Abstract
Objective
Urinary tract infection is a common pediatric problem with the potential to produce long-term morbidity. Therefore, appropriate diagnosis and prompt treatment is required. However, studies about magnitude of uropathogenicity and antimicrobial resistance pattern of pediatric urinary tract infection (UTI) are lacking in resource limited countries including Ethiopia. This study was aimed to determine bacterial isolates, antimicrobial susceptibility pattern among pediatric patients with UTI.
Material and methods
A cross- sectional study was conducted. Pathogenic bacterial isolates were identified by culture and biochemical methods following standard procedures. Antimicrobial susceptibility testing of the isolates for commonly used antibiotics was done using the standard disc diffusion method on Muller Hinton agar. Associations between dependent and independent variables were measured using chi-square test and within 95% confidence interval. P values <0.05 were considered as statistically significant.
Results
A total of 310 pediatric patients were included in the study, and 82 (26.45%) bacterial isolates were detected. Gram- negative bacteria were predominant etiologic agents of UTI in this study. E. coli was the most frequently occurring pathogen (n=45; 54.88%) followed by S. aureus and P.aeruginosa (n=8; 9.75% for both), P. vulgaris, P.aeruginosa (n=4; 4.88%, for both) and Enterococcus species (n=3; 3.66%). All K. pneumoniae, P. mirabilis, and K. ozanae straines were 100% resistance to ampicillin, followed by P. aeruginosa (87.5%) and E. coli (69%). While all Gram- positive bacterial isolates were 100% sensitive to ciprofloxacin. Malnutrition, history of catherization and previous history of UTI were independently associated with UTI (p=0.000).
Conclusion
There was a high prevalence of uropathogenic bacteria and drug resistance particularly to ampicillin (72%) and tetracycline (37.80%). This condition indicates that antibiotic selection should be based on knowledge of the local prevalence of bacterial organisms and antibiotic sensitivities rather than empirical treatment.
Keywords: Pediatric, symptomatic, urinary tract infection
Introduction
Urinary tract infection (UTI) is a significant public health problem and one of the common sources of infections in children. If not treated early, the complication may lead to renal scaring, end stage renal failure and hypertension. It causes significant morbidity and considerable mortality.[1] In these days, the significance of UTI has been increasingly important, particularly the role of UTI as an occult cause of febrile illness in young children.[2]
Patients’ symptoms are dysuria, urgent and frequent urination, flank pain, along with malodorous and/or cloudy urine. Signs of infection include the presence of blood (hematuria) or white blood cells (pyuria) in urine. Unfortunately, children often lack these symptoms.[3] UTI is the presence of bacteria (bacteriuria) in urine and defined as the growth of a single pathogen of ≥105 colony forming units/ml of clean catch midstream urine.[4] However, since asymptomatic colonization of the urinary tract can occur, other features such as the presence of inflammatory markers or follow-up cultures may be needed to definitively diagnose a UTI.[4]
It is well documented that, UTIs are present in 1% of boys and 3–8% of girls. In the first few months of age, UTI is more common in boys with rates of 2.7% compared with 0.7% in their counterparts. Majority of infections in boys occur within the first three months of life but by school age, its rate decreases in boys and increases in girls.[5] Some reports have shown a 10–12 fold increased risk of UTI in uncircumcised boys compared with circumcised boys.[6]
Research findings show that recurrent UTI is around 12–30% which is common in infants <6 months, and in patients with severe vesico-ureteric reflux and abnormal nuclear renal scans at the onset of the first infection. UTIs manifesting febrile presentations have been reported in infants aged <8 weeks (7.5%), <1 year (5,3%), and of children aged <2 (4.1%), and <5 (1.7%) years.[7] In children with genitourinary abnormalities diagnosis of pediatric UTI must be considered. Besides, accurate and early diagnosis and management of UTI can provide patients with an improved long-term prognosis.[8,9] Different researches show that age, sex, hospitalization and malnutrition are the possible associated etiologic factors of UTI in pediatric patients.[10]
Antimicrobial resistance among urinary tract isolates has recently been reported with an increased frequency all over the world. It is becoming a challenge in low-income countries, because of high prevalence of infection, irrational use of antibiotics, and poor infection prevention practices.[11] Pediatric UTI and drug resistance are emerging problems in developing countries where diagnostic facilities are very poor. However, pediatric UTI is not well studied in Ethiopia. Therefore, this study is conducted to determine bacterial isolates, antimicrobial susceptibility pattern and associated factors among pediatric patients suspected for UTI in The University of Gondar Hospital.
Material and methods
Study area
The study was conducted at The University of Gondar Hospital (UOGH). It is a referral and teaching hospital which provides healthcare services over five million population located in Gondar town, Amhara national regional state, and 750 km far from Addis Ababa (Capital city of Ethiopia).
