast, a vaginal film has: less volume to leak from the vagina or dilute innate endogenous antimicrobial factors in vaginal fluid; much smaller size for discreteness of use and portability; and less packaging to dispose of post-application. Vaginal films, like the nonoxynol 9 contraceptive film, have proven more desirable than other dosage formulations, including gels, tablets, and even vaginal rings18,19,20,21.
Two prior studies have demonstrated the acceptability and pharmacokinetic (PK) equivalence of a fast-dissolving dapivirine vaginal film22,23. In this current study (FAME 05) we evaluated the single-dose, multi-compartment pharmacokinetics (blood, cervical tissue [CT], cervicovaginal fluid [CVF], and rectal fluid [RF]) and pharmacodynamics (PD, ex vivo HIV tissue explant challenge) of a fast-dissolving TFV film compared to the TFV 1% gel formulation used in prior clinical trials. A companion study, FAME 04, involved one week of daily dosing of the same TFV film and gel products with additional safety, immunological, and microbiome assessments24.
MATERIALS AND METHODS
Study design and participants
This was a two-arm, single site randomized crossover study of two TFV formulations, conducted at the Drug Development Unit (DDU) of the Johns Hopkins Hospital in Baltimore, MD. The protocol was approved by the Johns Hopkins Medicine Institutional Review Board (IRB00046617) and registered with Clinicaltrials.gov (NCT02280109). Ten healthy, HIV-negative women between the ages of 18 and 45 years were recruited to participate. All participants provided informed consent prior to screening and study procedures. After a baseline evaluation to determine eligibility, each participant was randomized to one of two sequences of a single vaginal dose of one of two study products, either gel then film or film then gel. The study products were TFV 1% gel (a unit dose equivalent to 40 mg in 4 ml of gel) and tenofovir vaginal film (40 mg). The 2-by-2 inch TFV films were composed of hydroxypropyl methyl cellulose, hydroxyethylcellulose, sodium carboxymethylcellulose, and glycerin. These products are not labeled for HIV prevention by FDA.
At the first study visit after qualification, the investigational product was applied by a gynecologist during a pelvic exam. The TFV gel was applied using a polyethylene vaginal applicator (HTI Plastics, Lincoln, NE). The TFV film was placed, unfolded, in the midvagina during a speculum exam. The participant remained recumbent for approximately 30 minutes after each dose. Participants returned for additional safety and PK sampling visits for the next 12 hours and 24, 48, 72, and 168 hours after dosing. Serial blood samples were collected post-dose for plasma TFV concentration (0, 0.5, 1, 2, 4, 5, 8, 12, 24, 48, 72, and 168 hours) and peripheral blood mononuclear cell (PBMC) TFV diphosphate (TFV-DP) (0, 2, 4, 8, and 24 hours) concentrations. CVF samples from midvagina, external cervical os, and posterior vaginal fornix were collected using a Dacron swab (Cardinal Health, McGraw Park, IL) 5, 72, and 168 hours after dosing. RF was collected at 5, 72, and 168 hours after dosing using an anoscope and Dacron swab. At 5 and 72 hours after dosing, a pair of CT biopsies for PK and PD readouts were collected using Tischler forceps.
Participants were seen on day 14 for a safety evaluation. The second product was dosed during a similar point in a later menstrual cycle (avoiding menses) followed by the same sampling schedule. A final set of cervical biopsies was collected at least 12 weeks before or after the second product dosing as negative control for the explant HIV challenge.
Clinical assessment
Participants were assessed for adverse events (AEs) at each study visit. AEs were graded based on the Division of AIDS (DAIDS) Table for Grading Adult and Pediatric Adverse Events, Version 1.0,(December 2004, Clarification dated August 2009) and the Female Genital Grading Table for Use in Microbicide Studies (Appendix 1 to DAIDS Table for Grading Adult and Pediatric Adverse Events, Version 1.0, December 2004, Clarification dated August 2009).
Pharmacokinetic sample analysis
TFV in plasma, CVF, RF, and CT biopsy homogenate as well as TFV-diphosphate (TFV-DP) concentrations in PBMC and CT homogenate were measured using ultra-performance liquid chromatographic-tandem mass spectrometry (LC-MS/MS) that have been previously described25,26. These methods were validated according to FDA Bioanalytical Method Validation Guidance27. The lower limits of quantification (LLOQs) for these assays are: plasma TFV 0.31 ng/mL, PBMC TFV-DP 50 fmol/sample or median 2 fmol/106 cells (based on number of cells analyzed), CVF TFV 0.625 ng/swab or median 0.005 ng/mg (based on swab weights), RF TFV 0.625 ng/swab or median 0.2 ng/mg (based on swab weights), CT TFV 0.05 ng/sample or median 0.003 ng/mg (based on biopsy weights), CT TFV-DP 50 fmol/sample or median 3 fmol/mg (based on biopsy weights).
