Abstract
Objective
We aimed to compare the oncological outcomes of patients with variant urothelial histologies (VH) with pure urothelial histology (PUH) in bladder cancer (BC) patients.
Material and methods
This study includes 223 patients who underwent radical cystectomies (RCs) between September 2006 and July 2016 with complete follow-up data A retrospective screening was performed to identify the patients with PUH and VH. The primary outcomes of interest were pathological stage of disease at RC and disease-specific survival (DSS). For comparison of categorical variables, Fisher’s exact test and Pearson chi- square and for continuous variables Wilcoxon rank-sum and Mann-Whitney U tests were used. Kaplan-Meier (KM) method was used for survival analysis and log-rank test was used for comparison of survival rates. Predictors of survival were detected with mulitivariable Cox-proportional hazards model including the variables such as gender, age, existence of VH, lymph node dissection (LND) type and pathological stage of the disease.
Results
A moderate-degree correlation was detected between VH and pathological stages of RC (r=0.45, p<0.001). In PUH group, 39 (25.8%) of 151 patients died after a median follow-up of 20 (0–107) months; whereas 37 (51.4%) of 72 patients with VH died after a median follow-up of 16.5 (0–104) months (p<0.001). In terms of pathological stage, the number of patients with PUH and VH were at stages pT0–2 (n=100; 66.2% vs. n=19; 26.4%), pT3–4 (n=35; 23.2% vs. 38; 52.8%, and in 16 (10.6%) and 15 (20.8%) patients with LN positivity, respectively (p<0.001). KM survival analysis revealed a significantly decreased DSS in patients with VH compared to PUH (p<0.001). Meanwhile, pathological disease stage and existence of VH were found to be associated with decreased DSS in the multivariate model.
Conclusion
The present study revealed that VH is associated with advanced pathological tumor stage at RC and decreased DSS compared to patients with PUH in patients with BC.
Keywords: Bladder cancer, histology, squamous, staging, survival
Introduction
Bladder cancer (BC) is a serious health problem worldwide in that it is the 9th most common cancer and the 13th cause of death from cancer.[1] According to the GLOBOCAN, the highest incidence rates were observed in men in Southern and Western Europe, North America, as well as certain countries in Northern Africa and Western Asia.[2] Turkey is one of the five countries with the highest age-standardized incidence (16.6/100.000) as well as the highest age-standardized death rate (6.6/100.000).[3] According to the data by Turkish Ministry of Health, Department of Fight Against Cancer, BC is the 3rd frequent cancer type after lung and prostate cancers in men.[4] Since BC is known as the most expensive cancer ever treated, it imposes a significant cost on Turkish health economy.[5]
Bladder cancer is generally evaluated in two categories as non-muscle invasive BC (NMIBC) that is confined to mucosa and muscle invasive BC (MIBC) that invades detrusor muscle. The latter one is more aggressive and likely to metastasizes either to associated lymph nodes or distant organs.[6] The gold-standard treatment of patients with MIBC is radical cystectomy (RC) in compliance with thorough loco-regional (pelvic) lymph node dissection (LND) and urinary diversion. Stage, and histology of the disease and histology is known as the most important factors for survival in MIBC.
Currently, histology is the only reliable determining factor for tumor biology. BC generally develops from urothelium; a specific form of epithelium that covers the inner surface of bladder and much of the urinary tract. However, in 10–25% of the cases, BC histology is different from the pure urothelial histology (PUH) and it is called “variant histology” (VH) referring to all pathologies in BC other than PUH.[7] Evidence from a retrospective analyses revealed that, patients with VH have more aggressive disease in comparison with PUH and poor response to existing therapies such as RC [7]. However, recent studies question this acceptance and noted that VHs might not be associated with decreased survival rates.[8,9] Accordingly, the aim of this retrospective report is to investigate the relationship between VH and oncological outcomes after RC such as pathological stage of BC and disease specific survival (DSS).
