Abstract
Natriuretic peptides (NP) are important predictors of outcomes in patients with acute myocardial infarction (AMI), but can change over time. The association of patterns of NP changes after AMI is less clear. We measured N terminal pro brain natriuretic peptide (NT-proBNP) during the AMI admission and at 1 month in a prospective AMI registry. Outcomes included 6 month dyspnea scores, 1-year readmission and 2-year mortality. An elevated NT-proBNP was defined using age-specific criteria. Patients were classified into 4 groups (Low/Low (referent group), Low/High, High/Low, High/High) based on NT-proBNP value at enrollment and 1 month. The incremental predictive value of NT-proBNP was determined after adjusting for 6-month GRACE risk score, diabetes and ejection fraction<40%. Among 803 patients, 303 (38%) were Low/Low, 240 (30%), were High/High, 230 (29%) were High/Low, and 30 (3.7%) were Low/High. Two-year mortality was highest in High/High patients but similar in the High/Low and Low/Low patients (13.1% vs 2.7% and 2.3%, respectively). Similarly, hospital readmission was significantly more likely in the High/High vs the High/Low and Low/Low groups (44.7% vs 19.8% and 22.3%, respectively). After adjustment, mortality was significantly higher in the High/High group (HR 4.02, 95% CI 1.67, 9.66) compared to the Low/Low group, although readmission was no longer different (HR 1.37, 95% CI 0.93, 2.03). In conclusion, persistently elevated NT-proBNP assessed 1 month after discharge was associated with a higher risk of mortality among AMI patients, whereas those in which NT-proBNP improved had similar outcomes as those with persistently low NT-proBNP. Post-discharge risk stratification using NT-proBNP has the potential to identify higher-risk patients after AMI.
Keywords: myocardial infarction, natriuretic peptide, outcomes
Patients who present with acute myocardial infarction (AMI), either ST elevation (STEMI) or non-ST elevation MI (NSTEMI), have variable prognoses mandating better methods for risk stratification to better identify high-risk patients for more aggressive treatment. Among cardiac biomarkers, the natriuretic peptides (NP), released from cardiac myocytes in response to increased wall stretch and wall tension (3), have demonstrated prognostic value in AMI patients (4-10) and are recommended as part of the risk stratification process for AMI patients (1,2). Increased levels of natriuretic peptides during an AMI admission are strongly associated with short- and long-term risk of death and subsequent heart failure (4-11). Current guidelines indicate that measuring NPs during an AMI hospitalization is reasonable to identify patients at increased risk of death or heart failure after discharge (2). Most studies reporting on outcomes have measured NPs at a single time point during the initial hospitalization (7, 8, 9). Because NPs can change significantly over time (4-7), earlier assessment may be less predictive of longer-term outcomes (4-6). Whether assessment of NP values shortly after discharge better reflect prognosis has not been well studied. The goal of this study was to assess the role of serial changes in N terminal pro brain natriuretic peptide (NT-proBNP)(initial hospitalization and 1 month after discharge) for assessing longer-term outcomes in patients with acute AMI.
METHODS
Details of the Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) registry have been previously described (12). Briefly, TRIUMPH was a large, prospective, multicenter registry of patients with AMI (STEMI or NSTEMI) who were enrolled at 1of 24 sites across the United States within 24 hours of presentation. All participants provided informed consent, and the protocols were approved by the institutional review board at each participating site. Patients who agreed to participate in a biomarker substudy underwent an in-home visit 30 days after discharge (12). Between April 2005 and December 2008, 4,340 patients were enrolled in TRIUMPH, 803 of whom participated in the biomarker sub-study and had serial NT-proBNP values available for analysis.
Trained data collectors performed detailed baseline chart abstractions and interviews to document patients’ medical history, clinical comorbidities, presenting ECG, inpatient processes of care, laboratory results, and treatments. All patients were asked to donate blood specimens at the time of enrollment in TRIUMPH. Because TRIUMPH was designed to investigate 1-year (as opposed to in-hospital) outcomes, fasting blood specimens were acquired as close to discharge as possible. Dyspnea was assessed using the Rose Dyspnea Scale (13), a 4-item questionnaire that assesses patients’ level of dyspnea with common activities. Each activity is assigned 1 point. Scores range from 0 to 4, with 0 indicating no dyspnea with activity and increasing scores indicating more limitations due to dyspnea. Follow-up was attempted on all survivors at 1, 6, and 12 months. An in-home visit was performed by trained medical personnel at 1 month, and 12-month follow-ups and trained administered the follow-up interviews at that time.
