Abstract
Purpose
To estimate the heart rate variability (HRV)-related lifetime cardiovascular disease (CVD) risk.
Methods
We followed 9,744 participants without baseline CVD, and used a life table approach to estimate lifetime CVD risk (coronary heart disease, heart failure and stroke) from 45 through 85 years according to several HRV measures [the standard deviation of RR intervals (SDNN), the root mean square of successive differences of successive RR intervals (RMSSD), the mean of all normal RR intervals (meanNN), low (LF) and high (HF) frequency power, and the LF/HF ratio].
Results
During 192,110 person-years of follow-up, we documented 2,856 CVD events. Cox regression analyses with the false discovery rate method correction showed independent associations of SDNN, meanNN, LF, and LF/HF in women with CVD. Lifetime CVD risks in the lowest compared with in the highest tertile were significantly increased in men for LF/HF [51.3% (95% confidence interval, 47.3–54.7) vs. 43.9% (40.1–47.2)], and in women for SDNN [39.4% (36.0–43.0) vs. 29.9% (26.3–33.0)], meanNN [39.3% (35.7–42.7) vs. 28.9% (25.7–31.7)], LF [39.4% (35.9–43.0) vs. 30.0% (26.2–33.2)], and LF/HF [37.6% (33.9–40.9) vs. 30.0% (26.8–32.7)].
Conclusions
Greater HRV was modestly associated with lower lifetime CVD risk.
Keywords: heart rate variability, lifetime risk, cardiovascular disease, epidemiology
INTRODUCTION
Heart rate variability (HRV) is a well-established marker of autonomic nervous system function–both sympathetic and parasympathetic (1). Reduced HRV, which reflects sympatho-vagal imbalance (i.e. increased sympathetic or reduced vagal activity), is associated with cardiovascular risk factors such as physical inactivity, hypertension, and diabetes (2–4), and cardiovascular disease (CVD), itself (5). Simple and convenient commercial devices and telephone or computer applications to assess HRV have become increasingly available to the public, and exercisers often now monitor HRV to identify training stress (6, 7). Anecdotal comments on the World Wide Web suggest that some people monitor HRV because they believe it is a marker of cardiovascular health. Thus, it seems important to further understand whether there is a meaningful association between HRV and long-term risk of CVD.
One way to do this may be to calculate HRV-related lifetime risk of CVD. Lifetime risk estimates, that is, absolute risks from a certain age through death, can readily convey the burden of CVD in a population (8). Yet, to the best of our knowledge, no study has estimated lifetime risks of CVD as it relates to HRV.
Therefore, the objectives of the present study were (i) to reevaluate the association between HRV in middle-age and incidence of CVD (coronary heart disease, heart failure and stroke) using Cox proportional hazards regression, and (ii) to estimate the lifetime risks of CVD from age 45 through age 85 years in relation to HRV in a large biracial cohort study.
MATERIALS AND METHODS
Study Design, Setting, and Population
The Atherosclerosis Risk in Communities (ARIC) Study is a population-based longitudinal prospective study of CVD (9). In 1987–1989, 15,792 mostly Caucasian or African American men and women aged 45 to 64 years were recruited from 4 U.S. communities: Forsyth County, North Carolina; Washington County, Maryland; suburban Minneapolis, Minnesota, and Jackson, Mississippi (African Americans only). Various demographic characteristics, health behaviors, and cardiovascular conditions were measured at the baseline home interview and clinic examination. Participants were followed through 2013 by clinical examinations and telephone interview for CVD events. All institutional review boards of the collaborating institutions approved the study protocol, and written informed consent was provided by each participants.