Material and methods
The present study includes 223 patients who underwent RC between September 2006 and July 2016 for MIBC or patients with NMIBC who failed intravesical treatment and complete follow-up data were available for DSS. All patients were preoperatively evaluated with abdomino-pelvic computed tomography (CT) or magnetic resonance imaging (MRI) for local clinical staging. Meanwhile, non-contrast thorax CT and bone scintigraphy or PET-CT were used to determine distant organ metastasis. Ethical committee approval was not obtained for this study which is not mandatory for retrospective studies in our institution. However, informed consent was obtained from all study population.
Radical cystectomy was performed in a standardized fashion together with loco-regional (pelvic) LND and subsequent urinary diversion. Standard LND, including removal of external iliac, obturator fossa, internal iliac and common iliac lymph nodes up to the ureteric crossing was applied to all cases after 2010. Before this date, a limited LND including obturatory lymph nodes were the common practice at our institution.
Pathological analyses were performed by pathologists specialized in the histopathology of the urinary system. (YO, IK). BC pathologies other than pure urothelial histology (PUC) were categorized as VH. When more than one VH were found, the predominant histological type was taken into consideration. Pathological staging was performed according to the 2010 Tumor-Node-Metastasis classification by 7th American Joint Committee on Cancer (AJCC).
Clinical and radiological follow-ups of the patients consisted of a baseline visit at 3 months of the surgery including abdomino-pelvic CT or MRI as well as thorax CT. After this initial visit, all patients were clinically and radiologically followed up at least twice a year. Neoadjuvant and adjuvant chemotherapy were offered to most of the patients with good renal function. However, chemotherapy regimens varied and thus not included in the statistical analyses.
To facilitate statistical analysis, 3 pathological categorizations were done. In the first categorization, patients were evaluated in three disease groups at final pathology (RC) as T0–2 (vesical), T2–4 (extravesical) and LN positive disease. In the second one, T staging at RC was grouped as organ confined (T0–T2) versus non-organ confined disease (pT3–pT4 and/orLN (+)). Finally, patients were also grouped as NMIBC and MIBC according to RC pathologies. Meanwhile as a clinical categorization, patients were divided into two groups as patients aged between 24 and 65 (adults, n=130) years and over 65 years (seniors, n=132). In addition, patients with squamous differentiation as a VH were evaluated separately and compared with patients with PUH.
Statistical analysis
In the present report, Fisher’s exact and Pearson chi-square tests for categorical variables and Wilcoxon rank-sum and Mann-Whitney U tests for continuous variables were used to compare demographic and pathological characteristics of the groups. Meanwhile, Spearman’s test was used to power the correlation between RC pathology stages and existence of VH. As the primary outcome of the study, DSS for patients with and without VH was estimated with Kaplan-Meier method and compared with log-rank test. Meanwhile, multivariate model was created by using the parameters such as gender, adults vs. seniors, LND type and stage of disease at final pathology. Hazard ratios (HRs) were calculated with 95% confidence intervals (CI) and in all calculations p values <0.05 was considered to be significant. All analyses were performed with Statistical Package for the Social Sciences (IBM SPSS Statistics, Armonk, NY, USA) version 22.0.
Results
The mean age of the overall patient cohort (n=223) was 64.2±8.7 (41–85) years; whereas 204 (91.5%) of those patients were men. Local pathological stages after RC were reported as carcinoma in situ (CIS) (n=8; 3.6%), pT0 (n=28; 12.6%), pTa (n=25; 11.2%), pT1 (n=15; 6.7%), pT2 (n=36; 16.1%), pT3 (n=77; 34.5%), and pT4 (n=34; 15.2%) in indicated number of patients. Also 151 (67.7%) PUH and 72 (32.3%) VH patients were detected. Patients with VH, had pT0 (7.1%), pTa (4.0%), pT1 (6.7%), pT2 (25%), pT3 (50.6%) and pT4 (58.8%) disease. A moderate-degree correlation was detected between VH and increasing degree of final pathological stages (r=0.45, and p<0.001). Most of the VHs were squamous differentiation (66.7%, Table 1). In overall cohort, patients underwent limited 169 (75.8%), and standard LND 54 (24.2%). The median number of LNs was found to be 8 (mean 9.15±6.7 [1–36]) for the overall cohort. The median follow-up of the patient cohort was 19 (mean 32.1±29.9, [0–107]) months. Within this period, 76 of 223 (34%) patients died of BC.