Blood samples were spun rapidly and frozen, then shipped overnight for measurement of NT-proBNP (Roche) at a centralized core laboratory (Clinical Reference Laboratories, Lenexa KS). An elevated NT-proBNP was defined using age-specific criteria: age ≤50, NT-proBNP >450 pg/ml; age 50-75, NT-proBNP >900 pg/ml; and >75, NT-proBNP >1800 pg/ml (14). Patients were classified into 4 groups based on whether their NT-proBNP value was elevated at enrollment and at 1 month follow up: Low/Low (referent group), Low/High, High/Low, and High/High.
The primary endpoint was 2-year all-cause mortality. Secondary endpoints included 1-year all cause readmission and 6-month Dyspnea scores. Mortality was determined through a query of the Social Security Death Master File. Hospitalizations that occurred during the initial 12 months were reviewed for cardiovascular events, including AMI, heart failure, or revascularization procedures. Chart abstractions were sent to 2 cardiologists who independently classified the reason for hospitalization. If there was disagreement between the 2 cardiologists, the record was adjudicated by a third senior cardiologist, and if disagreement persisted, up to 5 cardiologists independently reviewed the charts until consensus was obtained.
The associations of different NT-proBNP categories with mortality and rehospitalization events were examined using Kaplan-Meier survival curves and Cox proportional hazards models, stratified by site. Hierarchical linear regression models, where patients were clustered within site, were used to model 6-month dyspnea scores. All models were adjusted for clinical variables, which included the 6-month Global Registry of Acute Coronary Events (GRACE) risk score (15), diabetes and left ventricular ejection fraction (LVEF) <40%, while the 6-month dyspnea outcome was additionally adjusted for baseline Rose dyspnea score. The only covariate with missing data was the GRACE score, which was missing in 2.5% of patients. All tests for statistical significance were 2-tailed, with an α level of .05. Statistical analyses were conducted using SAS software, release 9.2 and R version 2.11.1. This research was funded by the National Institutes of Health through the National Heart, Lung, and Blood Institute SCCOR in Diabetic Heart Disease (P50HL077113). The authors are responsible for the study design, conduct, and analysis, and drafting and editing of the final manuscript.
RESULTS
A total of 803 patients had both baseline and 1-month NT-proBNP values for analysis. When compared to patients who did not participate in the biomarker substudy, patients who had Nt-proBNP were more likely to be white and to undergo PCI but were less likely to have chronic kidney disease or heart failure (Table 1). Because only 30 (3.7%) patients were classified as Low/High, they were excluded from further analysis due to the small sample size. Of the remaining 773 patients who formed the study cohort, 39.2% had low NT-BNP levels at both assessments and were classified as Low/Low (referent), 29.8% as High/Low, and 31.0% as High/High. Baseline factors differed across NT-proBNP groups (Table 2). Patients classified as High/High had more cardiovascular risk factors and higher GRACE scores, and were more likely to have an LVEF <40%. Patients classified as High/Low had intermediate risk characteristics as compared with the other 2 cohorts.
Table 1.
Comparison of patients who were and were not included in the Biomarker Substudy
| Included (N=803) | Not included (N=3462) | P-Value | |
|---|---|---|---|
| Age | 59±12 | 59±13 | 0.79 |
| Male | 546 (68) | 2299 (66) | 0.39 |
| White | 594 (74) | 2275 (66) | <0.001 |
| Diabetes | 228 (20) | 1075 (31) | 0.14 |
| Hypertension | 533 (66) | 2303 (67) | 0.94 |
| CKD | 42 (5.2) | 266 (7.7) | 0.015 |
| Prior MI | 166 (21) | 722 (21) | 0.91 |
| Prior CHF | 53 (6.6) | 305 (8.8) | 0.04 |
| Prior PCI | 154 (19) | 685 (20) | 0.70 |
| In-Hospital PCI | 570 (71) | 2219 (64) | <0.01 |
| Prior CABG | 82 (10) | 400 (12) | 0.28 |
| In-Hospital CABG | 73 (9.1) | 324 (9.4) | 0.81 |
| EF<40% | 129 (16) | 650 (19) | 0.07 |
| GRACE Score | 97±27 | 99±30 | 0.08 |
Data presented as N (%) or mean ± standard deviation. Abbreviations: CABG coronary artery bypass surgery, CHF congestive heart failure, CKD chronic kidney disease, EF ejection fraction, MI myocardial infarction, PCI percutaneous coronary intervention
Table 2.