Main Exposure: Heart Rate Variability
The main exposure of interest was HRV. The European Society of Cardiology and the North American Society of Pacing and Electrophysiology have documented standards and procedures for HRV measurement (1). Briefly, heart rate is measured from the intervals between R waves of successive heartbeats (RR interval); HRV reflects the magnitude of RR interval variation over time. Protocols for data processing and analysis in ARIC were previously published (10) and are summarized in Supplementary Table I and II. ARIC measured HRV using a 2-minute electrocardiogram (ECG) at baseline. All data were collected on supine, resting participants, reflect short-term daytime HRV, and were analyzed using ECG software (time-domain) or a previously developed computer algorithm (frequency-domain). HRV measures are commonly divided into time- and frequency-domain measurements. Time-domain measures are calculated directly from heart rate or the duration between successive RR intervals. Frequency-domain measures are calculated from spectral imaging of the ECG recording. In this study, we evaluated 3 time-domain and 3 frequency-domain measures of HRV. The time-domain measures included; (i) the standard deviation of all normal-to-normal RR intervals (SDNN), which characterizes overall HRV; (ii) the root mean square of successive differences in normal-to-normal RR intervals (RMSSD), which is thought to reflect parasympathetic nervous system activity; and (iii) the mean of all RR intervals (meanNN), which measures both sympathetic and parasympathetic influences. The frequency-domain measures included; (i) low frequency power (LF, 0.04–0.15 Hz), considered to include both sympathetic and parasympathetic activities; (ii) high frequency power (HF, 0.15–0.40 Hz), thought to reflect parasympathetic activity; and (iii) low and high frequency power ratio (LF/HF), which estimates the balance between sympathetic and parasympathetic activity. The reliability and validity of our short duration HRV metrics were previously described (11).
Other Cardiovascular Risk Factors
We assessed other potential CVD risk factors, including race (white or African American), body mass index, hypertension (systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥90 mmHg or hypertension medication use), diabetes mellitus (a fasting blood glucose ≥126 mg/dl, non-fasting blood glucose ≥200 mg/dl, a self-reported physician diagnosis of diabetes, or use of antidiabetic medication in the past 2 weeks) (12), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), smoking status (current, former or never), alcohol drinking status (current, former or never), physical activity, educational attainment (grade school, high school without graduation, high school with graduation, vocational school, college with or without graduation, or graduate or professional school) and heart rate. The Baecke questionnaire was used to query participants regarding their frequency of and number of hours walking and participating in as many as 4 sports during the previous year (13). Each exercise or sport activity was converted into metabolic equivalents of task (METs) according to the Compendium of Physical Activities (14). Previous studies evaluated the reliability and validity of the Baecke questionnaire (15). Moderate activities were defined as those involving a workload of 3–6 METs and vigorous activities were those involving a workload of >6 METs. Physical activity levels were categorized into the following three levels: “recommended” (≥75 minutes/week of vigorous intensity or ≥150 minutes/week of any combination of moderate + vigorous intensity), “intermediate” (1–74 minutes/week of vigorous intensity or 1–149 minutes/week of any combination of moderate + vigorous intensity), or “poor” (0 minutes/week of moderate or vigorous intensity).
Confirmation of Cardiovascular Disease
For the present study, we defined an incident CVD event as the first-ever coronary heart disease, heart failure, or stroke. Annual telephones captured participant’s hospitalizations and deaths related to possible CVD (16). Lists of discharges from local hospitals and death certificates were also surveyed from state vital statistics offices for potential CVD events. ARIC staffs validated CVD outcomes by reviewing medical records and recorded information. Incident coronary heart disease was defined as a definite or probable myocardial infarction, definite coronary death, or coronary revascularization procedure. Incident heart failure was defined as a hospitalization with an International Classification of Diseases-9th Revision (ICD-9) discharge code of 428 (428.0 to 428.9) among the primary or secondary diagnoses or else a death certificate with an ICD-9 code of 428 or an ICD-10 code of I50 among the listed or underlying causes of death (16). A previous study has shown a sensitivity of 93% for heart failure (16). Definite or probable strokes were classified by computer algorithm and physician review based on the National Survey of Stroke. When there were disagreements, they were resolved by a second physician (16).
Statistical Analysis
SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses.
We excluded participants who reported or had electrocardiographic evidence of prebaseline CVD (n=1,553), participants taking medication known to affect HRV (β-blockers, antiarrhythmics, or digoxin; n=1,540), participants with missing or poor quality HRV data (n=2,461), and participants whose outcome status was missing (n=151). We further excluded non-white participants in Washington County or Minneapolis and non-white/black participants in Forsyth County (n=64) due to small numbers in order to allow multivariable adjustment for race and study site (17), and participants with missing data on any covariates (n=279). After exclusions, 9,744 participants (4,140 men and 5,604 women) were available for these analyses.
