Abstract
Background:
Individuals with chronic kidney disease (CKD) have low levels of physical activity and physical function. Although guidelines endorse exercise counseling for individuals with CKD, it is not yet part of routine care.
Objective:
We investigated the effect of attending a real-life exercise counseling clinic (ECC) on physical function in individuals with CKD.
Design:
Retrospective analysis of prospectively collected observational data with quasi-experimental design.
Setting and Participants:
Patients with all stages of CKD registered in a large provincial renal program were eligible. The exposed cohort who attended the ECC between January 1, 2011, and March 15, 2014, included 214 individuals. The control cohort included 292 individuals enrolled in an observational study investigating longitudinal change in frailty during the same time period.
Predictor/Factor:
Attendance at an ECC.
Outcomes and Measurements:
Change in physical function as measured by Short Physical Performance Battery (SPPB) score, physical activity level (Human Activity Profile [HAP]/Physical Activity Scale for the Elderly [PASE]), and health-related quality of life (HRQOL; EQ5D/VAS) over 1 year.
Results:
Eighty-seven individuals in the ECC cohort and 125 participants in the control cohort completed 1-year follow-up. Baseline median SPPB score was 10 (interquartile range [IQR]: 9-12) and 9 (IQR: 7-11) in the ECC and control cohorts, respectively (P < .01). At 1 year, SPPB scores were 10 (IQR: 8-12) and 9 (IQR: 6-11) in the ECC and control cohorts, respectively (P = .04). Mean change in SPPB over 1 year was not significantly different between groups: −0.33 (95% confidence interval [CI]: −0.81 to 0.15) in ECC and −0.22 (95% CI: −0.61 to 0.17) in control (P = .72). There was no significant difference in the proportion of individuals in each cohort with an increase/decrease in SPPB score over time. There was no significant change in physical activity or HRQOL over time between groups.
Limitations:
Quasi-experimental design, low rate of follow-up attendance.
Conclusions:
In this pragmatic study, exercise counseling had no significant effect on change in SPPB score, suggesting that a single exercise counseling session alone is inadequate to improve physical function in CKD.
Keywords: exercise counseling, chronic kidney disease, physical function, functional status, physical activity, health-related quality of life, health promotion, exercise
Abrégé
Contexte:
Les personnes atteintes d’insuffisance rénale chronique (IRC) ont des capacités physiques réduites et sont généralement peu actives physiquement. Bien que les recommandations aillent dans le sens d’encourager ces patients à adopter un programme d’exercices, on observe que cela ne fait toujours pas partie de la routine de soins.
Objectif de l’étude:
Mesurer l’effet de la fréquentation d’une clinique de consultation en entraînement (CCE) sur la condition physique des individus atteints d’IRC.
Type d’étude:
Il s’agit d’un modèle d’étude quasi expérimental sous forme d’une analyse rétrospective de données observationnelles colligées prospectivement.
Cadre de l’étude et participants:
Étaient admissibles tous les patients atteints d’IRC, peu importe le stade, inscrits à un vaste programme de santé rénale provincial. La cohorte exposée, soit les patients ayant fréquenté une CCE entre le 1er janvier 2011 et le 15 mars 2014, était composée de 214 sujets. La cohorte contrôle était constituée de 292 individus participant à une étude observationnelle qui évaluait les changements longitudinaux de fragilité physique pendant la même période.
Facteur prédictif:
La fréquentation d’une CCE.
Mesures:
Pendant un an, on a mesuré le niveau d’activité physique, la qualité de vie relative à l’état de santé et les changements dans les capacités physiques des participants (test SPPB - Short Physical Performance Battery Score).
Résultats:
Seuls 87 patients de la cohorte exposée et 125 de la cohorte contrôle ont complété le suivi. Les médianes initiales au test SPPB étaient de 10 (EI: 9-12) et de 9 (EI: 7-11) respectivement (p < 0,01). Après un an, les scores au test SPPB étaient pratiquement inchangés: médiane de 10 (EI: 8-12) pour la cohorte exposée et de 9 pour la cohorte contrôle (EI: 6-11) (p = 0,04). Pendant l’année du suivi, la variation moyenne du score au test SPPB a été semblable dans les deux groupes: −0,33 (IC 95 % −0,81 à 0,15) dans la cohorte exposée et −0,22 (IC 95 % −0,61 à 0,17) dans le groupe contrôle (p = 0,72). Au fil du temps, la proportion d’individus ayant présenté une diminution ou une augmentation du score au test SPPB était similaire dans les deux groupes; et aucun changement significatif dans le niveau d’activité physique ou la qualité de vie relative à l’état de santé n’avait été observé entre les groupes.
Limites de l’étude:
Les résultats sont limités par le modèle quasi expérimental de l’étude et la faible participation au suivi sur un an.
Conclusion:
Cette étude pragmatique démontre que le fait de consulter pour un programme d’entraînement n’a que peu d’effet sur le score obtenu au test SPPB. Cette observation suggère qu’une seule séance de consultation en vue d’adopter un programme d’entraînement n’est pas suffisante pour améliorer la condition physique des patients atteints d’IRC.
What was known before
Physical function and physical activity levels are low in individuals with chronic kidney disease (CKD) and worsen with CKD progression. Exercise counseling has been recommended for individuals with CKD to increase physical activity behavior and improve physical function.
What this adds
As the only study to date to investigate the role of exercise counseling alone on physical function in individuals with CKD, this study addresses an important knowledge gap. In addition, this study provides valuable information regarding the true clinical effectiveness of such a program due to its pragmatic nature and evaluation of a “real-world” clinical program.
