Abstract
The phenolic contents of plant foods are commonly quantified by the Folin-Ciocalteu assay based on gallic acid equivalents (GAEs). However, this may lead to inaccuracies since gallic acid is not always representative of the structural heterogeneity of plant phenolics. Therefore, product-specific standards have been developed for the phenolic quantification of several foods. Currently, maple-derived foods (syrup, sugar, sap/water, and extracts) are quantified for phenolic contents based on GAEs. Since lignans are the predominant phenolics present in maple, herein, a maple phenolic lignan-enriched standard (MaPLES) was purified (by chromatography) and characterized (by UFLC-MS/MS with lignans previously isolated from maple syrup). Using MaPLES and secoisolariciresinol (a commercially available lignan), the phenolic contents of the maple-derived foods increased threefold compared to GAEs. Therefore, lignan-based standards are more appropriate for phenolic quantification of maple-derived foods vs. GAEs. Also, MaPLES can be utilized for the authentication and detection of fake label claims on maple products.
Keywords: maple, phenolics, lignans, gallic acid equivalents (GAEs), Folin-Ciocalteu assay
TOC image
INTRODUCTION
The United States is the world’s largest consumer of sweeteners, including corn sweeteners (mainly, high fructose corn syrup) and other edible syrups.1 Despite having a wide choice of sweeteners, there is increasing consumer interest in natural sweeteners among which pure maple syrup is highly regarded. Maple syrup is a plant-derived natural sweetener obtained by boiling the sap collected from mainly the sugar maple (Acer saccharum) species. Worldwide, maple syrup is only produced in northeastern North America, primarily in the province of Quebec (in Canada), followed by the New England states (in the United States). Thus, it is not surprising that this agricultural product is of significant economic importance to this region of the world. Moreover, maple syrup has a positive consumer acceptance worldwide and its production is deeply linked with the tradition and culture of both the indigenous and modern peoples of eastern North America. Also, with more consumer interest in locally produced foods, and growing skepticism for artificial ingredients and flavors, there has been increased public and scientific attention on maple-derived food products.2
The most commonly consumed form of maple-derived foods is maple syrup which is produced by boiling maple sap (ca. 40 L sap yields 1 L syrup). This is followed by maple sugar which is produced by slow boiling of maple syrup until a solid texture is obtained which is then crushed and sieved to the desired particle size. In addition, in recent years, other value-added maple-derived food products have emerged in the market. These include pasteurized and sterilized maple water (i.e. maple sap which is ca. 98% water) which is being marketed as a functional beverage and has attracted considerable public3 and scientific attention.4–6 Also, phenolic-enriched maple syrup-derived extracts are being developed for potential nutraceutical and functional food applications.7 This recent diversification in maple-derived food product portfolio is driven, in part, by several recent published in vitro6–15 and animal studies16–20 supporting the potential biological effects of maple phytochemicals against oxidation, inflammation, diabetes, and neurodegenerative diseases.21, 22
Given the premium price and popularity of maple-derived foods, the industry is faced with fake labeling claims and counterfeit products such as artificial maple flavored and caramel colored, and simulated corn fructose-based sweeteners that falsely claim to contain maple syrup. Consequently, the Food and Drug Administration (FDA) in the United States has recently been petitioned by maple producers in North America regarding fake maple labels on food products with similar petitions being planned for provincial and federal agencies in Canada.23 Therefore, given the increasing public and scientific attention on maple-derived foods, there is an urgent need to develop appropriate product-specific standards to support research authenticity, reproducibility, and future health based studies on maple. To date, maple-derived foods are being quantified for phenolic contents based on the widely used industrial standard, namely, gallic acid equivalents (GAEs), by the Folin-Ciocalteu assay (further discussed below).6, 7, 11, 15, 17, 19 In addition, to date, there are no chemically characterized product-specific standard/s available for the authentication of maple products.