Pharmacodynamic ex vivo HIV explant challenge
As previously described, two CT biopsies were briefly exposed to HIV-1 BaL in the laboratory, and HIV infection was measured over the culture period by HIV-1 p24 ELISA assay (Alliance, Perkin Elmer) of culture supernatant (4, 7, 10, and 14 days after inoculation)28,29. The cumulative p24 antigen was calculated from the sum of all 4 p24 antigen supernatant concentrations for each biopsy, then divided by the original biopsy weight. The unit of analysis was this weight-adjusted cumulative p24 antigen averaged for each pair of biopsies which were taken at each scheduled biopsy time..
Data Analysis
Concentration-time data and PK-PD relationships were visually examined (SigmaPlot, version 13, Systat Software, San Jose, CA). Non-compartmental analysis of concentration data estimated PK parameters including peak concentration (Cmax), time to peak concentration (Tmax), area under the concentration-time curve to last sample (AUClast), and time to last concentration (Tlast) (Phoenix® WinNonlin® version 6.4, Certara USA, Inc., Princeton, NJ). Readouts were summarized using non-parametric descriptive statistics (median, interquartile range), the Friedman test for comparisons among readouts, the Wilcoxon rank sum test for paired comparisons, and the Spearman test for correlations between matrix concentrations (IBM SPSS Statistics v. 24, Armonk, NY). P values less than 0.05 were considered statistically significant. A PK-PD relationship was first assessed with linear regression of log transformed TFV and TFV-DP concentrations in plasma, CT and CVF (each individually as single independent variables) with log-transformed cumulative p24 concentrations (dependent variable) (IBM SPSS). Iterative model fitting using an Emax model (2 to 4 parameter Hill equation) with log-transformed PK and PD was performed to assess PK-PD relationships (Phoenix WinNonlin).
RESULTS
Subjects
Ten women enrolled in the study, ranging in age from 22 to 45 years (median 33.5). Five women self-identified as African American, four as white (one as Hispanic/Latina), and one as Asian. All participants completed all study visits and evaluations.
Adverse events
Of 22 adverse events captured during the study, none were serious nor related to study product (Table 1). All resolved by the end of follow up. Fourteen adverse events occurred after gel dosing, while 7 occurred after film dosing (p=0.20); one participant experienced an adverse event in the washout period between film and gel dosing.
TABLE 1.
Adverse Events
| DAIDS Category | Film | Gel |
|---|---|---|
| Systemic | 2, nasal congestion, fatigue | 1, upper respiratory infection |
| Chemistry | 0 | 4, hyperglycemia (non-fasting) (N=3), elevated AST |
| Hematology | 3, anemia (2 Gr 2) | 2, anemia |
| Urinalysis | 0 | 1, hematuria |
| Gastrointestinal | 1, constipation | 3, nausea, heartburn, diarrhea (N=2) |
| Neurologic | 1, headache | 2, headache 2 |
| Gynecological Pain | 0 | 1, dysmenorrhea |
Nine of 10 research participants reported total 22 adverse events, 21 during dosing period which are indicated. None were attributed to study product. No Grade 3 or 4 events were reported. All grade 1, except hematology (3 Grade 2 in parentheses). No differences in adverse event number when comparing Film to Gel (Wilcoxon, P=0.20). Initial number indicates number of participants experiencing the adverse event; if multiple symptoms are included in a category, the number of participants affected by a particular symptom is indicated (unless only one participant was affected)
Pharmacokinetics
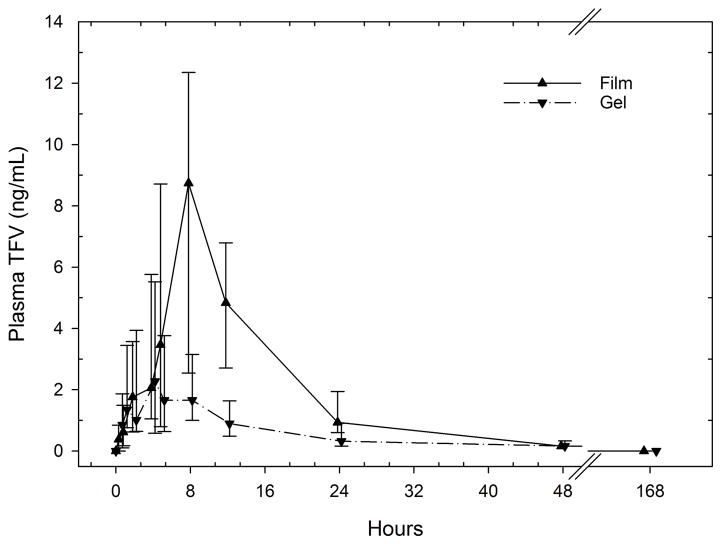
Following dosing of each product, plasma TFV concentrations reached a peak between 1 and 12 hours, then fell to undetectable concentrations (<0.31 ng/mL) by 48 hours in all but one participant (film 0.39 ng/mL and gel 0.51 ng/mL) (Figure 1). Tmax occurred later after film dosing, median (IQR) 8 hours (8, 8), when compared to gel dosing, 4 (2, 8) (p=0.02). TFV Cmax was higher in 9 of 10 participants after film dosing, when compared to gel dosing(Table 2, p=0.08). Plasma TFV concentration vs. time curves were similar before 8 hours, but separated (statistically significant) between 8 and 24 hours after dosing, during which time concentrations following film dosing continued to rise and were greater by 3.1- to 4.4-fold when compared to individually-paired gel dosing (p<0.02). Overall, plasma TFV non-compartmental Cmax and AUClast were higher after film than gel dosing (Table 2). Finally, Tlast was later after film dosing, 24 hours (24, 24), when compared to gel dosing, 18 hours (12, 24) (p=0.06). PBMC TFV-DP concentrations were below limits of assay quantification in all samples tested.
