Abstract
Objective
Cisplatin is an effective antineoplastic agent used in cancer therapy. However, the use of cisplatin is restricted due to its toxic side effects. Alleviation of its side effects which restricts cisplatin use is highly important. We aimed to investigate the effects of curcumin, vitamin E and their combination in cisplatin induced testicular apoptosis.
Material and methods
Thirty-five Wistar albino male adult rats, weighing 300–350 g were divided randomly into five groups including seven rats in each as control, cisplatin, curcumin, vitamin E, and curcumin + vitamin E. On the posttest 5th day, rats were sacrificed, and their testes were removed. 4–5 μm sections from formalin fixed paraffin embedded testis tissues were stained both hematoxylin-eosin to analyze histologically and immunohistochemically to determine the expression of the apoptotic pathway proteins (Bax, Cas-3, Bcl-2).
Results
Increased histological damage with cisplatin administration was reduced in treatment, especially in combination therapy. Cas-3 and Bax protein immunostaining intensities H-scores were significantly increased but Bcl-2 was slightly decreased in the cisplatin group compared to the control. In all treatment groups Bax, Cas-3 decreased compared to cisplatin group however Bcl-2 decreased in the curcumin and vitamin E groups. Bax/Bcl-2 was the highest in the cisplatin, and decreased in all treatment groups in favor of control.
Conclusion
Cas-3 expression increased by cisplatin administration suggests that cisplatin causes apoptosis of germ cells. According to the present findings, cisplatin mainly caused testicular apoptosis through the Cas-3 and Bax apoptotic protein pathways. Cisplatin-induced testicular apoptosis can be prevented by administration of curcumin, vitamin E, and combination therapy.
Keywords: Apopitozis, cisplatin, curcumin, immunohistochemistry, testis, vitamin E
Introduction
Cisplatin (cis-diamminedichloroplatinum II) is an effective antineoplastic agent used successfully in the treatment of many types of cancer including testicular, ovarian, gastric, pulmonary, prostate, head, and neck, bladder and cervical cancers, lymphoma, and osteosarcoma.[1,2] However, its ototoxicity, nephrotoxicity, myelotoxicity, and gastrointestinal toxicity, and serious side effects involving neurologic, hematologic, and reproductive system restrict its clinical use.[3,4] Multiple number of studies have indicated the roles of reactive oxygen radicals (ROSs), and oxidative stress (OS) on reproductive toxicity of cisplatin.[4]
Though oxygen is indispensable for the survival of living organisms, during its metabolism, extremely reactive intermediate products which are known as the sources of free radicals are formed. Reactive molecules released during conversion of foods into energy are called as free radicals. These reactive oxygen metabolites damage components of cells including lipid, protein, DNA, mitochondria, endoplasmic reticulum. Mammalian cells have innate antioxidant defense mechanisms including non-enzymatic, and endogenous enzymatic systems as superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), and glutathione (GSH) so as to keep formation of free radicals under control, and to withstand their harmful effects.[5,6] In some cases of stress, as a result of disruption of the balance between prooxidants, and oxidants in living organisms, OS is released. OS may cause macromolecular, and cellular damage, and lead to serious outcomes, even death of the organism.[5] In various studies performed with rat testes, it has been demonstrated that cisplatin treatment leads to a marked increase in apoptosis of germ cells, histopathological damage, and decrease in levels of biochemical antioxidant enzymes including SOD, GPX, CAT, and GSH.[3,4,7]
Cisplatin is very widely used in the cancer treatment either as a monotherapy or combination therapy with other agents.[8,9] Despite promising results especially in the treatment of testicular malignancy, long-lasting testicular dysfunction is one of the widely seen adverse effects of cisplatin.[10] Besides, side effects of cisplatin on testes have been demonstrated in many animal experiments. These adverse side effects sepecially related to destruction of spermatogenes have been reported as decrease in spermatogenesis, loss of testicular volume, and weight, and germ cell apoptosis.[7]
Alleviation of toxicity of cisplatin which is used as an anticancer drug conveys critical importance, while molecular, and cellular mechanism of cisplatin toxicity has not been understood yet. Induction of apoptosis by cisplatin may occur through many pathways involving in excessive production of ROS. Available evidence demonstrate that increases in ROS production, and expression of endogenous antioxidant molecules support cisplatin toxicity.[11–14] As a result of OS, priorly, apoptotic cell loss occurs.[15]
Cisplatin may produce reactive oxygen species, and inhibit antioxidant enzymes in normal tissues. Some findings suggest that accumulation of reactive oxygen particles mediates cisplatin toxicity. Therefore, many preservative agents have been used in combination with cisplatin to decrease damage incurred by cisplatin without altering its antitumoral effectiveness.[16]
Protection against cisplatin-induced testicular damage essentially is based on reinforcement of intracellular survival mechanisms. Some studies performed have demonstrated protective effects of antioxidant substances on cisplatin-induced toxicity.[4,7]
Curcumin is a polyphenol isolated from the roots of the plant turmeric. Antioxidant, anti-free radical, antineoplastic, and antifungal effects of curcumin have been reported. The effects of curcumin are dependent on interactions with multiple number of molecular targets including growth factors, kinases, transcription factors, inflammatory factors, apoptosis-related factors, adhesion molecules, enzymes related to cellular proliferation. Curcumin has not any known toxic effect.[14,17,18]
Vitamin E is an alpha-tocopherol with the highest antioxidant activity. Alpha-tocopherol is a membrane-specific antioxidant because of its lipophilic activity, and it is situated in the membrane-rich cell components. It has anticancer, cardioprotective, neuroprotective, antidiabetic, osteoprotective, immunomodulator, and gastroprotective effects. As a very potent antioxidant, it forms the first defense line which protects unsaturated fatty acids in the structure of phospholipids from the effects of free radicals. It removes the lipid peroxyl radicals, and terminates lipid peroxidation chain reactions. Therefore it is also known as chain-breaking antioxidant. Vitamin E is one of the major lipid soluble antioxidants which prevent lipid peroxidation maintaining membrane integrity.[19]
In cancer patients receiving cisplatin treatment serum antioxidant concentrations decrease. Cisplatin chemotherapy cycles are related to decreases in the circulatory levels of vitamins C, and E, ceruloplasmin, and uric acid.[14,20] Therefore in this study, curcumin, vitamin E, and combination of both were administered to rats as an antioxidant support.