Background
Kidney disease progression is associated with worsening functional status across the spectrum of chronic kidney disease (CKD).1-3 Physical function is an important domain in health-related quality of life (HRQOL) and an important criterion for frailty.4,5 Physical activity also declines with worsening CKD.1,6 Low levels of physical activity and physical function have been associated with a 1.5- to 2-fold increased risk of hospitalization, institutionalization, and mortality rates in individuals with CKD and those on dialysis.7-11
Physical activity is a potentially modifiable risk factor. Randomized controlled trials demonstrate improved physical function when physical activity is increased through exercise programming in individuals with CKD.12,13 Kidney Disease Outcomes Quality Initiative (KDOQI) and Kidney Disease: Improving Global Outcomes (KDIGO) guidelines indicate that patients with CKD should be counseled regarding the benefits of maintaining regular exercise and physical activity.14,15 However, counseling and education regarding the benefits of physical activity and exercise are often missed as part of routine care in the CKD population.16,17 Although numerous barriers to exercise including fear of injury, fatigue, shortness of breath, and lack of motivation have been identified, the majority of patients with CKD indicate that they would exercise if counseled to do so.18-20
In the general population, physical activity and exercise counseling have been shown to be effective at increasing self-reported physical activity.21,22 Although some of these counseling initiatives have enrolled participants with comorbidities including heart disease and diabetes mellitus, the effect of this type of intervention in the CKD population is unknown.
We investigated the effect of attendance at an exercise counseling clinic (ECC), a preexisting clinical program in our center, on physical function over 1 year in individuals with CKD. We hypothesized that voluntary participation in the ECC would result in maintenance of physical function at 1 year as compared with individuals with CKD who did not attend the ECC, in whom we anticipated physical function would decline. As a secondary objective, we evaluated predictors of change in physical function over time.
Materials and Methods
The University of Manitoba Health Research Ethics Board approved this study protocol (HS15231). Research was conducted according to principles outlined in the Declaration of Helsinki.
Study Design
A retrospective analysis of prospectively collected data with quasi-experimental pretest-posttest nonequivalent control design was performed.
Study Population
The exposed population included adults (>/= 18 years old) with any stage of CKD, registered in the Manitoba Renal Program (MRP), who attended the MRP ECC for a baseline assessment between January 1, 2011, and March 15, 2014, and consented for inclusion of their clinic data in the ECC Research Database. The MRP is the sole renal care provider for individuals with CKD throughout the province of Manitoba (population 1.27 million).23
The control population included adults with CKD registered in the MRP and enrolled in a contemporaneous longitudinal observational study investigating frailty in CKD between January 2012 and March 15, 2014, but who did not attend the ECC.24 Participants in this group attended their usually scheduled renal clinic appointments where they underwent baseline and yearly assessments of physical function, physical activity level, and HRQOL as part of the frailty study. No standard exercise programming or physical activity counseling occurred as part of renal clinics or the frailty study protocol.
Description of ECC
An active clinical program, the ECC is situated in a medical fitness facility specializing in chronic disease management. Individuals with any stage of CKD registered within the MRP are eligible for referral to the ECC. Referral can be made by any physician or allied health professional affiliated with the MRP. Throughout clinic, the importance and benefits of physical activity are emphasized. Attendees complete questionnaires to assess medical history, current symptoms, physical activity level, HRQOL, and level of physical fitness prior to their visit. At clinic, a nephrologist first assesses individual contraindications for exercise. Attendees then undergo a brief battery of physical performance measures including Short Physical Performance Battery (SPPB) and grip strength. Subsequently, patients are counseled by a certified exercise physiologist (CEP; Canadian Society for Exercise Physiology, Ottawa, Ontario, Canada) regarding barriers, motivators, and goals for exercise using the Transtheoretical (Stages of Change) Model.25,26 This culminates in the prescription of an individualized exercise plan using the FITT (Frequency, Intensity, Time, and Type) exercise prescription model which generally includes a combination of aerobic and resistance exercise whenever possible. Following the initial visit, attendees receive motivational counseling by telephone, which includes review of the initial exercise prescription, identification and trouble shooting of barriers to exercise participation, and reminder regarding yearly follow-up. These phone calls were scheduled to occur at 1, 3, 6, and 9 months following the initial appointment for participants included in this study. Attendees are scheduled for voluntary yearly follow-up appointments at the ECC, at which functional status and physical performance measures are reassessed and exercise plan is modified as deemed necessary and appropriate during one on one counseling.
Outcome Measures
Primary outcomes
Change in physical function from baseline to 1 year was measured by SPPB. SPPB is a composite score of 4-m gait speed, balance, and sit-to-stand testing.27 Total SPPB score (range: 0-12) is correlated with other measures of disability and is predictive of functional status, nursing home admission, and mortality in multiple populations, including individuals with CKD.27-29 Lower scores represent poorer physical function, and patients can be classified into severe (0-3), moderate (4-6), mild (7-9), or minimal (10-12) limitations in physical function based on SPPB score.
Change in grip strength over time served as a second measure of physical function. Grip strength was measured in a standing position using a Jamar hand dynamometer held in line with the forearm at the level of the thigh away from the body and squeezed vigorously with maximum force.30 Two trials were performed in each hand. Maximum for each hand and maximum total (right and left hand) were recorded.