Participants were followed from the baseline (1987–1989) to the first endpoint: incident CVD, death, loss to follow-up, or administratively censored at December 31, 2013. Firstly, we calculated sex-specific hazard ratios (HRs) for time to first CVD event and corresponding 95% confidence intervals (CIs) after adjustment for potential confounding factors using Cox proportional hazard models. Model 1 adjusted for age, and race/ARIC field center; and Model 2 additionally for body mass index, prevalent hypertension, prevalent diabetes, high- and low-density lipoprotein, smoking status, drinking status, physical activity, educational attainment, and heart rate (except for meanNN). In order to account for multiple tests, we evaluated the association between HRV measures and CVD risk using the false discovery rate method. P-values adjusted for the false discovery rate method <0.05 were regarded as significant. Next, we estimated sex-specific remaining lifetime risks of incident CVD from age 45 years through age 85 years in relation to HRV, using a modified version of survival analysis (18). This method uses survival age as the time scale, combines data on participants entering the observation periods at different ages, and accounts for varying durations of follow-up on individuals. In addition, it adjusts for competing risk of non-CVD deaths in order to yield an accurate estimate of age-specific hazards, incidence rates, cumulative incidences, and survival probabilities (18). The multiple-decrement life-table approach treats death as a true competing event, and the decedent’s risk for subsequent events is set to zero (19). This is a more appropriate assumption when attempting to determine the public health burden of disease because decedents can no longer be at risk for the disease of interest. In a standard Kaplan-Meier analysis, it is assumed that all subjects eventually get the disease, and those who die are treated as censored observations. Thus, a standard Kaplan-Meier method usually overestimates the remaining lifetime risk (20). We considered the difference of lifetime risks between two groups as significant when their 95% CIs didn’t overlap.
RESULTS
As shown in Table 1, men and women in lower levels of SDNN were more likely to be older, and they had higher prevalences of hypertension and diabetes, and higher body mass index. Correlation coefficients between pairs of HRV measurements are reported in Supplementary Table III. Most pairs of HRV measures were moderately highly correlated, with the exceptions of pairs involving meanNN with frequency-domain measures or pairs including LF/HF.
Table 1.
Baseline Characteristics of Men (n=4,140) and Women (n=5,604) According to SDNN, ARIC, 1987–1989.
| Men SDNN tertile (Range, ms) |
Women SDNN tertile (Range, ms) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 1 (2.3–29.4) | 2 (29.5–42.5) | 3 (42.6–278.8) | p for trend | 1 (2.3–27.1) | 2 (27.2–39.2) | 3 (39.3–394.0) | p for trend | |