Our group has conducted extensive phytochemical studies on maple-derived food products (syrup, sap, and extracts) which have led to the isolation and structure elucidation (by NMR and mass spectrometry) of a wide diversity of phenolic compounds.6–10 Among the different chemical types of phenolic compounds identified in maple, the lignan sub-class predominates including compounds such as secoisolariciresinol (Seco), a prototypical lignan found in flaxseed which has been extensively studied for its health benefits,24, 25 as well as several other new and known lignan molecules.8, 9
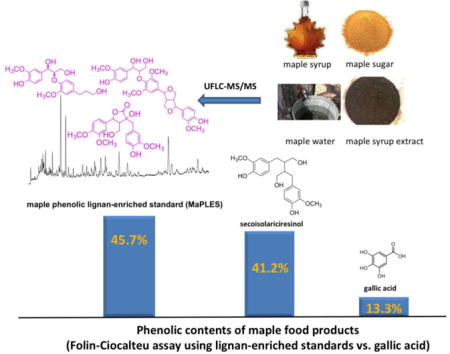
To date, the majority of published reports on maple-derived food products have quantified the phenolic contents of their study materials based on GAEs by the Folin-Ciocalteu assay.6, 7, 11, 17, 19 However, given the mono-phenolic structure of gallic acid, this standard is not always representative of the structural homogeneity and diversity of complex plant phenolics. In fact, the extensive use of GAEs by academic and industry research laboratories is reported to lead to inaccuracies and underestimation of phenolic contents for several foods including grape seeds,26 chocolate,27 pomegranate,28 and cranberry.29–31 This has led to the development of product-specific standards for the phenolic quantification of these aforementioned food products.26–31 Based on these reports, and given that gallic acid is not structurally representative of maple lignans, herein we sought to develop a maple product-specific standard for: 1) the phenolic quantification and, 2) authentication, of maple-derived food products. For this project, we were able to leverage our group’s access to authentic lignan standards (previously isolated and identified by NMR from maple syrup)6–9 to purify (by chromatography) and characterize (by UFLC-MS/MS) the maple phenolic lignan-enriched standard (MaPLES). Also, given that the Folin-Ciocalteu reaction is independent, and that analyses of phenolic mixtures can be recalculated in terms of any other standards,32 several maple-derived food materials were quantified for phenolic contents based on MaPLES and Seco compared to GAEs. The findings from the current study support the use of lignan-based standards, rather than gallic acid, for the quantification of phenolic contents of maple-derived food products.
MATERIAL AND METHODS
Chemicals
Sodium carbonate was purchased from Fisher Scientific (Fair Lawn, NJ, USA). ACS grades of methanol, ethyl acetate, and dimethylsulfoxide (DMSO) were obtained from Pharmco-AAPER through Wilkem Scientific (Pawcatuck, RI, USA). The lignan standards used for UFLC-MS/MS characterization were previously isolated and identified (by NMR and mass spectrometry) from maple syrup and maple sap by our laboratory.6–9 Secoisolariciresinol (Seco), gallic acid, Folin-Ciocalteu reagent, and LC-MS grade of methanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). The C18 resin was purchased from Varian (Palo Alto, CA, USA).
Ultra-Fast Liquid Chromatography Mass Spectrometry (UFLC-MS)
UFLC-MS/MS analyses were performed on a SHIMADZU Prominence UFLC system (Marlborough, MA, USA) consisting of three LC-20AD pumps, a DGU-20A degassing unit, SIL-20AC auto sampler, CTO-20AC column oven and CBM-20A communication bus module. Chromatographic separation was performed on a Waters CORTECS® C18 column (150 mm × 2.1 mm i.d., 2.7 μm. Milford, MA, USA) with a Waters CORTECS® VanGuard™ Pre-Column (5 mm × 2.1 mm i.d., 2.7 μm. Milford, MA, USA). The mobile phase consisted of methanol (A) and 0.1% (v/v) formic acid/ water (B) with a gradient elution of 20% A at 0-1 min, 20-25% A at 1-6 min, 25-51% A at 6-214 min. The column temperature was 40 °C, flow rate was 0.25 mL/min, and injection volume was 2.00 μL. Mass spectrometry was performed using a QTRAP 4500 system from Applied Biosystems/MDS Sciex (Framingham, MA, USA) coupled with an electrospray ionization (ESI) interface. Nitrogen was used in all cases. In this study, the parameters were optimized as follows: ESI voltage, 4500 V; nebulizer gas, 50; auxiliary gas, 50; curtain gas, 20; turbo gas temperature, 550°C; declustering potential, 45V; entrance potential 10V; collision energy, 22 eV. The samples were analyzed with an IDA (Information-Dependent Acquisition) method, which can automatically select candidate ions for MS/MS. The mass range was set from m/z 100 to 1000 and the mass range for product ion scan was m/z 100-1000. The data were acquired and processed using Analyst 1.6.2 software.
Analytical High Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD)
All HPLC-DAD analyses were performed on a Hitachi Elite LaChrom system (Pleasanton, CA, USA) consisting of a L2130 pump, a L-2200 autosampler, and a L-2455 Diode Array Detector, operated by EZChrom Elite software. Chromatographic separation was performed on an Alltima C18 column (250 × 4.6 mm i.d., 5 μM; Alltech). The mobile phase consisted of methanol (A) and 0.1% (v/v) trifluoroacetic acid/ water (B) with a gradient elution of 0−30 min, from 5% to 33.4% A, 30−80 min, from 33.4% to 71% A, 80−85 min, from 71% to 100% A, 85−86 min, from 100% to 5% A, 86−94 min, 5% A. The column temperature was at room temperature, the flow rate was at 0.75 mL/min, the injection volumes were 20 μL, and the detection wavelength was 280 nm.