Secondary outcomes
Change in HRQOL was measured using the EQ5D-3L and EuroQol Visual Analogue Scale (EQ-VAS) (EuroQol, Rotterdam, The Netherlands). This self-administered tool consists of a descriptive system containing 5 questions in 3-point Likert-type format which assess how significantly the domains of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression are affected in daily life.31 The EQ-VAS asks individuals to rate their current state of health from “worst imaginable” to “best imaginable” on a 100-point scale. The EQ5D-3L and EQ-VAS have been extensively used and validated in various populations including CKD.32,33
Change in self-reported physical activity behavior over 1 year was measured using the 94-item Human Activity Profile (HAP) in the ECC cohort. This self-administered questionnaire identifies activities that the individual is still doing and those that the individual has stopped doing to calculate a maximal activity score (MAS) and adjusted activity score (AAS).34 HAP-MAS has been well correlated with objective measures of physical activity and has been widely used in various populations, including those with CKD.34-36
Change in self-reported physical activity behavior over time was measured by the Physical Activity Scale for the Elderly (PASE) in the control cohort.37 This interviewer-administered questionnaire measures physical activity over 7 days by recording frequency and duration of leisure activities and housework resulting in the calculation of a weighted activity score. The PASE has been validated using accelerometry and has been used in the aging North American population and in individuals with CKD.37-39
Trained individuals performed outcome assessments at each clinic/study visit in both study groups. Demographic and clinical characteristics were collected at baseline during clinic and study visits and from patient charts for both cohorts. These included age, gender, race, cause of kidney failure, stage of kidney disease, renal replacement therapy modality (if appropriate), comorbidities, hemoglobin, estimated glomerular filtration rate (eGFR), and serum calcium, phosphate, and parathyroid hormone.
Data Analysis
Comparison of baseline characteristics between the ECC and control cohorts was performed using the 2-tailed Student t test or Mann-Whitney U test for continuous variables and chi-square test for categorical variables, as appropriate. Similar analyses were also performed comparing the characteristics of individuals in each group who returned for 1-year follow-up with those who did not.
Change in outcome measures from baseline to 1 year was compared between cohorts using Mann-Whitney U and Student t tests for continuous variables, depending on data distribution. The proportion of individuals who had a significant change in SPPB between study groups was compared using the chi-square test. Change was defined as an increase or decrease in SPPB score of at least 1 point at 1 year. This magnitude of difference is clinically relevant using anchor-based methods.40
Multiple logistic regression using stepwise selection was performed to determine significant predictors of change in SPPB score over time. Participation in the ECC was the primary predictor in this model, and other predictors included baseline SPPB, age, sex, weight, race (Caucasian vs not), diabetes status, other comorbidities, hemoglobin, albumin, calcium, phosphate, and change in physical activity level.
The change in proportion of individuals identifying a problem in EQ5D-3L domains over 1 year was compared between and within groups using the chi-square test. Similarly, due to the different measures used for physical activity in the study cohorts, the proportion of individuals in whom physical activity level increased over 1 year was compared between study cohorts. Significant change in physical activity was defined as an increase or decrease of 0.5 times the standard deviation from baseline HAP score (ECC cohort) or PASE (control cohort) score.
For all analyses, 2-sided P < .01 was considered statistically significant. Statistical analysis was performed using SAS 9.3 (SAS, Cary, North Carolina).
Results
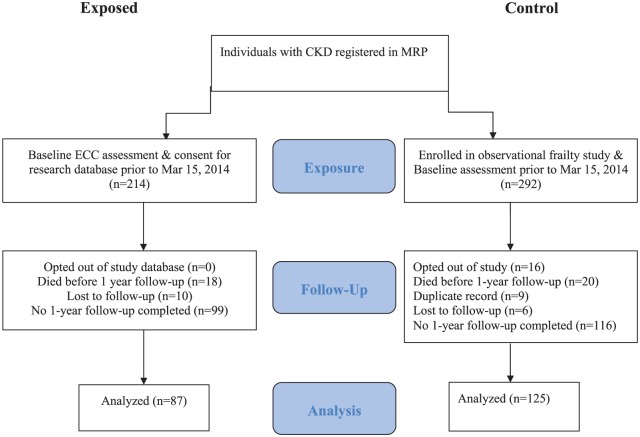
A total of 506 patients were eligible for inclusion. Of 214 individuals who received baseline assessment in the ECC prior to March 15, 2014, 87 (41%) returned for follow-up assessment. Similarly, of 292 individuals who received baseline assessment in the control cohort, 125 (43%) had a 1-year follow-up assessment (Figure 1).
Figure 1.
Patient flow diagram.
Note. CKD = chronic kidney disease; MRP = Manitoba Renal Program; ECC = exercise counseling clinic.
Baseline characteristics in those who attended the ECC and those in the control cohort were broadly similar (Table 1). However, the ECC cohort had a larger proportion of individuals who were on dialysis. By design, no individuals with renal transplant were enrolled in the frailty study. Individuals in the ECC cohort were also significantly younger (60 vs 68 years old in the control group; P < .01), had a higher body mass index (BMI; 30.4 vs 28.3 in the control group; P = .02), and had a lower proportion of individuals with arthritis (33% vs 48%; P = .03).
Table 1.
Comparison of Baseline Characteristics Between Study Cohorts.