In this study, the hypothesis which suggests that individual or combined administration of curcumin, and vitamin E may preclude cisplatin-induced testicular apoptosis because of their intrinsic biochemical and antioxidant contents, has been proposed. The aim of this study was also to evaluate the antiapoptotic effects of curcumin, vitamin E, and curcumin + vitamin E combination on cisplatin-induced apoptosis in testes of rats using immunohistochemical analyses of expressions of apoptotic (Bax, Cas-3) and antiapoptotic proteins (Bc1–2).
Material and methods
Experimental animals
For this study approval of Local Ethics Committee of Gaziosmanpaşa University Animal Experiments (2016 HADYEK-21) was obtained. A total of 35 nearly 3-month old Wistar albino strain healthy male rats weighing 300–350 g were used in the study. The rats were divided randomly into five groups including seven rats in each group as control, experimental and three different treatment groups as follows:
Group 1: Control group - physiologic saline (SF) administered group.
Group 2: Cisplatin-experiment group.
Group 3: Cisplatin + Curcumin− treatment group.
Group 4: Cisplatin + Vitamin E− treatment group.
Group 5: Cisplatin + Curcumin + Vitamin E-treatment group.
All rats were kept in standard plastic cages under an average temperature of 22°C, 50–60%, relative humidity, 12-hour night, and 12-hour light cycles, with a free access to food, and water.
Experimental procedure
On the first day of the experiment, single dose of intraperitoneal cisplatin (16 mg/kg, ip) was administered to all rats (excl. the control group) through intraperitoneal route as indicated in the literature.[14,21] At the same day each treatment group received curcumin, vitamin E, and their combinations in that order. Curcumin was administered through intraperitoneal route at a dose of 200 mg/kg for 5 days at the same time of the day. Vitamin E was also given the first day as a single dose of 50 mg/kg ip. Curcumin plus vitamin E combination was administered using their individual doses also through intraperitoneal route for 5 days. While control group received initially physiologic saline at a dose of 16 mL/kg through intraperitoneal route. On the fifth day of the experiment, rats were sacrificed under deep anesthesia, and their testes were extracted. Following routine histological monitoring, testes were blocked in paraffin. Testicular tissue sections from blocked in paraffin were stained with hematoxylin-eosin using immunohistochemical staining protocol, and subjected to microscopic analysis. For the anesthesia of the rats ketamine (60 mg/kg/ip), and xylazine (10 mg/kg/ip) were used.
Histological procedures
Immediately following their extraction, testes were immersed in 4% buffered neutral formaldehyde solution, and fixed for 48 hours. Then they were subjected to routine histological follow-up procedures, and embedded in paraffin blocks. Tissue sections in paraffin blocks were cut using rotary microtome (LeicaRM2135, Germany) as 4–5 μm thick slices, and placed on ground glass frozen slides for staining with hematoxylin-eosin, and on poly-L-lysine coated slides for immunohistochemical staining.
From every animal in each group an average of 8 consecutive sections of testicular tissues stained with hematoxylin-eosin were examined under light microscope, and evaluated histopathologically.
Immunohistochemical analyses
Indirect immunohistochemical staining protocol was applied so as to determine immunohistochemical expressions of apoptotic pathway proteins (Bax, Bcl2, Acas3) in testicular sections. Under light microscope intensities of immunostaining were evaluated. The results obtained were transformed into H-scores which are semiquantitative expressions of immunostaining intensity, and compared statistically. Immunohistochemical staining protocol was applied, and briefly described as follows.