| Participants, n (%) | 1,378 | 1,376 | 1,386 | 1,862 | 1,879 | 1,863 | ||
| Age, years | 55.4±5.7 | 53.7±5.7 | 53.0±5.6 | <0.001 | 54.6±5.7 | 53.3±5.6 | 52.4±5.5 | <0.001 |
| African American, % | 21.3 | 20.0 | 24.1 | 0.071 | 26.6 | 24.9 | 28.0 | 0.359 |
| Body mass index, kg/m2 | 27.4±4.3 | 26.9±3.7 | 26.9±3.8 | 0.002 | 27.7±6.1 | 27.0±5.8 | 27.1±5.4 | 0.003 |
| Hypertension, % | 21.9 | 14.0 | 12.8 | <0.001 | 22.7 | 16.0 | 15.1 | <0.001 |
| Diabetes, % | 13.0 | 7.6 | 6.1 | <0.001 | 12.6 | 8.1 | 5.9 | <0.001 |
| HDL cholesterol, mg/dL | 45.7±14.7 | 45.5±13.5 | 45.6±13.6 | 0.932 | 58.6±17.7 | 58.1±16.8 | 59.4±16.9 | 0.172 |
| LDL cholesterol, mg/dL | 139.4±36.8 | 137.8±36.9 | 138.7±36.2 | 0.652 | 136.2±40.5 | 135.5±40.1 | 132.5±39.0 | 0.004 |
| Current smoker, % | 30.0 | 25.4 | 26.9 | 0.072 | 23.5 | 24.0 | 25.2 | 0.240 |
| Current drinker, % | 66.1 | 66.4 | 67.2 | 0.554 | 48.8 | 52.2 | 53.0 | 0.010 |
| Physical inactivity, % | 35.8 | 31.8 | 31.8 | 0.027 | 39.0 | 36.6 | 36.0 | 0.061 |
| High school graduation, % | 76.3 | 80.5 | 78.6 | 0.142 | 77.2 | 79.8 | 81.2 | 0.003 |
| Heart rate, bpm | 70.8±11.3 | 65.3±8.5 | 61.5±8.3 | <0.001 | 74.5±10.5 | 69.0±8.5 | 65.0±8.0 | <0.001 |
| RMSSD, ms | 15.6±6.3 | 25.4±8.8 | 45.9±30.6 | <0.001 | 15.7±6.3 | 25.8±8.8 | 45.8±27.9 | <0.001 |
| MeanNN, ms | 868.4±134.6 | 933.8±121.9 | 993.6±136.4 | <0.001 | 821.3±113.4 | 882.4±110.2 | 937.1±116.8 | <0.001 |
| LF, ms2 | 12.4±12.5 | 28.5±27.8 | 70.1±89.4 | <0.001 | 10.6±10.5 | 22.5±20.2 | 57.5±76.5 | <0.001 |
| HF, ms2 | 5.5±6.2 | 11.3±11.8 | 34.8±78.1 | <0.001 | 6.7±6.2 | 14.4±13.3 | 37.6±62.7 | <0.001 |
| LF/HF | 3.2±3.0 | 3.6±3.3 | 3.6±3.6 | <0.001 | 2.1±2.0 | 2.2±2.0 | 2.4±2.4 | <0.001 |
ARIC, Atherosclerosis Risk in Communities Study; SDNN, standard deviation of all normal-to-normal RR intervals; RMSSD, root mean square of successive differences in normal-to-normal RR intervals; MeanNN, mean of all normal-to-normal RR intervals; LF, low frequency power; HF, high frequency power.
Values are mean ± standard deviation for continuous variables and % for categorical variables.
During 1987–2013 and a median of 24 years of follow-up, 9,744 participants (4,140 men and 5,604 women) provided 192,110 person-years of observation. We identified 2,856 incident CVD events, and 1,646 non-CVD deaths.
In Cox regression analyses, the age- and race-adjusted model generally showed inverse dose-response relations between middle-age HRV and CVD incidence (Model 1 in Table 2). Further adjustments for other CVD risk factors attenuated the associations. The false discovery rate method showed that only SDNN, meanNN, LF, and LF/HF in women were significantly associated with CVD risk (asterisks in Table 2).
Table 2.
Hazard Ratios, Lifetime Risks and 95% Confidence Intervals from Age 45 Years to 85 Years of Cardiovascular Disease According to Heart Rate Variability, ARIC, 1987–2013.