| Variable | ECC cohort (n = 87) | Control cohort (n = 125) | P value |
|---|---|---|---|
| Age, y | 60.0 (53.0-69.0) | 68.1 (56.3-77.0) | <.01 |
| Sex, female | 34 (39.1%) | 54 (43.2%) | .55 |
| CKD, eGFR <30 mL/min/1.73 m2 | 26 (29.9%) | 83 (66.4%) | <0.01 |
| CKD, eGFR ≥30 mL/min/1.73 m2 | 13 (14.9%) | 15 (12.0%) | |
| HD | 21 (24.1%) | 11 (8.8%) | |
| PD | 13 (14.9%) | 16 (12.8%) | |
| Transplant | 14 (16.1%) | 0 (0.0%) | |
| Race, Caucasian | 58 (73.4%) | 90 (72.0%) | .84 |
| BMI, kg/m2 | 30.4 (26.0-36.3) | 28.3 (25.0-31.4) | .02 |
| SBP, mmHg | 140 (125-152) | 134 (121-148) | .29 |
| DBP, mmHg | 77 (69-84) | 73 (66-82) | .23 |
| Ischemic heart disease | 17 (19.5%) | 25 (20.0%) | .93 |
| Congestive heart failure | 7 (8.1%) | 18 (14.4%) | .16 |
| Peripheral vascular disease | 11 (12.6%) | 19 (15.2%) | .60 |
| Hypercholesterolemia | 56 (64.4%) | 88 (70.4%) | .35 |
| Stroke | 11 (12.6%) | 15 (12.0%) | .89 |
| Diabetes | 47 (54.0%) | 73 (58.4%) | .53 |
| Hypertension | 80 (92.0%) | 105 (84.0%) | .09 |
| Arthritis | 29 (33.3%) | 57 (48.3%) | .03 |
| Hemoglobin, g/L | 116 (106-127) | 116 (108-124) | .68 |
| Creatinine, µmol/L | 266 (144-589) | 274 (191-447) | .42 |
| eGFR, mL/min/1.73 m2 | 18.0 (8.0-36.0) | 19.0 (10.0-26.0) | .50 |
| Calcium, mmol/L | 2.41 (2.32-2.51) | 2.31 (2.23-2.42) | <.01 |
| Phosphate, mmol/L | 1.36 (1.12-1.82) | 1.35 (1.14-1.57) | .79 |
| Albumin, g/L | 35.0 (32.5-38.0) | 36.0 (33.0-39.0) | .25 |
| PTH, ng/L | 170.0 (95.0-372.0) | 126.0 (68.0-199.0) | <.01 |
Note. Values in parentheses indicate 95% confidence interval for continuous variables and proportion for categorical variables. ECC = exercise counseling clinic; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; HD = hemodialysis; PD = peritoneal dialysis; BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; PTH = parathyroid hormone.
No significant differences in baseline characteristics of follow-up attendees and non-attendees in the ECC cohort were identified. However, stage of CKD and dialysis modality were significantly different between control attendees and non-attendees (P < .01) and non-attendees had lower hemoglobin (110 vs 116 g/L), lower eGFR (13 vs 19 mL/min/1.73 m2), and higher serum phosphate (1.63 vs 1.35 mmol/L) than control group attendees (P < .01). See Supplemental Data Tables S1a and S1b.
Receipt of follow-up phone calls in the ECC group did not impact attendance at 1-year follow-up, as the proportions receiving at least 1 follow-up phone call in the year after attending ECC baseline visit were similar in both groups (78% of follow-up attendees vs 68% of non-attendees; P = .11).
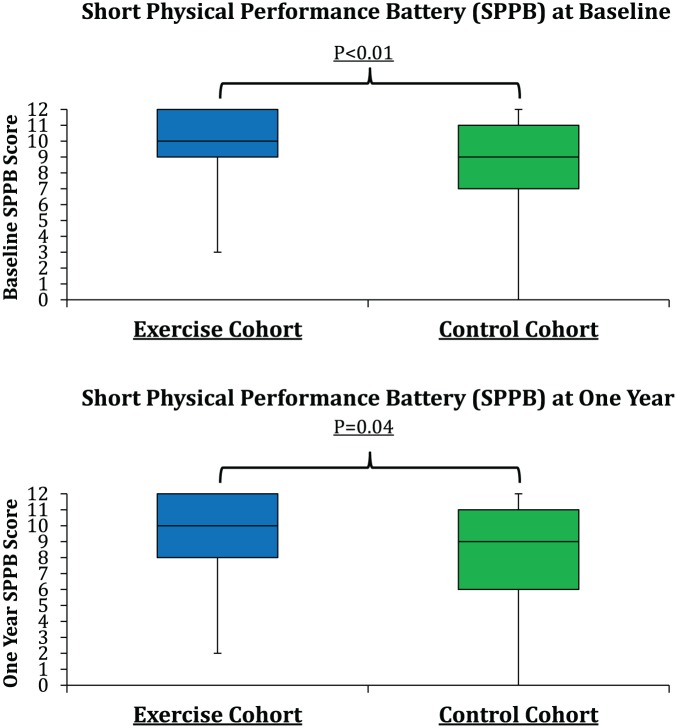
Change in SPPB Score Over 1 Year
Median SPPB scores were 10 (interquartile range [IQR]: 9-12) and 9 (IQR: 7-11) at baseline and 10 (IQR: 8-12) and 9 (IQR: 6-11) at 1-year follow-up in the ECC and control cohorts, respectively (Figure 2 and Table 2). Baseline (P < .01) and follow-up scores (P = .04) were higher in the ECC cohort than in the control cohort. Mean change in SPPB score over time was −0.33 (95% confidence interval [CI]: −0.81 to 0.15) and −0.22 (95% CI: −0.61 to 0.17) for the ECC and control cohorts, respectively (P = .72). The number of individuals in each group who had an increase (>1 point) in SPPB score at 1 year was 26 of 87 (30%) in the ECC group and 39 of 125 (31%) in the control group (P = .84). Similarly, the proportion of individuals with a decline of more than 1 point in SPPB score over 1 year in each group was not significantly different between cohorts (39% in ECC and 31% in control group, respectively; P = .41). In those individuals who scored below 10 on baseline SPPB, there was a trend for higher proportion improving by 1 point or more at 1 year in the ECC group (34% vs 15% in the control group; P = .03); however, mean change in SPPB was not significantly different, −0.24 (SD: 2.14) and −0.01 (SD: 2.55), for the ECC and control groups, respectively, in this subpopulation (P = .61). No significant change in the individual components of SPPB score (gait speed, balance, and chair stand) was noted over time in either group (data not shown). In addition, no significant change in mean or median SPPB was noted between those in the ECC group who received follow-up phone calls in the year following baseline visit (n = 68; mean: 0.4; median: 0) and those who did not (n = 19; mean: 0, median: 0) (P = .91).