Table 1.
Rates of histopathological types detected in our study
| Histology | Frequency | % |
|---|---|---|
| Squamous differentiation | 48 | 66.6 |
| Squamous cell carcinoma | 6 | 8.3 |
| Signet ring pattern | 4 | 5.5 |
| Sarcomatoid carcinoma | 3 | 4.2 |
| Small cell differentiation | 3 | 4.2 |
| Sarcomatoid differentiation | 2 | 2.8 |
| Nested pattern | 2 | 2.8 |
| Micropapillary pattern | 2 | 2.8 |
| Large cell undifferentiated pattern | 1 | 1.4 |
| Plasmocytoid variant | 1 | 1.4 |
| Total | 72 | 100 |
In more detail, the median follow-up for patients with PUH and VH were 20 (mean 35±31 [0–107]) and 16.5 (mean 25.8±26.6 [0–104]) months, respectively (p=0.02). During their follow-up, 39 (25.8%) of 151 patients with PUH, and 37 (51.4%) of 72 in patients with VH died (p<0.001). The patients with PUH, had pT0–2 (n=100; 66.2%), pT3–4 (n=35; 23.2%) and LN positive (n=16; 10.6%) diseases. This figure was 19 (26.4%), 38 (52.8%) and 15 (20.8%) patients for the same pathological groups in patients with VH (p<0.001). Moreover, the rate of non-organ confined disease (pT3–4 and/or LN (+) disease) at RC pathology was found to be significantly higher in patients with VH in comparison with patients with PUH (Table 2). On the contrary, identification of NMIBC at RC pathology was significantly lower in the former group in comparison with the latter group (Table 2). Of note, there was no statistical difference between the patients with PUH and VH patients in terms of mean total LN count (median 8 vs. 8.5 [mean 9.18±6.6 vs. 9.08±6.9], p=0.82).
Table 2.
Demographic and final pathological characteristics (n=223) of the follow-up cohort.
| PUH | VH | p | Squamous differentiation | p | |
|---|---|---|---|---|---|
| Patients, n | 151 | 72 | - | 48 | - |
|
| |||||
| Age (years) | 64.6±8.5 | 63.3±9.1 | 0.52* | 63 | 0.67* |
|
| |||||
| Gender | 0.62** | 0.60** | |||
| Female | 12 (7.9) | 7 (9.7) | 5 (10.4%) | ||
| Male | 139 (92.1) | 65 (90.3) | 43 (89.6%) | ||
|
| |||||
| Adults vs. seniors | 0.15** | 0.12** | |||
| Adults | 72 (47.7) | 42 (58.3) | 29 (60.4%) | ||
| Seniors | 79 (53.2) | 30 (41.7) | 19 (39.6%) | ||
|
| |||||
| Histopathological stage of the disease (%) | <0.001** | <0.001** | |||
| Organ confined (pT0–pT2) | 94 (62.3) | 14 (19.4) | 9 (18.8%) | ||
| Non-organ confined (pT3–pT4 and/orLN(+)) | 57 (37.7) | 58 (80.6) | 39 (81.3%) | ||
|
| |||||
| LN involvement | 0.06** | 0.31** | |||
| Positive | 16 (10.6) | 15 (20.8) | 8 (16.7%) | ||
| Negative | 135 (89.4) | 57 (79.2) | 40 (83.3%) | ||
|
| |||||
| NMID vs. MID at final pathology | <0.001** | <0.001** | |||
| NMID | 73 (48.3) | 3 (4.2) | 3 (6.3%) | ||
| MID | 78 (51.7) | 68 (95.8) | 45 (93.8%) | ||
Independent samples t-test,
Pearson chi-square test.