Cut slices placed on adhesive microscope slides were left overnight in incubator at 60°C, and the next day they were awaited for 5 minutes in three separate xylene series for deparaffinization. The slides were passed through alcohol series with decreasing dilutions from 100% down to 70%, then they were rehydrated for 5 minutes in distilled water. The slides were placed in citric acid solution, and subjected to 360 watt microwave for 5 minutes for antigen retrieval. Following shock cooling procedure, they were left in distilled water for 10 minutes. The slides were left in 3% hydrogen peroxide (H2O2) solution for 10 minutes, and washed 3-fold each time for 5 minutes with phosphate buffer solution (PBS). The periphery of the sections were circumscribed with hydrophobic PAP pen, then non-immune blocking serum was dropped on them, and incubated for 15 minutes under humid, and dark environment. After removal of the blocking agent without washing, primary antibodies Cas-3, Bax, Bcl-2 (1:50, Santa Cruz) were dropped on the slides. They were placed in a humid closed box, and left overnight under dark at ± 4°C in a refrigerator. The next day the slides were three times washed in PBS. Biotinylated secondary antibody was dropped on the sections, and incubated in a closed humid box under dark and room temperature for 30 minutes. The slides were washed thrice each time for 5 minutes, and 3-amino 9-ethylcarbazole (AEC) chromogen solution was dropped on the preparations. Within 5–10 minutes a reaction occurred, then the slides were immersed in PBS, and the reaction was terminated. After thrice application of PBS each time for 5 minutes, the sections were immersed in distilled water, and opposite staining was performed with hematoxylin. The slides were rinsed in distilled water, and excess water was removed, then water-based solution was dropped on the slides and covered with coverglasses. For negative control, instead of primary antibody, PBS was dropped on some sections, and immune reaction was not observed on these sections.
Immunohistochemically stained sections were examined under light microscope at 40× magnification in order to detect immunoreactive cells. Staining intensity scoring scale based on antigen-antibody reaction was used to determine expression levels of Cas-3, Bax, and Bcl2. Criteria used for scoring have been given in Table 1 in detail. Categorical variables for each type of protein were counted on five random areas of five sections according to their staining intensities. The results obtained were converted into H-scores using [∑Pi(i+l)] formula (i: staining intensity score; Pi: percentage of stained cells).
Table 1.
Criteria used for the scoring of immune reactivities (staining intensities) of Cas-3, Bax, Bcl-2 proteins
| Skor | Immune reactivity |
|---|---|
| 0+ | Negative staining |
| 1+ | Weakly stained |
| 2+ | Moderate staining |
| 3+ | Strong staining |
Preparation of curcumin extract solution
Since curcumin is a molecule in the form of solid powder, we priorly prepared a solution from this powder form. We subjected the powder through processing procedures as we described in our previously published article.[22] Briefly, 2.5 g dry curcumin powder (Sigma-Aldrich) was placed in successive dilutions of dimethylsulfoxide (DMSO), and ethanol, and dissolved with the aid of magnetic mixer under room temperature. Priorly, DMSO curcumin solution was centrifuged at 5000 rpm for 10 minutes. After removal of DMSO, the remaining pellet was mixed with alcohol food grade 96%, and the procedure was repeated. Following removal of ethanol, the pellet was diluted in 25 mL sterile saline solution, and 2.5 g/25 mL stock curcumin solution was prepared. Thus prepared curcumin solution could be easily delivered through intraperitoneal route at doses calculated based on body weights of the rats.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Science (IBM SPSS Statistics; Armonk, NY, ABD) for Windows, version 20. The fitness of continuous variables to normal distribution pattern was evaluated using Kolmogorov-Smirnov test. Data were expressed as mean ± standard error of mean (SEM). Comparison of weighted mean values of H-scores of immunostaining intensities of Cas-3, Bax, and Bcl-2 proteins was realized using One-way ANOVA post-hoc Tukey HSD test, while comparison of Bax and Bcl-2 ratios was performed using Least Significant Differences (LSD) test. P values less than 0.05 was considered to be statistically significant.
Results
Microfilms of hematoxylin-eosin stained sections of testicular tissue demonstrating the effects of cisplatin and also curcumin, vitamin-E, and their combinations given to alleviate the effects cisplatin on testicular histology are shown in Figure 1. In overall histopathological evaluation, morphology of testicular seminiferous tubuli, interstitial areas, and germinal epithelium had a normal histological appearance (Figure 1a). However, some degenerative abnormalities were seen in the testicular tissue of the cisplatin group including regressed maturation of germinal cells, arrest of mitotic division of germ cells in the spermatogenic series, desquamation, and shedding of germ cells into tubular lumen, enlargement of the interstitial area, vascular bleeding, patchy areas of disrupted epithelial integrity (Figures 1c, and d). In the groups which received treatment to decrease the harmful effects of cisplatin on testicular tissue, especially in the curcumin plus vitamin-E group, alleviation of these deleterious effects was observed (Figure 1b).
Figure 1. a–d.
