Abstract
Background
Structural equation modeling (SEM) can help understanding complex functional relationships among obesity, non-alcoholic fatty liver disease (NAFLD), family history of obesity, targeted metabolomics and pro-inflammatory markers. We tested two hypotheses: 1) If obesity precedes an excess of free fatty acids that increase oxidative stress and mitochondrial dysfunction, there would be an increase of serum acylcarnitines, amino acids and cytokines in obese subjects. Acylcarnitines would be related to non-alcoholic fatty disease that will induce insulin resistance. 2) If a positive family history of obesity and type 2 diabetes are the major determinants of the metabolomic profile, there would be higher concentration of amino acids and acylcarnitines in patients with this background that will induce obesity and NAFLD which in turn will induce insulin resistance.
Methods/Results
137 normoglycemic subjects, mean age (SD) of 30.61 (8.6) years divided in three groups: BMI<25 with absence of NAFLD (G1), n = 82; BMI>30 with absence of NAFLD (G2), n = 24; and BMI>30 with NAFLD (G3), n = 31. Family history of obesity (any) was present in 53%. Both models were adjusted in SEM. Family history of obesity predicted obesity but could not predict acylcarnitines and amino acid concentrations (effect size <0.2), but did predict obesity phenotype.
Conclusion
Family history of obesity is the major predictor of obesity, and the metabolic abnormalities on amino acids, acylcarnitines, inflammation, insulin resistance, and NAFLD.
Introduction
Nearly a third of the world’s population is either obese or overweight [1,2]. Mexico ranks second worldwide in prevalence of combined overweight and obesity in adults at 71.3% [3–5]. Long-term weight loss maintenance is still a great challenge despite advances in the modalities to treat obesity [6].
The classic paradigm proposes obesity as imbalance of positive energy intake, which results in expansion of adipose tissue, and subsequent proinflammatory process due to release of cytokines, C reactive protein (CRP), interferon gamma (IFNγ), Tumor Necrosis Factor alpha (TNFα), among many other molecules; additionally, increase of free fatty acids induces lipotoxicity [7–9]. As a consequence, patients with obesity have a higher risk of insulin resistance (IR), lipid dysmetabolism, Type 2 Diabetes (T2DM), Non-alcoholic Fatty Liver Disease (NAFLD), and other chronic complications [9].
Interestingly, not all patients with obesity develop T2DM or NAFLD, furthermore, patients with high genetic predisposition to obesity can acquire NAFLD more easily with minimal environmental exposure. This suggests either additive or synergistic interactions among multiple risk factors that include environment, lifestyle, genome, epigenome, metabolome, and other key factors that play a role in its pathogenesis [10].
Over the past decade, strong emphasis was placed on identifying genetic risk factors for obesity [11–13]. While these efforts yielded some remarkable results, some recent studies have suggested that the use of clinical family history may be more informative than genotyping single candidate genes because it captures both genetic risk as well as the epigenetic markers that are associated with the life style of parents and to the second degree grandparents [14]. A powerful method to assess such interrelationship between genetics, epigenome and the phenotype is metabolomics, which uses highly sensitive technologies such as mass spectroscopy to analyze low-molecular weight intermediates (< 1 kDa) in biological fluids or tissues such as blood, urine, saliva, etc.[15,16]. Recent studies have documented that obese, insulin resistant, and NAFLD, when compared with normal subjects, have differences in blood metabolite profiles, such as glucose, lipids, acylcarnitines and amino acids [17–25]. While metabolic profiling yields copious data, traditional methods of analyses such as cluster analyses and standard linear models can fail to determine functional relationships between the examined variables [26].
In this study, we computed Structural Equation Models (SEM) [27] to integrate multidimensional data into single framework to unravel their interrelationship in the context obesity and related traits. While SEM have been used to reconstruct phenotype networks in genetics, behavioral, and social science [27,28], to the best of our knowledge, it has not been applied to the metabolomics field. Here we used SEM to address the interrelationships between obesity, NAFLD, different degrees of family history of obesity, targeted metabolomics and pro-inflammatory markers.