PUH: pure urothelial histology; VH: variant histology; NMID: non-muscle invasive disease; MID: muscle invasive disease; LN: lymph node
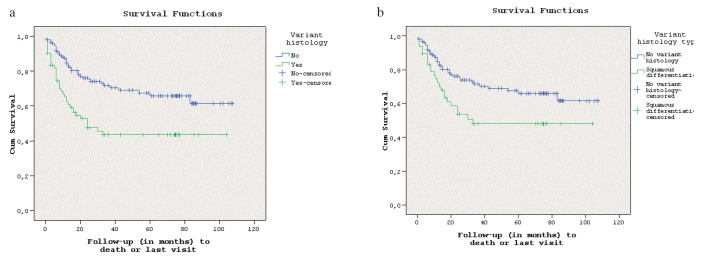
Kaplan-Meier survival analysis revealed that BC patients with PUH lives significantly longer than patients with VH (Log-rank test, p<0.001, Figure 1a). One year and 4-years estimated DSS was calculated to be 76% and 67%; 53% and 43% for patients with PUH and patients with VH, respectively. The multivariate Cox regression analysis revealed that final disease stage (T0–T2 vs. T3–4 vs. LN (+) disease) and having VH is associated with worse DSS; whereas gender, age (adult vs. seniors) and LND type were found to have no relationship with DSS of these patients (Table 3).
Figure 1. a, b.
(a) Kaplan-Meier survival analysis of PUH vs. VH for DSS (log-rank test, p=0.01). (b) Kaplan-Meier survival analysis of PUH vs. squamous differentiation for DSS (log-rank test, p=0.01)
PUH: pure urothelial histology; VH: variant histology; DSS: disease-specific survival
Table 3.
Multivariate Cox regression analyses for the predictors of disease specific survival
| Covarites | Whole cohort (n=223) | Whole cohort excluding patients with VH other than squamous differantiation (n=199) | ||
|---|---|---|---|---|
|
|
|
|||
| Disease specific survival | ||||
|
| ||||
| HR (95% CI) | p | HR (95% CI) | p | |
| Gender (male vs. female) | 0.98 (0.41–2.3) | 0.97 | 0.7 (0.24–1.9) | 0.50 |
|
| ||||
| Age (adults vs. seniors) | 1.2 (0.7–1.9) | 0.36 | 1.3 (0.82–2.3) | 0.21 |
|
| ||||
| VH vs. PUH | 1.7 (1.0–2.8) | 0.029 | 1.4 (0.82–2.6) | 0.19 |
|
| ||||
| LN dissection type (limited vs. standard) | 1.3 (0.76–2.3) | 0.30 | 1.4 (0.78–2.8) | 0.22 |
|
| ||||
| Stage of disease | ||||
|
| ||||
| pT0–2 | Reference | - | Reference | |
|
| ||||
| pT3–4 | 2.0 (1.1–3.6) | 0.01 | 2.0 (1.1–3.8) | 0.02 |
|
| ||||
| LN positive | 3.9 (1.9–7.8) | <0.001 | 4.2 (1.8–8.4) | <0.001 |
LN: lymph node; PUH: pure urothelial histology; VH: variant histology
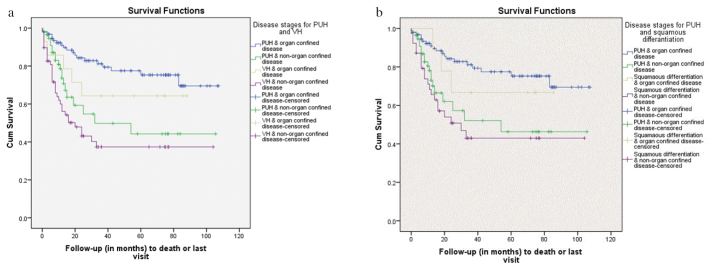
When survivals of BC patients with PUH were compared according to disease stages; 1 and 4 years of DSS were found to be 92% and 77% for patients with organ confined disease and 78% and 50% for patients with non-organ confined disease. For patients with VH, 1 and 4 years of DSS were found to be 86% and 64% for patients with organ confined disease; whereas these rates were 60% and 36% for non-organ confined disease. In pairwise comparisons with Wilcoxon rank-sum test, DSS was significantly better in patients with PUH in patients with non-organ confined disease in comparison with patients with VH (p=0.038). Meanwhile, no statistically significant difference was detected between the survivals of patients with organ confined disease in terms of having PUH or VH (p=0.161) (Figure 2a).