Purification of the Maple Phenolic Lignan-Enriched Standard (MaPLES)
Maple syrup (grade C code 14-032428 / baril 882-1; 40 L) was shipped frozen to our laboratory by the Federation of Maple Syrup Producers of Quebec (Longueuil, Quebec, Canada) and stored at −20 °C until extraction and purification as follows. Briefly, maple syrup (1551 g) was dissolved in deionized water (2 L) and partitioned with ethyl acetate (2 L×3). The combined ethyl acetate fractions were concentrated in vacuo to yield a maple syrup ethyl acetate extract (280.9 mg) which was purified by C18 chromatography (glass column: 350 mm in height and 50 mm in diameter) eluted with a gradient solvent system of 5%, 15%, 30%, 40%, 50%, 60% and 80% of methanol:H2O (v:v) to yield seven fractions, A-G, respectively. Based on analytical HPLC-DAD profiling, and by comparison of retention times (tR) to lignan standards previously isolated from maple syrup by our laboratory,6–9 fractions C and D were combined to yield the maple phenolic lignan-enriched standard (MaPLES; 64.1 mg) (chromatograms provided in Supporting Information). MaPLES (2 mg/mL) was dissolved in 50% methanol:H2O (1:1, v/v) and the lignan standards (solubilized in DMSO) were diluted in 50% methanol:H2O (1:1, v/v). All samples were filtered (0.22μm, 25 mm i.d.), and stored at −20 °C prior to analyses.
Maple-Derived Food Products
The maple-derived food products (see Table 2) were provided by the Federation of Maple Syrup Producers of Quebec (Longueuil, Quebec, Canada) and consisted of maple water (Wahta™, Rougemont, Quebec, Canada),6 maple syrup (Grade C; code 14-032428 / baril 882-1),8, 9 maple sugar (Lot SG 300315-C / baril 882-1), and a food grade phenolic-enriched maple syrup-derived extract (MSX).7 The maple sugar was produced from the same batch/grade of maple syrup by a slow boiling process to yield a solid which were then crushed and sieved to the desired particle size.
Table 2.
Quantification of phenolic contents of maple-derived food products by the Folin-Ciocalteu assay based on gallic acid, secoisolariciresinol and MaPLES equivalents (as % or g/100 g).
| gallic acid | secoisolariciresinol | MaPLES | |
|---|---|---|---|
| maple water | 0.0003 | 0.0009 | 0.0010 |
| maple syrup | 0.03 | 0.11 | 0.12 |
| maple sugar | 0.09 | 0.29 | 0.32 |
| maple syrup-derived extract (MSX)a | 13.26 | 41.15 | 45.74 |
The food grade maple-syrup derived extract (MSX) has been chemically characterized as previously reported.7
Phenolic Quantification of Maple-Derived Food Products
The maple-derived food products were quantified for phenolic contents using the Folin-Ciocalteu method as previously reported.7, 8 Briefly, each sample (200 μL) was diluted with methanol:H2O (1:1, v/v; 3 mL) and incubated at room temperature (for 10 min) with 200 μL of the Folin-Ciocalteu reagent. Subsequently, 600 μL of a 20% sodium carbonate solution was added to each tube with vigorous mixing. Each sample was then incubated at 40 °C for an additional 20 min and then immediately cooled to room temperature using an ice bath. The standards (MaPLES, Seco, and gallic acid) and samples were processed identically. UV absorptions were recorded on a Beckman Coulter DU® 800 spectrophotometer (Fullerton, CA, USA) at 755 nm and phenolic contents were calculated from the respective curves for each standards which were as follows: MaPLES: y=1.5634x+0.0045, R2=0.999 (52.5–420 μg/mL); Seco: y=1.8001x−0.0083, R2=0.999 (62.5-1000 μg/mL); gallic acid: y=5.5801x−0.0079, R2=0.999 (62.5-527 μg/mL).
RESULTS AND DISCUSSION
Purification of Maple Phenolic Lignan-Enriched Standard (MaPLES)
Our group has previously isolated (by chromatography) and identified (by NMR and mass spectrometry) a large diversity of phenolic compounds from maple water, maple syrup and maple syrup-derived extracts.6–10 We reported that lignans are the major sub-class of phenolic compounds found in these maple-derived food products. Furthermore, our laboratory has developed protocols for the extraction (by partitioning with different organic solvents) and purification (by column chromatography) of phenolic-enriched extracts of maple syrup.6–10 Therefore, using these existing protocols, we generated an ethyl acetate extract of maple syrup which was subsequently purified by medium pressure liquid chromatography (with C18 resin) to yield seven fractions, A-F. The lignan-enriched fractions, namely C-D, were combined (based on analytical HPLC-DAD by comparison to the previously isolated lignan standards) to yield MaPLES (details provided in Supporting Information).
