Abstract
Biotic stress, as a result of plant-pathogen interactions, induces the accumulation of reactive oxygen species in the cells, causing severe oxidative damage to plants and pathogens. To overcome this damage, both the host and pathogen have developed antioxidant systems to quench excess ROS and keep ROS production and scavenging systems under control. Data on ROS-scavenging systems in the necrotrophic plant pathogen Rhizoctonia solani are just emerging. We formerly identified vitamin B6 biosynthetic machinery of R. solani AG3 as a powerful antioxidant exhibiting a high ability to quench ROS, similar to CATALASE (CAT) and GLUTATHIONE S-TRANSFERASE (GST). Here, we provide evidence on the involvement of R. solani vitamin B6 biosynthetic pathway genes; RsolPDX1 (KF620111.1), RsolPDX2 (KF620112.1), and RsolPLR (KJ395592.1) in vitamin B6 de novo biosynthesis by yeast complementation assays. Since gene expression studies focusing on oxidative stress responses of both the plant and the pathogen following R. solani infection are very limited, this study is the first coexpression analysis of genes encoding vitamin B6, CAT and GST in plant and fungal tissues of three pathosystems during interaction of different AG groups of R. solani with their respective hosts. The findings indicate that distinct expression patterns of fungal and host antioxidant genes were correlated in necrotic tissues and their surrounding areas in each of the three R. solani pathosystems: potato sprout-R. solani AG3; soybean hypocotyl-R. solani AG4 and soybean leaves-R. solani AG1-IA interactions. Levels of ROS increased in all types of potato and soybean tissues, and in fungal hyphae following infection of R. solani AGs as determined by non-fluorescence and fluorescence methods using H2DCF-DA and DAB, respectively. Overall, we demonstrate that the co-expression and accumulation of certain plant and pathogen ROS-antioxidant related genes in each pathosystem are highlighted and might be critical during disease development from the plant’s point of view, and in pathogenicity and developing of infection structures from the fungal point of view.
Introduction
The nectrotrophic fungus Rhizoctonia solani Kühn (teleomorph Thanatephorus cucumeris, Frank, Donk) is an economically devastating plant pathogen with a wide host range. R. solani is classified into fourteen anastomosis groups (AGs) based on hyphal fusion [1], and strains belonging to AG3 and AG4 are root infecting pathogens that cause damping-off and stem rot of potato (Solanum tuberosum L.) and soybean (Glycine max (L.) seedlings, respectively [2–5]. Isolates of the R. solani AG1-IA complex can infect aerial portions of the plant as in the case of soybean leaves causing rhizocotnia foliar blight (RFB) [3, 6, 7]. The common elements in disease development of R. solani isolates are the close association of fungal hyphae with the host epidermis forming branches known as infection cushions or aggregates, penetration of the epidermis, inter- and intracellular colonization and breakage of plant tissue by the production of hydrolytic enzymes, which eventually leads to the development of browning and necrosis associated with oxidative burst and death of tissue [4, 8]. Current cultural and chemical controls are not completely effective to manage Rhizoctonia diseases and the diseases remain a persistent problem. Furthermore, resistance to R. solani in any plant species does not exist.
One of the earliest plant responses following pathogen recognition is the hypersensitive response, leading to the production of reactive oxygen species, primarily superoxide (O2-) and H2O2, at the site of attempted invasion [9]. The produced ROS activates plant defense responses, including programmed cell death, or functions as second messengers in the induction of various plant defense-related genes [10, 11]. In the case of necrotrophic fungi, ROS plays a central role during their interaction with their plant hosts by stimulating the plant’s basal defense responses [12–15]. Several studies showed that the onset of basal resistance in plants to R. solani is tied with ROS-scavenging mechanisms, accumulation of metabolites related to vitamin B6 biosynthetic pathway, oxylipins production and cell wall bound phenolic compounds [16–21].
Different strategies of oxidative stress response systems are deployed by biotrophic and necrotrophic fungi during the infection process. Biotrophic pathogens such as rust fungi respond to oxidative stress by containing and suppressing the oxidative burst, while necrotrophic pathogens such as Botrytis cincerea rely on the exploitation of the oxidative burst in plants to its own advantage and in some cases contribute to it [22]. Hence, the ability of necrotrophic fungi to surpass or manipulate ROS-related plant defenses is crucial for disease progression, and ROS detoxification is essential to the sensitivity of the necrotrophic fungus while encountering its host. Detoxification systems, such as the NOX complex is a good example of how necrotrophic fungi such as B. cinerea and Alternaria alternata can sustain a reduced redox states within subcellular microenvironments [22]. In recent years, vitamin B6 was recognized as a strong antioxidant displaying a great capacity to quench ROS matching that of tocopherols or ascorbic acid, and may have a function in stress alleviation in fungi and plants [23, 24]. Data on ROS-scavenging systems in R. solani are just emerging. To date, the upregulation of R. solani genes particularly, PYRIDOXAL REDUCTASE (PLR AKR8; DW520695), and PYRIDOXAL-5-PHOSPHATASES and TRANSAMINASES of the vitamin B6 salvage biosynthetic pathway, was reported in R. solani hyphae in association with a mycoparasite or an antagonistic bacteria, respectively [25, 26].
In our previous publication, we fully characterized two genes of the de novo vitamin B6 biosynthetic pathway; RsolPDX1 (KF620111.1) and RsolPDX2 (KF620112.1) genes, and one gene RsolPLR (KJ395592.1) of the vitamin B6 salvage biosynthetic pathway of R. solani AG3. Upon exposure to the ROS-generating chemicals, paraquat and H2O2, the vitamin B6 genes exhibited differential regulation and their transcript abundance levels were mostly higher than levels to the well-recognized antioxidant genes, CATALASE (CAT) and GLUTATHIONE S-TRANSFERASE (GST) [27]. These results implicated the role of vitamin B6 genes of the de novo and the salvage biosynthetic pathways function as antioxidants against oxidative stress [27].
Gene expression studies focusing on oxidative stress responses from both the plant and the pathogen sides during plant-R. solani interactions are very limited. Foley, Kidd [28] identified a number of wheat-derived and R. solani AG8-derived genes involved in ROS production and redox regulation, and whose expression was affected during infection. Their study highlighted the need to understand the ROS-scavenging system interplay of fungal and host derived genes involved in ROS/redox regulation at the site of infection and the surrounding areas. However our knowledge on the role of vitamin B6 genes as antioxidants in other AGs of R. solani is limited.
In this study, we first provided indirect evidence on the functionality of RsolPDX1 and RsolPDX2 of R. solani AG3, and their involvement in vitamin B6 de novo biosynthesis pathway via heterologous complementation of the yeast, Saccharomyces cerevisiae strains Δsnz1 and sno1Δ. Second, we showed that the antioxidant genes encoding vitamin B6 (i.e., PDX, PLR), CAT and, GST of R. solani AG3 and potato are differentially induced and transcriptionally regulated at the site of infection (i.e., necrotic tissues, and in the surrounding areas) during R. solani AG3-potato sprout interaction (pathosystem I). Third, we extended our study to investigate whether differential and spatial expression patterns and transcriptional regulation of vitamin B6 genes and other antioxidant genes also occur in the pathogen and host upon R. solani AG4 infection of soybean hypocotyl (pathosystem II) and also upon R. solani AG1-IA infection of soybean leaves (pathosystem III).
Materials and methods
Yeast complementation assays- heterologous expression of R. solani AG3 PDX1 and PDX2 and growth assays
Full-length clones of RsolPDX1-AG3 and RsolPDX2-AG3 were obtained by primer pairs (RsolAG3-PDX1-CDS-F/R and RsolAG3-PDX2-CDS—F/R) (Table 1) from cDNA of R. solani AG3 isolate Rs114 (ATCC 10183. The PCR products of the two genes were purified using the QIA quick PCR Purification Kit (Qiagen, Toronto, ON, Canada), and sub-cloned into the pDrive cloning vector (Qiagen) to obtain pDrive-PDX1-AG3 and pDrive-PDX2-AG3. The clones were digested with NotI and ligated into a NotI-digested yeast shuttle vector pFL61 (Addgene, Cambridge, MA, USA) [29] to obtain pFL61-PDX1-AG3 and pFL61-PDX2-AG3. All clones were sequenced to confirm the presence of the target genes. To determine whether PDX1-AG3 and PDX2-AG3 encode functional proteins, the Saccharomyces cerevisiae mutant strains (Δsnz1:disrupted pdx1 and Δsno1:disrupted pdx2), obtained from the European Saccharomyces cerevisiae Archive for functional analysis (EUROSCARF; http://wwwrz.uni-frankfurt.de/FB/fb16/mikro/EUROSCARF/), were transformed using a lithium acetate-based method [30] with the construct pFL61-expression vector or with pFL61 alone (negative control), and tested for their capacity to complement the defect function of the snz1 and sno1.
Table 1. List of primers used in this study.