Patient characteristics based on Nt-proBNP category
| Low/Low (N=303) |
High/Low (N=230) |
High/High (N=240) |
P-Value | |
|---|---|---|---|---|
| Age | 57±10 | 60 ± 12 | 60 ± 11 | 0.053 |
| Male | 229 (76) | 142 (62) | 161 (67) | < 0.001 |
| White | 216 (71) | 188 (82) | 172 (72) | 0.010 |
| Prior Diabetes | 74 (24) | 54 (24) | 87 (36) | 0.001 |
| Prior Hypertension | 195 (64) | 147 (64) | 163 (68) | 0.011 |
| Prior CKD | 5 (1.7) | 9 (3.9) | 27 (11) | < 0.001 |
| Prior MI | 58 (19) | 40 (17.4) | 59 (25) | 0.123 |
| Prior CHF | 6 (2.0) | 13 (5.7) | 30 (13) | < 0.001 |
| Prior PCI | 60 (20) | 32 (14) | 55 (23) | 0.083 |
| In-Hospital PCI | 218 (72) | 169 (74) | 158 (66) | 0.009 |
| Prior CABG | 24 (7.9) | 21 (9.1) | 33 (14) | 0.122 |
| In-Hospital CABG | 16 (5.3) | 19 (8.3) | 29 (12) | < 0.001 |
| EF<40% | 13 (4.3) | 40 (17.4) | 72 (30) | < 0.001 |
| GRACE Score | 92±24 | 98±28 | 104±28 | < 0.001 |
Abbreviations as in Table 1
Patients with High/High NT-proBNP levels had more than a 4-fold higher mortality at two years (p<0.001) as compared with the Low/Low and High/Low groups (13.1% vs 2.3% and 2.7%, respectively; Figure 1). In adjusted models, 2-year mortality remained significantly higher in the High/High patients, with no significant difference between the Low/Low and High/Low groups (Figure 2).
Figure 1.
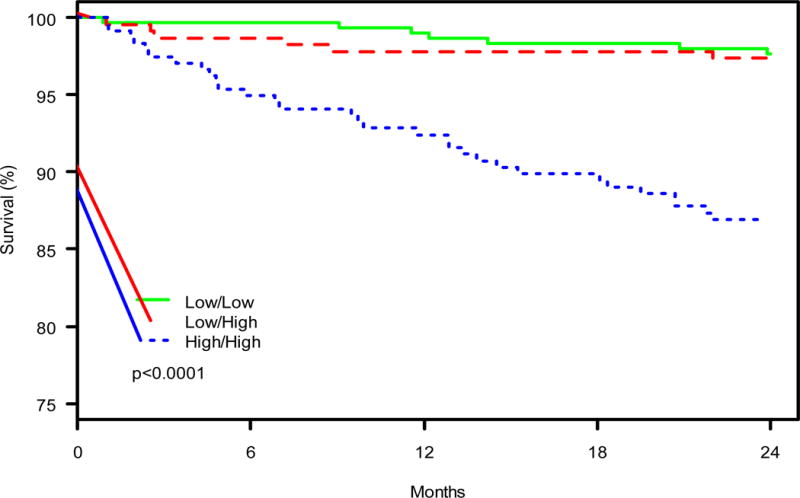
Kaplan Meier plot for 2 Year Mortality–Green (solid) line indicates Low/Low group; red (dashed) line indicates Low/High group; and blue (dotted) line indicates High/High group.
Figure 2.
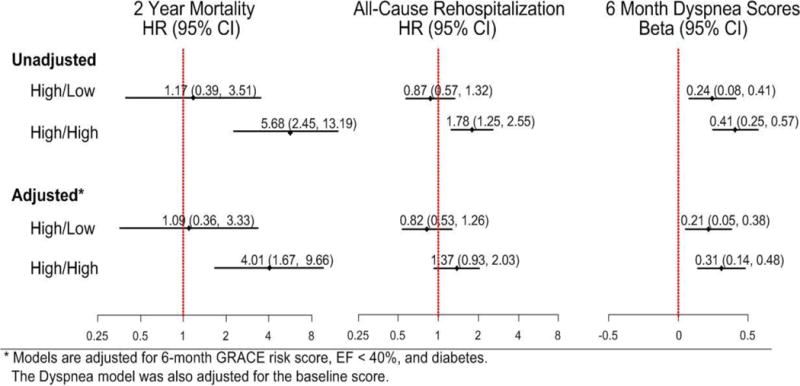
Odds Ratios for the Outcomes. Outcomes included 2 year mortality, readmission and 6 month Dyspnea scores
Six-month Dyspnea scores were also significantly correlated with NT-proBNP categories (Figure 2), with patients having persistently elevated NT-proBNP levels having significant worse dyspnea scores 6 months after discharge. This association persisted after adjustment for GRACE score, diabetes and LVEF.