UFLC-MS/MS Characterization of MaPLES
Nineteen lignans (structures shown in Figure 1) were identified in MaPLES by UFLC-MS/MS analyses (mass spectral data provided in Supporting Information). Among these lignans, seventeen compounds (1-10, 12-18) were identified by comparison to lignan standards previously isolated and identified (by NMR) from maple syrup by our laboratory,6–10 and one compound (11; for which we lacked a standard due to limitations in sample quantity) was tentatively identified based on its pseudo molecular ion [M+Na]+. In addition, compounds 17 and 17′ exist as a pair of isomers which corresponded with the retention times (tR) and pseudo molecular ions [M+Na]+ of standards obtained from our previous isolation studies. A detailed description of the identification of the nineteen lignans in MaPLES is provided below.
Figure 1.
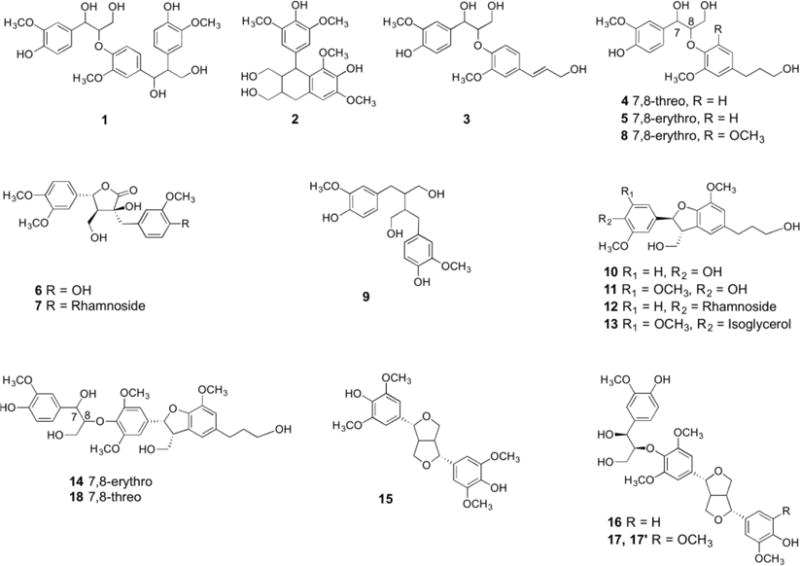
Compounds identified in the maple phenolic lignan-enriched standard (MaPLES) by UFLC-MS/MS based on comparison to authentic lignan standards previously isolated from maple syrup.8, 9
The total ion chromatography (TIC) of the lignan standards and MaPLES are shown in Figures 2A and 2B, respectively, with accompanying identities of the compounds shown in Table 1. By comparison of tR, pseudo molecular ions [M+Na]+, and MS/MS spectral data of the unknown peaks in MaPLES with the authentic lignan standards (corresponding data for the standards are provided in Supporting Information), compounds 1-10, 12-16 and 18 were identified as leptolepisol D (1), lyoniresinol (2), guaiacylglycerol-β-O-4′-coniferyl alcohol (3), threo-guaiacyl-glycerol-β-O-4′-dihydroconiferyl alcohol (4), erythro-guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol (5), 5-(3′′,4′′-dimethoxyphenyl)-3-hydroxy-3-(4′-hydroxy-3′-methoxybenzyl)-4-(hydroxymethyl)dihydrofuran-2-one (6), [3-[4-[(6-deoxy-α-L-mannopyranosyl)oxy]-3-methoxyphenyl]methyl]-5-(3,4-dimethoxyphenyl)-dihydro-3-hydroxy-4-(hydroxymethyl)-2(3H)-furanone (7), erythro-1-(4-hydroxy-3-methoxy-phenyl)-2-[4-(3-hydroxypropyl)-2,6-dimethoxyphenoxy]-1,3-propanediol (8), secoisolariciresinol (9), dehydroconiferyl alcohol (10), icariside E4 (12), sakuraresinol (13), acernikol (14), syringaresinol (15), buddlenol E (16), and 2-[4-[2,3-dihydro-3-(hydroxymethyl)-5-(3-hydroxypropyl)-7-methoxy-2-benzofuranyl]-2,6-dimethoxyphenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol (18), respectively. In the absence of a standard (due to limited quantities obtained from our previous isolation studies), compound 11 [at tR = 116.31 min] was tentatively identified based on the pseudo molecular ion [M+Na]+ at m/z 413 as 5′-methoxy-dehydroconiferyl alcohol. Compounds 17 and 17′ exist as a pair of isomers with two distinct peaks at tR = 200.50 min (17) and tR = 202.07 min (see Figure 2B) but with the same pseudo molecular ion [M+Na]+ at m/z 637 and the same MS/MS fragment ions at m/z 441. Therefore, compounds 17 and 17′ were identified as a pair of isomers of (1S,2R)-2-[2,6-dimethoxy-4-[(1S,3aR,4S,6aR)-tetrahydro-4-(4-hydroxy-3,5-dimethoxyphenyl)-1H,3H-furo[3,4-c]furan-1-yl]phenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol, which have previously been isolated from maple syrup by our laboratory.9 Based on the lignan standards available to us, we were only able to identify nineteen of these compounds in MaPLES. However, as shown in Figure 2B, there are several other compounds present in MaPLEs which are currently unidentified. These unidentified peaks are most likely phenolic compounds including other lignans previously isolated from maple syrup but for which we lacked authentic standards (due to limited samples obtained in our previous studies). Nevertheless, MaPLES is representative of the majority of lignans present in maple syrup.