Figure 2. a, b.
(a) Kaplan-Meier survival analysis of patients with PUH and VH for DSS according to the disease stages (organ confined vs. non-organ confined disease, p<0.01, log-rank test). (b) Kaplan-Meier survival analysis of patients with PUH and squamous differentiation for DSS according to the disease stages (organ confined vs. non-organ confined disease, p<0.01, log-rank test)
PUH: pure urothelial histology; VH: variant histology; DSS: disease-specific survival
In the present study, 48 patients had squamous differentiation with a mean age of 63.0±9.1 (41–85) years. Of these patients, 43 (89.6%) were men. After a median follow-up of 29.2 (0–104) months, 23 (47.9%) of them died of BC. This difference, in comparison with patients with PUH was statistically significant (p=0.007). Of patients with squamous differentiation, 11 (22.9%), 29 (60.4%) and 8 (16.7%) had pT0–2 disease, pT3–4 disease and LN positive disease, respectively. This figure was statistically different from patients with PUH patients (p<0.001). Meanwhile, the rate of non-organ confined disease at RC was found to be significantly higher; while the rate of NMIBC at RC was found to be significantly lower in patients with squamous differentiation compared to patients with PUH (Table 2).
Comparison of survivals of patients with PUH and squamous differentiation revealed a statistically significant difference in favor of patients with PUH (p=0.01, Figure 1b). One year and 4-year estimated DSS was calculated to be 76% and 67%; 58% and 47% for patients with PUH and patients with squamous differentiation, respectively (p<0.001). In the multivariate Cox regression analysis, it was noted that stage (T0–T2 vs. T3–4 vs. LN (+) disease) was found to be associated with worse DSS; while gender, age (adult vs. seniors), having squamous differentiation and LND type were found to have no relationship with survival of the patients (Table 3).
In patients with squamous differentiation, 1 and 4 years of DSS was found to be 100% and 67% in patients with organ-confined disease, 68% and 42% in patients with non-organ confined disease. The KM curves of patients with PUH and squamous differentiation for each of the stage groups were given in Figure 2b. There was no statistically significant difference in term of DSS between the patients with PUH and squamous differentiation for organ-confined (p=0.53) and non-organ confined disease (p=0.30).
Discussion
It is known that the existence of VH in TUR specimens is associated with higher disease stages. By using a cohort over 28.000 patients, Ploeg et al.[10] reported that 7.7% of patients undergoing TURBT had variant non-urothelial histology; whereas only 23% of them were diagnosed at T1 stage. Meanwhile, the rate of MIBC was 52.5% in patients with PUH; whereas this rate was over 70% in all other non-urothelial VHs. Similarly, Shapur et al.[11] found that 57 (72%) of 79 patients with VH were found to have MIBC; whereas patients with VH were only 10.4% of the study cohort. Moreover, 5-year muscle invasion free survival rates were 84.4% and 63.1% (p=0.02) for patients with PUH vs. VH. On the other hand, Monn et al.[12] reported that 61% of patients with PUH had evidence of muscle invasion on TURBT compared with 79.6% of those with VH (p=0.001). Similar to our study, patients with VH were found to be at higher pathological stages at RC in comparison with PUH (p<0.001). Congruently, the present study has clearly shown that patients with VH present at a higher pathological stage at RC compared to patients PUH. Of patients with PUH, 37.8% patients had non-organ confined disease; whereas this figure was 80.6% for patients with VH (p<0.001, Table 2). Moreover, we detected a significant correlation between final pathological stage at RC and existence of VH. For this reason, we believe that patients with VH might get benefit multimodality treatment strategy depending on individual histology because of their aggressive oncological features in spite of conflicting results in the literature on survival of these patients.