In unadjusted analyses, 1-year all-cause hospital readmission was frequent in all groups, but was significantly higher in patients with persistently elevated NT-proBNP levels (44.7% vs. 19.8% in High/Low and 22.3% in Low/Low patients ; Figure 2). However, after adjustment for GRACE score, DM and LVEF, the increased risk of rehospitalizations was no longer statistically significant when compared with Low/Low patients (HR 1.37, 95% CI 0.93, 2.03) (p=0.11).
DISCUSSION
We examined the association of baseline and 30-day NT-proBNP levels with mortality, readmissions and dyspnea. Among AMI patients, we found that compared with those who had persistently low NT-proBNP levels, those with persistently elevated levels had a significantly higher risk of 2 year mortality. In contrast, patients who had an elevated NT-proBNP during the initial admission that subsequently normalized (almost half of those with an initially elevated NT-proBNP) had a similar mortality risk as those without an NT-proBNP elevation at either time point. These data suggest that follow-up NT-proBNP levels are more associated with subsequent clinical events and dyspnea than those obtained at the time of hospitalization. In-hospital biomarker risk stratification may not be necessary in lieu of post-discharge testing.
An important finding was that after correcting for LVEF, which was not done in most prior studies (4,7,8,9), NT-proBNP remained independently associated with mortality and dyspnea. Although NP levels correlate with LVEF, the correlation has been variable (11, 16). The incremental value of NPs to LVEF may include their association with infarct expansion (17) and left ventricular dilation (10). In addition, increased NP levels also occur in other cardiac functional and structural abnormalities, such as diastolic dysfunction (18, 19), left ventricular hypertrophy (20) and valvular abnormalities (21) that have been associated with cardiac events after AMI (16). Therefore, NPs may serve as a simple biomarker for identifying multiple underlying cardiac abnormalities that lead to worse outcomes.
We found that persistently elevated levels of NT-proBNP were associated with higher dyspnea scores at 6 months. This may reflect persistent volume overload or pulmonary edema in our cohort, given the known association of increased NP levels with these conditions. Natriuretic peptide levels have been shown to correlate with echocardiographic evidence of diastolic dysfunction and increased filling pressures (18-20). More frequent dyspnea as reflected in higher dyspnea scores has important implications. After adjusting for sociodemographic and clinical factors, higher dyspnea scores remained strongly associated with worse QOL, with a significantly increased risk of 1-year rehospitalization and mortality (22). Other studies have also found that dyspnea is associated with worse quality of life (23-25).
Assessment of NPs during hospitalization is one of the few variables to consistently identify patients with a higher risk for hospital readmission (26). This is important, as it is reflected in quality metrics and reimbursement. Although we found that NT-proBNP was not an independent predictor of readmission, the effect size was clinically important and we were likely underpowered to define its independent effect. As a simple single marker that is readily available and easily interpreted, NT-proBNP may still be a useful marker of high-risk patients.
Few prior studies have reported on using serial NP measurements to predict outcomes in patients after AMI (4,5,6). Morrow et.al. measured BNP levels during admission and at 4 month follow-up in 3,490 patients (4). Only 140 (4.0%) patients had elevated levels at both assessments. In contrast, we found that almost 60% of patients had elevated BNP levels on initial assessment, with 31% having persistently elevated values. In another study, Lindahl et.al. measured NT-proBNP at the time of randomization, at 6 weeks, 3 and 6 months in 1,216 patients (5). The ability of NT-proBNP to predict mortality increased at each follow up period, suggesting later measurement better reflect long term risk. Similar to prior studies, we found that patients who had a persistently elevated BNP were at the highest risk for adverse outcomes, with a more than 4-fold higher risk of death or new heart failure over 2 year follow-up. In contrast to most of the prior studies, we included a broader, more heterogenous patient population that likely better reflects clinical practice than those enrolled in clinical trials (4-6). In addition to mortality, we also assessed endpoints that are important to the patient, such as dyspnea and readmission. We also used recommended criteria to define an elevated NT proBNP level, rather than a single level.