Figure 1.
Plasma TFV vs. time by product (median [IQR]). Nominal sampling times offset for clarity.
TABLE 2.
Pharmacokinetic parameter estimates by matrix, analytes, and product (median [IQR]).
| Matrix-Analyte | Cmax | AUClast | ||||||
|---|---|---|---|---|---|---|---|---|
| Film | Gel | Film/Gel Ratio | p value | Film | Gel | Film/Gel Ratio | p value | |
| Plasma TFV | 10.4 (3.9, 13.6) | 2.9 (1.5, 5.5) | 2.5 (1.8, 3.5) | a0.084 | 113 (56, 163) | 32 (13, 56) | 3 (2.6, 3.9) | 0.084 |
| CVF Exocervix TFV | 1,588 (631, 3,342) | 1,056 (461, 1,986) | 1.3 (0.8, 2.8) | . | 57,683 (23,631, 122,052) | 38,181 (16,872, 71,792) | 1.3 (0.9, 2.8) | . |
| CVF Fornix TFV | 3,351 (1,872, 5,287) | 1,186 (935, 2,250) | 3.1 (1.4, 5.6) | 0.084 | 122,474 (67,857, 202,087) | 43,488 (36,109, 83,017) | 3.1 (1.4, 5.2) | . |
| CVF Mid-vaginal TFV | 2,186 (1,140, 5,813) | 1,109 (532, 1,789) | 2.9 (1.1, 9) | 0.014 | 79,354 (42,185, 213,176) | 40,259 (20,027, 64,548) | 2.9 (1.1, 8.9) | 0.014 |
| CT TFV | 28 (7, 52) | 8.7 (5.9, 14.0) | 2.2 (0.9, 7) | 0.037 | 1,035 (263, 1,896) | 285 (187, 510) | 4 (1.2, 8.6) | 0.014 |
| CT TFV-DP | 160 (27, 485) | 40 (21, 93) | 3.6 (0.4, 15.1) | 0.049 | 6,637 (1,043, 19,936) | 1,509 (596, 2,521) | 3.8 (2.2, 10) | 0.014 |
| RF TFV | 0.50 (0.09, 1.85) | 2.65 (1.27, 30.20) | 0.08 (0.03, 0.33) | 0.004 | 4.7 (2.9, 49.6) | 90 (31, 522) | 0.08 (0.02, 0.54) | 0.098 |
BLQ, below lower limits of assay quantitation
Cmax units: TFV, plasma ng/mL, CVF ng/mg, RF ng/mg, CT ng/mg; TFV-DP fmol/mg
AUC units: TFV, plasma ng-hr/mL, CVF ng-hr/mg, RF ng-hr/mg, CT ng-hr/mg; TFV-DP fmol-hr/mg
P values greater than 0.1 are not shown.
Comparison of plasma concentrations at 8, 12, and 24 hours are greater with film compared to gel (p<0.02)
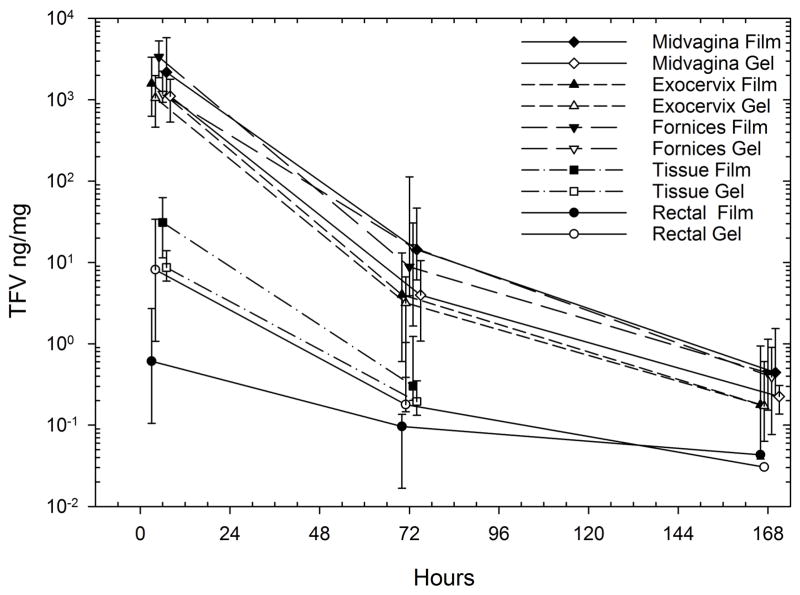
CVF TFV concentrations sampled from all 3 intra-vaginal sites declined from 5 hours to 168 hours after dosing, but remained detectable throughout the sampling interval (Figure 2). Only 1 of 10 participants had any CVF TFV concentration that was below the limits of assay quantitation (BLQ) 168 hours after dosing (exocervix, gel dosing). Mid-vagina CVF TFV Cmax and AUC (both p=0.014), as well as concentrations at all 3 sample times, were significantly higher after film compared to gel dosing (p≤0.02) (Table 2). The differences ranged from 3.1-fold (2.0, 3.8) higher at 168 hours to 4.4-fold (2.9, 7.7) higher at 24 hours. Forniceal CVF TFV trended toward higher concentrations after film than gel at 5 hours (p=0.08), but none of the exocervical CVF TFV concentrations differed between study products. When comparing among CVF sampling sites at any given time for the same product, forniceal samples were higher than exocervical samples, with a Cmax and AUClast forniceal:exocervical ratio of 2.0 (1.2, 6.9) and 2.0 (1.2, 6.8), respectively (both p≤0.01). Variability of samples (indicated by the range of quartiles) was also greater for film compared to gel. CVF concentrations between sites were highly correlated (rho>0.94, p<0.001)
Figure 2.