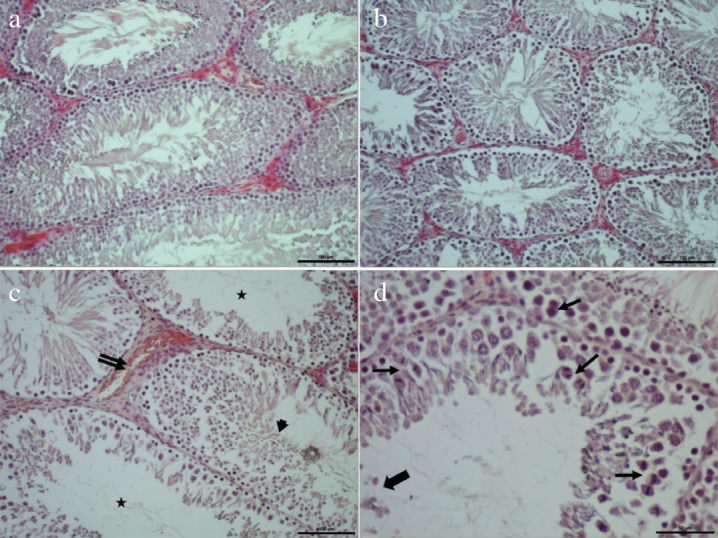
Normal histological appearance of seminiferous tubuli, interstitial tissue, and seminiferous epithelium in the control group (a). Histological appearance of the testicular tissue with decreased tissue damage in the curcumin plus vitamin E combination group (b). Deficient maturation of germinal cells (arrow head), interstitial dilation, and vascular bleeding (double arrow), empty tubuli or tubuli containing scarce number of spermatozoa (asterix) (c); representative histological appearance of destructive changes in the cisplatin group (d) where desquamation, and shedding of germinal epithelial cells into tubular lumen (thick arrow), disordered germinal epithelium, arrest of germ cell division (thin arrows) are seen (Bar: a, b, c 50 μm; d, 100 μm. HE)
Mean H-scores of each apoptotic protein as estimated based on the results of immunohistochemical analyses are shown in Table 2. Microphotos of immunohistochemical stainings representing expressions of apoptotic protein of each group are shown in Figure 2.
Table 2.
Immunohistochemical staining severity scores of apoptotic, and anti-apoptotic pathway proteins
| Control | CRC+Vit-E | CRC | Vit-E | CISP | |
|---|---|---|---|---|---|
| Bax | 8.42±2.59a | 10.81±1.24a | 17.74±2.86a | 24.16±4.01a | 116.97±9.65b |
| Bcl-2 | 117.53±11.39a | 81.22±4.98ab | 45.33±7.12b | 49.86±4.38b | 108.35±29.29ab |
| Acas-3 | 7.83±1.66a | 11.45±1.82a | 13.72±1.69a | 23.17±3.84a | 120.15±7.98b |
Letters on values express similarities (the same letter), and differences (different letter) between groups as for parametres indicated in each row.
CRC: curcumin; Vit-E: vitamin E; CISP: cisplatin. ±:SEM
Figure 2.
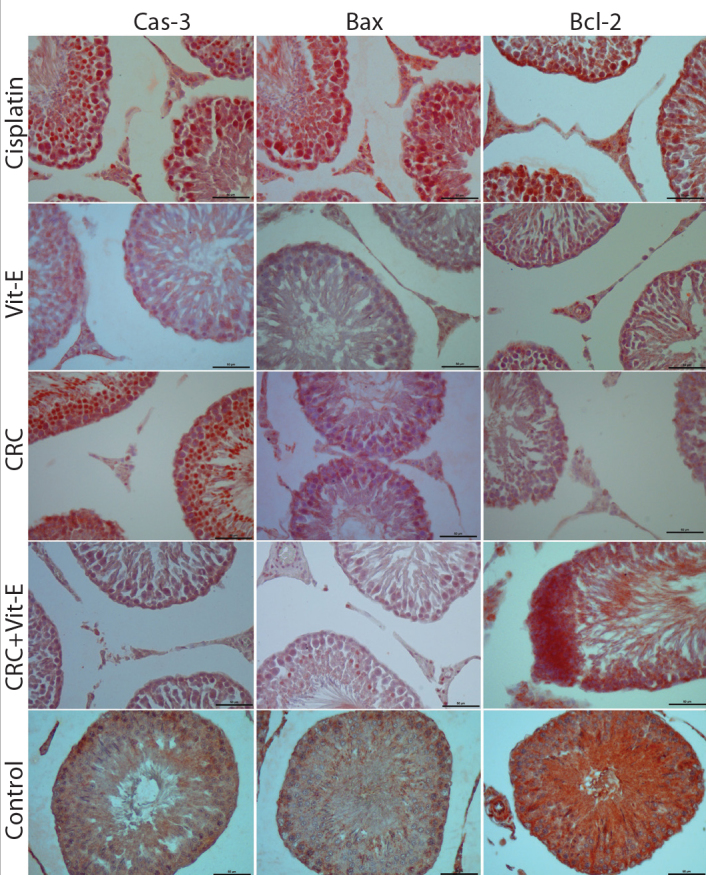
Representative microscopic images of immunohistochemical expressions of Cas-3, Bax, Bcl-2 proteins in all groups (Crc: curcumin, Bar=50 μm). Generally, Cas-3, and Bax expressions are increased in cisplatin, decreased in the control, and at intermediate levels in the treatment groups being closer to those of the contain group. While for Bcl-2 expression the reverse is true. However in the cisplatin group an increase-though not at a great extent- is seen.
At microscopic analyses of immunohistochemical expression of Bax protein, markedly increased immunostaining intensity of Bax relative to the control was observed. However in all treatment groups, decreased immunostaining intensities of Bax protein relative to the control group was seen. Highest H-scores of immunostaining intensities of Bax protein were detected in the cisplatin group which were statistically significantly different from those of the control group (p<0,001). In the comparisons of weighted mean H-scores of the treatment groups, all groups were statistically comparable among themselves, and relative to the control group (p>0.05). However their weighted mean H-scores were significantly different from those of the cisplatin group (p<0.001).