Study design and period
Cross-sectional study was conducted from February 1, 2015 to June 05, 2015.
Population
Source population
All pediatric patients who were attending The University of Gondar Hospital.
Study population
Pediatric patients with symptomatic UTI, attending The University of Gondar Hospital during the study period consisted the study population.
Operational definition
Pediatric patients are patients aged 0–18 years.
Symptomatic UTI is associated with the patients complaining of dysuria, fever, urgent and frequent urination, flank pain, along with malodorous and/or cloudy urine and the amount of bacteria on the urine is greater than 100,000/mL of midstream urine.
Inclusion criteria
Pediatric patients with symptomatic UTI.
Exclusion criteria
Pediatric patients with symptomatic UTI less than 2 years and currently on antibiotherapy were excluded.
Study variables
Dependant variables
Presence or absence of bacterial isolates and antimicrobial susceptibility pattern.
Independent variables
Age, sex, prior UTI, history of catheterization & malnutrition.
Sample size determination and sampling technique: A single proportion formula was used to calculate sample size, n=z2 p(1-p)/d2
p=50%, Z=1.96 d=0.05=385. However, the total population <10,000, so sample size was calculated by a formula: Nf= no/1+no/N. During the study time, 1260 pediatric patients expected to be attended the hospital. N=1260, Nf= no/1+no/N=295. With 5% contingency added for non-response rate, the final sample size consisted of 310 pediatric patients. A total of 310 pediatric patients with symptomatic UTI in the hospital during the study period were consecutively included in the study.
Data collection
Using a pretested structured questionnaire form, information was collected by experienced nurses on sociodemographic information (age, gender, and residence) and clinical information (fever, pain during micturition, and abdominal pain) and risk factors such as malnutrition and previous UTI. These information was gathered through interview with the guardian’s interview, revising medical records, clinical and laboratory diagnosis.
The questionnaire form was translated to Amharic and re-translated back to English to make the reliability of the data. The instrument’s reliability and validity was also confirmed in the pre-test study.
Sample collection
Urine specimens were collected from each pediatric patient. The guardians were instructed how to collect a clean-catch mid-stream urine specimen. Accordingly, about 10 ml urine specimen was collected from each patient in a sterile screw-capped, wide-mouth container and labelled with the unique sample number, date and time of collection. Immediately, it was delivered to bacteriology laboratory and processed. Isolation and Identification of bacterial UTI: Using calibrated wire loop (0.001 mL) samples were inoculated in to Cystine Lysine Electrolyte Deficient medium (CLED). After overnight incubation at 37°C for 24–48 hours colonies were counted to check significant growth. Colony counts yielding bacterial growth of 105 CFU/mL of urine were regarded as significant for bacteriuria.
Colonies from CLED were subcultured into MacConkey agar and blood agar plates (BAP) (Oxoid) and incubated at 37°C for 24–48 hours. Identification of bacteria was done using colony characteristics, Gram reaction of the bacteria and biochemical tests following standard procedure.[12,13]
Antimicrobial susceptibility testing
All identified pure bacterial isolates were subjected to in vitro susceptibility testing using Kirby Bauer disk diffusion method as described in Clinical Laboratory Standard Institution (CLSI) guideline and interpreted accordingly. From a pure culture 3–5 identical colonies of bacteria were taken and transferred to a tube containing 5 mL sterile nutrient broth (Oxoid) and mixed gently until a homogenous suspension was formed and incubated at 37°C until the turbidity of the suspension become adjusted to a McFarland standard 0.5% Baso4. A sterile cotton swab was used and the excess suspension was removed by gentle rotation of the swab against the surface of the tube. The swab was then used to distribute the bacteria evenly over the surface of Muller Hinton agar (PH=7.2–7.4) (oxoid) following discs were used: - ceftriaxone, tetracycline, nitrofurantin, gentamycin, ampicillin, ticracilin, picracilin, and trimethoprim-sulphamethaxzole for Gram- negative bacteria, whereas vancomycin, nitrofarantin, erythromycin, ciprofloxacilin, trimethoprime/sulfamethoxazole, chloramphinicol and tetracyclin for pathogenic Gram- positive cocci. The criteria used to select the antimicrobial agents were based on both their availability for the management of UTIs and CLSI guideline. The plates were then incubated at 37°C for 24 hours. Diameters of the zone of inhibition around the discs were measured to the nearest millimetre using a metal calibre, and the isolates were classified as susceptible and resistant.[13]
Quality control
The reliability of the study findings were guaranteed by implementing quality control (QC) measures throughout the whole process of the laboratory work. All materials, equipment and procedures were adequately controlled. Pre-test was done at polyclinic. Culture media was tested for sterility and performance. Pre-analytical, analytical and post-analytical stages of quality assurance that were incorporated in standard operating procedures (SOPs) of the microbiology laboratory of University Gondar were strictly followed. International control strains: American Type Culture Collection (ATCC) such as S. pneumoniae ATCC 49619, S. aureus ATCC 25923, E. coli ATCC25922 and E. faecalis ATCC 29212, were used in this study to control the performance of the media. To standardize the inoculum density of bacterial suspension for a susceptibility test, 0.5 McFarland standard was used.[14] The data entry was checked by double entry.