Cervicovaginal and rectal fluid, vaginal tissue homogenate TFV concentrations vs. time by product (median [IQR]). Nominal sampling time offset for clarity.
TFV and TFV-DP concentrations in CT homogenates were also higher 5 hours after film dosing, 28 ng/mg (7, 52) and 160 fmol/mg (27, 485), respectively, when compared to gel dosing (both p<0.05). This resulted in paired film:gel ratios of 3.0 (1.1, 7.3) for TFV and 3.7 (2.0 17.8) for TFV-DP (p≤0.04). Concentrations were not different 72 hours after dosing.
Using molar concentrations of TFV and TFV-DP, the combined (film and gel) TFV-to-TFV-DP ratio in CT was 532 (332, 999) and not different between products. At 5 hours after dosing when all matrices were available and detectable, combined (film and gel) CVF tenofovir concentrations were 2 log10 and 5 log10 greater than tissue and plasma concentrations, respectively (p<0.001). CT TFV-DP correlated modestly with plasma TFV concentrations (rho=0.522, p<0.001) and correlated highly with both CVF (rho≥0.818, p<0.001) and CT (rho=0.92, p<0.001) concentrations.
Unlike TFV concentration and time differences (film greater than gel) at all other anatomic sites, RF TFV concentrations 5 hours after gel dosing were 12-fold (3, 39) greater than after film dosing (p=0.004). The RF concentration distribution 5 hours after gel, 2.5 ng/mg (0.5, 26), overlapped the 5 hour CT homogenate TFV concentration for both film, 31 ng/mg (11, 63), and gel, 9 ng/mg (6, 17). RF Tmax values were more common at 5 hours, but higher concentrations were seen 72 hours post-dose in 4 participants after film and one participant after gel. RF concentrations correlated least well with all other matrices (rho <0.44, p>0.01).
Pharmacodynamics (HIV-1 p24 measurement)
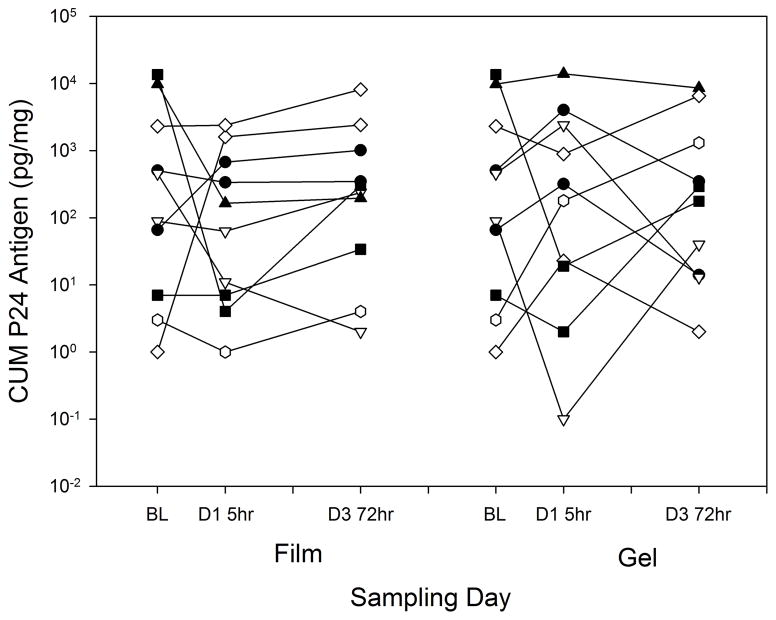
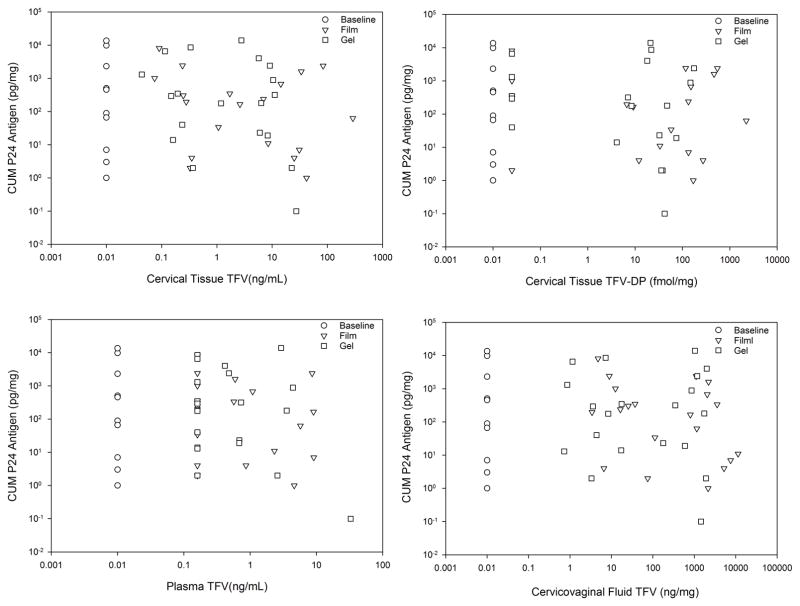
Cumulative p24 antigen in the CT ex vivo HIV challenge assay was not different among values for baseline and both times (5 and 72 hours) for both film and gel formulations (p=0.4) (Figure 3). Pairwise testing showed statistically significant differences only for the film formulation which increased between the 5 and 72 hour sampling times (p<0.01). Linear regression of cumulative P24 against drug concentrations (pooling concentrations for both film and gel dosing within each anatomic matrix) indicated no statistically significant PK-PD relationships (Figure 4). Excluding baselines, any imputed values, or both did not result in any statistically significant regression slopes. Similarly, sigmoid Emax PK-PD modelling was not successful.
Figure 3.
Explant ex vivo HIV challenge over time by product. Control (no drug) biopsy is indicated as “BL” (baseline), for comparison, even though the biopsy may have followed the study product dosing by 12 weeks.
Figure 4.