Microscopic analyses of immunohistochemical expression of Cas-3 which is another apoptotic protein, revealed marked increase in immunostaining intensity in the cisplatin group. Similarly, in all treatment groups immunostaining intensities decreased relative to the control group. In compliance with these results the highest H score was detected in the cisplatin group which was statistically significantly different from that of the control, and treatment groups (p<0.001). H-scores of treatment groups were statistically significantly comparable both among themselves, and also similar to those of the control group (p>0.05), but different from those of the cisplatin group (p<0.001). Interestingly, immunohistochemical expressions of both apoptotic proteins in especially curcumin plus vitamin-E combination group were closer to those of the control group.
In the immunohistochemical analysis of antiapoptotic protein Bcl-2 expression, the highest immunostaining intensity was observed in the control group. As a striking finding, similar increases in Bcl-2 expressions were observed both in the cisplatin, and control groups. In the curcumin plus vitamin-E combination treatment group, immunostaining intensity of this protein increased in favour of the control group. In other treatment groups, weaker immunostaining intensities were detected. In parallel with these findings, mean H-scores of cisplatin, control, or curcumin plus vitamin-E combination groups were found to be statistically comparable. (p>0.05). With this respect, curcumin, and vitamin-E groups were comparable to cisplatin (p>0.05), but different from the control group (p=0.24, and p=0.37, respectively).
Bax/Bcl-2 H-score ratios were at their highest, and lowest levels in the cisplatin, and control groups, respectively. Cisplatin group was statistically significantly different from other groups (p<0.001). Bax/Bcl-2 ratios of all treatment groups were statistically comparable to those of the control, but different from those of the cisplatin group (p>0.05). however only vitamin E group was significantly different form both of the control, and the cisplatin groups (p=0.35, and p=0.001, respectively) (Figure 3).
Figure 3.
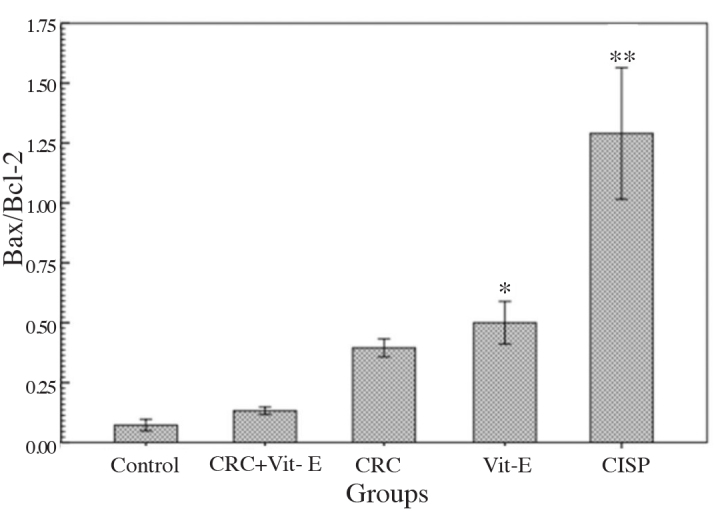
Comparative H-scores of Bax and Bcl-2 proteins in each group relative to the control
*p=0.35; cisplatin p=0.001, **control, and treatment groups p<0.001. CRC: curcumin; Vit-E: vitamin E; CISP: cisplatin
Discussion
A marked increase in the incidence of cancer in all the world is a known fact. According to the data of cancer statistics declared by TR Ministry of Health, total incidence of cancer in our country in the year 2015 was 210.2 per 100,000 population (male, 24.6/100,000, and female, 173.6/100,000). In the treatment of cancer, treatment alternatives include, radiotherapy, surgical intervention, and chemotherapy. For the cancer chemotherapy, taxanes, 5-fluorouracil (5FU), bleomycin, etoposide, and cisplatin are being widely used.
After use of chemotherapeutic agents, adverse side effects as nausea, vomiting, leukopenia, hair loss, diarrhea, nephrotoxicity, and hepatotoxicity are frequently seen. Despite these acute adverse effects, thanks to increase in the number of alternatives of chemotherapy, and diagnostic modalities within the last 2–3 decades, curative response, and 5–10-year-survival rates have increased. At the beginning, the first priority was to survive, and other bodily functions remained in the background. Accordingly, reproductive functions were less frequently considered. However with increase in curative responses, posttreatment reproductive functions started to become more important. It is known that spermatogenesis can be impaired reversibly, and irreversibly after chemotherapy dependent on the duration, and dose of the chemotherapeutic drugs. Based on the degree of testicular involvement, spermatogenesis may recover up to 2 years of treatment. Minimization of spermatogenetic impairment is very important.
Cisplatin is one of the very effective chemotherapeutic drugs used in different cancer types. However its clinical use is restricted because of its many toxic side effects.[1] Especially its adverse effects on male reproductive system target interstitial Leydig cells, seminiferous tubuli epithelium, Sertoli cells, and particularly germ cells leading to reproductive system toxicity.[23] Therefore, prevention of side effects of cisplatin conveys importance, and the ways of preventing side effects of cisplatin in clinical use is still one of the issues of debate. In this study, it has been immunohistochemically detected that cisplatin-induced testicular apoptosis is realized through these Cas-3, and Bax apoptotic protein pathways, and when antioxidant molecules as curcumin, and vitamin E are given individually or especially in combination toxic effects of cisplatin may be precluded with possible reduction in cell death.