| Target organism | Primer | Sequence (5′ → 3′) | Annealing temperature (°C) | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| PDX1 (Pyridoxine biosynthesis gene) | |||||
| Soybean | GMa- PDX1.1-F | TACAGTGTACGGCAACGGTGCA | 59 | 235 | XM_003542937.3 |
| GM-PDX1.1-R | GTCCTTGATGAGCTGCGGG | ||||
| GM-PDX1.2-F | TACAGTCTACGGCAACGGCGCC | 54 | 235 | NM_001251112.2 | |
| GM-PDX1.2-R | ATCCTTGATGAGCTGCGGA | ||||
| Potato | STb-PDX1.1-qF | CTGTGACTATTCCTGTAAT | 55 | 83 | [37] |
| ST- PDX1.1-qR | GTAATCTACTCCGATAGC | ||||
| ST-PDX1.2-qF | TGCTCTAATCCTTACAAG | 55 | 162 | ||
| ST-PDX1.2-qR | GTAGGTCTCATCACTAAC | ||||
| R. solani AG3 | 5'GSP1-(PDX1) | TGACGAACAGCCTCGACAACATTTCC | 55 | 219 | [27] |
| 3'GSP2-(PDX1) | TCCGTTTGTCTGTGGGGCTACATCTCTC | ||||
| R. solani AG4 | RsolcAG8-PDX1- F | ATGATCAAAGAGATCGTGGAC | 53 | 243 | AVOZ01001318.1 |
| RsolAG8-PDX1-R | GATCATTGCTGCACCTTCGGA | ||||
| R. solani AG1-IA | RsolAG1-IA-PDX1-F | ATCTCTGCCTACTACCAATG | 51 | 131 | AFRT01001225.1 |
| RsolAG1-IA-PDX1-R | TGCCCCCATCCGTGTTCTGAG | ||||
| PDX2 (Pyridoxine biosynthesis gene) | |||||
| Soybean | GM-PDX2-F | TTAGGAGTGAAAGGTGTGGAG | 53 | 328 | XM_014774652.1 |
| GM-PDX2-R | TTTGGAGACGAGCTCTGGCAC | ||||
| Potato | ST-PDX2-qF | ATTCCAATCCTGCTATTC | 55 | 185 | [37] |
| ST-PDX2-qR | CACAATATCAGAAGTTCCT | ||||
| R. solani AG3 | 3'GSP1-(PDX2) | AATCTTGCTTGCCTCTGGTGGTGTTG | 55 | 225 | [27] |
| 5'GSP1-(PDX2) | ATCCCATTAAATGGTCGGTCCTCATCA | ||||
| R. solani AG4& R. solani AG1-IA | RsolAG8-PDX2-F | ATTATATCACGCGTGACACC | 53 | 207 | JATN01000314.1 |
| RsolAG8-PDX2-R | GGCGCCTTCAACACCACCAGA | ||||
| PLR (PYRIDOXAL REDUCTASE) | |||||
| Soybean | GM-PLR-F | TCCTCCAAAGCCTGAACCCGA | 53 | 115 | XM_003527250.3 |
| GM-PLR-R | TAGAAGGAGTTCGTTGGTGT | ||||
| Potato | ST-PLR-F | GCCGCCCAAACCCGAACCCGA | 58 | 118 | XM_006358747.2 |
| ST-PLR-R | AAGGAGGATCTCGTTAGTGT | ||||
| R. solani AG3 | PLR AKR8 F | GAAAGCCTCCTCTTGGAATCT | 58 | 300 | [25] |
| PLR AKR8 R | GGGTAAGATTGGATCGATTGGG | ||||
| R. solani AG4 & R. solani AG1-IA | RsolAG1-IA-PLR-F | AAGGGGCACTAACCGGAAAGC | 58 | 194 | AFRT01000983.1 |
| RsolAG1-IA-PLR-R | ATGTTGCCCGAGAGAGGAGAC | ||||
| CATALASE | |||||
| Soybean | GM-CAT d-F | ACATGTTCACCTTTTTATTTG | 47 | 149 | NM_001253092 |
| GM-CAT-R | CTTTATACCACTAGTGGTCTT | ||||
| Potato | ST-CAT-F | ATGTTCACTTTCCTCTTCGAC | 52 | 147 | AY442179.2 |
| ST-CAT-R | CTTGACACCACATGTGGGCTT | ||||
| R. solani AG3 | RsolAG3-CAT-F | ACCAGAAGTGTTAGTCCAGCGG | 56 | 190 | [27] |
| RsolAG3-CAT-R | CATCCGGTCACAGCAGCGTA | ||||
| R. solani AG4& R. solani AG1-IA | RsolAG1-IA-CAT-F | TGCGGGACTCATGCTGCTCTC | 55–54 | 114 | AFRT01002805.1 |
| RsolAG1-IA-CAT-R | TCCTTGGTGCCTTTCCTATCC | ||||
| GLUTATHIONE S-TRANSFERASES | |||||
| Soybean | GM-GST e-F | TCCATTTGGGATGAGGGTCAG | 53 | 208 | NM_001251762 |
| GM-GST-R | ATTTCTGTCATTCCAAACCTCC | ||||
| Potato | ST-GST-F | TCCTTTTAGCCATAGAGTTGA | 51 | 200 | POTPR1A |
| ST-GST-R | CTTCAAATGCCTCATCAATGC | ||||
| R. solani AG3 | RsolAG3-GST-F | AGAAGACGAGGCAAATGCGA | 57 | 256 | [27] |
| RsolAG3-GST-R | ATCTCTTCAACCGCCTTCCAGT | ||||
| R. solani AG4 & R. solani AG1-IA | RsolAG1-IA-GST-F | TACAAACGCATACCTTATCGC | 53 | 217 | ELU40094.1 |
| RsolAG1-IA-GST -R | TTGGAGCCGGGTAAGTTTTG | ||||
| Reference genes | |||||
| Soybean | UKN2f _F | GCCTCTGGATACCTGCTCAAG | 58 | 79 | [38] |
| UKN2_R | ACCTCCTCCTCAAACTCCTCTG | ||||
| Potato | ST-Actin7-qF | GGCTATGTATGTTGCTAT | 55 | 186 | [37] |
| ST-Actin7-qR | ATCTTCATCAGGTTATCAG | ||||
| R. solani AG3 | G3PDHg -F | GGTATTATTGGATACACTGA | 55 | 129 | [25] |
| G3PDH -R | TTAAGCCTCAGCGTCTTTCT | ||||
| R. solani AG1-IA | RsolH3h _F | CTTCCAATCATCGGCAGTCCTC | 52 | 76 | [34] |
| RsolH3 _R | ATTGGTATCTTCGAACAAAGACACGAG | ||||
| R. solani AG4 | GM-RS-4i | CGGTTCATCTGCATTTACCTT | 55 | 88 | [39] |
| GM-RS3-R | AGTGTTATGCTTGGTTCCACT | ||||
| Transformation | |||||
| R. solani AG3 |
RsolAG3-PDX1-CDS-F RsolAG3-PDX1-CDS-R |
GCGGCCGCATCATGTCTGCTCCTGTGTC GCGGCCGCCACTCACATAGTCAGCCCAA |
55 | 969 | KF620111.1 |
| R. solani AG3 | RsolAG3-PDX2-CDS-F | GCGGCCGCTCGATGACTAGAACTGAAAC | 55 | 747 | KF620112.1 |
| RsolAG3-PDX2-CDS-R | GCGGCCGCGGTTCACTTGGCAAAGCTC | ||||
a Glycine max
b Solanum tuberosum
c Rhizoctonia solani
d Catalase
e Glutathione S-Transferase
f Hypothetical protein unknown 2
g G3PDH: Glyceraldehyde-3-phosphatedehydrogenasegen
h Histone 3
i ITS1 of 5.8S rRNA
For vitamin B6 growth-dependence assays: Yeast cultures grown to exponential phase (2 × 107 cells mL−1) followed by two serial 10-fold dilutions corresponding to the wild-type BY4742, and transformants were spotted on minimal selective SC medium (Difco Laboratories, MI, USA) medium lacking vitamin B6. The mutants, Δsnz1 and Δsno1 are sensitive to the superoxide generator menadione, and this sensitivity is corrected by the addition of vitamin B6 [31]. Transformed yeast cells were grown on minimal SC medium to the post-diauxic phase for 20h followed by a treatment with 40 mM menadione (-Aldrich Canada, Mississauga, ON, Canada) for 3 h in the presence or absence of 2μg mL−1 of vitamin B6 and spotted on SC medium lacking Uracil and vitamin B6. Pictures were captured from plates that were incubated for 3 d at 30°C [32].
Biological material and inoculation
Fungal material
Starter cultures of R. solani AG3 isolate Rs114 (ATCC 10183), AG4 isolate A76 obtained from M. Cubeta, North Carolina University, USA, and AG1-IA strain ROS-2A4 obtained from P. Ceresini, University of São Paulo State (UNESP), Brazil were recovered from cryogenic storage at −80°C on potato dextrose agar (PDA; Difco Laboratories) and grown for 3 days at 24°C in the dark and used as a source of inoculum for plant infection. Cultures of R. solani strains grown on half-strength PDA overlaid with cellophane membrane were used for the visualization of ROS in their hyphae.
Plant material
Certified potato tubers (Solanum tuberosum var. Russet Burbank, susceptible to R. solani AG3), obtained from the Potato Research Center (NB, Canada) and propagated at the Macdonald Campus Farm (Ste. Anne de Bellevue, Canada), were kindly provided by D.J. Donnelly (McGill University). Potato tubers were grown without light at 4°C to induce sprout formation. For sprout induction, uniform-sized tubers were surface sterilised and sprouted for 12 days under controlled conditions following the methods of Aliferis and Jabaji [17], Chamoun, Samsatly [33]. Prevention of browning of the sprout tips was achieved by applying 1.5 mL solution of CaNO3 (0.5M) to sprouts of 3 cm in length. Inoculation of sprouts was performed when sprouts were 8 cm in length.
Soybean (Glycine max) cv. Williams 82 seeds, a susceptible cultivar to R. solani AG-4, were sterilized according to Aliferis, Faubert [16]. Pre-germinated seeds were planted in Cone-tainers® (Stuewe & Sons, Inc., Oregon, USA) filled with 130 mL of sterile turf:perlite (1:1, v/v) and incubated in the dark in a growth chamber in order to produce etiolated seedlings with longer lengths of hypocotyls (approximately 15 cm in height). Hypocotyls were inoculated with AG4 isolate A76 as previously published [16].
For unifoliate leaf inoculation with R. solani AG1-IA, pre-germinated soybean seeds in Cone-tainers® as described above were grown under controlled conditions of temperature, humidity and light following the method of Copley, Aliferis [34]. Fully expanded soybean unifoliate leaves were detached from 2 week-old plants, and used for inoculation.
Inoculation of potato sprouts, soybean hypocotyls and soybean leaves
Excised sprouts and etiolated hypocotyls were arranged horizontally in sterile Pyrex trays (40 cm × 26 cm) lined with wet sterile Whatman No. 1 paper. Each treatment replicate consisted of 4 excised potato sprouts or 4 etiolated hypocotyls. There were 3 biological replicates per treatment. Inoculation was performed by sandwiching horizontally the sprouts (8 cm in length) or hypocotyls (15 cm in length) of each treatment replicate between two PDA strips (2 cm × 8 cm) of a 3-day-old R. solani AG3 Rs114 or R. solani AG4 isolate A76 cultures, respectively, with the bottom edge of the strips placed at 4 cm from the basal part of the potato sprouts or hypocotyl sprouts according to the method of Aliferis and Jabaji [17], Chamoun, Samsatly [33]. Potato sprouts or soybean hypocotyls sandwiched with sterile PDA strips served as controls. Moist, sterile absorbent cotton wool was placed at the base of the sprouts or the hypocotyls of each replicate treatment to maintain humidity. Pyrex trays were sealed with Saran Wrap, and placed in a growth chamber under photosynthetically inactive black light (365 nm) at 24°C.
Fully expanded unifoliate soybean leaves were placed into sterile Pyrex trays as described above. Inoculation was performed by placing PDA plugs (5 mm) of 3-day-old R. solani AG1-IA culture disc, or with sterile PDA alone (control) at the center of leaves. Each replicate treatment consisted of 5 unifoliate leaves. Trays were wrapped with plastic membrane, placed in growth chambers under conditions of day/night temperatures, light cycles and humidity as we previously described in Copley, Aliferis [34].