Figure 2.
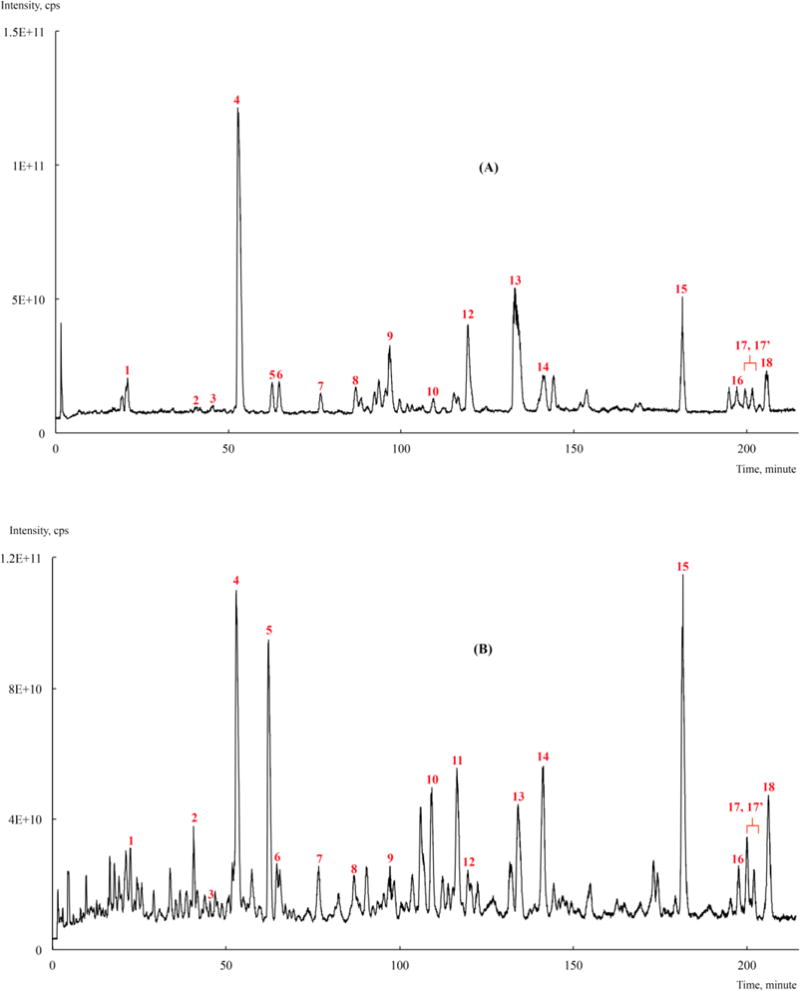
(A) The total ion chromatography (TIC) of lignan standards previously isolated from maple syrup.8, 9 (B) TIC of the maple phenolic lignan-enriched standard (MaPLES).
Table 1.