Reactive oxygen species developing as a result of normal metabolism, or more frequently due to ischemic conditions, radiation, inflammation, ageing, chemical substances, drugs, and exposure to electromagnetic fields, induce oxidative stress. OS attacks at double-bond containing groups of lipid, and protein structures, and double-bonds of bases in DNA. As a result, macromolecules as intracellular lipid, protein and DNA are damaged, and apoptosis induced by cellular injury occurs.[24]
Since testes contain greater amounts of polyunsaturated membrane lipids, they are among the target organs of OS.[3] Therefore, testes are more frequently effected by many oxidative agents as cisplatin. On the other hand, since testes have low oxygen pressures because of their weaker vascular support, they can defend themselves, despite to a lesser degree against oxidative damage. This condition confers the testes the ability to resist against OS.[25]
Testicular tissue, and spermatogenesis are impaired after development of oxidative damage secondary to many factors including radiotherapy, varicocele, chemotherapy, torsion, infections, and smoking. Multiple number of studies have demonstrated destructive changes in testicular tissue induced by cisplatin. Also in our study, histological impairment was detected in the testes of the rats which received cisplatin as shown in Figure 1.
The present study have indicated that treatment with curcumin, and vitamin E either singly or in combination may have protective antiapoptotic effects against cisplatin-induced testicular apoptosis. This condition was immunohistochemically demonstrated by decrease in the increased expression of caspase-3 which is an indicator of apoptosis of testicular cells induced by cisplatin, decrease in Cas-3, and Bax expressions, and elevated expression of antiapoptotic protein Bcl-2 expression following administration of antioxidant molecules (Figure 2). Besides analyses of general histological images of hematoxylin-eosin stained sections of testicular tissue tend to support this assertion (Figure 1).
Decreased expression of endogenous antioxidants has been reportedly correlated with increased oxidative damage caused by cisplatin treatment. In another study, it has been demonstrated that cisplatin may lead to production of excess amounts of free radicals, and destructive changes in DNA.[13]
Apoptosis is a controlled, and programmed cell death which may become apparent under normal growth and development or as a component of a response to exposure to radiation, toxins or ischemia. Apoptosis has been asserted probably as main outcome of cisplatin-induced toxicity. This argument was found to be compliant with the results of the study. In connection with this assumption, Freitas et al.[21] detected that application of cisplatin at a dose of 16 mg/kg induced apoptosis three days later, as was revealed in our study. Therefore in this experimental study we administered the same dose of cisplatin to the rats.
Curcumin is a multifaceted molecule with anti-inflammatory, antioxidant, and antitumoral activities which may limit side effects of some chemotherapeutic drugs. The mechanism of cellular protection provided by curcumin has not been completely understood. Some evidence suggest that curcumin may demonstrate activity as a free radical repellent, while in some other studies the authors advocate that it encourages formation of free radicals in tumor cells with resultant apoptosis of cancer cells.[26,27]
As is known, curcumin is very well tolerated by the organism. Any evidence of hepatotoxicity was not detected in animals treated with curcumin. Moreover it has been reported that it could alleviate hepatotoxicity induced by other drugs including cisplatin.[18] However, the available data suggest that mitochondrial dysfunction developing in patients treated with cisplatin adversely contribute to both hepatotoxicity, and nephrotoxicity.[17,18]
It has been reported that vitamin E contains tocopherol, tocotrienol, and free radical scavengers, and limits cisplatin-induced nephrotoxicity, and endothelial cell toxicity.[19,20] Vitamin E also protects fatty acids which comprise unsaturated phospholipid membrane from damage incurred by free radicals, and it is a potential inhibitor of lipid peroxidation reactions.[20,28]
Salehi et al.[17] reported that curcumin-dexamethasone combination has a histoprotective effect. Fetoni et al.[18] demonstrated that curcumin had alleviated cisplatin-induced ototoxicity in in vivo setting by increasing expression of oxygenase-1 enzyme. Kalkanis et al.[27] demonstrated that vitamin E supplementation had decreased cisplatin-induced toxicity in rats. In another study, supplementation of vitamin E, and dexamethasone given through intratympanic route was reported to be very effective in alleviation of side effects of cisplatin treatment as supported by our study results.[20]
In another study the anti-cytotoxic effectiveness of curcumin on neural cell culture exposed to cisplatin, and also antigenotoxic effectiveness of different doses of curcumin against genotoxicity induced by cisplatin were investigated. Three different concentrations of curcumin observedly decreased micronuclei density stimulated substantially by cisplatin. This neuronal model demonstrated that antioxidant drugs (curcumin), and chemotherapeutic drugs can be used in combination to decrease genotoxic effects of cancer therapy.[29]
In another study the effects of vitamins E, B, C, and L-carnitine on cisplatin-induced ototoxicity were investigated, and as a result, the researchers observed that these vitamins, and prominently, and especially vitamin E, and L-carnitine had decreased cisplatin-induced ototoxicity.[30]
In our previous study where we investigated protective effects of curcumin, and vitamin E in cisplatin-induced ototoxicity, similar to the result of this study, we detected that these antioxidant molecules alleviated cellular apoptosis induced by cisplatin administration.[14,21]
Based on these literature information, we can say that curcumin, and vitamin E may prevent increased apoptosis caused by cisplatin-induced testicular toxicity thanks to their antioxidant, free-radical scavenger and similar activities. Therefore, these antioxidant molecules were administered both individually, and in combination, and their effects on cisplatin-induced testicular toxicity were evaluated using immunohistochemical analyses of apoptotic pathway proteins.