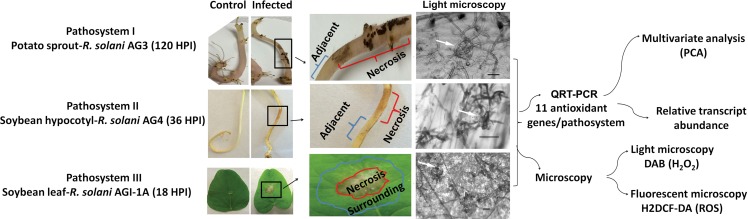
PDA strips or plugs were removed 120 h, 36h and 18h post-inoculation (HPI) of plant tissue to reveal visible necrotic lesions with infection zones on sprouts, hypocotyls and leaves, respectively. These time points were selected in order to capture the onset of infection cushions and the development of mycelial aggregates that were stereoscopically confirmed (Fig 1). Necrotic tissues containing infection zones plus a one-cm area away from necrotic tissues were harvested. Similar areas and amounts of tissues were harvested from control samples. Harvested tissues were flash-frozen with liquid nitrogen and stored at −80°C. Each treatment (infected or control) consisted of three biological replications.
Fig 1. Flow chart of the experimental set up for disease development in the three pathosystems.
I. potato sprouts-R. solani AG3; II. soybean hypocotyls-R. solani AG4; III. soybean leaves-R. solani AG1-IA. Sprouts and hypocotyls in pathosystems I and II, were sandwiched between two PDA strips of a 3-day-old R. solani AG3 or AG4 cultures, respectively. Sprouts or hypocotyls sandwiched with sterile PDA strips served as controls. Leaves in pathosystem III were inoculated with 3-day-old plugs of R. solani AG1-IA. Samples were taken at 120 HPI (Hour post-inoculation) (pathosystem I), 36 HPI (pathosystem II), and 18 HPI (pathosystem III) from necrotic lesions and adjacent or surrounding areas. Corresponding segments were taken from controls. Typical initials of infection cushions with mycelial mass aggregates are clearly visible under the light microscope (white arrows). Transcriptional abundance of 11 antioxidant genes from both the plant and pathogen of each pathosystem was analyzed in necrotic tissues and their surrounding areas followed by multivariate analysis (PCA). The three pathosystems were examined for ROS accumulation using fluorescent (H2DCF-DA) and DAB staining methods in control and infected sprouts, hypocotyls and leaves along with mycelia of control R. solani. Bar = 50 μm.
RNA extraction, primer design and quantitative RT-PCR
Total RNA was isolated from 100 mg of flash frozen pulverized tissue obtained from the necrotic lesions and their surrounding areas of respective treatments and corresponding areas of control soybean hypocotyls, leaves, or potato sprouts. Extraction was done using the TRIZOL reagent (Generay Biotech, Shanghai, China) following the manufacturer's instructions. RNA concentration and purity was spectrophotometry measured using ND1000 (NanoDrop, Wilmington, Delaware), and RNA quality was verified by gel electrophoresis. RNA (500 ng) was reverse transcribed using the Quantitect Reverse transcriptase kit™ (Qiagen). QRT-PCR assays were conducted on fungal and plant target antioxidant genes encoding GST, CAT, the de novo vitamin B6 biosynthesis genes; a synthase (PDX1) and a glutaminase, (PDX2), and the vitamin B6 salvage pathway encoding gene, PYRIDOXAL REDUCTASE (PLR) and appropriate internal reference genes for the three pathosystems (Table 1) using Stratagene Mx3000 (Stratagene, Cedar Creek, USA). Primer names were preceded by the plant or fungus abbreviation: GM, Glycine max, ST, Solanum tuberosum, or Rsol, R. solani. Primer sets were designed based on sequences from NCBI, and were checked for specificity to amplify only their target gene. QRT-PCR conditions were optimized for each primer set, and products were confirmed by sequencing. For primer pairs that were used on more than one AG, they were checked for their ability to amplify with comparable efficiencies in the different pathosystems. Reverse transcription PCR assays were performed on three biological replicates and two technical replicates. PCR assay conditions were performed as previously described [27, 35] using suitable annealing temperature for each primer pair (Table 1). In all QRT-PCR assays, routine negative and positive controls were performed at every run. No template control served as negative control. Also, cDNA of uninfected plant host or R. solani alone served as negative controls when amplifying fungal genes or plant genes, respectively. Positive controls for each run consisted of cDNA of R. solani alone or plant host alone when amplifying the fungal or plant genes, respectively. The relative transcript abundance levels of the plant and fungal-derived genes were estimated and normalized against their respective reference genes according to Zhao and Fernald [36]. In the case of R. solani AG-3, expression of the antioxidant genes was normalized using G3PDH. This choice was based on the lowest coefficient variation when compared to Tubulin and Histone using the statistical tool Bestkeeper (http://www.gene-quantifiaction.info).
Statistical analyses
Significance of the relative transcript abundance between treatments and controls were analyzed by two-way analysis of variance (ANOVA), and when necessary by least significant differences (LSD) at P < 0.05 using the SPSS statistical package v. 22.0, (IBM Corp., Armonk, NY, USA). Transcript changes were deemed statistically and biologically significant if P<0.05 and fold changes were > +1.5 or > -1.5.
In order to find potential correlations and trends between relative abundance of plant and fungal genes expressed in necrotic tissues and their surrounding areas of each pathosystem, a multivariate analysis of the data was performed using the SIMCA-P+ v.12.0 software (Umetrics, MKS Instruments Inc., Andover, MA, USA). The data matrix consisted of biological replicates of control plant tissues, and tissues of necrotic lesions and areas surrounding them (columns; X variables) and the variables for relative transcript abundance of antioxidant genes (rows; Y variables). Data were obtained from the analysis of relative transcript abundance of 11 genes for potato sprouts-R. solani AG3, soybean leaves-R. solani AG1-IA and soybean hypocotyls-R. solani AG4 [11 rows x 9 columns]. For the evaluation of data and detection of outliers, principal component analysis (PCA) was performed. To determine which of the genes were most affected in each pathosystem, PCA loading coefficient plot for the effect of the relative transcript abundance of genes on tissue type, and a loading biplot was built for the visualization of correlations between the X and Y variables using the pc (corr) (i.e., correlation scaled loadings of the correlation between Y variables and X scores based on the variable importance of X as scores and loadings) setting in SIMCA-P+. For data normalization, mean-centering and Pareto [PAR] scaling were used.
Optical and fluorescence microscopy
Association of the detected ROS that accumulated during disease development, with changes in the transcript abundance of the genes was visualized during disease development in the three pathosystems. Cellular and extracellular ROS accumulation was visualized by 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) and 3,3′-diaminobenzidine (DAB) staining methods in control and infected soybean leaves and hypocotyls, and potato sprouts, along with mycelia of control R. solani AG1-IA, AG4, AG3 grown on half-strength PDA overlaid with cellophane membrane. For ROS detection, samples were incubated with 10 μM H2DCF-DA, a specific ROS molecular-detection probe, in H2O for 30 min. Samples were then washed with pre-warmed (28°C) H2O for 30 min to remove the non-internalized probe. To visualize H2O2 accumulation in situ, DAB staining was performed, by treating the samples DAB as described in Pogany, von Rad [40] with some modifications. Whole leaves of soybean were treated with DAB for 24h, whereas thin shavings (approximately 2 x 0.5 cm) of soybean hypocotyls and potato sprouts were obtained with a blade and treated for 4h. All samples were cleared with saturated 15.1 M of chloral hydrate solution, and examined under Zeiss SteREO Discovery.V20 microscope (Carl Zeiss Canada Ltd., Toronto, Ontario, Canada). Fluorescence detection from plant and fungal tissues were read at an excitation wavelength of 470 nm using a GFP filter.
Results
Functional characterization of RsolPDX1 and RsolPDX2 by yeast heterologous complementation provided proof of the ability of R. solani AG3 PDX1 and PDX2 to encode functional enzymes
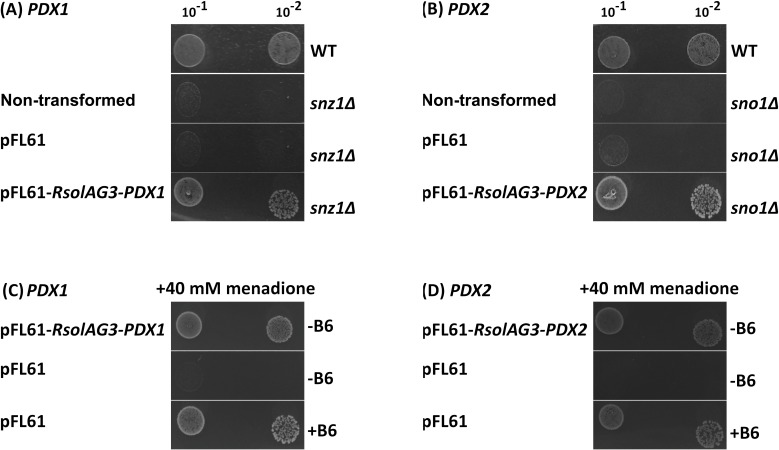
To determine whether RsolPDX1 and RsolPDX2 encode functional enzymes, the full coding sequence of each gene was cloned into the yeast expression vector pFL61 and transformed into S. cerevisiae strains defective in either snz1 (yeast functional PDX1 homolog; Δsnz1) or sno1 (yeast functional PDX2 homolog; Δsno1) and therefore unable to grow in media lacking vitamin B6 [31, 32]. Thus, growth of the transformed mutant S. cerevisiae cells on media lacking vitamin B6 will confirm the complementarity of the R. solani homologs to yeast vitamin B6 genes in addition to their role in vitamin B6 biosynthesis. On media not amended with pyridoxine, growth of S. cerevisiae strains Δsnz1 and Δsno1 expressing RsolPDX1 and RsolPDX2, was evident (Fig 2A and Fig 2B). In contrast, the non-transformed strains or the empty vector-transformed Δsnz1 or Δsno1 mutant cells did not grow on media lacking pyridoxine. Complementation of the Δsnz1 mutant by Rsol-PDX1 and Δsno1 mutant by Rsol-PDX2 demonstrated that Rsol-PDX1 and Rsol-PDX2 are functional, and that they can replace the yeast SNZ1 and SNO1, respectively, implying that they are involved in de novo vitamin B6 biosynthesis.
Fig 2. Complementation of the yeast Δsnz1 and Δsno1 mutants by RsolPDX1 and RsolPDX2, respectively.