Based on the aforementioned concepts, we tested two hypotheses:
If obesity precedes an excess of free fatty acids that perhaps increase oxidative stress and mitochondrial dysfunction, there will be increased acylcarnitines (AC) in blood in obese patients. Also, there will be an increase of amino acids (AC) and cytokines (INFL). Acylcarnitines will be related to non-alcoholic fatty disease (NAFLD) that will induce insulin resistance (IR). (Obesity→AC+AA → NAFLD → IR and INFL).
If a positive family history of obesity (FHOB) is the major determinant of the metabolomic profile, then those subjects will exhibit higher concentration of AC and AA that will induce obesity and NAFLD, which in turn will derive on IR. In this model, we are proposing that obesity is a consequence of previous alteration of fatty acids and amino acids metabolism (FHOB→High AA and AC → Obesity & NAFLD → IR).
Material and methods
Study sample
We conducted a cross-sectional study with 137 consecutive patients aged between 18 to 45 years who were recruited from January to October 2012 at the outpatient clinic, Hospital General de Mexico, Mexico City. Subjects were divided into 3 groups: Group 1 BMI<25 (G1), Group 2 BMI>30 (G2) and, group 3 (BMI>30 with NAFLD) (G3). We had made previous simulations, and a priori anticipated unbalanced sample size groups. Family history of diabetes/obesity was recorded, parental first-degree relative was defined as “Direct”, meanwhile, second-degree relatives were “Indirect”. We excluded patients with pregnancy, those who smoke, or consumed more than 10 grams of alcohol/week, those with clinical history of known hepatotoxic medications, diagnosis of cancer, any acute or chronic infectious disease, hypertension, diabetes, chronic kidney disease or any pathological condition during the general examination or laboratory tests.
The study protocol was approved by the Human Ethics Committee at the Hospital General de México, an informed consent was obtained from all subjects. The investigation was conducted according to the principles expressed in the Declaration of Helsinki.
Procedure
Subjects were invited to participate, if they accomplished inclusion criteria, and signed the informed consent. During the first visit, we recorded blood pressure, BMI and bioelectric impedance parameters using a Quantum IV–Body Composition Analyzer (RJL Systems, USA). Within a week but on different date, patients were instructed to fast 8 hours before a 8 am oral glucose tolerance test (OGTT) with 75 g of glucose. Baseline blood sample was drawn for for glucose, creatinine, urea (to discharge kidney disease), total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, alanine aminotransferase (AST), and aspartate aminotransferase (ALT) with an AU480 Chemistry System (Beckman Coulter, USA). During the OGTT, we also measured insulin levels through ELISA with an Abnova™ Kit using the V1.24 device (Multiskan Ascent, USA) with intra and inter-assay coefficients of variation (CV) ranging between 1.8 to 2.9%. The Matsuda insulin sensitivity index was calculated as described elsewhere (35). Inflammatory markers measured in blood were IL-6 and TNFα using ELISA with Bioplex-Pro™ Cytokine assays and the Bio-Plex Pro II wash station with a magnetic plate carrier (Bio Rad, USA), CV:4–19%. C Reactive protein was measured with an immunoenzyme assay using microplates (Monobind Inc, USA).
Plasma samples from fasting subjects were used to determine the profiles of 31 endogenous acylcarnitines and 7 amino acids were using Quattro Micro API (MicroMass) tandem mass spectrometer (MS-MS). All procedures for sample preparation and MS-MS analysis were performed by NeoBase no derivatized kit (PerkinElmer, USA) according to the manufacturer's protocol. Briefly, plasma was dried in filter papers and single disks were punched from each spot using a 3 mm punch. One disk was used per well. Using a multichannel pipette, 190 μL of extraction solution containing a mixture of the respective stable isotope-labeled internal standards, was added to each well. The plate was covered with aluminum foil, shaken at 650 x g and incubated for 30 min at 30°C. The plate was finally placed in the auto-sampler for the analysis. Finally, we performed a hepatic ultrasound with the Voluson Pro VTM ultrasound system (GE, USA) with a 3.5MHz transductor. Hepatic ultrasounds have a sensitivity and specificity for NAFLD detection of 80 to 90% (36). Presence of NAFLD was determined if 3 parameters were present: 1) high hepatic echotexture, 2) high attenuation, 3) low portal and hepatic vein visualization.