Statistical analysis and interpretation
Data were entered using IBM Statistical Package for the Social Sciences (IBM SPSS Statistics; Armonk, NY, USA) version 20 software, analyzed, tabulated and summarized. The results were presented by bar graph and tables. Strength of association was measured using chi-square test. Within 95% confidence interval, p values of < 0.05 were considered as statistically significant.
Ethical considerations
The study was conducted after ethical approval of the proposal by institutional review board. The parents/guardians of the children gave their written informed consent for their children to participate in this study. The children also verbally assented to participate in this study. Only those volunteers and participants who had full right to continue or withdraw from the study were requested to give urine specimens. Information obtained in each course of the study was kept confidential. For each confirmed case, the responsible clinician of the patient was informed and treatment was started as per the guideline of the hospital and antibiotic susceptibility result.
Results
Sociodemographic characteristics and UTI positivity
A total of 310 pediatric patients were included in this study. The mean age of the study participants’ was 10.24±3.91 years. Majority of the study participants 163 (52.5%) were of school age (7–12 years). The study population consisted of 149 (49.06%) male and 161 (60.94%) female patients. Most of the study participants 160 (51.6%) were urban residents. Clinical anamnesis of the patients showed that they had a history of UTI (n=96; 30.9%) catheterization (n=15; 4.84%) and malnutrition (n=75; 24.20%) (Table 1). Sex, history of previous episode of UTI, history of catheterization and malnutrition had significant association with UTI (p value=0.000) (Table 1).
Table 1.
Sociodemographic characteristic of study participants in relation to UTI
| Characteristics | No. tested (%) | Positive No. (%) | Negative No. (%) | Chi-square | p | |
|---|---|---|---|---|---|---|
| Sex | Male | 149 (49.06) | 25 (16.78) | 124 (83.22) | 14.11 | 0.000 |
| Female | 161 (51.94) | 57 (35.40) | 104 (64.60%) | |||
|
| ||||||
| Age in years | 2–6 | 54 (17.42) | 16 (26.63) | 38 (70.37) | 2.53 | 0.283 |
| 7–12 | 163 (52.58) | 37 (22.67) | 126 (77.33) | |||
| 13–18 | 93 (30) | 29 (31.18) | 64 (68.82) | |||
|
| ||||||
| Residence | Urban | 160 (51.61) | 43 (26.88) | 117 (73.12) | 0.155 | 0.694 |
| Rural | 150 (48.39) | 39 (26) | 111 (74) | |||
|
| ||||||
| Previous episodes of UTI | Yes | 96 (30) | 39 (40.6) | 57 (59.40) | 13.776 | 0.000 |
| No | 214 (70) | 43 (21) | 171 (79) | |||
|
| ||||||
| Previous catheterization | Yes | 15 (4.84) | 11 (73.33) | 4 (16.77) | 15.19 | 0.000 |
| No | 295 (95.16) | 71 (24.07) | 224 (75.93) | |||
|
| ||||||
| Presence of malnutrition | Yes | 75 (24.20) | 33 (44) | 42 (56) | 14.67 | 0.000 |
| No | 235 (75.80) | 49 (20.85) | 186 (79.15) | |||
Prevalence of urinary tract infection
The overall prevalence of culture- confirmed UTI was 26.45% (n=82 patients). Gram- negative bacteria comprised 82.93% (n=68) of the isolates. E. coli 45 (54.88%) was the most frequent isolated bacteria followed by P. aeuroginosa, S. aureus (9.75% for both), K. pneumonea and P. vulgaris (4.88%; for both) (Figure 1).
Figure 1.