Yeast cultures grown to exponential-phase followed by two serial 10-fold dilutions were placed onto vitamin B6-deficient SC (SC-B6) agar plates composed of (A) wild-type (WT), Δsnz1mutant, Δsnz1 transformed with either pFL61 alone or the RsolPDX1 expression vector (pFL61-RsolPDX1), (B) wild-type (WT), Δsno1mutant, Δsno1 transformed with either pFL61 alone or the RsolPDX2 expression vector (pFL61-RsolPDX1) (left colonies: 10−1ml-1 right colonies: 10−2ml-1). (C) Δsnz1 mutant yeast transformed either with pFL61 alone or with the RsolPDX1 expression vector (pFL61-RsolPDX1), and (D) Δsno1 mutant yeast transformed either with pFL61 alone or with the RsolPDX2 expression vector (pFL61-RsolPDX2) were both grown to the post-diauxic phase in SC-Uracil medium followed by a 180 min treatment with 40 mm menadione in the presence (+B6) or absence (-B6) of 2 μg ml−1 of vitamin B6. Following menadione treatment, two serial 10-fold dilutions using SC-Uracil medium (left colonies: 10−1ml-1, right colonies: 10−2 ml-1) were placed in SC-Uracil-B6 plates. Pictures were obtained after 3 d incubation at 30°C.
Δsnz1 and Δsno1 display sensitivity to the superoxide generator menadione, and this sensitivity is corrected by the addition of vitamin B6 [31]. Thus, it is expected that vectors harbouring Rsol-PDX1 or Rsol-PDX2, but not the mutants transformed with the empty vector, are resistant to menadione (Fig 2C and Fig 2D). Unsurprisingly, growth defect restoration of the empty vector-transformed mutants (pFL61-Δsnz1, pFL61-Δsno1) was achieved by the addition of 2 μg ml−1 of pyridoxine. In conclusion, these data indicate that that Rsol-PDX1 and Rsol-PDX2 confers resistance to ROS to the cells.
Distinct expression of host and pathogen antioxidant genes in necrotic tissues and surrounding areas
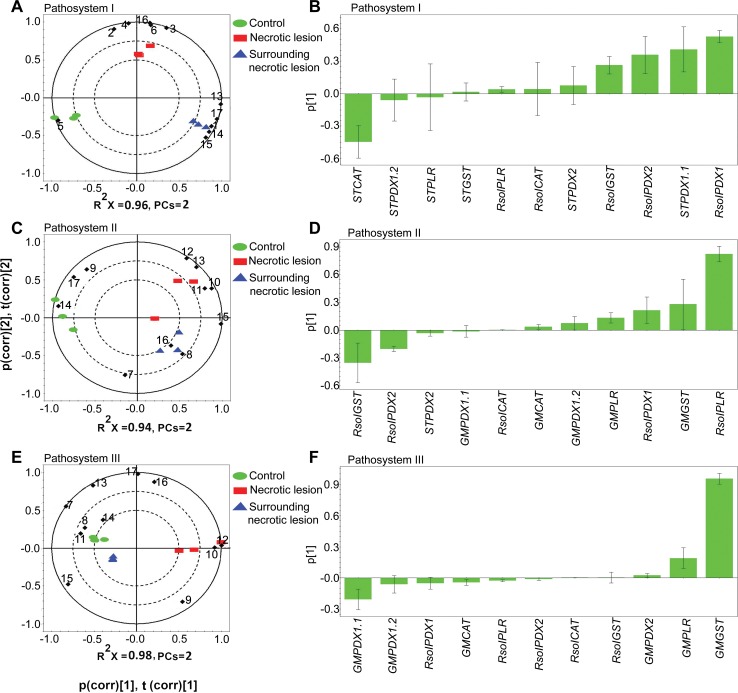
For the discovery of trends of plant and fungal antioxidant encoding genes, in the three pathosystems, PCA revealed tight groups with no outliers (P<0.05) (S1 Fig). Antioxidant genes that influenced the separation of treatments (i.e., plant control, infected plant necrotic tissues and surrounding tissues) for each pathosystem were selected based on strong loading coefficients obtained from the loadings plots (Fig 3) and designated to have an effective antioxidant role in plant-pathogen interactions of each pathosystem. The strength of the correlations was pathosystem-dependent. Additionally, selected genes were highly associated with the distinct plant tissue type as revealed by the PCA- loading biplots (Fig 3).
Fig 3.
Principal component analysis (PCA) loading biplots for the effect of 11 antioxidant genes relative transcript abundance on control, infected and surrounding tissues in (A) pathosystem I, (C) pathosystem II, and (E) pathosystem III (P< 0.05). Principal component analysis (PCA) loading coefficient plots for the effect of 11 antioxidant genes relative transcript abundance on control, infected and surrounding tissues in (B) pathosystem I, (D) pathosystem II, and (F) pathosystem III. The scaled loading vectors pc (corr) and t (corr) are displayed for the first and the second component. Outer ellipses represent the Hotelling’s T2 at a 95% confidence interval. R2X represents the fraction of the sum of squares of the two principal components. ST: Solanum tuberosum, GM: Glycine max. Diamond represents genes. 1 = STPDX1.1, 2 = STPDX1.2, 3 = STPDX2, 4 = STPLR, 5 = STCAT, 6 = STGST, 7 = GMPDX1.1, 8 = GMPDX1.2, 9 = GMPDX2, 10 = GMPLR, 11 = GMCAT,12 = GMGST, 13 = RsolPDX1, 14 = RsolPDX2, 15 = RsolPLR, 16 = RsolCAT,17 = RsolGST.
R. solani AG3 infection of potato sprouts significantly activates the fungal RsolAG3-CAT and RsolAG3-GST in addition to the de novo vitamin B6 genes of both the pathogen and the host
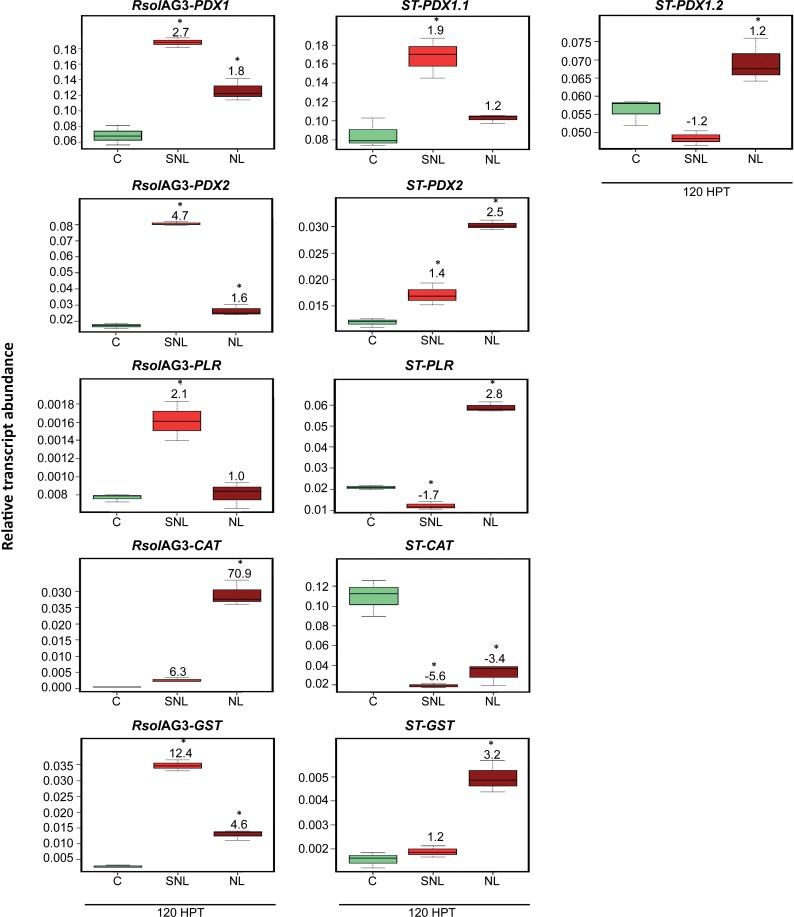
A remarkable gene upregulation of the fungal and plant vitamin B6 de novo pathway genes was observed during disease development of R. solani AG3 on potato sprouts. Based on PCA loading biplots and coefficients, the model showed that the loading coefficient values (p) of RsolAG3-PDX1 (p, 54%) ST-PDX1.1 (p, 40%), RsolAG3-PDX2 (p, 35%), and RsolAG3-GST (p, 25%), were tightly linked with tissues of surrounding areas of necrotic lesions. On the other hand, the potato CATALASE, ST-CAT (p, -45%) was negatively associated with necrotic tissues and their surrounding areas (Fig 3A and Fig 3B). Significant fold increases of transcript abundances of the above fungal genes ranged from a minimum of 1.6 (P = 0.0029) to a maximum of 4.7 (P< 0.0001) depending on the gene and the plant’s area sampled (i.e., necrotic tissue vs surrounding or adjacent tissue) for RsolAG3-PDX1 and RsolAG3-PDX2 (Fig 4). Interestingly, the plant homologue ST-PDX1.1 was differentially upregulated and tissue-dependent (Fig 4). ST-PDX1.1 had a significant increase (1.9 folds; P = 0.0006) in only the tissues surrounding the necrotic area. Significant activation of fungal RsolAG3-CAT (70.9 folds; P< 0.0001) and RsolAG3-GST (12.4 folds; P< 0.0001) was observed in necrotic tissues and surrounding areas, respectively.
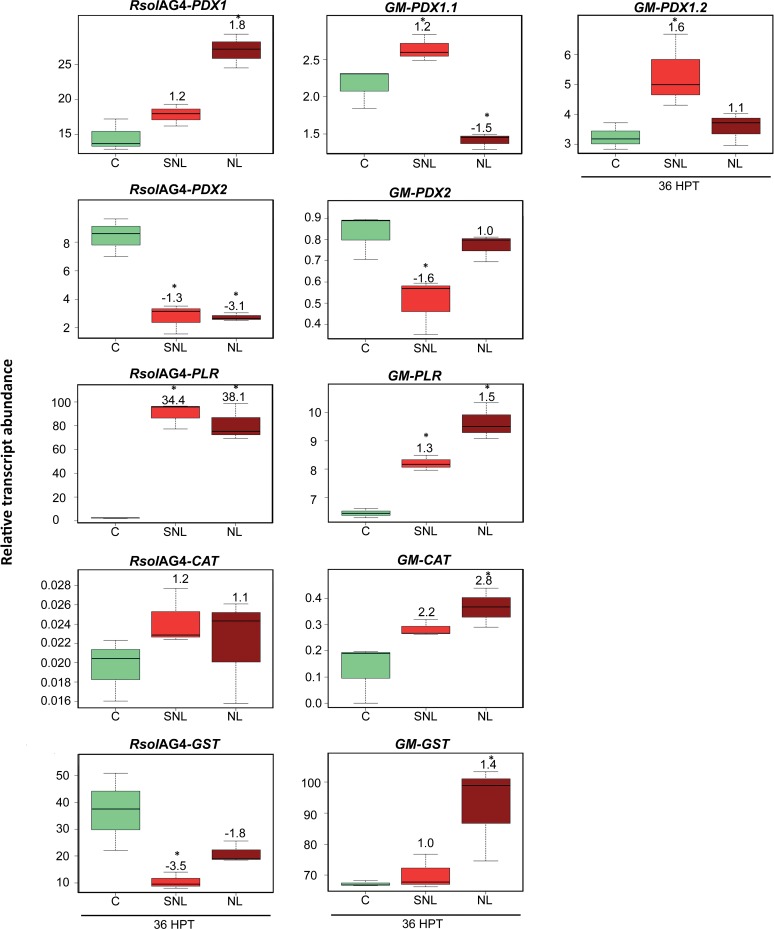
Fig 4. Relative transcript abundance of antioxidants in pathosystem I.