Statistical analysis
We described and contrasted demographic, serum biochemical values for the three described groups (Method section); those variables with skewed distributions were normalized using log10 transformation. Contrasts by group were computed using Chi-square or one way-ANOVA with Fisher post hoc tests according to variable dimension. For metabolic and inflammatory markers, we computed False discovery Rate (FDR) with the Benjamini–Hochberg procedure [29].
We used Partial Least Squares Discriminant Analysis (PLS-DA) to visually discriminate metabolites and inflammatory markers between the 3 groups. The quality of PLS-DA was assessed using 3 different parameters: R2, Q2 and accuracy. The goodness of fit was quantified by R2 and the predictive ability was indicated by Q2.
To assess the significance of class discrimination a permutation test was conducted where the model was run 1000 times. Variable Importance in Projection (VIP) was calculated as a weighted sum of squares of the PLS loadings taking into account the amount of explained Y-variation.
In order to test the two described hypotheses we used SEM, a generalization of both regression and factor analysis. The rationale for using SEM is that the covariance matrix of the observed variables is a function of a set of parameters that were defined a priori. If the model is correct and the parameters are known, then the population covariance matrix would be exactly reproduced by SEM (except for sampling variation). The hypothetical relation among the variables in our models was built based on the conjunction of previous reported data from the literature validated by simulation models that were generated in advance and modified according to modification indexes. To avoid hormonal effects and physiological changes from adolescence and senescence we only included patients between 18 to 45 years old, yet to conserve the required illness diversity for analysis of biological variance. Age did not have any rol as observed neither latent variable for SEM.
The general SEM model can be decomposed into two sub models: a measurement and a structural model. By convention, when graphically representing the model the observed variables are enclosed by rectangles or squares and latent variables are enclosed by ovals or circles. Residuals are always unobserved variables (latent factors) and are represented by ovals or circles. In this study, the root mean square error of approximation (RMSEA) was used to evaluate the goodness-of-fit of any model, where a value <0.9 was considered acceptable for our models. Standardized β values >0.2 were relevant in the pathophysiology of our models [27,30–32].
We considered sample size according to the minimum number of patients needed to have good cohesion of factors in our models. For this we did a priori Monte Carlo simulations modeling with beta distribution considering parameters α = 1 and β = 7, to increase the skewness of simulated data. Maximum and minimum values were obtained considering 3 standard deviations of the mean of values obtained from previous studies. We expected at least 20% difference on the effect size of the concentration of amino acids between lean patients and patients with obesity. We also considered an effect size of at least 20% in acylcarnitine concentrations between patients with and without NAFLD. With 137 patients, we had good adjustment of our models that included effect size between 19 and 100%.
All statistical analyses were done using SPSS 18.0, Amos and Metaboanalyst 3.0[33].
Results
We included 137 normoglycemic subjects, with a mean age of 30.61 (SD 8.6) years (Table 1), seventy percent were women. Group 1 (BMI<25) had 82 subjects; Group 2 (BMI >30) and Group 3 (BMI>30 and NAFLD) group had 24 and 31 subjects, respectively. Family history of obesity (any) was present in 53% of the total population, while family history of diabetes was present in 66%. Table 1 shows mean values of clinical, inflammatory and metabolomic parameters of the 3 groups and Table 2 shows the different degrees of family history of obesity and diabetes.
Table 1. Clinical and metabolomic parameters of patients according to 3 groups.