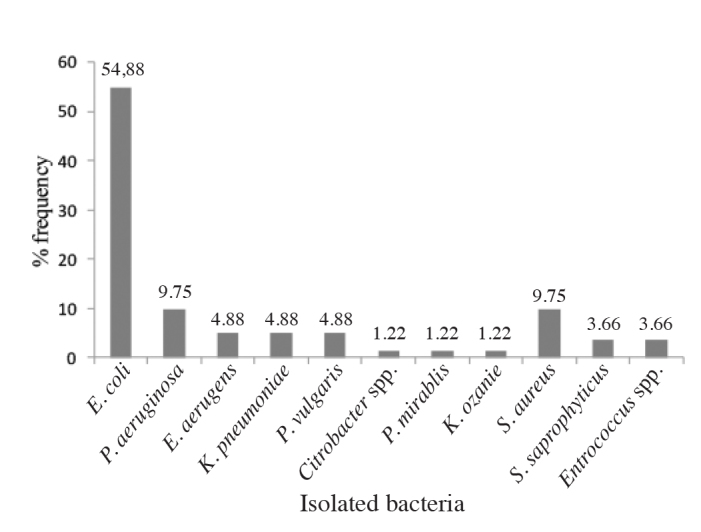
Bacterial isolates obtained from pediatric patients
Antimicrobial susceptibility patterns of bacterial uropathogens
The overall Gram- negative bacteria were sensitive to ampicillin (n=19; 27.94%), ciprofloxacin (n=55; 80.88%), chloramphenicol (n=45; 75%), ceftriaxone (n=48; 80%), gentamycin (n=54; 79.41%), nitrofurantin (n=62; 91.18%), co-trimoxazole (n=43; 72%) and tetracycline (n=35; 58%). Antimicrobial resistance levels for the Gram- negative bacteria, causing UTI were ranging from 0 to 100%. E. coli were resistant to ampicillin (69%), tetracycline (44%), co-trimoxazole (27%) and ceftriaxone (24%). All of 8 (100%) strains of P. aeruginosa were 100% sensitive to gentamycin, nitrofurantin, piperacillin and ticarcillin Whereas all K. pneumoniae, P. mirabilis, Citrobacter spp. and K. ozanea were 100% resistance to ampicillin (Table 2).
Table 2.
Antimicrobial susceptibility pattern of Gram-negative bacteria isolated from urine cultures obtained from pediatric patients
| BI | F | DS | AMP | CIP | CAF | CRO | GEN | NIT | SXT | TTC | CFP | PIC | TIC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | ND | ND | ND | |||
| E. coli | 45 | S | 14 (31) | 37 (82) | 35 (78) | 34 (76) | 36 (80) | 40 (89) | 33 (73) | 25 (56) | ND | ND | ND |
| R | 31 (69) | 8 (18) | 10 (22) | 11 (24) | 9 (20) | 5 (11) | 12 (27) | 20 (44) | ND | ND | ND | ||
|
| |||||||||||||
| Citrobacter | 01 | S | 0 (0) | 1 (100) | 1 (100) | 1 (100) | 0 (0) | 1 (100) | 1 (100) | 1 (100) | ND | ND | ND |
| R | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (100) | 0 (100) | 0 (100 | ND | ND | ND | ||
|
| |||||||||||||
| Entrobacteraerogen | 04 | S | 2 (50) | 3 (75) | 3 (75) | 4 (100) | 2 (50) | 4 (100) | 3 (75) | 4 (100) | ND | ND | ND |
| R | 2 (50) | 1 (25) | 1 (25) | 0 (0) | 2 (50) | 0 (0) | 1 (25) | 0 (0) | ND | ND | ND | ||
|
| |||||||||||||
| P. vulgaris | 04 | S | 2 (50) | 3 (75) | 2 (50) | 4 (100) | 4 (100) | 3 () | 4 (100) | 2 (50) | ND | ND | ND |
| R | 2 (50) | 1 (25) | 2 (5) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 2 (50) | ND | ND | ND | ||
|
| |||||||||||||
| P. mirabils | 01 | S | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | ND | ND | ND |
| R | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | ND | ND | ND | ||
|
| |||||||||||||
| K.pneumoniae | 04 | S | 0 (0) | 2 (50) | 3 (75) | 4 (100) | 3 (75) | 4 (100) | 2 (50) | 1 (25) | ND | ND | ND |
| R | 4 (100) | 2 (50) | 1 (25) | 0 (0) | 1 (25) | 0 (0) | 2 (50) | 3 (75) | ND | ND | ND | ||
|
| |||||||||||||
| K.ozaenae | 01 | S | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 1 (100) | 1 (100) | 0 (0) | 1 (100) | ND | ND | ND |
| R | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | ND | ND | ND | ||
|
| |||||||||||||
| P.aeruginosa | 08 | S | 1 (12.5) | 7 (87.5) | ND | ND | 8 (100) | 8 (100) | ND | ND | 7 (87.5) | 8(100) | 8(100) |
| R | 7 (87.5) | 1 (12.5) | ND | ND | 0 (0) | 0 (0) | ND | ND | 1 (12.5) | 0(0) | 0(0) | ||
|
| |||||||||||||
| Total | 68 | S | 19 (28) | 55 (80.88) | 45 (75) | 48 (80) | 54 (79.41) | 62 (91.18) | 43 (72) | 35 (58) | 7 (87.5) | 8(100) | 8(100) |
| R | 49 (72) | 13 (19.12) | 15 (25) | 12 (20) | 14 (20.59) | 6 (8.82) | 17 (28) | 25 (42) | 1 (12.5) | 0 (0) | 0 (0) | ||
AMP: ampicillin; CAF: chloramphenicol; NIT: nitrofurantoin; CIP: ciprofloxacin; SXT: co-trimoxazole; CRO: ceftriaxone; GN: gentamicin; TTC: tetracycline; TIC: ticracin; CFP: cefepiem; P: cefepime; PIC:pepracillin; ND: not done; F: frequency; BI: bacterial isolate; DS: drug sensitivity pattern; S: sensitivity; R:resistance
The overall Gram- positive bacterial isolates were sensitive to ciprofloxacin 14 (100%), erythromycin and vancomycin (n=12; 86%), clindamycin, nitrofurantin and co-trimoxazole (n=10;71%), chloramphenicol (n=9; 64%), and tetracycline (n=8; 43%) (Table 3).