The results we obtained indicate that cisplatin upregulates immune expressions of apoptotic pathway proteins Cas-3 and Bax, while downregulates immune expression of antiapoptotic protein Bcl-2 leading to testicular apoptosis. However downregulation of Bcl-2 does not occur at a very low level as expected. This condition may be explained by the assumption that the cells activate their own dynamics against cisplatin toxicity, and increase Bcl-2 expression with an attempt to survive. Vitamin E, and curcumin demonstrate their protective effect by increasing expressions of apoptotic proteins (Cas-3, Bax), and anti-apoptotic Bcl-2 protein against cisplatin-induced apoptosis. This condition was revealed by decreasing Bax and Bcl-2 ratios in the treatment groups especially those treated with combination of these two substances. In summary, our available results demonstrate that administration of curcumin, and vitamin E may be a beneficial adjunctive treatment for the protection of testicular germ cells during cisplatin treatment.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Gaziosmanpaşa University School of Medicine.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - F.G.; Design - F.G.; Supervision - F.G., F.E.; Resources - F.G., F.E.; Materials - F.G.; Data Collection and/or Processing - F.G.; Analysis and/or Interpretation - F.G.; Literature Search - F.G., F.E.; Writing Manuscript - F.G.; Critical Review - F.E., F.G.; Other - F.G., F.E.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Lirdi LC, Stumpp T, Sasso-Cerri E, Miraglia SM. Amifostine protective effect on cisplatin-treated rat testis. Anat Rec (Hoboken) 2008;291:797–808. doi: 10.1002/ar.20693. https://doi.org/10.1002/ar.20693. [DOI] [PubMed] [Google Scholar]
- 2.Roldan-Fidalgo A, Martin Saldana S, Trinidad A, Olmedilla-Alonso B, Rodriguez-Valiente A, Garcia-Berrocal JR, et al. In vitro and in vivo effects of lutein against cisplatin-induced ototoxicity. Exp Toxicol Pathol. 2016;68:197–204. doi: 10.1016/j.etp.2016.01.003. https://doi.org/10.1016/j.etp.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Beytur A, Ciftci O, Oguz F, Oguzturk H, Yilmaz F. Montelukast attenuates side effects of cisplatin including testicular, spermatological, and hormonal damage in male rats. Cancer Chemother Pharmacol. 2012;69:207–13. doi: 10.1007/s00280-011-1692-y. https://doi.org/10.1007/s00280-011-1692-y. [DOI] [PubMed] [Google Scholar]
- 4.Kaya K, Ciftci O, Cetin A, Dogan H, Basak N. Hesperidin protects testicular and spermatological damages induced by cisplatin in rats. Andrologia. 2015;47:793–800. doi: 10.1111/and.12332. https://doi.org/10.1111/and.12332. [DOI] [PubMed] [Google Scholar]
- 5.Gonsette RE. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci. 2008;274:48–53. doi: 10.1016/j.jns.2008.06.029. https://doi.org/10.1016/j.jns.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Martinez Y, Li X, Liu G, Bin P, Yan W, Mas D, et al. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 2017;19:1–8. doi: 10.1007/s00726-017-2494-2. https://doi.org/10.1007/s00726-017-2494-2. [DOI] [PubMed] [Google Scholar]
- 7.Anand H, Misro MM, Sharma SB, Prakash S. Protective effects of Eugenia jambolana extract versus N-acetyl cysteine against cisplatin-induced damage in rat testis. Andrologia. 2015;47:194–208. doi: 10.1111/and.12247. https://doi.org/10.1111/and.12247. [DOI] [PubMed] [Google Scholar]
- 8.Pectasides D, Pectasides E, Papaxoinis G, Skondra M, Gerostathou M, Karageorgopoulou S, et al. Testicular function in poor-risk nonseminomatous germ cell tumors treated with methotrexate, paclitaxel, ifosfamide, and cisplatin combination chemotherapy. J Androl. 2009;30:280–6. doi: 10.2164/jandrol.108.006437. https://doi.org/10.2164/jandrol.108.006437. [DOI] [PubMed] [Google Scholar]
- 9.Takeshita H, Chiba K, Kitayama S, Moriyama S, Omura R, Noro A. Triplet chemotherapy with paclitaxel, gemcitabine, and cisplatin as second-line therapy for advanced urothelial carcinoma. Mod Chemother. 2013;2:1. https://doi.org/10.4236/mc.2013.21001. [Google Scholar]
- 10.Colpi GM, Contalbi GF, Nerva F, Sagone P, Piediferro G. Testicular function following chemo-radiotherapy. Eur J Obstet Gynecol Reprod Biol. 2004;113(Suppl 1):S2–6. doi: 10.1016/j.ejogrb.2003.11.002. https://doi.org/10.1016/j.ejogrb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Yumusakhuylu AC, Yazici M, Sari M, Binnetoglu A, Kosemihal E, Akdas F, et al. Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol. 2012;76:404–8. doi: 10.1016/j.ijporl.2011.12.021. https://doi.org/10.1016/j.ijporl.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Bilmez ZEB, Aydin S, Sanli A, Altintoprak N, Demir MG, Erdogan BA, et al. Oxytocin as a protective agent in cisplatin-induced ototoxicity. Cancer Chemoth Pharm. 2016;77:875–9. doi: 10.1007/s00280-016-2978-x. https://doi.org/10.1007/s00280-016-2978-x. [DOI] [PubMed] [Google Scholar]