| Men HRV tertile |
Women HRV tertile |
|||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Time-domain measures | ||||||
| SDNN, range | 2.3–29.4 | 29.5–42.5 | 42.6–278.8 | 2.3–27.1 | 27.2–39.2 | 39.3–394.0 |
| HR, model 1 | 1.18 (1.04–1.34)* | 1.05 (0.92–1.19) | 1 | 1.43 (1.25–1.64)* | 1.11 (0.96–1.27) | 1 |
| HR, model 2 | 1.06 (0.93–1.21) | 1.02 (0.90–1.16) | 1 | 1.20 (1.04–1.39)* | 1.05 (0.91–1.20) | 1 |
| Lifetime risk, % | 49.1 (45.3–52.5) | 46.0 (42.1–49.4) | 45.8 (41.5–49.5) | 39.4 (36.0–43.0)† | 31.5 (28.0–34.4) | 29.9 (26.3–33.0) |
| RMSSD, range | 1.9–18.8 | 18.9–29.6 | 29.7–420.2 | 2.0–18.8 | 18.9–30.5 | 30.6–420.1 |
| HR, model 1 | 1.11 (0.97–1.25) | 1.00 (0.88–1.14) | 1 | 1.18 (1.03–1.35)* | 0.96 (0.84–1.10) | 1 |
| HR, model 2 | 1.04 (0.90–1.19) | 1.02 (0.90–1.17) | 1 | 1.03 (0.83–1.20) | 0.96 (0.83–1.11) | 1 |
| Lifetime risk, % | 47.5 (43.8–50.8) | 47.5 (43.5–51.0) | 46.4 (41.8–50.1) | 36.2 (33.0–39.1) | 32.7 (29.0–36.0) | 32.5 (28.6–35.7) |
| MeanNN,, range | 512.9–867.9 | 868.0–980.9 | 981.0–1575.0 | 471.4–823.8 | 823.9–923.1 | 923.2–1587.2 |
| HR, model 1 | 1.34 (1.19–1.52)* | 1.11 (0.98–1.26) | 1 | 1.52 (1.34–1.74)* | 1.16 (1.01–1.33) | 1 |
| HR, model 2 | 1.15 (1.01–1.30) | 1.04 (0.92–1.18) | 1 | 1.34 (1.17–1.53)* | 1.12 (0.98–1.29) | 1 |
| Lifetime risk, % | 49.8 (45.8–53.2) | 46.7 (42.8–50.2) | 44.4 (40.3–47.8) | 39.3 (35.7–42.7)† | 32.5 (29.0–35.4) | 28.9 (25.7–31.7) |
| Frequency-domain measures | ||||||
| LF, range | 0.0–11.3 | 11.4–31.8 | 31.9–961.0 | 0.0–9.2 | 9.3–26.1 | 26.2–1440.0 |
| HR, model 1 | 1.25 (1.10–1.42)* | 1.19 (1.05–1.35) | 1 | 1.50 (1.31–1.72)* | 1.15 (0.99–1.32) | 1 |
| HR, model 2 | 1.15 (1.01–1.30) | 1.15 (1.01–1.30) | 1 | 1.26 (1.10–1.45)* | 1.11 (0.96–1.27) | 1 |
| Lifetime risk, % | 49.6 (45.9–52.9) | 48.0 (43.9–51.4) | 43.0 (38.7–46.5) | 39.4 (35.9–43.0)† | 31.6 (28.3–34.5) | 30.0 (26.2–33.2) |
| HF, range | 0.0–4.4 | 4.5–12.2 | 12.3–1700.0 | 0.0–6.0 | 6.1–16.0 | 16.1–1150.0 |
| HR, model 1 | 1.05 (0.92–1.19) | 0.94 (0.83–1.07) | 1 | 1.20 (1.05–1.37)* | 1.10 (0.96–1.27) | 1 |
| HR, model 2 | 1.05 (0.92–1.19) | 0.98 (0.86–1.11) | 1 | 1.15 (1.00–1.32) | 1.15 (1.01–1.32) | 1 |
| Lifetime risk, % | 49.2 (45.5–52.5) | 44.4 (40.5–47.8) | 47.4 (42.7–51.1) | 36.0 (32.6–39.7) | 34.4 (30.7–37.5) | 31.4 (27.5–34.7) |
| LF/HF, range | 0.1–1.7 | 1.8–3.6 | 3.7–33.5 | 0.0–1.1 | 1.2–2.2 | 2.3–29.4 |
| HR, model 1 | 1.29 (1.14–1.46)* | 1.08 (0.96–1.23) | 1 | 1.41 (1.23–1.61)* | 1.14 (0.99–1.31) | 1 |
| HR, model 2 | 1.14 (1.01–1.29) | 1.00 (0.88–1.14) | 1 | 1.18 (1.03–1.35)* | 1.07 (0.94–1.23) | 1 |
| Lifetime risk, % | 51.3 (47.3–54.7)† | 45.8 (41.6–49.3) | 43.9 (40.1–47.2) | 37.6 (33.9–40.9)† | 33.8 (30.0–36.9) | 30.0 (26.8–32.7) |
ARIC, Atherosclerosis Risk in Communities Study; HRV, heart rate variability; SDNN, standard deviation of all normal-to-normal RR intervals; RMSSD, root mean square of successive differences in normal-to-normal RR intervals; MeanNN, mean of all normal-to-normal RR intervals; LF, low frequency power; HF, high frequency power; and HR, hazard ratio.