Table 3.
Antimicrobial susceptibility pattern of Gram-positive bacteria isolated from urine cultures obtained from pediatric patients
| BI | F | DS | CIP | CAF | CLIN | ERY | NIT | SXT | TTC | VAN |
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | 8 | S | 8 (100) | 6 (77) | 7 (87.5) | 7 (87.5) | 6 (77) | 7 (64.5) | 6 (77) | 8 (100) |
| R | 0 (0) | 2 (23) | 1 (12.5) | 1 (12.5) | 2 (12.5) | 1 (12.5) | 2 (23) | 0 (0) | ||
|
| ||||||||||
| S. saprophyticus | 3 | S | 3 (100) | 3 (100) | 1 (33.33) | 2 (66.66) | 2 (66.66) | 2 (66.66) | 3 (100) | 2 (66.66) |
| R | 0 (0) | 0 (0) | 2 (66.66) | 1 (33.33) | 1 (33.33) | 1 (33.33) | 0 (0) | 1 (33.33) | ||
|
| ||||||||||
| Enterococcus species | 3 | S | 3 (100) | 1 (33.33) | 2 (66.66) | 3 (100) | 2 (66.66) | 1 (33.33) | 2 (66.66) | 2 (66.66) |
| R | 0 (0) | 2 (66.66) | 1 (33.33) | 0 (0) | 1 (33.33) | 2 (66.66) | 1 (33.33) | 1 (33.33) | ||
|
| ||||||||||
| Total | 14 | S | 14 (100) | 9 (64) | 10 (71) | 12 (86) | 10 (71) | 10 (71) | 8 (57) | 12 (86) |
| R | 0 (0) | 5 (36) | 4 (29) | 2 (14) | 4 (29) | 4 (29) | 6 (43) | 2 (14) | ||
CIP: ciprofloxacin; CAF: chloramphenicol; CLIN: clindamycin; NIT: nitrofurantoin; SXT: co-trimoxazole; GN: gentamicin; TTC: tetracycline; VAN: vancomycin; F: frequency; BI: bacterial isolate; DS: drug sensitivity pattern; S: sensitive; R: resistance
Multidrug resistance patterns of bacterial isolates
Among a total of isolates (n=82), multidrug resistance (resistance against two and more than two drugs) was detected in 48 (58.54%) isolated bacterial urophatogens including 40 (82.98%) Gram- negative and 8 (17.02%) Gram- positive bacteria (Table 4).
Table 4.