Figure 2.
Median SPPB score over time between study cohorts.
Note. SPPB = Short Physical Performance Battery.
Table 2.
Comparison of Baseline Outcome Measures of Follow-up Attendees and Non-Attendees.
| A. Exercise counseling cohort | |||
|---|---|---|---|
| Outcome measure | 1-y follow-up (n = 87) | No follow-up (n = 109) | P value |
| SPPB score | 10 (9-12) | 10 (9-12) | .73 |
| EQ-VAS | 66 (50-75) | 60 (50-70) | .24 |
| HAP-MAS | 68 (58-77) | 68 (57-81) | .79 |
| PASE | X | X | |
| Grip strength, kg | 52 (40-61) | 51 (39-62) | .69 |
| B. Control cohort | |||
| Outcome measure | 1-y follow-up (n = 125) | No follow-up (n = 122) | P value |
| SPPB score | 9 (7-11) | 10 (6-12) | .59 |
| EQ-VAS | 70 (50-80) | 60 (50-75) | .05 |
| HAP-MAS | X | X | |
| PASE | 85 (50-126) | 53 (9-109) | <.01 |
| Grip strength, kg | 45 (32-64) | 45 (34-60) | .76 |
Note. Data presented as median (interquartile range). SPPB = Short Physical Performance Battery; EQ-VAS = EuroQol Visual Analogue Scale; HAP = Human Activity Profile; MAS = maximal activity score; PASE = Physical Activity Scale for the Elderly.
Change in Grip Strength
Median combined grip strength at baseline was 52 kg (IQR: 40-61) in the ECC group and 45 kg (IQR: 34-60) in the control group (P = .01). Significant decline in mean grip strength over time occurred in the ECC cohort (−3.0 kg; 95% CI: −7 to 3) as compared with the control cohort (4.0 kg; 95% CI: −2 to 10) (P < .01) (Table 3).
Table 3.
Change in SPPB, Strength, EQ-VAS, and Physical Activity Over 1 Year.
| Outcome | ECC cohort (n = 87) | Control cohort (n = 125) | P value |
|---|---|---|---|
| % SPBB improved by ≥1 point (n) | 30 (26) | 31 (39) | .84 |
| % SPPB same or better (n) | 61 (53) | 69 (86) | .23 |
| % SPPB decline by ≥1 point (n) | 39 (34) | 31 (39) | .41 |
| Mean change in SPPB score (95% CI) | −0.33 (−0.9, 0.15) | −0.22 (−0.61 to 0.17) | .72 |
| Mean change in grip strength, kg (95% CI) | −3.0 (−7.0, −3.0) | 4.0 (−2.0 to 10.0) | <.01 |
| Mean change in EQ-VAS (95% CI) | 0 (−10, 10) | 0 (−10 to 20) | .61 |
| Follow-up median HAP-MAS score (IQR) | 72 (58-80) | X | X |
| Follow-up median PASE score (IQR) | X | 63 (31-117) | X |
| % Improved physical activity (n) | 32 (28) | 30 (37) | .68 |
Note. SPPB = Short Physical Performance Battery; EQ-VAS = EuroQol Visual Analogue Scale; ECC = exercise counseling clinic; CI = confidence interval; HAP = Human Activity Profile; MAS = maximal activity score; IQR = interquartile range; PASE = Physical Activity Scale for the Elderly.
Physical Activity
Regardless of performance level, there was no significant difference in the proportion of individuals in whom physical activity level improved between ECC (38%) and control cohorts (32%) (P = .40).
HRQOL
HRQOL at baseline as measured by median EQ-VAS score was 66 (IQR: 50-75) and 70 (IQR: 50-80) in the ECC and control cohorts, respectively (P = .37). There was a trend for median EQ-VAS score to increase in both groups over 1 year (70 and 75 in ECC and control groups, respectively), but mean change in EQ-VAS score was not significantly different between study groups (Table 3). Although restrictions reported with EQ5D-3L in Mobility and Anxiety/Depression were similar between study cohorts at baseline, individuals in the ECC group endorsed more restrictions in Self-Care (20% vs 9%; P = .02), Usual Activities (47% vs 25%; P < .01), and Pain/Discomfort (67% vs 51%; P = .02) than in the control group, respectively. The differences between groups in these domains remained similar at 1 year (Table 4). At 1 year, no significant differences in proportion of individuals who experienced worsening symptoms between cohorts were noted in any EQ5D-3L domains.
Table 4.
Proportion of Each Cohort Endorsing Any EQ5D-3L Symptoms Over Time.
| EQ5D-3L domain | Baseline |
1-y follow-up |
||||
|---|---|---|---|---|---|---|
| ECC (n = 87) | Control (n = 125) | P value | ECC (n = 87) | Control (n = 125) | P value | |
| Mobility (EQ5D-M) | 50.0% | 41.5% | .23 | 49.4% | 54.8% | .44 |
| Self-Care (EQ5D-S) | 19.8% | 8.5% | .02 | 16.1% | 7.3% | .04 |
| Usual Activity (EQ5D-U) | 46.5% | 24.6% | <.01 | 42.0% | 25.8% | .02 |
| Pain/Discomfort (EQ5D-P) | 67.1% | 50.9% | .02 | 72.8% | 47.6% | <.01 |
| Anxiety/Depression (EQ5D-A) | 40.0% | 29.7% | .13 | 38.8% | 30.7% | .23 |
Note. ECC = exercise counseling clinic.