UFLC-MS/MS data for compounds identified in MaPLES by comparison of retention times (tR) and mass spectral data with authentic lignan standards previously isolated and identified (by NMR) from maple syrup.8, 9
| No. | Compounds | tR (min) | [M+Na]+ (m/z) | MS/MS (m/z) |
|---|---|---|---|---|
| 1 | leptolepisol D | 20.96 | 539 | 539, 491 |
| 2 | lyoniresinol | 40.76 | 443 | –a |
| 3 | guaiacylglycerol-β-O-4′-coniferyl alcohol | 45.53 | 399 | 399, 201 |
| 4 | threo-guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol | 52.85 | 401 | 401, 203 |
| 5 | erythro-guaiacylglycerol-β-O-4′-dihydroconiferyl alcohol | 62.19 | 401 | 401, 203 |
| 6 | 5-(3′′,4′′-dimethoxyphenyl)-3-hydroxy-3-(4′-hydroxy-3′-methoxybenzyl)-4-(hydroxymethyl)dihydrofuran-2-one | 64.03 | 427 | 427, 409, 379, 365, 217 |
| 7 | [3-[4-[(6-deoxy-α-L-mannopyranosyl)oxy]-3-methoxyphenyl]methyl]-5-(3,4-dimethoxyphenyl)-dihydro-3-hydroxy-4-(hydroxymethyl)-2(3H)-furanone | 76.78 | 573 | 573, 525, 511, 427, 409, 379 |
| 8 | erythro-1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-hydroxypropyl)-2,6-dimethoxyphenoxy]-1,3-propanediol | 86.54 | 431 | 431, 233, 219 |
| 9 | secoisolariciresinol | 96.89 | 385 | –a |
| 10 | dehydroconiferyl alcohol | 109.28 | 383 | –a |
| 11 | 5′-methoxydehydroconiferyl alcohol | 116.31 | 413 | –a |
| 12 | icariside E4 | 119.74 | 529 | 529, 383 |
| 13 | sakuraresinol | 133.10 | 487 | 487, 411, 395, 381 |
| 14 | acernikol | 141.29 | 441 | –a |
| 15 | syringaresinol | 180.76 | 609 | –a |
| 16 | buddlenol E | 197.45 | 607 | 607, 411, 395 |
| 17, 17′ | (1S,2R)-2-[2,6-dimethoxy-4-[(1S,3aR,4S,6aR)-tetrahydro-4-(4-hydroxy-3,5-dimethoxyphenyl)-1H,3H-furo[3,4-c]furan-1-yl]phenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | 200.50 202.07 |
637 637 |
637, 441, 425 637, 441, 425 |
| 18 | 2-[4-[2,3-dihydro-3-(hydroxymethyl)-5-(3-hydroxypropyl)-7-methoxy-2-benzofuranyl]-2,6-dimethoxyphenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | 206.31 | 609 | 609, 413 |
Phenolic Quantification of Maple-Derived Food Products with Lignan-Enriched Standards (MaPLES and Seco) vs. GAEs by the Folin-Ciocalteu Assay
As shown in Table 2, several maple-derived food products, maple water,6 maple syrup,8, 9 maple sugar, and a previously chemically characterized food grade phenolic-enriched maple syrup-derived extract (MSX)7 were quantified for phenolic contents based on lignan-enriched standards (i.e. MaPLES and Seco) equivalents vs. GAEs.
As expected, the trend in phenolic contents among the food products was MSX > maple sugar > maple syrup > maple water. Thus, the phenolic content of maple water (i.e. maple sap) was the lowest since ca. 40 L of maple sap is concentrated (by boiling) to produce ca. 1 L of maple syrup. Similarly, maple syrup is further concentrated to yield maple sugar, and MSX is a highly concentrated and purified phenolic-enriched extract derived from maple syrup.7 Overall, the most striking result was that the phenolic contents of the four maple-derived food products were threefold higher based on the lignan-enriched standards compared to GAEs. However, since the Folin-Ciocalteu reagent can react with reducing substances (vitamins, nitrogen-containing compounds, etc.) apart from phenols,32 further studies using a diversity of maple products (increased sample size and types) are warranted since the four maple products were evaluated in this study as ‘neat/natural’ forms. Nevertheless, our observations are similar to those reported for several other phenolic-rich plat foods including grape seeds, chocolate, pomegranate and cranberry, wherein their respective product-specific generated standards yielded higher phenolic contents compared to GAEs.26–31
In conclusion, in the current study, we have purified and chemically characterized a maple phenolic lignan-enriched standard (MaPLES) which contains the majority of the natural occurring lignan constituents found in maple syrup. Based on MaPLES, and the commercially available lignan standard, Seco, we found that the use of GAEs grossly underestimates the phenolic contents of several maple-derived food products. Therefore, lignan-based standards are more appropriate for the phenolic quantification of maple-derived foods rather than GAEs. However, since GAEs are commonly used as an industrial standard for measuring phenolic contents of many foods, a precise and validated GAE/MaPLES ratio will be required for comparison of the phenolic contents of maple products with those of food products obtained with GAEs. Lastly, given that the maple syrup industry is currently faced with counterfeit and fake labelling claims on products,23 MaPLES can also be utilized for the authentication of maple-derived food products. Our group will be pursuing multi-laboratory and other validation studies as previously reported,28 as well as further refinement of UFLC-MS/MS methods, to evaluate the phenolic content and authenticity of commercially available maple food products using MaPLES.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the Federation of Quebec Maple Syrup Producers (Longueuil, Quebec, Canada) and Agriculture and Agri-Food Canada for funding of this research project. We would like to thank Dr. Alvin Bach II for assistance with accumulating mass spectrometry data. This research was made possible by the use of instruments made available through an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430.