Multidrug resistance pattern of bacterial isolates among pediatric patients
| Bacterial isolate | Antimicrobial pattern | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total (%) | R0 | R1 | R2 | R3 | R4 | R5 | |
| Gram-positive | 14 (17.07) | 2 (14.28) | 4 (28.57) | 5 (35.71) | 1 (7.14) | 0 (0) | 2 (14.28) |
| S. aureus | 8 (57) | 2 (23) | 3 (35.5) | 2 (23) | 1 (12.5) | 0 (0) | 0 (0) |
| S. saprophyticus | 3 (21.5) | 0 (0) | 0 (0) | 2 (66.66) | 0 (0) | 0 (0) | 1 (33.33) |
| Entrococcus species | 3 (21.5) | 0 (0) | 1 (33.33) | 1 (33.33) | 0 (0) | 0 (0) | 1 (33.33) |
|
| |||||||
| Gram- negative | 68 (82.93) | 9 (13) | 10 (15) | 19 (27.94) | 10 (15) | 7 (10) | 4 (6) |
| Citrobacter | 1 (1.33) | 0 (%) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| Entrobacteraerogens | 4 (6) | 1 (25) | 2 (25) | 0 (0) | 1 (25) | 0 (0) | 0 (0) |
| K.pneumonae | 4 (6) | 0 (0) | 1 (25) | 0 (0) | 1 (25) | 2 (50) | 0 (0) |
| K.ozanea | 1 (1.33) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| E. coli | 45 (66) | 6 (13) | 10 (22) | 14 (31) | 7 (16) | 4 (9) | 4 (9) |
| P.vulgaris | 4 (6) | 1 (25) | 1 (25) | 1 (25) | 1 (25) | 0 (0) | 0 (0) |
| P.mirabils | 1 (1.33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
| P.aerogunesa | 8 (12) | 1 (12.5) | 5 (62.5) | 2 (23) | 0 (0) | 0 (0) | 0 (0) |
|
| |||||||
| Total | 82 (100) | 11 (13.41) | 13 (15.85) | 24 (29.26) | 11 (13.41) | 7 (8.53) | 6 (7.31) |
R0- No antibiotic resistance, R1- Resistance to one, R2-Resistance to two, R3-Resistance to three, R4- Resistance to four, ≥R5-resistance to five and more than five antimicrobials.
Discussion
Urinary tract infection in a pediatric patient is a significant source of morbidity and considerable mortality. It is generally agreed that pediatric patient with UTI requires further investigation and treatment to minimize future complications. The prevalence of pediatric UTI in our study was 26.45%. This finding is in line with the study done in Gondar hospital which was 28.1%.[15] However, our finding was higher than that found in a study which was conducted in Beheshti Hospital, Iran (18.4%).[4] The difference may be due to our study population consisting of only symptomatic patients with UTIs.
The sex distribution of patients in our study is consistent with those of other reported studies, showing statistically predominance of females with UTI (69.51% of the culture-positive cases). This rate is comparable to those reported from many countries.[16,17] Increased incidence of infection in female is related to difference between the male and female genitourinary system anatomy i.e proximity of the female urethra to the anal region that consists of many microbial flora. The major cause of female UTI is the shorter and wider urethra through which the bacteria, from fecal flora may readily enter into the bladder and kidney.[18]
Urinary tract infections are frequently caused by a single pathogen namely E. coli.[2,15,19] In our study also we isolated only E. coli as a single UTI pathogen in 54.88% of the cases. In our country in various studies E. coli has been found to be a common pathogen causing UTI.[15,19]
E. coli, P. aeuroginosa, S. aureus, K. pneumonae and P. vulgaris were the most common bacterial pathogens causing UTI in children in our study. More or less similar observations were made in pediatric cases of UTI in different studies and countries, though the proportion and the prevalence varied.[20,21] Gram- negative bacteria were more prevalent (82.92%) in this study which is in agreement with previous studies.[4,15,20] Gram- negative bacteria are the dominant bacteria that cause UTI. This could be due to the presence of unique structure in Gram- negative bacteria which facilitates attachment to the uroepithelial cell, with resultant high prevalence in the gastrointestinal tract. Its this unique structural characteristics prevent elimination of bacteria with urinary lavage, and allow its multiplication and tissue invasion ensuing in invasive infection and pyelonephritis. In this study E. coli was the predominant bacteria causing UTI in pediatric patients. This is in agreement with other studies done both in pediatric and adults.[15,19] In our study, the second predominant isolates were S. aureus and K. pneumoniae with a similar prevalence. This study result was similar to that of the study conducted in Gondar[15] where S. aureus was the second predominant isolate. In Jima[19] K. pneumoniae was the second predominant isolate. S. aureus was more prevalent among Gram- positive bacteria in this study, in contrary to other study S. saprophyticus was the most prevalent.[22] This may be due to lack of sexual activity in the pediatric age group which predisposes the female to the possibility of contracting UTI by S. saprophyticus.
In this study, it had been tried to assess the susceptibility patterns of the isolated bacteria to different antibiotics in vitro disc diffusion. Gradually increasing antimicrobial resistance among uropathogen isolates (mostly ampicillin, tetracycline and cotrimoxazole) has been reported from many countries.[4,15,20,22] This may be due to frequent prescribing antimicrobial agents and easy accessibility to antibiotics in every hospital in most countries. These antimicrobial agents are also frequently used in empirical treatment of UTI in our hospital. In our study, the frequency of resistance to ampicillin was 72%. This is comparable to 77% resistance to ampicillin, found in a study done in children in Tanzania.[23] However, this resistance rate to ampicillin is lower than 96% prevalence reported from Iraq.[20] In present study, it was found that all K. pneumoniae, P. mirabilis, Citrobacter and K. ozanea isolates were 100% resistance to ampicillin. This implies that ampicillin had been more prevalently used previously and could not be the choice of drug for empirical treatment of UTI particularly in the study area.