Cumulative P24 Antigen v. TFV Concentration across all matrices by product. Regression lines are not shown since no linear regression fitting demonstrated statistically significant slopes (none different from slope=0). Baseline values arbitrarily imputed as 0.01 units in each panel. Concentration values below the lower limit of assay quantitation (LLOQ) calculated for each individual sample are imputed as LLOQ/2 for the median calculated LLOQ.
DISCUSSION
We demonstrated the feasibility of a single dose TFV fast-dissolving film (40 mg) to achieve concentrations of TFV in plasma, CVF, and activated TFV-DP in CT which exceed those after a single dose of TFV 1% gel during the day of dosing; both film and gel had similar concentrations 3 and 7 days later. Supported by the acceptability of the film established in the companion study, FAME 04, and the theoretical adherence advantages of films over other products, we believe this study helps to advance TFV film as a potentially viable product for extended safety and efficacy testing as a topical microbicide.
In contrast to FAME 04, a 7 dose study of the same film and gel, we report greater concentrations in all matrices (at some time points) after film compared to gel dosing. Temporally richer FAME 05 sampling (2, 4, 5, 8, and 12 hours) could detect the later peaking and higher 8–24 hour plasma TFV differences with film, which indirectly indicate differences in CVF and tissue TFV. Complementarily, FAME 04 (with pre-dose and 2 hour post-dose sampling) was more analytically rich with readouts beyond PK and larger sample size. The other study difference was that the film was folded in half prior to dosing in FAME 04, whereas in FAME 05, the film was not folded. While folding may ease self-administration, it can reduce the dissolution rate of the film in vitro; though the effect of folding on clinical dissolution has not been evaluated (authors unpublished data).
In addition, our FAME 05 concentrations in all matrices were below those in FAME 04. For example, our CT TFV-DP was 40 fmol/mg (20, 93) and 169 fmol/mg (84, 506) 5 hours after gel and film dosing, respectively, whereas, FAME 04 reported 222 fmol/mg (71, 556) and 937 fmol/mg (56, 1456) 2 hours after dosing gel and film, respectively. Accumulation of drug with the 7 daily doses in FAME 04 compared to the single dose in FAME 05 explains this difference. Vaginal tissue concentrations rise even higher with longer daily dosing – 2,000 fmol/mg (4 fmol/0.2 μL) after 2 weeks reported by Schwartz, et al., and 1,807 fmol/mg (591, 5860) after 6 weeks in MTN-001, both of which studies dosed the same TFV 1% gel daily30,31. Together, these studies indicate tissue accumulation continues through 2 weeks after which steady-state is achieved. Continuing accumulation of tissue TFV-DP for weeks of dosing is expected based on every 24 hour dosing and a longer cervicovaginal tissue TFV-DP half-life, 53 hours (45, 68); accordingly, steady-state (6 half-lives) would require (IQR) 11 days to 17 days, consistent with the similarity in 2-week Schwartz, et al. and 6 week MTN-001 reports30–32.