Model 1: Adjusted for age, sex, and race/ARIC field center.
Model 2: Adjusted for Model 1 + body mass index, hypertension, diabetes, HDL cholesterol, LDL cholesterol, smoking status, drinking status, physical activity level, educational attainment, and heart rate (except for meanNN).
P-values adjusted for the false discovery rate method<0.05.
Significantly increased lifetime risks compared to the highest tertile,
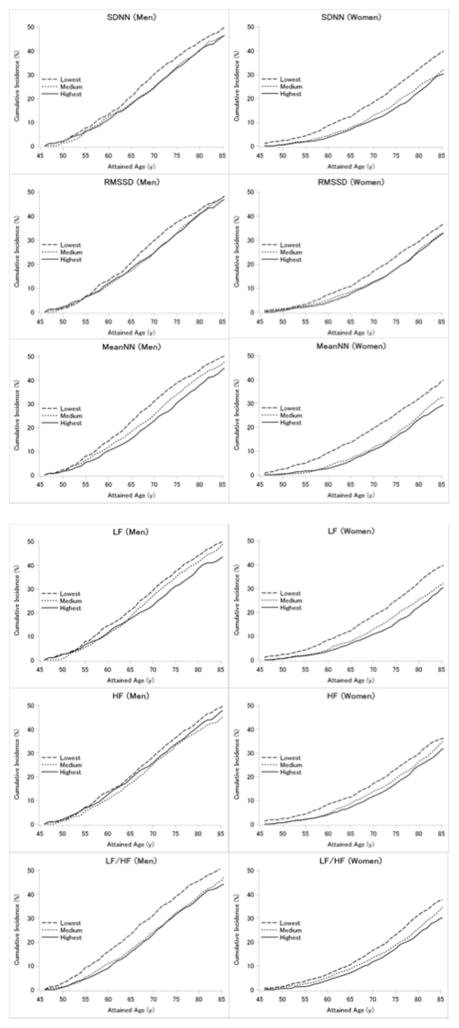
The overall lifetime risks of CVD from age 45 years to age 85 years were 47.0% (95% confidence interval, 44.7–49.0) for men and 33.7% (31.7–35.5) for women, respectively. As shown in Table 2 and the Figure, HRV in middle age displayed inverse dose-response relations with lifetime risk of CVD. Lifetime risks of CVD in the lowest vs. highest tertiles of HRV were approximately 49% vs. 45% for men, and 38% vs. 30% for women, respectively. Lifetime risks of CVD were significantly increased in the lowest compared with the highest tertile in men for LF/HF (the lowest vs. the highest=51% vs. 44%) and in women for SDNN (39% vs. 30%), meanNN (39% vs. 29%), LF (39% vs. 30%), and LF/HF (38% vs. 30%).
Figure.
Sex-specific risk estimates of cardiovascular disease risk from age 45 to 85 years in relation to heart rate variability, ARIC, 1987–2013. SDNN, standard deviation of all normal-to-normal RR intervals; RMSSD, root mean square of successive differences of successive RR intervals; MeanNN, mean of all normal-to-normal RR intervals; LF, low frequency power; and HF, high frequency power.
DISCUSSION
In this population-based prospective cohort study in the U.S., firstly, we observed inverse associations between HRV measures in middle age and CVD incidence using Cox regression models, particularly in women. Secondly, we found inverse dose-response relations between HRV and lifetime risk of CVD from age 45 years to age 85 years. Furthermore, several HRV measures, specifically LF/HF for men and SDNN, meanNN, LF, and LF/HF for women, were significantly inversely associated with lifetime risk of CVD. Although an inverse association between HRV and CVD risk is well established, this is the first estimate of how HRV measures translate into lifetime CVD risk.