Subgroup Analysis
To address potential confounding, subgroup analysis of the 110 control participants and 60 ECC attendees with eGFR <30 mL/min/1.73 m2 who were not renal transplant recipients was performed. Similar to the analysis of the whole study cohort, no significant difference in change in SPPB, HRQOL, or physical activity level over 1 year was identified between study groups (data not shown). However, a higher proportion of individuals with baseline SPPB >10 declined in the ECC group as compared with the control group (20% vs 7%; P = .01). Decline in grip strength at 1 year was significantly greater in the ECC cohort (−3; IQR: −7 to 4) as compared with the control cohort (5; IQR: −2 to 10) in this subgroup analysis (P < .01).
Predictors of Change in Physical Function
In our multivariate stepwise regression model, only baseline SPPB score (odds ratio [OR]: 0.83; 95% CI: 0.75-0.93) was a significant predictor of improvement in SPPB score at 1 year (Table 5). Not surprisingly, increasing eGFR was protective (OR: 0.95; 95% CI: 0.91-0.98) and the presence of diabetes (OR: 3.57; 95% CI: 1.68-7.63) predicted decline in SPPB score over 1 year (Table 5). Interestingly, higher serum calcium level was also associated with decline in SPPB (OR: 1.39; 95% CI: 1.12-1.72). Exposure to exercise counseling did not predict change in SPPB score over time in the above models.
Table 5.
Significant Predictors of Change in SPPB Over 1 Year.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| A. Improvement in SPPB (≥1 point) | |||
| Weight, kg | 0.98 | 0.97-1.00 | .04 |
| Baseline SPPB | 0.83 | 0.75-0.93 | <.01 |
| Area under the ROC curve (95% CI): 0.707 (0.629-0.785) | |||
| B. Decline in SPPB (≥1 point) | |||
| eGFR (per unit increase) | 0.95 | 0.91-0.98 | <.01 |
| Diabetes | 3.57 | 1.68-7.63 | <.01 |
| Calcium (per 0.1-unit increase) | 1.39 | 1.12-1.73 | <.01 |
| PO4 (per unit increase) | 0.25 | 0.08-0.80 | .02 |
| Albumin (per unit increase) | 0.93 | 0.86-1.00 | .04 |
| Area under the ROC curve (95% CI): 0.746 (0.671-0.821) | |||
Note. Model selection methods used: stepwise Selection. Variables considered: exercise counseling/control group, age, sex, modality, race, weight, SBP, DPB, IHD, lung disease, CHF, PVD, cholesterol, stroke, diabetes, hypertension, arthritis, hemoglobin, creatinine, eGFR, calcium, PO4, albumin, physical activity change, baseline SPPB. SPPB = Short Physical Performance Battery; OR = odds ratio; CI = confidence interval; ROC = receiver operating characteristic; eGFR = estimated glomerular filtration rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; IHD = ischemic heart disease; PVD = peripheral vascular disease; CHF = congestive heart failure; PO4 = phosphate.
Discussion
This pragmatic evaluation of a preexisting clinical program did not demonstrate a statistically significant change in physical function as measured by SPPB score over 1 year in individuals with CKD who attended an ECC when compared with a contemporaneous control group. In addition, no statistically significant difference in the proportion or rate of decline in SPPB was observed between groups. The lack of improvement (and actual decline) in grip strength observed in the ECC cohort supports our observation that a single exercise counseling session alone does not improve or help maintain physical function in individuals with CKD.
Previous studies examining the association between exercise counseling interventions and physical activity levels have found that physical activity improved significantly following the intervention.21,22,41-44 Recognizing that physical function is unlikely to change without a corresponding change in physical activity, the lack of improvement in physical activity level in either the ECC or control cohorts may explain our negative findings. However, several other explanations for our negative findings exist. First, few studies investigating exercise counseling interventions have reported outcomes other than change in self-reported physical activity level.21,22,42-44 It is possible that although exercise counseling may increase exercise behavior with subsequent increase in physical activity in some settings, these increases may not be substantial enough to result in a sustained improvement in physical function over time.
Although our counseling methods were similar to previous interventional studies of exercise counseling with the creation of individualized exercise plan, discussion of goals, and motivational telephone calls following the counseling visit, no formal physician prescription was distributed to attendees. Details and frequency of motivational telephone calls that each individual received after attending the exercise clinic were not well documented in our clinical program. Many individuals may have only received 1 out of 4 scheduled phone calls. These factors may, at least in part, explain the differing results of our study from other activity and exercise counseling trials performed in the general population.21,22,44
As well, the overall level of physical function was significantly higher at baseline in the ECC group than in the control group. The lack of observed improvement in SPPB in the ECC cohort may be due to a ceiling effect of the SPPB. This is supported by the results of our regression analysis. Based on the number of individuals with a baseline and follow-up score of 12 in this study, a ceiling effect could modify the proportion of individuals with an improvement in SPPB score in each group by as much as 30%. However, we observed no significant effect of the ECC even when only those individuals with baseline SPPB score of less than 10 were analyzed, suggesting that a ceiling effect may not completely explain our negative results. To avoid this concern in the future, a tool that is responsive to change at higher levels of physical function or a focus on lower functioning groups should be considered for future investigations.