Vitamin B6 (PDX1, PDX2, and PLR), GST, and CAT genes of the pathogen R. solani AG3 (RsolAG3) and the host, Solanum tuberosum (ST) in control and in infected and surrounding tissues at 120 HPI during R. solani AG3 and potato sprouts interaction. C: control; R. solani AG3 grown alone or potato sprouts inoculated with sterile with PDA SNL: tissue surrounding necrotic lesion. NL: necrotic lesion. Asterisks indicate significant relative transcript abundance ratios between the control and interaction using least significant difference (LSD) test (P < 0.05). Fold change is calculated in relation to the control at 120 HPI. PDX1 and PDX2: Pyridoxine biosynthesis genes, PLR: PYRIDOXAL REDUCTASE, CAT: CATALASE, GST: GLUTATHIONE S-TRANSFERASE.
Fungal-derived vitamin B6 genes and RsolAG4-GST are differentially regulated during soybean root and stem rot disease development caused by R. solani AG4
The fungal vitamin B6 genes along with RsolAG4-GST play a significant role in soybean-R. solani AG4 pathosystem compared to their plant’s counterpart during disease development (Fig 3D). A strong positive loading coefficient value (p, 80%) was observed for RsolAG4-PLR (Fig 3D) with significant upregulation of transcript abundance in necrotic tissues and surrounding areas by 38.1 fold (P = 0.0001) and 34.4 fold (P = 0.0001), respectively (Fig 5). RsolAG4-PDX1 was moderately linked (p, 20%) with necrotic tissue showing an increase of transcript abundance by 1.8 fold (P = 0.0004) (Fig 5). However, RsolAG4-GST (p, -35%) and RsolAG4-PDX2 (p, -20%) were negatively associated with necrotic tissue and surrounding areas. (Fig 3C and Fig 3D).
Fig 5. Relative transcript abundance of antioxidants in pathosystem II.
Vitamin B6 (PDX1, PDX2, and PLR), GST, and CAT genes of both the pathogen R. solani AG4 (RsolAG4) and the host, Glycine max, (GM) in control and infected and surrounding tissues at 36 HPI during R. solani AG4 and soybean hypocotyls interaction. C: control; R. solani AG4 grown alone or soybean hypocotyl inoculated with sterile PDA. SNL: tissue surrounding necrotic lesion. NL: necrotic lesion. Asterisks indicate significant relative transcript abundance ratios between the control and the interaction using least significant difference (LSD) test (P < 0.05). Fold change is calculated in relation to the control at 36 HPI. PDX1 and PDX2: Pyridoxine biosynthesis genes, PLR: PYRIDOXAL REDUCTASE, CAT: CATALASE, GST: GLUTATHIONE S-TRANSFERASE.
Among the plant-derived genes, GM-GST (p, 25%) and GM-PLR (p, 15%) were moderately linked with necrotic tissue (Fig 3C and Fig 3D). GM-PLR and GM-GST showed an increase of 1.5 (P = 0.0001) and 1.4 (P = 0.0183) folds, respectively, in necrotic tissue (Fig 5). The soybean GM-CAT encoding gene was positively upregulated in the necrotic tissues with 2.8 (P = 0.0109) fold increase (Fig 5). The de novo plant vitamin B6 genes showed an interesting pattern where both GM-PDX2 and GM-PDX1.1 were significantly downregulated by 1.6 (P = 0.0092) and 1.5 fold (P = 0.0036), respectively (Fig 5) while GM-PDX1.2 was significantly upregulated in areas surrounding to necrotic tissues (1.6 fold; P = 0.0203) (Fig 5).
Major plant-derived vitamin B6 genes and GM-GST play a prominent role during soybean RFB disease development caused by R. solani AG1-IA
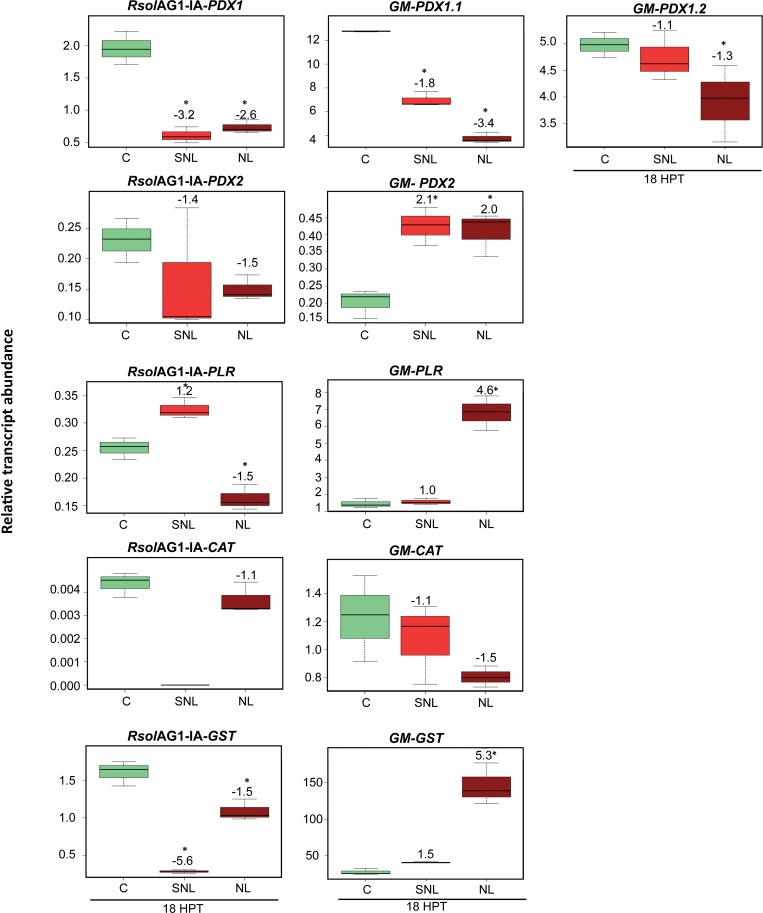
Upon fungal challenge, the relative transcripts abundance of the soybean PYRIDOXAL REDUCTASE (GM-PLR) and GLUTATHIONE S-TRANSFERASE (GM-GST) was highly upregulated whereas their corresponding fungal genes were notably downregulated (Fig 6). PCA analysis showed that leaf soybean GM-GST (p, 95%), and GM-PLR (p, 20%), were closely linked with the necrotic tissue, with significant increased abundance levels of GM-GST and GM-PLR by 5.3 (P = 0.0001) and 4.6 (P< 0.0001) fold, respectively (Fig 6). On the other hand, GM-PDX1.1 (p, -20%) was negatively linked with necrotic tissue and surrounding areas (Fig 3E and Fig 3F), and displayed a significant downregulation with fold changes of 3.4 (P< 0.0001) and 1.8 (P< 0.0001) fold, respectively (Fig 6). Although not supported by high loading coefficient values in the PCA loading analysis, the fungal genes RsolAG1-IA-PDX1 (one component of de novo vitamin B6 biosynthesis genes) and RsoAG1-IA-GST exhibited significant down-regulation with fold changes of 2.6 (P = 0.0001) and 1.5 (P = 0.0024), respectively, in necrotic tissues, and with fold changes of 3.2 (P = 0.0001) and 5.6 (P< 0.0001), respectively, in the surrounding areas (Fig 6).
Fig 6. Relative transcript abundance of antioxidants in pathosystem III.
Vitamin B6 (PDX1, PDX2, and PLR), GST, and CAT genes of both pathogen R. solani AG1-IA (RsolAG1-IA) and the host, Glycine max (GM) in control and in infected and surrounding tissues at 18 HPI during R. solani AG1-IA and soybean leaves interaction. C: control; R. solani AG1-IA grown alone or soybean leaf confronted with sterile PDA. SNL: tissue surrounding necrotic lesion. NL: necrotic lesion. Asterisk indicates significant relative transcript abundance ratios between the control and interaction using least significant difference (LSD) test (P < 0.05). Fold change is calculated in relation to the control at 18 HPI. PDX1 and PDX2: Pyridoxine biosynthesis genes, PLR: PYRIDOXAL REDUCTASE, CAT: CATALASE, GST: GLUTATHIONE S-TRANSFERASE.
Accumulation of ROS in R. solani mycelia is related to transcriptional regulation of vitamin B6 machinery and other antioxidant genes
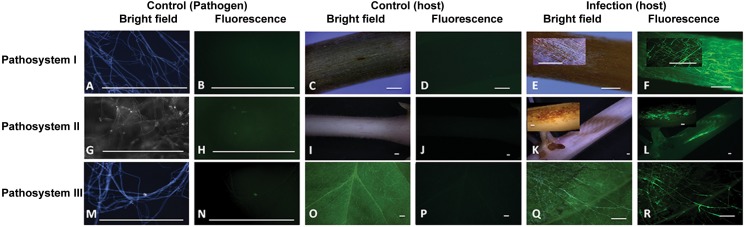
The presence of ROS in the fungal hyphae and plant tissues was detected by fluorescent H2DCF-DA and non-fluorescent DAB methods. Absence of fluorescence was observed in control hyphae grown on half-strength PDA (Fig 7B, Fig 7H and Fig 7N), while an intense green fluorescence was detected in R. solani AG3, AG4, and AG1-IA, hyphae during disease development (Fig 7F, Fig 7L and Fig 7R). Tissues of control and infected potato sprouts, soybean hypocotyls and leaves did not display any green fluorescence (Fig 7D, Fig 7J and Fig 7P) and (Fig 7F, Fig 7L and Fig 7R), respectively.
Fig 7. Intracellular production of ROS during disease development in pathosystems I-III.
ROS production as visualized using H2DCF-DA in control and infected potato sprouts, soybean hypocotyls and leaves along with mycelia of control R. solani AG-3, AG-4, AG1-IA, grown on half-strength PDA overlaid with cellophane membranes. (A-B) Control mycelia with no evidence of endogenous ROS at 120 h, (G-H) 36 h, and (M-N) 18 h upon growth on PDA membrane overlaid with cellophane membranes. (C-D) Control S. tuberosum sprout, (I-J) G. max hypocotyl and (O-P) G. max leaf with no evidence of endogenous ROS. Mycelia of R. solani during disease development in pathosystems I-III (Fig E-F, Fig K-L, and Fig Q-R, respectively). Bar = 500 μm.