Disease specific survival was one of the primary outcomes of the present study and Cox multivariate regression analysis revealed that the presence of VH is associated with 1.7 times of worse DSS (p=0.029). Rogers et al.[13] compared urothelial and non-urothelial histologies of 955 patients who underwent RC and reported that patients with non-urothelial carcinoma and non-squamous carcinoma such as adenocarcinoma, small cell carcinoma or carcinosarcoma had significantly increased risk for progression and death compared with PUH or squamous cell carcinoma (p<0.001). Three and 5 year DSSs were found to be 71.9±2% and 67.9±2%; 27.6±9% and 18.4±8% for the groups, respectively. Recently, Soave et al.[14] reported the relationship between preoperative circulating tumor cells (CTC) count, BC histopathology and survival of 188 patients. They reported that patients with non-squamous differentiation had inferior recurrence free survival (RFS), compared to patients with PUH and patients with squamous cell differentiation (p=0.016). Moreover, the presence of CTC and non-squamous cell differentiation were associated with decreased RFS and DSS after a median follow-up of 25 months. However, it is worth mentioning that BC histology was not an independent factor for RFS and CSS.
Despite this evidence in the literature addressing the relationship between VH and survival, some studies did not confirm DSS disadvantage of VH. Wasco et al.[15] evaluated the oncological outcomes of 295 subsequent RC patients and reported that VH did not predict DSS on multivariate logistic model (p=0.68). This situation was attributed to the limited follow-up time leading a very few cancer specific deaths that limited the statistical power. Meanwhile similar to our study, the predictors of DSS were found as stage of disease and LN status. In another study, Moschini et al.[16] retrospectively evaluated 1.067 patients who underwent RC and noted the predictors for disease recurrence, cancer specific mortality (CSM) and overall mortality (OM) as Charlson comorbidity index score, stage of disease, positive LN count and having small cell variant. Meanwhile, they found no difference in terms of disease recurrence, CSM and OM between the various histologic variants except small cell histology after a median follow-up of 6.5 years after surgery. Consequently, the impact of VHs on survival parameters is controversial and for overcoming the rarity of some subtypes for statistical comparisons, prospective multi-institutional studies are needed.
In our series, squamous variant was the predominant pathology among patients with VH. Generally, the oncological outcomes of the patients with squamous differentiation are conflicting. Indeed completely diverse outcomes have been reported in various studies. Antunes et al.[17] in a retrospective review of 113 patients reported that the disease-specific death (DSD) rates were significantly higher in patients with squamous differentiation (40%) in comparison with PUH (16%, p=0.002). Moreover, squamous differentiation was found to be a predictor of CSS in a multivariate model with a HR of 3.51 (p=0.003). On the contrary, an older retrospective series comprising of 531 patients found that squamous differentiation identified in the final surgical specimen is not one of the predictors of DSS in multivariate logistic regression model (p>0.05).[18] Recently, Kim et al.[9] reported that either squamous or glandular differentiation (n=186) was not significantly associated with the risk of death from BC in spite of the presence of significantly greater number of patients with pT3–4 (70% vs. 38%, p<0.0001) and LN (+) disease (20% vs. 15%, p=0.05) compared to patients with PUH (n=827). Similar outcomes were also reported by Ehdaie et al.[19] who followed up 67 patients with VH and compared them with 1.867 patients with PUH. They found no difference in KM survival analysis for CSS (p=0.17) between the groups as well as squamous differentiation was not found to be a predictor of CSS in a multivariate model. Lastly, Mitra et al. compared patients with squamous differentiation to patients with PUH by using an intensive match-pair analysis where the two groups are balanced with various parameters such as pathological stage, administration of intravesical agents, neoadjuvant or adjuvant chemotherapy. The authors reported no difference in terms of RFS (p=0.79) and overall survival (p=0.40) between the two groups after a median follow-up of 15.2 and 11.0 months, respectively.[8] In our series, we found that patients with squamous differentiation have significantly lower survival rates than the patients with PUH. However, in the multivariate model, this VH type was not found as a variable affecting survival. Thus, to our opinion, squamous differentiation is better to be considered as a milder form of VH and clinical decision- making process for patients with this histopathology should not differ from those patients with PUH.