- 13.Sagit M, Korkmaz F, Akcadag A, Somdas MA. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013;270:2231–7. doi: 10.1007/s00405-012-2254-6. https://doi.org/10.1007/s00405-012-2254-6. [DOI] [PubMed] [Google Scholar]
- 14.Soyalıç H, Gevrek F, Koç S, Avcu M, Metin M, Aladağ İ. Intraperitoneal curcumin and vitamin E combination for the treatment of cisplatin-induced ototoxicity in rats. Int J Pediatr Otorhinolaryngol. 2016;89:173–8. doi: 10.1016/j.ijporl.2016.08.012. https://doi.org/10.1016/j.ijporl.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Strich R. Programmed Cell Death Initiation and Execution in Budding Yeast. Genetics. 2015;200:1003–14. doi: 10.1534/genetics.115.179150. https://doi.org/10.1534/genetics.115.179150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yildirim NC, Kandemir FM, Benzer F. Beneficial effects of grape seed extract against cisplatın-induced testiculer damage in rabbits. Dig J Nanomat Biostruct. 2011;6:155–9. [Google Scholar]
- 17.Salehi P, Akinpelu OV, Waissbluth S, Peleva E, Meehan B, Rak J, et al. Attenuation of cisplatin ototoxicity by otoprotective effects of nanoencapsulated curcumin and dexamethasone in a guinea pig model. Otol Neurotol. 2014;35:1131–9. doi: 10.1097/MAO.0000000000000403. https://doi.org/10.1097/MAO.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 18.Fetoni AR, Eramo SLM, Paciello F, Rolesi R, Podda MV, Troiani D, et al. Curcuma Longa (Curcumin) Decreases In Vivo Cisplatin- Induced Ototoxicity Through Heme Oxygenase-1 Induction. Otol Neurotol. 2014;35:E169–E77. doi: 10.1097/MAO.0000000000000302. https://doi.org/10.1097/MAO.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80:1613–31. doi: 10.1016/j.bcp.2010.07.043. https://doi.org/10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paksoy M, Ayduran E, Sanli A, Eken M, Aydin S, Oktay ZA. The protective effects of intratympanic dexamethasone and vitamin E on cisplatin-induced ototoxicity are demonstrated in rats. Med Oncol. 2011;28:615–21. doi: 10.1007/s12032-010-9477-4. https://doi.org/10.1007/s12032-010-9477-4. [DOI] [PubMed] [Google Scholar]
- 21.De Freitas MR, Figueiredo AA, Brito GA, Leitao RF, Carvalho Junior JV, Gomes Junior RM, et al. The role of apoptosis in cisplatin-induced ototoxicity in rats. Braz J Otorhinolaryngol. 2009;75:745–52. doi: 10.1016/S1808-8694(15)30528-0. https://doi.org/10.1016/S1808-8694(15)30528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soyalıç H, Gevrek F, Karaman S. Curcumin Protects Against Acoustic Trauma in the Rat Cochlea. Int J Pediatr Otorhinolaryngol. 2017;99:100–6. doi: 10.1016/j.ijporl.2017.05.029. https://doi.org/10.1016/j.ijporl.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr. 2005;34:6–8. doi: 10.1093/jncimonographs/lgi006. https://doi.org/10.1016/j.ijporl.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Yan LJ. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014;2:165–9. doi: 10.1016/j.redox.2014.01.002. https://doi.org/10.1016/j.redox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. https://doi.org/10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007;43:568–80. doi: 10.1016/j.freeradbiomed.2007.05.009. https://doi.org/10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalkanis JG, Whitworth C, Rybak LP. Vitamin E reduces cisplatin ototoxicity. Laryngoscope. 2004;114:538–42. doi: 10.1097/00005537-200403000-00028. https://doi.org/10.1097/00005537-200403000-00028. [DOI] [PubMed] [Google Scholar]
- 28.Villani V, Zucchella C, Cristalli G, Galie E, Bianco F, Giannarelli D, et al. Vitamin E neuroprotection against cisplatin ototoxicity: Preliminary results from a randomized, placebo-controlled trial. Head Neck. 2016;38(Suppl 1):E2118–21. doi: 10.1002/hed.24396. https://doi.org/10.1002/hed.24396. [DOI] [PubMed] [Google Scholar]
- 29.Mendonca LM, Dos Santos GC, Antonucci GA, Dos Santos AC, de Bianchi ML, Antunes LM. Evaluation of the cytotoxicity and genotoxicity of curcumin in PC12 cells. Mutat Res. 2009;675:29–34. doi: 10.1016/j.mrgentox.2009.02.003. https://doi.org/10.1016/j.mrgentox.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Tokgoz SA, Vuralkan E, Sonbay ND, Caliskan M, Saka C, Besalti O, et al. Protective effects of vitamins E, B and C and L-carnitine in the prevention of cisplatin-induced ototoxicity in rats. J Laryngol Otol. 2012;126:464–9. doi: 10.1017/S0022215112000382. https://doi.org/10.1017/S0022215112000382. [DOI] [PubMed] [Google Scholar]