Multidrug resistance were seen in 58.53% of the isolated bacteria which raises serious concerns. This suggests a high resistance gene pool perhaps due to gross misuse, and inappropriate usage of the antimicrobial agents, incorrect administration of antimicrobial agents, and increase in the production of bacterial enzymes such as beta- lactamases, and lack of good controlling mechanism, which can increase the prevalence of multidrug resistant microorganisms.[23] Taken all together, these findings clearly show how resistant strains are expanding at an alarming rate in the area. With this trend, an antibiotic which was effective a year ago might not be used next year. This creates great burden especially to people living in resource-poor countries where they couldn’t ensure their daily bread let alone for medication. The cost of new antibiotics is also high which in turn poses great burden for poor countries.
On the other hand, some of isolated bacteria were sensitive to nitrofurantin, gentamycin, ciprofloxacin and ceftriaxone similar to other studies done in Ethiopia.[13,17] Low resistance were observed for these drugs because they are not easily accessible and the price is relatively expensive compared to others. Therefore, these drugs could be potentially an alternative empirical treatment of pediatric UTI.
In addition to the identification of the common bacterial agents and the susceptibility patterns in the present study, it has been attempted to assess the association of different sociodemographic and clinical parameters with development of bacterial infection. In our study, sex, malnutrition, previous history of UTI and history of catheterization were significantly associated with UTI (p=0.000) which was supported by other researchers.[24,25]
In present study, the overall prevalence of symptomatic pediatric UTI was 26.45%, and E. coli was the most predominant organism followed by S. aureus and K. pnemoniae. Most bacteria were resistant to ampicillin, tetracycline and cotrimoxazole, while others were sensitive to nitrofurantin, ciprofloxacin, eryrhromycin, gentamycin and vancomycin. Multidrug resistant bacteria were very prevalent. Occurrence of bacterial infection was positively associated with previous catheterization, malnutrition, sexual activity and previous episodes of UTI. Regular monitoring is required to establish reliable information about resistance patterns of urinary pathogens for optimal empirical therapy of patients with UTIs. Therefore, we suggest that empirical antibiotic selection should be based on the knowledge of local prevalence of bacterial organisms and antibiotic susceptibility patterns rather than on the universal guidelines. Further longitudinal studies are mandatory to assess prevalence of uropathogens, antimicrobial resistance patterns and associated risk factors.
Acknowledgements
We acknowledge University of Gondar Hospital Laboratory for funding this study. We greatly appreciate University of Gondar Hospital Laboratory Microbiology staff for cooperation during the study. We are also grateful to the patients who participated in this study.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of University of Gondar, College of Medicine and Health Sciences, School of Biomedical and Laboratory Science.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.A.; Design – B.A., B.Abebe, S.M.; Supervision – M.D., Z.A., A.G.; Data Collection and/or Processing – B.A., B.Abebe, A.S.; Analysis and/or Interpretation – B.A., M.D., A.G.; Literature Search – T.S., A.G.; Writing Manuscript – M.D., B.A., Z.A.; Critical Review – M.D., B.A., A.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Whiting P, Westwood M, Watt I, Cooper J, Kleijnen J. Rapid tests and urine sampling techniques for the diagnosis of urinary tract infection (UTI) in children under five years: a systematic review. BMC Pediatr. 2005;5:4. doi: 10.1186/1471-2431-5-4. https://doi.org/10.1186/1471-2431-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauchner H, Philipp B, Dashefsky B, Klein JO. Prevalence of bacteriuria in febrile children. Pediatr Infect Dis J. 1987;6:239–42. doi: 10.1097/00006454-198703000-00004. https://doi.org/10.1097/00006454-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein RA, Gaynes R, Edwards JR, System NNIS. Overview of Nosocomial Infections Caused by Gram-Negative Bacilli. Clin Infect Dis. 2005;41:848–54. doi: 10.1086/432803. https://doi.org/10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 4.Zorc JJ, Kiddoo DA, Shaw KN. Diagnosis and management of pediatric urinary tract infections. Clin Microbiol Rev. 2005;18:417–22. doi: 10.1128/CMR.18.2.417-422.2005. https://doi.org/10.1128/CMR.18.2.417-422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riccabona M. Urinary tract infections in children. Curr Opin Urol. 2003;13:59–62. doi: 10.1097/00042307-200301000-00010. https://doi.org/10.1097/00042307-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Roberts K, Akintemi B. The epidemiology and clinical presentation of urinary tract infections in children younger than 2 years of age. Pediatr Ann. 1999;28:644–9. doi: 10.3928/0090-4481-19991001-08. https://doi.org/10.3928/0090-4481-19991001-08. [DOI] [PubMed] [Google Scholar]