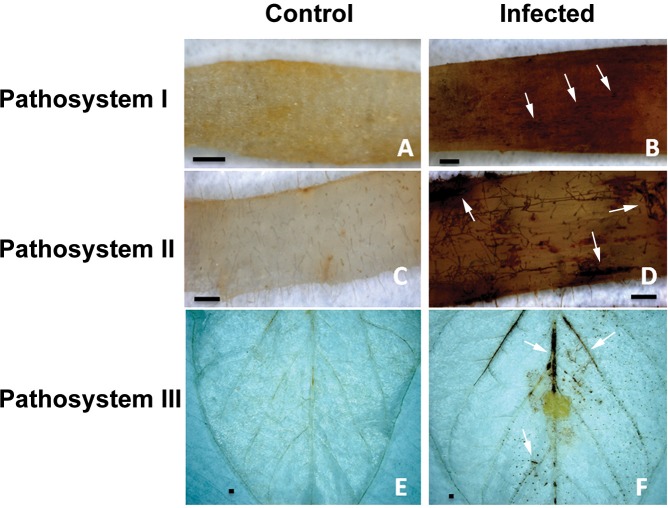
To demonstrate whether the generation of ROS was promoted by R. solani infection, plant tissues were assayed in situ for the production of H2O2 in response to infection, using DAB staining. DAB oxidizes in the presence of H2O2 generating a dark brown precipitate in plant tissues [41]. Tissues of infected potato sprouts and soybean hypocotyls and leaves displayed a strong brown precipitate specifically at necrotic and surrounding areas (Fig 8B, Fig 8D and Fig 8F). However, control uninfected tissues did not show any browning (Fig 8A, Fig 8C and Fig 8E).
Fig 8. Microscopic detection of H2O2 accumulation using DAB staining during disease development in pathosystems I-III.
H2O2 accumulation was visualized by DAB staining in control and infected potato sprouts and soybean hypocotyls and leaves. White arrows indicate dark brown precipitate developed at necrotic and surrounding areas. Control potato sprout (A), soybean hypocotyl (C), and soybean leaf (E) with no evidence of endogenous H2O2 at 120 HPT, 36h HPT, and 18h HPT, respectively. R. solani mycelia and S. tuberosum sprout (B), G. max hypocotyl (D), and G. max leaf (F) in pathosystems I-III with H2O2 accumulation. Bar = 500 μm.
Discussion
It is widely known that ROS management through the use of antioxidants is important for pathogenic microorganisms to prevent excessive oxidative stress [10, 11]. This research further supports our preceding study on the novel function of vitamin B6 genes in R. solani as an antioxidant stress protector against ROS that prevents expansion of oxidative stress [27]. Necrotrophic fungi can regulate intracellular levels of ROS for developmental and virulence purposes while the host uses ROS to hinder disease progression. A comprehensive understanding of plant responses to fungal pathogens exists [42, 43]. Gene expression studies focusing on oxidative stress responses from both the plant and the pathogen side during plant-R. solani interactions are very limited [28, 34]. This study is the first analysis of gene expression encoding vitamin B6 and several antioxidant genes in plant and fungal tissue of three pathosystems during interaction of different AG groups of R. solani with their respective hosts.
In a previous study, we fully characterized two vitamin B6 de novo biosynthetic pathway genes; RsolPDX1 (KF620111.1) and RsolPDX2 (KF620112.1) genes, and one gene, RsolPLR (KJ395592.1) of the vitamin B6 salvage biosynthetic pathway of R. solani AG3 [27]. The use of mutant yeasts and complementation with PDX1 or PDX2 proved to be a valuable tool in fungi [31, 32]. In this study, we confirmed that R. solani AG3 PDX1 and PDX2 are functionally exchangeable with yeast SNZ1 and SNO1, respectively, and are involved in the de novo vitamin B6 biosynthesis via successful complementation of the S. cerevisiae null mutant Δsnz1 phenotype with RsolAG3-PDX1, and the null mutant Δsno1 with RsolAG3-PDX2. Complementation of each of the S. cerevisiae null mutants (Δsnz1 and Δsno1) with its respective R. solani AG3 gene restored their function implying their ability of de novo production of vitamin B6 in yeast. This also suggests that RsolAG3-PDX1 and RsolAG3-PDX2 can interact with their respective yeast counterpart to form the PDX1–PDX2 complex [44]. Similar to other fungi, our complementation assays validated the role RsolAG3-PDX1 or RsolAG3-PDX2 in oxidative stress resistance through the reversion of the menadione sensitivity of the Δsnz1 or Δsno1 mutant strains, respectively [31, 32]. Taken together, our results demonstrated that R. solani AG3 is probably autotrophic to vitamin B6. However, in order to provide direct evidence of autotrophy to vitamin B6, in vitro assays are required to demonstrate the capability of vitamin B6 synthesis by R. solani AG3 PDX1–PDX2 protein complex. Complementation assays for PDX1/PDX2 of R. solani AG4 and AG1-IA were not attempted, as their respective genes are not characterized.
It is common knowledge that ROS is produced at the site of attempted invasion through an oxidative burst during disease development [9, 10, 28, 45]. Oxidative damage can be prevented in both the host and the pathogen when their antioxidant machinery is efficiently used [12, 46]. Both plants and fungi have ROS scavenging activities that detoxify ROS either directly through the action of enzymes such as; superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and peroxiredoxins (Prx), or indirectly via vitamins like ascorbic acid (vitamin C), tocopherols (vitamin E), and vitamin B6 [23, 46–48].
To describe trends (positive or negative) between the plant’s and fungal antioxidants genes (total 11 antioxidant/pathosystem) in response to stress during plant-pathogen interactions of each pathosystem, it necessitates the use of multivariate analysis. The application of PCA revealed tight groups with no outliers. PCA loading biplots demonstrated that several trends could be observed between the relative transcript accumulation of antioxidant genes and the plant’s areas sampled (necrotic tissues or surrounding areas) in each pathosystem. These results indicate the distinctive differences in regulation of fungal-derived and plant-derived antioxidant genes of each pathosystem in response to R. solani attack of different AGs.
Our results clearly showed that ROS formation was induced in hyphal cells of R. solani AG1-IA, AG3 and AG4 during disease development on their respective hosts. ROS production was also reported in R. solani AG 2–2 IV and AG-8, serious pathogens of sugar beet and wheat, respectively [21, 28]. These findings support the notion that several AGs produce ROS. The induced ROS were also linked to the regulation of antioxidant genes and their collective possible role in oxidative stress alleviation. A strong brown precipitate was detected in tissues of infected potato sprouts and soybean hypocotyls and leaves. Accumulation of H2O2 in single cells followed by their death was reported in various plant-fungus interactions [40, 49–51]. These data confirm that Rhizoctonia disease development in potato sprouts and soybean hypocotyls and leaves provokes an oxidative stress in fungal hyphae and plant tissues specifically in necrotic areas and could be related to the oxidative status of the fungus as well as the plant.
The differential expression of antioxidant genes in the host and the pathogen is an important determinant of disease outcome and pathogenicity. ROS is under tight control during disease progression where sometimes it can lead to cell death of fungal and plant tissues. Alternatively, pathogen-induced ROS themselves can act as signalling molecule promoting development of fungal structures or resistance in plant cells [11, 28]. In our study, comparison of the differential gene expression across the three pathosystems was not attempted, because the method of inoculation and plant tissue types are different in each pathosystem, resulting in variable infection intensities and different gene expression. Instead, we focused on understanding the expression patterns of fungal and host antioxidant genes in each pathosystem. Our results showed that distinct expression patterns of fungal and host antioxidant genes were associated in necrotic tissues and their surrounding areas for each of the three R. solani pathosystems.
During R. solani AG3 infection of potato sprouts, significant gene expression of both fungal and plant vitamin B6 de novo pathway genes was observed. Genes of the vitamin B6 de novo pathway of R. solani and potato (homolog ST-PDX1.1) were highly induced in the surrounding tissue and to a lesser extent in the necrotic tissue. Interestingly, the potato ST-PDX genes (i.e., ST-PDX1.1 and ST-PDX 1.2 homologs of ST-PDX1 and ST-PDX 2) are characterized according to their catalytic ability [52] and were expressed in all potato tissues [37]. The catalytic homolog ST-PDX1.1, but not the non-catalytic homolog ST-PDX 1.2, was induced in response to biotic and abiotic stress (this study, Moccand, Boycheva [52]). These results strongly indicate that ST-PDX1.1 plays a role in disease development. It has been reported that Arabidopsis (AT-PDX1.2) acts as a pseudoenzyme that can have a role of a positive regulator of vitamin B6 abundance during abiotic stress [52]. Additionally, it was shown that potato ST-PDX2 might be regulated by ST-PDX1.2, due to the unique (novel) ability of the latter to interact with AT-PDX2 [37, 53]. In our study, the upregulation of ST-PDX2 was not coupled by the upregulation of ST-PDX1.2 during disease development. Whether ST-PDX1.2 acts as a positive regulator of vitamin B6 abundance during potato interaction with R. solani merits further studies.
Activation of some of the antioxidant genes was tissue specific. Higher expression of host genes (ST-PDX2, ST-PLR and ST-GST) compared to their counterpart in the pathogen in necrotic tissues was detected. Tissue specificity response is supported by the findings of Denslow, Walls [54]. The increase in the transcript abundance of PDX1 was evident upon infection of tobacco leaves with P. syringae pv. phaseolicola at the region surrounding the infiltration area. However, levels of PDX1 were lower in the infiltration area where hypersensitive response has developed [54].
In Pathosystem II, both fungal- and plant-derived antioxidant genes played a significant role during disease development where some of them were substantially induced while others were downregulated. Interestingly, fungal and host genes encoding PLR, a member of the vitamin B6 salvage biosynthetic pathways, were among the driving antioxidant force during soybean hypocotyl-R. solani interaction. Both fungal-derived and soybean-derived PLR encoding genes were expressed in the necrotic region as well as in the surrounding tissue, however RsolAG4-PLR was highly expressed. Similarly, in other studies, transcriptional regulation of PLR was substantially induced in response to accumulation of ROS in R. solani mycelia parasitized by a mycoparasite or exposed to different abiotic stressors [25, 27, 55]. The results of our study, clearly indicate the involvement of RsolAG4-PLR as an efficient quencher of ROS. On the other hand, the upregulation of soybean PLR (GM-PLR), but not at the same extent as that of the pathogen, in the same areas may imply that the plant may also be limiting ROS damage to the soybean tissue.