In this study, we found that presence of any kind of VH is associated with decreased DSS; whereas squamous differentiation as a VH is not. Despite, we do not have data to explain this discrepancy, and we believe that less frequent variants of BC (other than squamous differentiation) as mentioned in Table 1 represent a more aggressive form of the disease. For this reason, multi-institutional studies with a central pathological evaluation should be done to understand the precise oncologic behavior of these rare VHs.
This study has some limitations that merit mentioning. Firstly, the retrospective nature of the study and the size of the patient cohort limit its value in comparison with other contemporary series with larger sample sizes. However as its major strength, this study includes one center experience that prevents variabilities in terms of pathological reporting. Secondly, median follow-up period (≈2 years) of the patient cohort also limits long-term predictions regarding the existence of VH at RC pathology specimens. This is partly due to referral nature of our practice which also shadows our accumulation of neoadjuvant or adjuvant chemotherapy data. Despite these weak points, we believe that our study represents a real life experience from a developing country which should recognize BC as a serious health problem. Thirdly, despite the number of LNs were not statistically different between groups; the majority of the population received limited LND which is currently considered undertreatment and might have affected survival outcomes. Finally, we did not evaluate the extent of VHs in the pathological specimens. However, 2 studies failed to show a significant relationship between the extent of VH and the oncological outcomes.[9,15]
In conclusion, patients with VH are more likely to demonstrate advanced histopathological stage at RC which is associated with decreased DSS. Therefore, an aggressive multimodal approach should be carried out for the treatment of these patients. Despite, the presence of squamous differentiation as a subgroup of VH is also associated with advanced pathological stage but not with decreased DSS.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Ö.Ş.; Design – Ö.Ş., A.B.; Supervision – Ö.Ş., S.E., F.Ö., M.T.; Resources – Ö.Ş.; Materials – A.B., Ö.Ş., S.E.; Data Collection and/or Processing – A.B., Ö.Ş., S.E.; Analysis and/or Interpretation – A.B., Ö.Ş., S.E.; Literature Search – Ö.Ş., T.T.; Writing Manuscript – A.B., Ö.Ş.; Critical Review – Ö.Ş., S.E., I.K.; Other – I.K., Y.Ö.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. https://doi.org/10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Mahdavifar N, Ghoncheh M, Pakzad R, Momenimovahed Z, Salehiniya H. Epidemiology, Incidence and Mortality of Bladder Cancer and their Relationship with the Development Index in the World. Asian Pac J Cancer Prev. 2016;17:381–6. doi: 10.7314/apjcp.2016.17.1.381. https://doi.org/10.7314/APJCP.2016.17.1.381. [DOI] [PubMed] [Google Scholar]
- 4.Aydin S, Boz MY. Rapid changes in the incidence of urinary system cancers in Turkey. Turk J Urol. 2015;41:215–20. doi: 10.5152/tud.2015.45548. https://doi.org/10.5152/tud.2015.45548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svatek RS, Hollenbeck BK, Holmang S, Lee R, Kim SP, Stenzl A, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66:253–62. doi: 10.1016/j.eururo.2014.01.006. https://doi.org/10.1016/j.eururo.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Sanli O, Dobruch J, Knowles MA, Burger M, Alemozaffar M, Nielsen ME, et al. Bladder cancer. Nat Rev Dis Primers. 2017;3:17022. doi: 10.1038/nrdp.2017.22. https://doi.org/10.1038/nrdp.2017.22. [DOI] [PubMed] [Google Scholar]