Five hours after a single TFV film dose, CT TFV-DP concentrations easily exceeded vaginal tissue TFV-DP concentrations associated with daily oral TDF dosing (estimated from combined MTN-001 and HPTN 066, 23 fmol/mg [17, 25]) which takes weeks to achieve after commencing daily oral dosing31,33. The CVF concentrations far exceeded CVF concentrations (>1,000 ng/mL or ~1 ng/mg) reported in CAPRISA 004 to be associated with 75% relative risk reduction in a post hoc analysis14. Finally, a single dose of the TFV film achieved similar CVF TFV concentrations achieved with single and multiple doses of TFV 1% vaginal gel in several prior studies, including in the subset of CAPRISA 004 women who dosed within one day of a CVF sampling visit14,30,31. Given these favorable concentration comparisons and the high levels of HIV protection achievable when adherence is high for both oral and topical dosing, this evidence recommends advancing the film formulation for further clinical development.
Development of the TFV film for use as a single episodic dose prior to HIV exposure might be considered, but depends on several key unknowns, namely, whether cervicovaginal tissue concentration of active drug is the single critical variable associated with PrEP efficacy, whether tissue concentrations associated with oral dosing are appropriate concentration targets, and how long the concentration needs to be maintained.
Protective tissue TFV-DP concentrations may be different with oral dosing compared to topical dosing, but no quantitative assessment based on clinical data is available to quantify the difference if it exists. This potential difference may be due to the addition of FTC to oral TDF dosing in the fixed dose combination; however, Partners PrEP failed to demonstrate a statistically significant difference between oral TDF alone and oral TDF/FTC in the only study to directly address this. Other variables (e.g., undetermined frequency of anal sex, lack of systemic concentrations as back-up to topical dosing) have been suggested to explain less than anticipated vaginal antiretroviral protection, but none of these explanations have been proven contributory, much less are there quantitative estimates of their relative contributions, if any.
The evidence for the association between cervicovaginal fluid or tissue concentration and PrEP efficacy relies on post hoc analyses. In an analysis of seroconversion rates across the six primary daily dosing PrEP efficacy randomized clinical trials, using oral vs. vaginal tissue TFV-DP concentration-based differences provided a much tighter sigmoid Emax PK-PD model fit than systemic drug concentrations alone1,3–7,34. Possibly arguing against this, adherence adjustments in three TFV vaginal gel trials (CAPRISA 004, FACTS 001, VOICE) all demonstrated improved 60–75% proteection in post hoc analyses2,7,8,12,14, despite estimates of higher tissue TFV-DP concentrations compared to oral dosing (discussed above). Further, these PK-guided adherence adjustments were dichotomous adjustments into adherent and poorly adherent cohorts and lacked quantitative adherence benchmarks (similar to STRAND and HPTN 066 for oral dosing) to fairly judge efficacy in highly adherent women33,35. Finally, it remains unclear how long protective concentrations must be sustained after HIV exposure. While film achieves tissue TFV-DP concentrations in 5 hours that are 7-fold greater than estimated steady-state tissue concentrations associated with 90% protection in Partners PrEP (see below), the tissue TFV-DP concentration falls to 40% of the clinical tissue IC90 by 72 hours after the single dose and may need additional doses for protection in the episodic dosing setting.
In a post hoc analysis of CAPRISA 004, CVF concentrations of >100 ng/mL and >1,000 ng/mL were associated with 65% and 76% protection, respectively, thus, demonstrating a concentration-response. Using these CVF concentrations as efficacy targets, however, is challenging because of the episodic dosing of gel in CAPRISA 004 wherein CVF samples were collected days or weeks after the dose resulting in concentrations at the time of sampling that were 1,000 to 10,000 times lower than the likely concentration at the time of the HIV exposure (~100,000 ng/mL or ~1,000 ng/mg)14. Despite uncertainty as to the CVF or tissue concentration target providing protection at the time of exposure, that the film achieves concentrations exceeding those of the gel and post hoc analyses indicating gel efficacy at least as high as 76% with episodic use, supports further development of the film.
Due to the absence of clinical trial data to clearly indicate the TFV-DP concentration, anatomic site, and duration best predicting efficacy, viral challenge in animal models and ex vivo human tissue explants have been used as surrogates. Macaque models repeatedly demonstrate protection from SHIV and SIV vaginal challenge with prior TFV dosing36–40. We used the explant challenge in FAME 05, but failed to demonstrate an antiviral effect. By contrast, FAME 04 demonstrated an antiviral effect with CT TFV-DP IC90 of 813 fmol/mg. The difference in explant results may largely be explained by the fact that all, but one, of the single dose FAME 05 CT concentrations fell well below the explant IC90 in multiple dose FAME 04. Accordingly, there were too few high concentrations to generate a statistically significant downward slope.