Footnotes
Supporting Information
The supporting information is available free of charge on the ACS Publications website at DOI:
The HPLC-DAD chromatograms of the maple syrup ethyl acetate extract and its fractions used for purification of MaPLES (Figures S1 and S2, respectively), the UFLC-MS/MS spectra of the nineteen compounds 1-16, 17 and 17′, and 18 identified in MaPLES (Figures S3–S21), and the UFLC-MS/MS data for the lignan standards previously isolated from maple syrup (Table S1).
Notes
The authors declare no competing financial interest.
References
- 1.United States Department of Agriculture Economic Research Service Sugar & Sweeteners. http://www.ers.usda.gov/topics/crops/sugar-sweeteners.aspx (accessed 23 March 2016 )
- 2.Arnaud CH. ACS meeting news: looking beyond the sugars in sweeteners. Chemical & Engineering News. 2014 Apr 14;92(5):10–13. [Google Scholar]
- 3.Schultz H. Maple water: the new coconut water? http://www.foodnavigator-usa.com/Suppliers2/Maple-water-The-new-coconut-water (accessed 23 March 2016 )
- 4.Legault J, Girard-Lalancette K, Grenon C, Dussault C, Pichette A. Antioxidant activity, inhibition of nitric oxide overproduction, and in vitro antiproliferative effect of maple sap and syrup from Acer saccharum. J Med Food. 2010;13:460–468. doi: 10.1089/jmf.2009.0029. [DOI] [PubMed] [Google Scholar]
- 5.Lagacé L, Leclerc S, Charron C, Sadiki M. Biochemical composition of maple sap and relationships among constituents. J Food Comp Anal. 2015;41:129–136. [Google Scholar]
- 6.Yuan T, Li L, Zhang Y, Seeram NP. Pasteurized and sterilized maple sap as functional beverages: chemical composition and antioxidant activities. J Funct Foods. 2013;5:1582–1590. [Google Scholar]
- 7.Zhang Y, Yuan T, Li L, Nahar P, Slitt A, Seeram NP. Chemical compositional, biological, and safety studies of a novel maple syrup derived extract for nutraceutical applications. J Agric Food Chem. 2014;62:6687–6698. doi: 10.1021/jf501924y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Seeram NP. Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds. J Agric Food Chem. 2010;58:11673–11679. doi: 10.1021/jf1033398. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Seeram NP. Further investigation into maple syrup yields 3 new lignans, a new phenylpropanoid, and 26 other phytochemicals. J Agric Food Chem. 2011;59:7708–7716. doi: 10.1021/jf2011613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Seeram NP. Quebecol, a novel phenolic compound isolated from Canadian maple syrup. J Funct Foods. 2011;3:125–128. [Google Scholar]
- 11.Thériault M, Caillet S, Kermasha S, Lacroix M. Antioxidant, antiradical and antimutagenic activities of phenolic compounds present in maple products. Food Chem. 2006;98:490–501. [Google Scholar]
- 12.Maisuria VB, Hosseinidoust Z, Tufenkji N. Polyphenolic extract from maple syrup potentiates antibiotic susceptibility and reduces biofilm formation of pathogenic bacteria. Appl Environ Microbiol. 2015;81:3782–3792. doi: 10.1128/AEM.00239-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Sarrías A, Li L, Seeram NP. Anticancer effects of maple syrup phenolics and extracts on proliferation, apoptosis, and cell cycle arrest of human colon cells. J Funct Foods. 2012;4:185–196. doi: 10.1002/ptr.3677. [DOI] [PubMed] [Google Scholar]
- 14.Nahar PP, Driscoll MV, Li L, Slitt AL, Seeram NP. Phenolic mediated anti-inflammatory properties of a maple syrup extract in RAW 264.7 murine macrophages. J Funct Foods. 2014;6:126–136. [Google Scholar]
- 15.Apostolidis E, Li L, Lee C, Seeram NP. In vitro evaluation of phenolic-enriched maple syrup extracts for inhibition of carbohydrate hydrolyzing enzymes relevant to type 2 diabetes management. J Funct Foods. 2011;3:100–106. [Google Scholar]