The interplay of ROS antioxidant genes produced by fungi and their hosts during their interaction was evident in R. solani AG1-IA-soybean leaves interaction (pathosystmem III). Major plant-derived antioxidant genes of soybean were upregulated in necrotic leaf tissues whereas the corresponding R. solani AG1-IA antioxidant genes showed notable downregulation. These results may possibly indicate that the elevation of antioxidant capacity of two of the three vitamin B6 genes, GM-PDX2 and GM-PLR as well as the enzymatic antioxidant, GLUTATHIONE S-TRANSFERASE (GM-GST) in soybean leaves against R. solani AG1-IA increased their tolerance to the development of necrosis and suppressed the spread of the pathogen. These results are supported by the over expression of the antioxidant VB6 genes (i.e., PDX) in Arabidopsis that led to increased tolerance to oxidative stress [24, 56]. Equally Arabidposis mutants with defects in vitamin B6 de novo biosynthetic pathway (PDX1.2 or PDX1.3) showed increased levels of disease of gray mold caused by Botrytis cinerea [57, 58]. Taken together, these results support the novel function of vitamin B6 genes as antioxidant stress protector against ROS to prevent oxidative stress.
In plant-necrotroph systems, the downregulation of antioxidant genes appears to play an important role during infection and initiation of the oxidative burst, leading to an overflow of ROS in infected areas. For example, in response to infection with Botrytis cinerea, leaves of ivy pelargonium (Pelargonium peltatum), showed a strong nitric oxide (NO) burst and H2O2 accumulation to arrest disease progression through NO-dependent reversible inhibition of catalase [59]. In our study, the plant’s GmPDX1.1, and ST-CAT in pathosystems I and III were substantially suppressed, respectively, which may lead to enhanced reactive oxygen species generation that typically accompanies infections caused by necrotrophs [60, 61].
Conclusions
In conclusion, we confirmed the role of R. solani AG3 de novo vitamin B6 genes through yeast complementation assay. In our previous study [27], we provided evidence that vitamin B6 genes of the de novo and the salvage biosynthetic pathways function as antioxidants against oxidative stress induced by ROS chemical inducers [27]. In this study, we extend our research to examine whether genes of vitamin B6 machinery are also implicated as antioxidants in response to oxidative stress formed during plant-pathogen interactions. Our findings present new evidence on: (i) the co-expression of VB6 genes along with other well-known antioxidant genes (i.e., CAT and GST) in both the plant and pathogen side during their interaction, and (ii) the differential transcriptional regulation of the genes in infected necrotic tissues and their surrounding areas of three pathosystems involving different plants and different AGs of R. solani.
It is also likely that the requirement of Vitamin B6 genes of the salvage pathway differs among different plant species in response to pathogen infection. It appears that both the pathogen and host employ unique expression strategy depending on the type of interaction as well as the type of tissue affected. Hence, the study offers novel insights into the biological correlation and identification of ROS antioxidant genes that can be used in soybean and potato breeding programs for resistance against R. solani. Overall, we demonstrate that the co-expression and accumulation of certain plant and pathogen ROS-antioxidant related genes in each pathosystem are highlighted and might be critical during disease development from the plant’s point of view, and in pathogenicity and developing of infection structures from the fungal point of view. Future studies are underway to test whether different potato cultivars containing variable amounts of vitamin B6 [37] exhibit differential degrees of susceptibility to R. solani AG 3, and that this susceptibility is correlated with content and differential gene expression of vitamin B6.
Supporting information
Principle component analysis score plots (PC1/PC2) for the effect of 11 antioxidant genes relative transcripts abundance on control, necrotic lesions and surrounding areas of necrotic lesions of Pathosystem I (A), II (B) and III (C). The ellipse represents the Hotelling T2 at a 95% confidence interval. Three biological replications were performed per treatment. Q2 (cum); cumulative fraction of the total variation of the X’s that can be predicted by the extracted components, R2X; the fraction of the sum of squares of the two principal components.
(TIF)
Acknowledgments
The authors thank D. Donnelley and K. Sabbally and for providing certified seed potatoes of Russet Burbank. We also acknowledge the J.-B Charron for the use of Zeiss SteREO Discovery.V20 microscope. Financial support, through a Discovery grant (RGPIN-2016-04805) to S. Jabaji, was provided by the Natural Sciences and Engineering research Council of Canada.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Discovery grant RGPIN-2016-04805 to S. Jabaji, provided by the Natural Sciences and Engineering Research Council of Canada, http://www.nserc-crsng.gc.ca/index_eng.asp.
References
- 1.García VG, Onco MP, Susan VR. Biology and systematics of the form genus Rhizoctonia. Span J Agric Res. 2006;4(1):55–79. [Google Scholar]
- 2.Buskila Y, Tsror Lahkim L, Sharon M, Teper-Bamnolker P, Holczer-Erlich O, Warshavsky S, et al. Postharvest dark skin spots in potato tubers are an oversuberization response to Rhizoctonia solani infection. Phytopathology. 2011;101(4):436–44. doi: 10.1094/PHYTO-09-10-0251 . [DOI] [PubMed] [Google Scholar]
- 3.Fenille RC, De Souza NL, Kuramae EE. Characterization of Rhizoctonia solani associated with soybean in Brazil. Eur J Plant Pathol. 2002;108(8):783–92. [Google Scholar]
- 4.Sneh B, Jabaji-Hare S, Neate S, Dijst G. Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control 1st ed: Springer Science & Business Media; 2013. [Google Scholar]
- 5.Tsror L. Biology, Epidemiology and management of Rhizoctonia solani on potato. J Phytopathol. 2010;158(10):649–58. doi: 10.1111/j.1439-0434.2010.01671.x [Google Scholar]
- 6.Ciampi MB, Meyer MC, Costa MJ, Zala M, McDonald BA, Ceresini PC. Genetic structure of populations of Rhizoctonia solani anastomosis group-1 IA from soybean in Brazil. Phytopathology. 2008;98(8):932–41. doi: 10.1094/PHYTO-98-8-0932 . [DOI] [PubMed] [Google Scholar]
- 7.Stetina KC, Stetina SR, Russin JS. Comparison of severity assessment methods for predicting yield loss to rhizoctonia foliar blight in soybean. Plant Dis. 2006;90(1):39–43. doi: 10.1094/pd-90-0039 [DOI] [PubMed] [Google Scholar]
- 8.Keijer J. The initial steps of the infection process in Rhizoctonia solani In: Sneh B JS, Neate S, Dijst G, editor. Rhizoctonia species: Taxonomy, molecular biology, ecology, pathology and disease control: Springer; 1996. p. 149–62. [Google Scholar]
- 9.Torres MA. ROS in biotic interactions. Physiol Plant. 2010;138(4):414–29. doi: 10.1111/j.1399-3054.2009.01326.x [DOI] [PubMed] [Google Scholar]
- 10.Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92(6):773–84. . [DOI] [PubMed] [Google Scholar]
- 11.Barna B, Fodor J, Harrach BD, Pogany M, Kiraly Z. The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol Biochem. 2012;59:37–43. doi: 10.1016/j.plaphy.2012.01.014 . [DOI] [PubMed] [Google Scholar]
- 12.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701 . [DOI] [PubMed] [Google Scholar]
- 13.Asai S, Yoshioka H. Nitric oxide as a partner of reactive oxygen species participates in disease resistance to necrotrophic pathogen Botrytis cinerea in Nicotiana benthamiana. Mol Plant Microbe Interact. 2009;22(6):619–29. doi: 10.1094/MPMI-22-6-0619 [DOI] [PubMed] [Google Scholar]
- 14.Pietrowska E, Rozalska S, Kazmierczak A, Nawrocka J, Malolepsza U. Reactive oxygen and nitrogen (ROS and RNS) species generation and cell death in tomato suspension cultures-Botrytis cinerea interaction. Protoplasma. 2015;252(1):307–19. doi: 10.1007/s00709-014-0680-6 ; PubMed Central PMCID: PMCPMC4287684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Kan JA. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006;11(5):247–53. doi: 10.1016/j.tplants.2006.03.005 . [DOI] [PubMed] [Google Scholar]
- 16.Aliferis KA, Faubert D, Jabaji S. A metabolic profiling strategy for the dissection of plant defense against fungal pathogens. PLoS One. 2014;9(11):e111930 doi: 10.1371/journal.pone.0111930 ; PubMed Central PMCID: PMCPMC4219818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aliferis KA, Jabaji S. FT-ICR/MS and GC-EI/MS metabolomics networking unravels global potato sprout's responses to Rhizoctonia solani infection. PLoS One. 2012;7(8):e42576 doi: 10.1371/journal.pone.0042576 ; PubMed Central PMCID: PMCPMC3411821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley RC, Gleason CA, Anderson JP, Hamann T, Singh KB. Genetic and genomic analysis of Rhizoctonia solani interactions with Arabidopsis; evidence of resistance mediated through NADPH oxidases. PLoS One. 2013;8(2):e56814 doi: 10.1371/journal.pone.0056814 ; PubMed Central PMCID: PMCPMC3581538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keshavarz-Tohid V, Taheri P, Taghavi SM, Tarighi S. The role of nitric oxide in basal and induced resistance in relation with hydrogen peroxide and antioxidant enzymes. J Plant Physiol. 2016;199:29–38. doi: 10.1016/j.jplph.2016.05.005 . [DOI] [PubMed] [Google Scholar]
- 20.Nikraftar F, Taheri P, Falahati Rastegar M, Tarighi S. Tomato partial resistance to Rhizoctonia solani involves antioxidative defense mechanisms. Physiol Mol Plant P. 2013;81:74–83. doi: 10.1016/j.pmpp.2012.11.004 [Google Scholar]
- 21.Taheri P, Tarighi S. A survey on basal resistance and riboflavin-induced defense responses of sugar beet against Rhizoctonia solani. J Plant Physiol. 2011;168(10):1114–22. doi: 10.1016/j.jplph.2011.01.001 . [DOI] [PubMed] [Google Scholar]
- 22.Heller J, Tudzynski P. Reactive oxygen species in phytopathogenic fungi: signaling, development, and disease. Annu Rev Phytopathol. 2011;49:369–90. doi: 10.1146/annurev-phyto-072910-095355 . [DOI] [PubMed] [Google Scholar]