As with selecting tissue concentration targets, interpreting explant challenge model IC90 results doesn’t map directly to a clinical IC90. Consider, in MTN-001, participants demonstrated high adherence to prescribed daily TDF (indicated by median pre-dose TFV serum concentration of 65 ng/mL), yet the vaginal tissue homogenate TFV-DP concentrations were below 25 fmol/mg31. With similar pre-dose serum TFV concentrations in one high adherence Partners PrEP cohort, >40 ng/mL serum TFV, the tissue TFV-DP concentrations are very likely similar41. This high adherence Partners PrEP subgroup had relative risk reduction of 89% for TDF only and 91% for TDF/FTC, approximating a clinical IC9041. [This assumes no sex differences in efficacy that Partners PrEP wasn’t powered to detect.] By comparison, the explant IC90 reported in FAME 04 is at least 1.5 log10 greater than the clinical IC90 from Partners estimated above. Similarly, the colon tissue explant IC90, 10,233 fmol/mg (RMP-02/MTN-006), is 1.7 to 2.5 log10 greater than the clinical IC90 for colon tissue TFV-DP estimated in iPrEx1,35,42,43. [The iPrEx clinical IC90 is based on 90% efficacy associated with 2 to 4 doses per week based on the STRAND study; in HPTN 066, 2 to 4 doses per week achieved colon tissue homogenate TFV-DP concentrations from 27 to 186 fmol/mg1,33,35.] Accordingly, both the cervicovaginal and colon explant challenge models appear too stringent and need recalibration to estimate the likelihood of clinical protection. In addition, as discussed above, whether the calibration for oral TDF (often complicated by concomitant dosing with emtricitabine) is relevant for topical TFV remains to be demonstrated and will require an efficacy trial.
Concluding, we demonstrated that a single dose of TFV 40 mg film achieved higher and more sustained concentrations in plasma, CVF, and CT compared to TFV 1% (40 mg) gel. Further, CT TFV-DP and CVF TFV concentrations exceed concentrations associated with high levels of protection with oral and vaginal dosing, respectively.. With its single dose advantages compared to TFV gel, and several theoretical advantages of films over gels which may favorably impact adherence, the film formulation remains a promising vaginal microbicide candidate as an alternative to oral PrEP. Longer duration safety and efficacy studies are needed to establish the anticipated adherence advantage and, possibly, superior efficacy of the TFV film formulation relative to gel or oral PrEP.
Acknowledgments
The authors thank the participants who volunteered for this study. The authors also thank the staff of the Johns Hopkins University Drug Development Unit, Clinical Pharmacology Analytical Laboratory and Charlene Dezzutti, PhD, University of Pittsburgh and Magee-Womens Research Institute. This study was funded, in part, by the National Institutes of Health, Division of AIDS, Integrated Pre-Clinical/Clinical Program for HIV Topical Microbicides (U19 AI082639) and the Clinical Pharmacology Training Program grant (T32GM066691). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIAID. This project has also been funded, in part, with federal funds from the National Institute of Allergies and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272200800014C.
Footnotes
Conflicts of Interest:
CWH has served on the scientific advisory board for and is currently receiving research funding from ViiV/GSK managed through Johns Hopkins. For the remaining authors none were declared.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, et al. Preexposure Prophylaxis for HIV Infection among African Women. The New England journal of medicine. 2012 doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rees H, Delany-Moretlwe SA, Lombard C, et al. FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women [Abstract 29LB]. Presented at: 22nd Conference on Retroviruses and Opportunistic Infections; 2015; Seattle. [Google Scholar]
- 9.Minnis AM, Gandham S, Richardson BA, et al. Adherence and acceptability in MTN 001: a randomized cross-over trial of daily oral and topical tenofovir for HIV prevention in women. AIDS and behavior. 2013;17(2):737–747. doi: 10.1007/s10461-012-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts ST, Heffron R, Ngure K, et al. Preferences for daily or intermittent pre-exposure prophylaxis regimens and ability to anticipate sex among HIV uninfected members of Kenyan HIV serodiscordant couples. AIDS and behavior. 2014;18(9):1701–1711. doi: 10.1007/s10461-014-0804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mack N, Evens EM, Tolley EE, et al. The importance of choice in the rollout of ARV-based prevention to user groups in Kenya and South Africa: a qualitative study. Journal of the International AIDS Society. 2014;17(3 Suppl 2):19157. doi: 10.7448/IAS.17.3.19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai JY, Hendrix CW, Richardson BA, et al. Pharmacological Measures of Adherence and Risk of HIV Acquisition in the VOICE Study. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. The New England journal of medicine. 2016;375(22):2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashuba AD, Gengiah TN, Werner L, et al. Genital Tenofovir Concentrations Correlate With Protection Against HIV Infection in the CAPRISA 004 Trial: Importance of Adherence for Microbicide Effectiveness. J Acquir Immune Defic Syndr. 2015;69(3):264–269. doi: 10.1097/QAI.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Preventing Unintended Pregnancy: The Contraceptive CHOICE Project in Review. J Womens Health (Larchmt) 2015;24(5):349–353. doi: 10.1089/jwh.2015.