- 7.Panaretto K, Craig J. Risk factors for recurrent urinary tract infection in preschool children. J Paediatr Child Health. 1999;35:454–9. doi: 10.1046/j.1440-1754.1999.355417.x. https://doi.org/10.1046/j.1440-1754.1999.355417.x. [DOI] [PubMed] [Google Scholar]
- 8.Winberg J, Bergstron T, Jacobsson B. Morbidity, age and sex distribution, recurrences and renal scarring in symptomatic urinary tract infection in childhood. Kidney Int Suppl. 1975;4:101–6. [PubMed] [Google Scholar]
- 9.Fluit C, Mark J, Franz-Josef S, Jacques A, Renu G, Verhoef J. Antimicrobial resistance among urinary tract infection (UTI) isolates in Europe: results from the SENTRY Antimicrobial Surveillance Program 1997. Antony Van Leeuwenhoek. 2000;77:147–52. doi: 10.1023/a:1002003123629. https://doi.org/10.1023/A:1002003123629. [DOI] [PubMed] [Google Scholar]
- 10.Sefton AM. The impact of resistance on the management of urinary tract infections. Int J Antimicrob Agents. 2000;16:489–91. doi: 10.1016/s0924-8579(00)00282-x. https://doi.org/10.1016/S0924-8579(00)00282-X. [DOI] [PubMed] [Google Scholar]
- 11.Turnidge J, Bell J, Biedenbach D, Jones R. Pathogen occurrence and antimicrobial resistance trends among urinary tract infection in the Asia -western pacific region. Int J Antimicrob Agent. 2002;20:7–10. doi: 10.1016/s0924-8579(02)00050-x. [DOI] [PubMed] [Google Scholar]
- 12.Chesbrougn M. Manual of medical microbiology. Low price ed. Britain: oxford press; 2000. pp. 251–60. [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-first Informational Supplement M100-S21. CLSI; Wayne, PA, USA: 2013. [Google Scholar]
- 14.Twaij M. Urinary tract infection in children: a review of its pathogenesis and risk factors. J R Soc Health. 2000;120:220–6. doi: 10.1177/146642400012000408. https://doi.org/10.1177/146642400012000408. [DOI] [PubMed] [Google Scholar]
- 15.Tessema B, Kassu A, Mulu A, Yismaw G. Predominant isolates of urinary tract pathogens and their antimicrobial susceptibility pattern in Gondar University teaching Hospital, Northwest Ethiopia. Ethiop Med J. 2007;45:61–5. [PubMed] [Google Scholar]
- 16.Marild S, Jodal U. Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Actapaediatr. 1998;87:549–52. doi: 10.1080/08035259850158272. https://doi.org/10.1111/j.1651-2227.1998.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 17.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dismon. 2003;49:53–70. doi: 10.1067/mda.2003.7. https://doi.org/10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 18.Jones RN, Inabo HI, Obanibi HBI. Antimicrobial susceptibility of some urinary tract clinical isolates to commonly used antibiotics. Afr J Biotechnol. 2006;5:487–9. [Google Scholar]
- 19.Getnet B, Wondwosen T. Bacterial uropathogen in urinary tract infection and antibiotic susceptibility pattern in Jima university specialized hospital Southwest Ethiopia. Ethiop J Health Sci. 2011;21:141–6. doi: 10.4314/ejhs.v21i2.69055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredrick F, Francis Joel M, Fataki M, Maselle Samuel Y. Aetiology, antimicrobial susceptibility and predictors of urinary tract infection among febrile under-fives in Tanzania. AJMR. 2013;7:1029–34. [Google Scholar]
- 21.Ayazi P, Mahyar A, Hashemi HJ, Khabiri S. Urinary tract infection in children. Iranian J Pediatr Soci. 2010;2:9–10. [Google Scholar]
- 22.Esmaeili M. Antibiotics for causative microorganisms of urinary tract infections. J Iranian Pediatr Dis. 2005;15:165–73. [Google Scholar]
- 23.Ndihokubwayo JB, Yahaya A, Desta AT, Ki-Zerbo G. Antimicrobial resistant in Africa region: Issues, challenges and action proposed. Africa Healt Motion. 2013;16:27–31. [Google Scholar]
- 24.Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468–74. doi: 10.1056/NEJM199608153350703. https://doi.org/10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 25.Stam WE. Catheter-associated urinary tract infection: epidemiology, pathogenesis, prevention. Am J Med. 1991;919:65–71. doi: 10.1016/0002-9343(91)90345-x. https://doi.org/10.1016/0002-9343(91)90345-X. [DOI] [PubMed] [Google Scholar]