Our study suggests that individuals with decreased HRV such as the lowest tertiles of HRV measures in this study might need to recognize themselves as those with increased lifetime risk of CVD [in this study, compared with those in the highest tertile, men in the lowest tertile of HRV had approximately 4% higher lifetime risk of CVD (49% vs. 45%), and women had 8% higher lifetime risk (38% vs. 30%)]. Since HRV measures are simple and non-invasive and thus are available to the public, if future studies can confirm abnormal ranges of HRV in relation to CVD risk, HRV measures might greatly contribute to CVD prevention.
HRV reflects autonomic nervous system function. Autonomic nervous system function can be impaired by several lifestyle factors such as smoking and physical inactivity, and comorbidities such as diabetes as well as aging (2–4, 21, 22). Impaired autonomic nervous system function has been suggested to trigger inflammation and arrhythmia (1, 23), elevate blood pressure (3), and disturb the dynamics between blood pressure and blood flow in the cerebral vessels (circulatory autoregulation) (24), which can increase CVD risk. Thus, these mechanisms may explain its inverse association with CVD observed in the present study, and HRV may be a marker for increased risk of CVD. Applications and devices measuring HRV are increasingly popular, particularly among athletes, to monitor autonomic nervous system function during exercise or training, and so the relation of HRV to CVD is of potential interest.
Although lower HRV was associated with higher lifetime risk of CVD, even those with higher HRV had a substantial lifetime risk of CVD (approximately 45% for men and 30% for women). A previous study estimated that the lifetime risks of CVD of men and women aged 45 years through 85 years who had one major risk factor (smoking, hypertension, diabetes, or hypercholesterolemia) were around 45% and 30%, respectively (20). Thus, although low HRV may be useful to identify individuals with high CVD risk, those with higher HRV also need additional risk assessments such as “Life’s Simple 7” recommended by American Heart Association and interventions to further reduce their lifetime risk of CVD. In addition, it is unclear whether attempting to optimize HRV over the long term would translate into any substantial cardiovascular health benefit. For answering this question, interventional studies (e.g. exercise) would be needed.
The present study demonstrated generally similar results for multiple HRV measures. Although each HRV measure is touted to reflect different autonomic nervous system functions, most measures were moderately correlated with each other. This suggests that the observed associations are not independent but reflect related pathophysiology.
HRV appeared to correlate with CVD risk in women somewhat better than in men. A previous study showed that HRV was significantly lower in women with untreated newly diagnosed hypertension compared with men (25). HRV might reflect end organ damages more sensitively in women than in men.
Some limitations of our study need to be mentioned. Estimates of lifetime risks of CVD should be interpreted carefully as risk markers examined may be to some degree confounded by other CVD risk factors. Even with such a proviso, our estimates of lifetime risk can help in understanding the association between HRV and CVD risk. A second potential limitation is that estimates of lifetime risk are subject to birth cohort effects, and therefore can change over time. Thirdly, we obtained HRV using a 2-minute ECG. Collection of long-term Holter ECG recordings is generally preferred to short-term ECG recordings, because longer recording reduces measurement variability (26), and HRV derived from short term ECG recordings may not represent the sympathetic and parasympathetic activity in a 24-hour period. However, a previous study has shown that 2- to 15-minute and 24-hour HRV measures are highly correlated (11). Finally, we could not conduct any correction such as the false discovery rate method for lifetime risk estimates. Thus, the association between HRV and lifetime risk (significant or not) may be to dome degree overestimated.
CONCLUSIONS
In the prospective population-based ARIC cohort, greater HRV in middle age was associated with modestly lower lifetime risk of CVD through age 85. Those with higher HRV still had a substantial lifetime risk of CVD, suggesting that they also need risk assessments and intervention to further reduce their lifetime risk of CVD.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
FUNDING SOURCES
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) via contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. The Nippon Foundation provided grants to support Dr. Kubota’s fellowship at School of Public Health, University of Minnesota.