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nel A, Bekker LG, Bukusi E, et al. Safety, Acceptability and Adherence of Dapivirine Vaginal Ring in a Microbicide Clinical Trial Conducted in Multiple Countries in Sub-Saharan Africa. PloS one. 2016;11(3):e0147743. doi: 10.1371/journal.pone.0147743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PloS one. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coggins C, Elias CJ, Atisook R, et al. Women’s preferences regarding the formulation of over-the-counter vaginal spermicides. AIDS. 1998;12(11):1389–1391. doi: 10.1097/00002030-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Raymond E, Alvarado G, Ledesma L, et al. Acceptability of two spermicides in five countries. Contraception. 1999;60(1):45–50. doi: 10.1016/s0010-7824(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 20.Nel AM, Mitchnick LB, Risha P, Muungo LT, Norick PM. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. J Womens Health (Larchmt) 2011;20(8):1207–1214. doi: 10.1089/jwh.2010.2476. [DOI] [PubMed] [Google Scholar]
- 21.El-Sahn M, Lucas J, Aikenheada M, Nemadeb R, Van Damme L. Understanding the Potential for Multipurpose Prevention of Pregnancy and HIV: Results from surveys assessing four hypothetical concept profiles of Multipurpose Prevention Technologies (MPTs) in Uganda, Nigeria and South Africa. Bill & Melinda Gates Foundation; Jun 6, 2016. [Google Scholar]
- 22.Bunge KE, Dezzutti CS, Rohan LC, et al. A Phase 1 trial to assess the safety, acceptability, pharmacokinetics and pharmacodynamics of a novel dapivirine vaginal film. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson JA, Marzinke MA, Bakshi RP, et al. Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B) AIDS research and human retroviruses. 2017;33(4):339–346. doi: 10.1089/aid.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunge KE, CSD, Hendrix CW, et al. Phase I Trial to Assess Safety, PK, and PD of Film and Gel Formulations of Tenofovir [Abstract 871]. Presented at: 23rd Conference on Retroviruses and Opportunistic Infections; 2016; Boston. [Google Scholar]
- 25.King T, Bushman L, Kiser J, et al. Liquid chromatography-tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;843(2):147–156. doi: 10.1016/j.jchromb.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 26.Keller MJ, Madan RP, Torres NM, et al. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PloS one. 2011;6(1):e16475. doi: 10.1371/journal.pone.0016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Center forDrug Evaluation and Research, Center for Verterinary Medicine. FDA. Guidance for Industry: Bioanalytical Method Validation. Rockville; 2001. [Google Scholar]
- 28.Abner SR, Guenthner PC, Guarner J, et al. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. The Journal of infectious diseases. 2005;192(9):1545–1556. doi: 10.1086/462424. [DOI] [PubMed] [Google Scholar]
- 29.Cummins JE, Jr, Guarner J, Flowers L, et al. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrobial agents and chemotherapy. 2007;51(5):1770–1779. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz JL, Rountree W, Kashuba AD, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PloS one. 2011;6(10):e25974. doi: 10.1371/journal.pone.0025974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PloS one. 2013;8(1):e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louissaint NA, Cao YJ, Skipper PL, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS research and human retroviruses. 2013;29(11):1443–1450. doi: 10.1089/aid.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendrix CW, Andrade A, Bumpus NN, et al. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066) AIDS research and human retroviruses. 2016;32(1):32–43. doi: 10.1089/aid.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendrix CW. Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell. 2013;155(3):515–518. doi: 10.1016/j.cell.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 35.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Science translational medicine. 2012;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobard C, Sharma S, Martin A, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. Journal of virology. 2012;86(2):718–725. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radzio J, Aung W, Holder A, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PloS one. 2012;7(12):e50632. doi: 10.1371/journal.pone.0050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuttall J, Kashuba A, Wang R, et al. Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrobial agents and chemotherapy. 2012;56(1):103–109. doi: 10.1128/AAC.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srinivasan P, Moss JA, Gunawardana M, et al. Topical Delivery of Tenofovir Disoproxil Fumarate and Emtricitabine from Pod-Intravaginal Rings Protects Macaques from Multiple SHIV Exposures. PloS one. 2016;11(6):e0157061. doi: 10.1371/journal.pone.0157061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith JM, Srinivasan P, Teller RS, et al. Tenofovir disoproxil fumarate intravaginal ring protects high-dose depot medroxyprogesterone acetate-treated macaques from multiple SHIV exposures. J Acquir Immune Defic Syndr. 2015;68(1):1–5. doi: 10.1097/QAI.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66(3):340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anton PA, Cranston RD, Kashuba A, et al. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS research and human retroviruses. 2012;28(11):1412–1421. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang KH, Hendrix C, Bumpus N, et al. A multi-compartment single and multiple dose pharmacokinetic comparison of rectally applied tenofovir 1% gel and oral tenofovir disoproxil fumarate. PloS one. 2014;9(10):e106196. doi: 10.1371/journal.pone.0106196. [DOI] [PMC free article] [PubMed] [Google Scholar]