Finally, our study was powered to detect a change in SPPB score of 1 point between groups. Even though the small difference in change in SPPB we observed between study groups is unlikely to be clinically significant based on previous publications, our sample size did not provide power to detect a statistically significant difference with this magnitude of change.40
The significant decline in grip strength in the ECC group over time as compared with the control group was unexpected. Baseline characteristics between attendees and non-attendees in the ECC group do not suggest that those in the ECC group who remained in the study were a sicker group than those in the control group, but this remains a possible explanation. Other possibilities include ascertainment bias and steroid myopathy as the ECC group included individuals who were transplant recipients (receiving prednisone), whereas the control cohort did not. Finally, the ECC group contained a higher proportion of hemodialysis (HD) patients, who have low physical function, which declines over time. The decrease in median grip strength seen in the ECC cohort fits within the 10% to 20% decline in physical function observed over the first year on HD.2,45
We observed no significant change in physical activity between study groups. The different measurement tools used for physical activity between study cohorts could have limited ability to detect a difference in this outcome. In addition, although validated using accelerometry in the dialysis population,36 the HAP measures the number, not the duration of different activities performed. Therefore, the score may not change significantly if the frequency or duration of an activity increases. Using accelerometry in a subset of individuals in each study group could mitigate the above issues in the future.
Most importantly, we believe that in the setting of known significant barriers to exercise in CKD, a single exercise counseling session alone is not adequate to increase physical activity to levels necessary for improvements in physical function and that exercise counseling accompanied by formal exercise programming is required to achieve this goal in the CKD population.18,19 This is supported by the significant improvement in physical function observed in intervention studies that combined exercise counseling with exercise programming in CKD.46-49
Limitations of this study include its quasi-experimental design, which may have resulted in selection bias as demonstrated by differences between the study cohorts in several baseline characteristics. Although internal validity is threatened in this study due to the low rate of follow-up attendance, which is common in clinical programs, analysis of baseline data between those who attended follow-up compared with those who did not demonstrates that characteristics were generally similar, decreasing the risk of this source of selection bias. Nonetheless, it is possible that those individuals in the ECC who did not return for follow-up were maintaining their exercise program and no longer felt that attendance at ECC was necessary. SPPB score may have improved in these individuals if still in the study. Similarly, in view of the differences in baseline characteristics between control group attendees and non-attendees, it is possible that non-attendees were a sicker group and may have had a decline in SPPB had they still been in the study.
A major strength of this study is that it was performed under pragmatic, real-life conditions and provides insight into the true clinical effectiveness of an intervention that has not been previously evaluated in the CKD population. In addition, the similarity of baseline characteristics such as age, gender, BMI, and comorbidities of the study population with that of prevalent dialysis patients in Canada supports external validity of this study.50 Finally, all assessors underwent identical formal training in outcome measurement techniques. This limits bias due to measurement variability related to the use of different outcome assessors in the study cohorts.
In conclusion, this quasi-experimental analysis of prospectively collected data from an ongoing clinical program showed no significant difference in physical function as measured by change in SPPB score at 1 year in individuals with CKD who attended an ECC compared with a convenience control group. Despite limitations in study design, these results suggest that exercise counseling alone is unlikely to improve physical function in individuals with CKD, a population with multiple comorbidities and significant barriers to exercise. Our study has both clinical and research implications. For programs considering the addition of physical activity promotion to clinical care, a simple exercise counseling structure appears insufficient and a more prescriptive approach combining counseling with exercise classes may be necessary. Future research should focus on randomized controlled trials to test the safety and efficacy of a combination of lifestyle education and structured exercise programming on exercise sustainability and prevention of adverse clinical outcomes in patients with CKD.
Supplementary Material
Footnotes
Ethics Approval and Consent to Participate: The University of Manitoba Research Ethics Board approved this study protocol. Written informed consent was obtained from participants prior to enrollment.
Consent for Publication: Consent for publication was obtained from all authors.
Availability of Data and Materials: Not available
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Serena Nelko was supported by a Mindel and Tom Olenick Research Studentship and the H.T. Thorlakson Foundation, internal funds, University of Manitoba, for her work on this project.
ORCID iDs: Clara J. Bohm  https://orcid.org/0000-0001-7710-7162
https://orcid.org/0000-0001-7710-7162
Navdeep Tangri  https://orcid.org/0000-0002-5075-6370
https://orcid.org/0000-0002-5075-6370
References
- 1. Finkelstein J, Joshi A, Hise MK. Association of physical activity and renal function in subjects with and without metabolic syndrome: a review of the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2006;48:372-382. doi: 10.1053/j.ajkd.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 2. Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293-1301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Painter P. Physical functioning in end-stage renal disease patients: update 2005. Hemodial Int. 2005;9:218-235. doi: 10.1111/j.1492-7535.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 4. Bohm C, Storsley L, Tangri N. The assessment of frailty in older people with chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24:498-504. doi: 10.1097/MNH.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 5. Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care. 1989;27:S217-S232. [DOI] [PubMed] [Google Scholar]
- 6. Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78:1164-1170. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol. 2009;4:1901-1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30:204-212. [DOI] [PubMed] [Google Scholar]