- 16.Nagai N, Ito Y, Taga A. Comparison of the enhancement of plasma glucose levels in type 2 diabetes Otsuka Long-Evans Tokushima fatty rats by oral administration of sucrose or maple syrup. J Oleo Sci. 2013;62:737–743. doi: 10.5650/jos.62.737. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe Y, Kamei A, Shinozaki F, Ishijima T, Iida K, Nakai Y, Arai S, Abe K. Ingested maple syrup evokes a possible liver-protecting effect-physiologic and genomic investigations with rats. Biosci Biotechnol Biochem. 2011;75:2408–2410. doi: 10.1271/bbb.110532. [DOI] [PubMed] [Google Scholar]
- 18.St-Pierre P, Pilon G, Dumais V, Dion C, Dubois M-J, Dubé P, Desjardins Y, Marette A. Comparative analysis of maple syrup to other natural sweeteners and evaluation of their metabolic responses in healthy rats. J Funct Foods. 2014;11:460–471. [Google Scholar]
- 19.Kamei A, Watanabe Y, Shinozaki F, Yasuoka A, Kondo T, Ishijima T, Toyoda T, Arai S, Abe K. Administration of a maple syrup extract to mitigate their hepatic inflammation induced by a high-fat diet: a transcriptome analysis. Biosci Biotechnol Biochem. 2015;79:1893–1897. doi: 10.1080/09168451.2015.1042833. [DOI] [PubMed] [Google Scholar]
- 20.Nagai N, Yamamoto T, Tanabe W, Ito Y, Kurabuchi S, Mitamura K, Taga A. Changes in plasma glucose in Otsuka Long-Evans Tokushima fatty rats after oral administration of maple syrup. J Oleo Sci. 2015;64:331–335. doi: 10.5650/jos.ess14075. [DOI] [PubMed] [Google Scholar]
- 21.Hawco CL, Wang Y, Taylor M, Weaver DF. A maple syrup extract prevents β-amyloid aggregation. Can J Neurol Sci. 2015:1–4. doi: 10.1017/cjn.2015.270. [DOI] [PubMed] [Google Scholar]
- 22.Ma H, Liu W, Nahar PP, DaSilva N, Wei Z, Pham PP, Vattem DA, Seeram NP. Phenolic-enriched maple syrup extract shows neuroprotective effects in murine microglial cells and delays β-amyloid aggregation induced neurotoxicity and paralysis of Caenorhabditis elegans. 251st American Chemical Society National Meeting; San Diego: The American Chemical Society; 2016. AGFD 20. [Google Scholar]
- 23.Bradley P. Maple syrup industry asks FDA to act against fake maple labels. http://wamc.org/post/maple-syrup-industry-asks-fda-act-against-fake-maple-labels#stream/0 (23 March 2016)
- 24.Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, Sun SZ. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br J Nutr. 2008;99:1301–1309. doi: 10.1017/S0007114507871649. [DOI] [PubMed] [Google Scholar]
- 25.Muir AD. Flax lignans-analytical methods and how they influence our understanding of biological activity. J AOAC Int. 2006;89:1147–1157. [PubMed] [Google Scholar]
- 26.Waterhouse AL, Ignelzi S, Shirley JR. A comparison of methods for quantifying oligomeric proanthocyanidins from grape seed extracts. Am J Enol Vitic. 2000;51:383–389. [Google Scholar]
- 27.Payne MJ, Hurst WJ, Stuart DA, Ou B, Fan E, Ji H, Kou Y. Determination of total procyanidins in selected chocolate and confectionery products using DMAC. J AOAC Int. 2010;93:89–96. [PubMed] [Google Scholar]
- 28.Martin KR, Krueger CG, Rodriquez G, Dreher M, Reed JD. Development of a novel pomegranate standard and new method for the quantitative measurement of pomegranate polyphenols. J Sci Food Agric. 2009;89:157–162. [Google Scholar]
- 29.Feliciano RP, Shea MP, Shanmuganayagam D, Krueger CG, Howell AB, Reed JD. Comparison of isolated cranberry (Vaccinium macrocarpon Ait.) proanthocyanidins to catechin and procyanidins A2 and B2 for use as standards in the 4-(dimethylamino)cinnamaldehyde assay. J Agric Food Chem. 2012;60:4578–4585. doi: 10.1021/jf3007213. [DOI] [PubMed] [Google Scholar]
- 30.Prior RL, Fan E, Ji H, Howell A, Nio C, Payne MJ, Reed J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J Sci Food Agric. 2010;90:1473–1478. doi: 10.1002/jsfa.3966. [DOI] [PubMed] [Google Scholar]
- 31.Krueger CG, Chesmore N, Chen X, Parker J, Khoo C, Marais JPJ, Shanmuganayagam D, Crump P, Reed JD. Critical reevaluation of the 4-(dimethylamino)cinnamaldehyde assay: cranberry proanthocyanidin standard is superior to procyanidin A2 dimer for accurate quantification of proanthocyanidins in cranberry products. J Funct Foods. 2016;22:13–19. [Google Scholar]
- 32.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