We did not have serial BNP values on all patients in the TRIUMPH study, which limited the power to more definitively assess some outcomes, such as readmission. It also may have impacted the generalizability of our findings, although we did not detect substantial differences between those that did and did not participate in biomarker testing. Finally, we can only describe an association and not causation. Future studies will be needed to more clearly define whether follow-up NP assessments can improve care and outcomes.
Acknowledgments
Funding: TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, and Blood Institute): Washington University School of Medicine SCCOR grant No. P50HL077113-01.
References
- 1.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ, American College of Cardiology.; American Heart Association Task Force on Practice Guidelines.; Society for Cardiovascular Angiography and Interventions.; Society of Thoracic Surgeons.; American Association for Clinical Chemistry 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Mair J, Hammerer-Lercher A, Puschendorf B. The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Chem Lab Med. 2001;39:571–588. doi: 10.1515/CCLM.2001.093. [DOI] [PubMed] [Google Scholar]
- 4.Morrow DA, de Lemos JA, Blazing MA, Sabatine MS, Murphy SA, Jarolim P, White HD, Fox KA, Califf RM, Braunwald E, Investigators Prognostic value of serial B-type natriuretic peptide testing during follow-up of patients with unstable coronary artery disease. JAMA. 2005;294:2866–2871. doi: 10.1001/jama.294.22.2866. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl B, Lindbäck J, Jernberg T, Johnston N, Stridsberg M, Venge P, Wallentin L. Serial analyses of N-terminal pro-B-type natriuretic peptide in patients with non-ST-segment elevation acute coronary syndromes: a Fragmin and fast Revascularisation during In Stability in Coronary artery disease (FRISC)-II substudy. J Am Coll Cardiol. 2005;45:533–541. doi: 10.1016/j.jacc.2004.10.057. [DOI] [PubMed] [Google Scholar]
- 6.Squire IB, Ørn S, Ng LL, Manhenke C, Shipley L, Aarsland T, Dickstein K. Plasma natriuretic peptides up to 2 years after acute myocardial infarction and relation to prognosis: an OPTIMAAL substudy. J Card Fail. 2005;11:492–497. doi: 10.1016/j.cardfail.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Heeschen C, Hamm CW, Mitrovic V, Lantelme NH, White HD, Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Investigators N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2004;110:3206–3212. doi: 10.1161/01.CIR.0000147611.92021.2B. [DOI] [PubMed] [Google Scholar]
- 8.de Lemos JA, Morrow DA, Bentley JA, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndrome. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 9.Morrow DA, de Lemos JA, Sabatine MS, Murphy SA, Demopoulos LA, DiBattiste PM, McCabe CH, Gibson CM, Cannon CP, Braunwald E. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol. 2003;41:1264–1272. doi: 10.1016/s0735-1097(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson JC, Groenning BA, Nielsen G, Fritz-Hansen T, Trawinski J, Hildebrandt PR, Jensen GB, Larsson HB, Sondergaard L. Left ventricular remodeling in the first year after acute myocardial infarction and the predictive value of N-terminal pro brain natriuretic peptide. Am Heart J. 2002;143:696–702. doi: 10.1067/mhj.2002.120293. [DOI] [PubMed] [Google Scholar]
- 11.Richards AM, Nicholls MG, Espiner EA, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107:2786–2792. doi: 10.1161/01.CIR.0000070953.76250.B9. [DOI] [PubMed] [Google Scholar]
- 12.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA, Cardiovascular Outcomes Research Consortium Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–4676. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 14.Hildebrandt P, Collinson PO, Doughty RN, Fuat A, Gaze DC, Gustafsson F, Januzzi J, Rosenberg J, Senior R, Richards M. Age-dependent values of N-terminal pro-B-type natriuretic peptide are superior to a single cut-point for ruling out suspected systolic dysfunction in primary care. Eur Heart J. 2010;31:1881–1889. doi: 10.1093/eurheartj/ehq163. [DOI] [PubMed] [Google Scholar]