ABBREVIATIONS
- HRV
heart rate variability
- CVD
cardiovascular disease
- ARIC
Atherosclerosis Risk in Communities
- SDNN
standard deviation of RR intervals
- RMSSD
root mean square of successive differences of successive RR intervals
- meanNN
mean of all normal RR intervals
- LF
low frequency power
- HF
high frequency power
- ECG
electrocardiogram
- ICD-9
International Classification of Diseases-9th Revision
Footnotes
DISCLOSURES
All authors have approved the final article.
CONFLICT OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Eur Heart J. 1996;17(3):354–81. [PubMed] [Google Scholar]
- 2.Soares-Miranda L, Sattelmair J, Chaves P, Duncan GE, Siscovick DS, Stein PK, et al. Physical activity and heart rate variability in older adults: the Cardiovascular Health Study. Circulation. 2014;129(21):2100–10. doi: 10.1161/CIRCULATIONAHA.113.005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension. 2003;42(6):1106–11. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder EB, Chambless LE, Liao D, Prineas RJ, Evans GW, Rosamond WD, et al. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005;28(3):668–74. doi: 10.2337/diacare.28.3.668. [DOI] [PubMed] [Google Scholar]
- 5.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102(11):1239–44. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 6.Nunan D, Jakovljevic DG, Donovan G, Hodges LD, Sandercock GR, Brodie DA. Levels of agreement for RR intervals and short-term heart rate variability obtained from the Polar S810 and an alternative system. Eur J Appl Physiol. 2008;103(5):529–37. doi: 10.1007/s00421-008-0742-6. [DOI] [PubMed] [Google Scholar]
- 7.Sawai A, Ohshige K, Tochikubo O. Development of wristwatch-type heart rate recorder with acceleration-pickup sensor and its application. Clin Exp Hypertens. 2005;27(2–3):203–13. [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353(9147):89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 9.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 10.Liao D, Barnes RW, Chambless LE, Heiss G. A computer algorithm to impute interrupted heart rate data for the spectral analysis of heart rate variability–the ARIC study. Comput Biomed Res. 1996;29(2):140–51. doi: 10.1006/cbmr.1996.0012. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder EB, Whitsel EA, Evans GW, Prineas RJ, Chambless LE, Heiss G. Repeatability of heart rate variability measures. J Electrocardiol. 2004;37(3):163–72. doi: 10.1016/j.jelectrocard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Kubota Y, McAdams-DeMarco M, Folsom AR. Serum uric acid, gout, and venous thromboembolism: The atherosclerosis risk in communities study. Thromb Res. 2016;144:144–8. doi: 10.1016/j.thromres.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 14.Kubota Y, Evenson KR, MacLehose RF, Roetker NS, Joshu CE, Folsom AR. Physical Activity and Lifetime Risk of Cardiovascular Disease and Cancer. Med Sci Sports Exerc. 2017;49(8):1599–605. doi: 10.1249/MSS.0000000000001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24(4):685–93. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 16.Kubota Y, Heiss G, MacLehose RF, Roetker NS, Folsom AR. Educational Attainment and Lifetime Risk of Cardiovascular Disease: the Atherosclerosis Risk in Communities Study. JAMA Intern Med. 2017 doi: 10.1001/jamainternmed.2017.1877. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota Y, London SJ, Cushman M, Chamberlain AM, Rosamond WD, Heckbert SR, et al. Lung function, respiratory symptoms and venous thromboembolism risk: the Atherosclerosis Risk in Communities Study. J Thromb Haemost. 2016;14(12):2394–401. doi: 10.1111/jth.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beiser A, D’Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19(11–12):1495–522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 20.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287(8):1003–10. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 21.Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76(8):562–64. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- 22.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98(8):772–76. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 23.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25(5):363–70. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke. 2010;41(1):102–9. doi: 10.1161/STROKEAHA.109.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavithran P, Madanmohan T, Nandeesha H. Sex differences in short-term heart rate variability in patients with newly diagnosed essential hypertension. J Clin Hypertens (Greenwich) 2008;10(12):904–10. doi: 10.1111/j.1751-7176.2008.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cygankiewicz I, Zareba W. Heart rate variability. Handb Clin Neurol. 2013;117:379–93. doi: 10.1016/B978-0-444-53491-0.00031-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.