- 9. Stack AG, Molony DA, Rives T, Tyson J, Murthy BV. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis. 2005;45:690-701. [DOI] [PubMed] [Google Scholar]
- 10. Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539-1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johansen KL, Kaysen GA, Dalrymple LS, et al. Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol. 2013;8:248-253. doi: 10.2215/CJN.08560812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;(10): 1-407CD003236. doi: 10.1002/14651858.CD003236.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Howden EJ, Coombes JS, Strand H, Douglas B, Campbell KL, Isbel NM. Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis. 2015;65:583-591. doi: 10.1053/j.ajkd.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 14. Kidney Disease: Improving Global Outcomes. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Supplements. 2013;3:1-150. [DOI] [PubMed] [Google Scholar]
- 15. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1-S153. [PubMed] [Google Scholar]
- 16. Johansen KL, Sakkas GK, Doyle J, Shubert T, Dudley RA. Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis. 2003;41:171-178. doi: 10.1053/ajkd.2003.50001. [DOI] [PubMed] [Google Scholar]
- 17. Delgado C, Johansen KL. Deficient counseling on physical activity among nephrologists. Nephron Clin Pract. 2010;116:c330-c336. doi: 10.1159/000319593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delgado C, Johansen KL. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant. 2012;27:1152-1157. doi: 10.1093/ndt/gfr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clarke AL, Young HM, Hull KL, Hudson N, Burton JO, Smith AC. Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant. 2015;30:1885-1892. doi: 10.1093/ndt/gfv208. [DOI] [PubMed] [Google Scholar]
- 20. Thompson S, Tonelli M, Klarenbach S, Molzahn A. A qualitative study to explore patient and staff perceptions of intradialytic exercise. Clin J Am Soc Nephrol. 2016;11:1024-1033. doi: 10.2215/CJN.11981115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orrow G, Kinmonth AL, Sanderson S, Sutton S. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. 2012;344:e1389. doi: 10.1136/bmj.e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Writing Group for the Activity Counseling Trial Research Group. Effects of physical activity counseling in primary care: the Activity Counseling Trial: a randomized controlled trial. JAMA. 2001;286:677-687. [DOI] [PubMed] [Google Scholar]
- 23.Manitoba’s Population Trends Past and Present. Winnipeg, Canada: Manitoba Bureau of Statistics; 2017. [Google Scholar]
- 24. Walker SR, Brar R, Eng F, et al. Frailty and physical function in chronic kidney disease: the CanFIT study. Can J Kidney Health Dis. 2015;2:32. doi: 10.1186/s40697-015-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390-395. [DOI] [PubMed] [Google Scholar]
- 26. Prochaska JO, DiClemente CC, Velicer WF, Rossi JS. Criticisms and concerns of the transtheoretical model in light of recent research. Br J Addict. 1992;87:825-828; discussion 833-835. [DOI] [PubMed] [Google Scholar]
- 27. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. [DOI] [PubMed] [Google Scholar]
- 28. Freire AN, Guerra RO, Alvarado B, Guralnik JM, Zunzunegui MV. Validity and reliability of the Short Physical Performance Battery in two diverse older adult populations in Quebec and Brazil. J Aging Health. 2012;24:863-878. doi: 10.1177/0898264312438551. [DOI] [PubMed] [Google Scholar]
- 29. Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89-96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Canadian Society for Exercise Physiology. Canadian Physical Activity, Fitness and Lifestyle Approach: CSEP Health and Fitness Program’s Health-Related Appraisal and Counselling Strategy. Ottawa, Ontario, Canada; Canadian Society for Exercise Physiology; 2003. [Google Scholar]
- 31. The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199-208. [DOI] [PubMed] [Google Scholar]
- 32. Gerard K, Nicholson T, Mullee M, Mehta R, Roderick P. EQ-5D versus SF-6D in an older, chronically ill patient group. Appl Health Econ Health Policy. 2004;3:91-102. [DOI] [PubMed] [Google Scholar]
- 33. Wasserfallen JB, Halabi G, Saudan P, et al. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2004;19:1594-1599. doi: 10.1093/ndt/gfh175. [DOI] [PubMed] [Google Scholar]
- 34. Fix AJ, Daughton DM. The Human Activity Profile Professional Manual. Lincoln: University of Nebraska; 1988. [Google Scholar]
- 35. Davidson M, de Morton N. A systematic review of the Human Activity Profile. Clin Rehabil. 2007;21:151-162. doi: 10.1177/0269215506069475. [DOI] [PubMed] [Google Scholar]
- 36. Johansen KL, Painter P, Kent-Braun JA, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59:1121-1127. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 37. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153-162. [DOI] [PubMed] [Google Scholar]
- 38. Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336-340. [PubMed] [Google Scholar]
- 39. Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The Physical Activity Scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643-651. [DOI] [PubMed] [Google Scholar]
- 40. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 41. Baumann S, Toft U, Aadahl M, Jørgensen T, Pisinger C. The long-term effect of screening and lifestyle counseling on changes in physical activity and diet: the Inter99 Study—a randomized controlled trial. Int J Behav Nutr Phys Act. 2015;12:33. doi: 10.1186/s12966-015-0195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conn VS, Hafdahl AR, Brown SA, Brown LM. Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns. 2008;70:157-172. doi: 10.1016/j.pec.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 44. Petrella RJ, Lattanzio CN, Shapiro S, Overend T. Improving aerobic fitness in older adults: effects of a physician-based exercise counseling and prescription program. Can Fam Physician. 2010;56:e191-e200. [PMC free article] [PubMed] [Google Scholar]
- 45. Knight EL, Ofsthun N, Teng M, Lazarus JM, Curhan GC. The association between mental health, physical function, and hemodialysis mortality. Kidney Int. 2003;63:1843-1851. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 46. Greenwood SA, Lindup H, Taylor K, et al. Evaluation of a pragmatic exercise rehabilitation programme in chronic kidney disease. Nephrol Dial Transplant. 2012;27(suppl 3):iii126-iii134. doi: 10.1093/ndt/gfs272. [DOI] [PubMed] [Google Scholar]
- 47. Rossi AP, Burris DD, Lucas FL, Crocker GA, Wasserman JC. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol. 2014;9:2052-2058. doi: 10.2215/CJN.11791113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol. 2013;8:1494-1501. doi: 10.2215/CJN.10141012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Vilsteren MC, de Greef MH, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant. 2005;20:141-146. doi: 10.1093/ndt/gfh560. [DOI] [PubMed] [Google Scholar]
- 50. Canadian Institute for Health Information. Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada, 2004 to 2013; Ottawa, ON CIHI: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.