- 15.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA, GRACE Investigators A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 16.Chen AA, Wood MJ, Krauser DG, Baggish AL, Tung R, Anwaruddin S, Picard MH, Januzzi JL. NT-proBNP levels, echocardiographic findings, and outcomes in breathless patients: results from the ProBNP Investigation of Dyspnoea in the Emergency Department (PRIDE) echocardiographic substudy. Eur Heart J. 2006;27:839–845. doi: 10.1093/eurheartj/ehi811. [DOI] [PubMed] [Google Scholar]
- 17.White M, Rouleau JL, Hall C, Arnold M, Harel F, Sirois P, Greaves S, Solomon S, Ajani U, Glynn R, Hennekens C, Pfeffer M. Changes in vasoconstrictive hormones, natriuretic peptides, and left ventricular remodeling soon after anterior myocardial infarction. Am Heart J. 2001;142:1056–1064. doi: 10.1067/mhj.2001.119612. [DOI] [PubMed] [Google Scholar]
- 18.Andersson B, Caidahl K, di Lenarda A, Warren SE, Goss F, Waldenström A, Persson S, Wallentin I, Hjalmarson A, Waagstein F. Changes in early and late diastolic filling patterns induced by long-term adrenergic beta-blockade in patients with idiopathic dilated cardiomyopathy. Circulation. 1996;94:673–682. doi: 10.1161/01.cir.94.4.673. [DOI] [PubMed] [Google Scholar]
- 19.Logeart D, Saudubray C, Beyne P, Thabut G, Ennezat PV, Chavelas C, Zanker C, Bouvier E, Solal AC. I Comparative value of Doppler echocardiography and B-type natriuretic peptide assay in the etiologic diagnosis of acute dyspnea. J Am Coll Cardiol. 2002;40:1794–1800. doi: 10.1016/s0735-1097(02)02482-8. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K, Burnett JC, Jougasaki M, Nishimura RA, Bailey KR, Saito Y, Nakao K, Redfield MM. Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension. 1996;28:988–994. doi: 10.1161/01.hyp.28.6.988. [DOI] [PubMed] [Google Scholar]
- 21.Sutton TM, Stewart RA, Gerber IL, West TM, Richards AM, Yandle TG, Kerr AJ. Plasma natriuretic peptide levels increase with symptoms and severity of mitral regurgitation. J Am Coll Cardiol. 2003;41:2280–2287. doi: 10.1016/s0735-1097(03)00486-8. [DOI] [PubMed] [Google Scholar]
- 22.Arnold SV, Spertus JA, Jones PG, Xiao L, Cohen DJ. The impact of dyspnea on health-related quality of life in patients with coronary artery disease: results from the PREMIER registry. Am Heart J. 2009;157:1042–1049. doi: 10.1016/j.ahj.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Wiklund I, Herlitz J, Hjalmarson A. Quality of life five years after myocardial infarction. Eur Heart J. 1989;10:464–472. doi: 10.1093/oxfordjournals.eurheartj.a059511. [DOI] [PubMed] [Google Scholar]
- 24.Melville MR, Packham C, Brown N, Weston C, Gray D. Cardiac rehabilitation: socially deprived patients are less likely to attend but patients ineligible for thrombolysis are less likely to be invited. Heart. 1999;82:373–377. doi: 10.1136/hrt.82.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pocock SJ, Henderson RA, Clayton T, Lyman GH, Chamberlain DA. Quality of life after coronary angioplasty or continued medical treatment for angina: three-year follow-up in the RITA-2 trial. Randomized Intervention Treatment of Angina. J Am Coll Cardiol. 2000;35:907–914. doi: 10.1016/s0735-1097(99)00637-3. [DOI] [PubMed] [Google Scholar]
- 26.Desai MM, Stauffer BD, Feringa HH, Schreiner GC. Statistical models and patient predictors of readmission for acute myocardial infarction: a systematic review. Circ Cardiovasc Qual Outcomes. 2009;2:500–507. doi: 10.1161/CIRCOUTCOMES.108.832949. [DOI] [PubMed] [Google Scholar]
- 27.Troughton RW, Frampton CM, Brunner-La Rocca HP, Pfisterer M, Eurlings LW, Erntell H, Persson H, O’Connor CM, Moertl D, Karlström P, Dahlström U, Gaggin HK, Januzzi JL, Berger R, Richards AM, Pinto YM, Nicholls MG. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J. 2014;35:1559–1567. doi: 10.1093/eurheartj/ehu090. [DOI] [PMC free article] [PubMed] [Google Scholar]