- 23.Mooney S, Leuendorf JE, Hendrickson C, Hellmann H. Vitamin B6: a long known compound of surprising complexity. Molecules. 2009;14(1):329–51. doi: 10.3390/molecules14010329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanderschuren H, Boycheva S, Li KT, Szydlowski N, Gruissem W, Fitzpatrick TB. Strategies for vitamin B6 biofortification of plants: a dual role as a micronutrient and a stress protectant. Front Plant Sci. 2013;4:143 doi: 10.3389/fpls.2013.00143 ; PubMed Central PMCID: PMCPMC3659326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamoun R, Jabaji S. Expression of genes of Rhizoctonia solani and the biocontrol Stachybotrys elegans during mycoparasitism of hyphae and sclerotia. Mycologia. 2011;103(3):483–93. doi: 10.3852/10-235 . [DOI] [PubMed] [Google Scholar]
- 26.Gkarmiri K, Finlay RD, Alstrom S, Thomas E, Cubeta MA, Hogberg N. Transcriptomic changes in the plant pathogenic fungus Rhizoctonia solani AG-3 in response to the antagonistic bacteria Serratia proteamaculans and Serratia plymuthica. BMC Genomics. 2015;16:630 doi: 10.1186/s12864-015-1758-z ; PubMed Central PMCID: PMCPMC4546130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samsatly J, Chamoun R, Gluck-Thaler E, Jabaji S. Genes of the de novo and salvage biosynthesis pathways of vitamin b6 are regulated under oxidative stress in the plant pathogen Rhizoctonia solani. Front Microbiol. 2015;6:1429 doi: 10.3389/fmicb.2015.01429 ; PubMed Central PMCID: PMCPMC4700284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley RC, Kidd BN, Hane JK, Anderson JP, Singh KB. Reactive oxygen species play a role in the infection of the necrotrophic fungi, Rhizoctonia solani in wheat. PLoS One. 2016;11(3):e0152548 doi: 10.1371/journal.pone.0152548 ; PubMed Central PMCID: PMCPMC4816451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minet M, Dufour ME, Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. The Plant Journal. 1992;2(3):417–22. [DOI] [PubMed] [Google Scholar]
- 30.Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16(5–6):339–46. doi: 10.1007/bf00340712 [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Navarro S, Llorente B, Rodriguez-Manzaneque MT, Ramne A, Uber G, Marchesan D, et al. Functional analysis of yeast gene families involved in metabolism of vitamins B1 and B6. Yeast. 2002;19(14):1261–76. doi: 10.1002/yea.916 . [DOI] [PubMed] [Google Scholar]
- 32.Benabdellah K, Azcon-Aguilar C, Valderas A, Speziga D, Fitzpatrick TB, Ferrol N. GintPDX1 encodes a protein involved in vitamin B6 biosynthesis that is up-regulated by oxidative stress in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2009;184(3):682–93. doi: 10.1111/j.1469-8137.2009.02978.x . [DOI] [PubMed] [Google Scholar]
- 33.Chamoun R, Samsatly J, Pakala SB, Cubeta MA, Jabaji S. Suppression subtractive hybridization and comparative expression of a pore-forming toxin and glycosyl hydrolase genes in Rhizoctonia solani during potato sprout infection. Molecular Genetics and Genomics. 2015;290(3):877–900. doi: 10.1007/s00438-014-0962-x [DOI] [PubMed] [Google Scholar]
- 34.Copley TR, Aliferis KA, Kliebenstein DJ, Jabaji SH. An integrated RNAseq-1H NMR metabolomics approach to understand soybean primary metabolism regulation in response to Rhizoctonia foliar blight disease. BMC Plant Biol. 2017;17(1):84 doi: 10.1186/s12870-017-1020-8 ; PubMed Central PMCID: PMCPMC5408482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chamoun R, Aliferis KA, Jabaji SH. Characterization and transcriptional regulation of Stachybotrys elegans mitogen-activated-protein kinase gene smkA following mycoparasitism and starvation conditions. Curr Genet. 2013;59(1–2):43–54. doi: 10.1007/s00294-012-0386-2 . [DOI] [PubMed] [Google Scholar]
- 36.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comp Biol. 2005;12(8):1047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mooney S, Chen L, Kuhn C, Navarre R, Knowles NR, Hellmann H. Genotype-specific changes in vitamin B6 content and the PDX family in potato. Biomed Res Int. 2013;2013:389723 doi: 10.1155/2013/389723 ; PubMed Central PMCID: PMCPMC3732595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libault M, Thibivilliers S, Bilgin D, Radwan O, Benitez M, Clough S, et al. Identification of four soybean reference genes for gene expression normalization. The Plant Genome. 2008;1(1):44–54. [Google Scholar]
- 39.Johanson A, Turner HC, McKay GJ, Brown AE. A PCR-based method to distinguish fungi of the rice sheath-blight complex, Rhizoctonia solani, R. oryzae and R. oryzae-sativae. FEMS Microbiol Lett. 1998;162(2):289–94. [DOI] [PubMed] [Google Scholar]
- 40.Pogany M, von Rad U, Grun S, Dongo A, Pintye A, Simoneau P, et al. Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis-Alternaria pathosystem. Plant Physiol. 2009;151(3):1459–75. doi: 10.1104/pp.109.141994 ; PubMed Central PMCID: PMCPMC2773049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thordal‐Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 1997;11(6):1187–94. [Google Scholar]
- 42.Conrath U. Priming of induced plant defense responses. Adv Bot Res. 2009;51:361–95. doi: 10.1016/s0065-2296(09)51009-9 [Google Scholar]
- 43.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–27. doi: 10.1146/annurev.phyto.43.040204.135923 . [DOI] [PubMed] [Google Scholar]
- 44.Gengenbacher M, Fitzpatrick TB, Raschle T, Flicker K, Sinning I, Muller S, et al. Vitamin B6 biosynthesis by the malaria parasite Plasmodium falciparum: biochemical and structural insights. J Biol Chem. 2006;281(6):3633–41. doi: 10.1074/jbc.M508696200 . [DOI] [PubMed] [Google Scholar]
- 45.Lehtonen MJ, Somervuo P, Valkonen JP. Infection with Rhizoctonia solani induces defense genes and systemic resistance in potato sprouts grown without light. Phytopathology. 2008;98(11):1190–8. doi: 10.1094/PHYTO-98-11-1190 . [DOI] [PubMed] [Google Scholar]
- 46.Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012;2012:1–26. doi: 10.1155/2012/217037 [Google Scholar]
- 47.Breitenbach M, Weber M, Rinnerthaler M, Karl T, Breitenbach-Koller L. Oxidative stress in fungi: its function in signal transduction, interaction with plant hosts, and lignocellulose degradation. Biomolecules. 2015;5(2):318–42. doi: 10.3390/biom5020318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mylona PV, Polidoros AN. ROS regulation of antioxidant genes. Reactive Oxygen Species and Antioxidants in Higher Plants. 2010:101. [Google Scholar]
- 49.Asselbergh B, Curvers K, Franca SC, Audenaert K, Vuylsteke M, Van Breusegem F, et al. Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 2007;144(4):1863–77. doi: 10.1104/pp.107.099226 ; PubMed Central PMCID: PMCPMC1949893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung KR. Stress response and pathogenicity of the necrotrophic fungal pathogen Alternaria alternata. Scientifica (Cairo). 2012;2012:635431 doi: 10.6064/2012/635431 ; PubMed Central PMCID: PMCPMC3820455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laluk K, Mengiste T. Necrotroph attacks on plants: wanton destruction or covert extortion? Arabidopsis Book. 2010;8:e0136 doi: 10.1199/tab.0136 ; PubMed Central PMCID: PMCPMC3244965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moccand C, Boycheva S, Surriabre P, Tambasco-Studart M, Raschke M, Kaufmann M, et al. The pseudoenzyme PDX1.2 boosts vitamin B6 biosynthesis under heat and oxidative stress in Arabidopsis. J Biol Chem. 2014;289(12):8203–16. doi: 10.1074/jbc.M113.540526 ; PubMed Central PMCID: PMCPMC3961649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner S, Bernhardt A, Leuendorf JE, Drewke C, Lytovchenko A, Mujahed N, et al. Analysis of the Arabidopsis rsr4-1/pdx1-3 mutant reveals the critical function of the PDX1 protein family in metabolism, development, and vitamin B6 biosynthesis. Plant Cell. 2006;18(7):1722–35. doi: 10.1105/tpc.105.036269 ; PubMed Central PMCID: PMCPMC1488916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denslow SA, Walls AA, Daub ME. Regulation of biosynthetic genes and antioxidant properties of vitamin B6 vitamers during plant defense responses. Physiol Mol Plant P. 2005;66(6):244–55. doi: 10.1016/j.pmpp.2005.09.004 [Google Scholar]
- 55.Morissette DC, Dauch A, Beech R, Masson L, Brousseau R, Jabaji-Hare S. Isolation of mycoparasitic-related transcripts by SSH during interaction of the mycoparasite Stachybotrys elegans with its host Rhizoctonia solani. Curr Genet. 2008;53(2):67–80. doi: 10.1007/s00294-007-0166-6 . [DOI] [PubMed] [Google Scholar]
- 56.Raschke M, Boycheva S, Crevecoeur M, Nunes-Nesi A, Witt S, Fernie AR, et al. Enhanced levels of vitamin B(6) increase aerial organ size and positively affect stress tolerance in Arabidopsis. Plant J. 2011;66(3):414–32. doi: 10.1111/j.1365-313X.2011.04499.x . [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Jin X, Ouyang Z, Li X, Liu B, Huang L, et al. Vitamin B6 contributes to disease resistance against Pseudomonas syringae pv. tomato DC3000 and Botrytis cinerea in Arabidopsis thaliana. J Plant Physiol. 2015;175:21–5. doi: 10.1016/j.jplph.2014.06.023 . [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Liu B, Li X, Ouyang Z, Huang L, Hong Y, et al. The de novo biosynthesis of vitamin B6 is required for disease resistance against Botrytis cinerea in tomato. Mol Plant Microbe Interact. 2014;27(7):688–99. doi: 10.1094/MPMI-01-14-0020-R . [DOI] [PubMed] [Google Scholar]
- 59.Floryszak-Wieczorek J, Arasimowicz M, Milczarek G, Jelen H, Jackowiak H. Only an early nitric oxide burst and the following wave of secondary nitric oxide generation enhanced effective defence responses of pelargonium to a necrotrophic pathogen. New Phytol. 2007;175(4):718–30. doi: 10.1111/j.1469-8137.2007.02142.x . [DOI] [PubMed] [Google Scholar]
- 60.Able AJ. Role of reactive oxygen species in the response of barley to necrotrophic pathogens. Protoplasma. 2003;221(1):137–43. [DOI] [PubMed] [Google Scholar]
- 61.Unger C, Kleta S, Jandl G, Tiedemann Av. Suppression of the Defence‐Related Oxidative Burst in Bean Leaf Tissue and Bean Suspension Cells by the Necrotrophic Pathogen Botrytis cinerea. Journal of Phytopathology. 2005;153(1):15–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Principle component analysis score plots (PC1/PC2) for the effect of 11 antioxidant genes relative transcripts abundance on control, necrotic lesions and surrounding areas of necrotic lesions of Pathosystem I (A), II (B) and III (C). The ellipse represents the Hotelling T2 at a 95% confidence interval. Three biological replications were performed per treatment. Q2 (cum); cumulative fraction of the total variation of the X’s that can be predicted by the extracted components, R2X; the fraction of the sum of squares of the two principal components.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.