- 7.Willis D, Kamat AM. Nonurothelial bladder cancer and rare variant histologies. Hematol Oncol Clin North Am. 2015;29:237–52. doi: 10.1016/j.hoc.2014.10.011. https://doi.org/10.1016/j.hoc.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Mitra AP, Bartsch CC, Bartsch G, Jr, Miranda G, Skinner EC, Daneshmand S. Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? An intensive case-control analysis. Urol Oncol. 2014;32:117–27. doi: 10.1016/j.urolonc.2012.08.017. https://doi.org/10.1016/j.urolonc.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Kim SP, Frank I, Cheville JC, Thompson RH, Weight CJ, Thapa P, et al. The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. J Urol. 2012;188:405–9. doi: 10.1016/j.juro.2012.04.020. https://doi.org/10.1016/j.juro.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Ploeg M, Aben KK, Hulsbergen-van de Kaa CA, Schoenberg MP, Witjes JA, Kiemeney LA. Clinical epidemiology of nonurothelial bladder cancer: analysis of the Netherlands Cancer Registry. J Urol. 2010;183:915–20. doi: 10.1016/j.juro.2009.11.018. https://doi.org/10.1016/j.juro.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Shapur NK, Katz R, Pode D, Shapiro A, Yutkin V, Pizov G, et al. Is radical cystectomy mandatory in every patient with variant histology of bladder cancer. Rare Tumors. 2011;3:e22. doi: 10.4081/rt.2011.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monn MF, Kaimakliotis HZ, Pedrosa JA, Cary KC, Bihrle R, Cheng L, et al. Contemporary bladder cancer: variant histology may be a significant driver of disease. Urol Oncol. 2015;33:18.e5–20. doi: 10.1016/j.urolonc.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Rogers CG, Palapattu GS, Shariat SF, Karakiewicz PI, Bastian PJ, Lotan Y, et al. Clinical outcomes following radical cystectomy for primary nontransitional cell carcinoma of the bladder compared to transitional cell carcinoma of the bladder. J Urol. 2006;175:2048–53. doi: 10.1016/S0022-5347(06)00317-X. https://doi.org/10.1016/S0022-5347(06)00317-X. [DOI] [PubMed] [Google Scholar]
- 14.Soave A, Riethdorf S, Dahlem R, Minner S, Weisbach L, Engel O, et al. Detection and oncological effect of circulating tumour cells in patients with variant urothelial carcinoma histology treated with radical cystectomy. BJU Int. 2017;119:854–61. doi: 10.1111/bju.13782. https://doi.org/10.1111/bju.13782. [DOI] [PubMed] [Google Scholar]
- 15.Wasco MJ, Daignault S, Zhang Y, Kunju LP, Kinnaman M, Braun T, et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urology. 2007;70:69–74. doi: 10.1016/j.urology.2007.03.033. https://doi.org/10.1016/j.urology.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Moschini M, Dell’Oglio P, Luciano R, Gandaglia G, Soria F, Mattei A, et al. Incidence and effect of variant histology on oncological outcomes in patients with bladder cancer treated with radical cystectomy. Urol Oncol. 2017;35:335–41. doi: 10.1016/j.urolonc.2016.12.006. https://doi.org/10.1016/j.urolonc.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Antunes AA, Nesrallah LJ, Dall’Oglio MF, Maluf CE, Camara C, Leite KR, et al. The role of squamous differentiation in patients with transitional cell carcinoma of the bladder treated with radical cystectomy. Int Braz J Urol. 2007;33:339–46. doi: 10.1590/s1677-55382007000300006. https://doi.org/10.1590/S1677-55382007000300006. [DOI] [PubMed] [Google Scholar]
- 18.Frazier HA, Robertson JE, Dodge RK, Paulson DF. The value of pathologic factors in predicting cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer. 1993;71:3993–4001. doi: 10.1002/1097-0142(19930615)71:12<3993::aid-cncr2820711233>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Ehdaie B, Maschino A, Shariat SF, Rioja J, Hamilton RJ, Lowrance WT, et al. Comparative outcomes of pure squamous cell carcinoma and urothelial carcinoma with squamous differentiation in patients treated with radical cystectomy. J Urol. 2012;187:74–9. doi: 10.1016/j.juro.2011.09.056. https://doi.org/10.1016/j.juro.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]