Mean (95% CI).
| G1 BMI<25 Mean (95% CI) number = 82 | G2 BMI>30; NAFLD (-) Mean (95% CI) number = 24 |
G3 BMI>30; NAFLD (+) Mean (95% CI) number = 31 |
p-value | FDR | Factor in SEM Model | ||
|---|---|---|---|---|---|---|---|
| Antropometric | |||||||
| Age | 28(26,29)b | 27(24,32)b | 36(33,39) a | <0.001 | NA | ||
| Gender (% Females) |
65 | 87 | 71 | 0.098 | NA | ||
| BMI | 22.2 (22,23)c | 32.9 (32,34)b | 36.2 (34,38)a | <0.001 | NA | Obesity | |
| Abdominal Circumference (cm) | 73.42(72,75)c | 85 (75,95)b | 102.48 (96,107)a | <0.001 | NA | Obesity | |
| %FAT | 29.7 (28,31)b | 40.88 (37,45)a | 41.63 (32,36)a | <0.001 | NA | Obesity | |
| Insulin Sensibility | |||||||
| Basal Glucose (mg/dl) | 86 (85,88)b | 88 (86,90)a,b | 91 (89,93)a | 0.003 | 0.009 | ||
| Glucose 30 min (mg/dl) | 123 (118,128)b | 126 (113,140)a,b | 142 (134,150)a | 0.004 | 0.01 | ||
| Glucose 60 min (mg/dl) | 109 (102,116)b | 118 (105,132)a,b | 135(123,147)a | 0.002 | 0.007 | ||
| Glucose 90 min (mg/dl) | 100 (96,106)b | 114 (103,126)a | 128 (120,136)a | <0.001 | <0.001 | ||
| Glucose 120min (mg/dl) |
99 (94,103) | 104(101,108)a,b | 116 (110,126)a | 0.001 | 0.002 | ||
| Matsuda Index | 6.1 (5.4,6.9)a | 4.8 (4,6)a | 3.1 (2.4,3.9)b | <0.001 | <0.001 | Matsuda Index | |
| Lipids and Liver Enzymes | |||||||
| Total Cholesterol (mg/dl) | 170 (164,176)b | 173(161,186)b | 190 (178,202)a | 0.008 | 0.02 | ||
| Triglycerids (mg/dl) | 93 (85,101)b | 114 (98,132)b | 170 (141,206)a | <0.001 | <0.001 | ||
| HDL- Cholesterol (mg/dl) | 48 (46,51)a | 44 (39,50)a,b | 49 (36,42)b | 0.001 | 0.002 | ||
| LDL- Cholesterol (mg/dl) | 100 (95,105)b | 110 (102,120,)a,b | 119 (110,129)a | 0.001 | 0.003 | ||
| ALT (U/L) | 19 (17,20)c | 21 (16,26)b | 31 (26,37)a | <0.001 | <0.001 | Fatty Liver | |
| AST (U/L) | 21 (20,23) b | 22 (19,25)a,b | 26 (23.29)a | 0.005 | 0.014 | Fatty Liver | |
| Inflammatory markers | |||||||
| CRP(mg/dl) | 1.9 (1.7,2.2)b | 4.2 (3.1,5.7)a | 4.8 (3.9,5.9)a | <0.001 | <0.001 | CRP | |
| IL-6 (pg/dl) | 1.15 (0.96,1.37) | 0.66 (0.33,1.3) | 0.85 (0.68,1.02) | 0.056 | 0.488 | INFL | |
| TNFα (pg/dl) | 1.14 (0.9,1.43) | 75 (0.41,1.3) | 0.9 (0.72,1.3) | 0.234 | 0.52 | INFL | |
| Amino acids | |||||||
| Arginine (μM) | 36.3 (33,40)b | 42.3(37,48.3)a,b | 45.2(39.2,52.1)a | 0.027 | 0.064 | AA1 | |
| Citruline (μM) | 9 (8.5, 9.5) | 9.5 (8.5,10.6) | 9.2(8.3,10.35) | 0.656 | 0.787 | AA2 | |
| Glycine (μM) | 101.8 (96.4,105.2) | 101.8 (92.5,112) | 99.6(92.4,107.3) | 0.926 | 0.936 | AA1 | |
| Alanine (μM) | 112 (106.9,117.6)b | 122.1(113.6,131.7)a,b | 128.3 (119.1,138.1)a | 0.006 | 0.016 | AA2 | |
| Leucine (μM) | 54.2 (50.6,57.9)b | 59.3 (54.4, 64.7)a,b | 63.6(57.9,69.9)a | 0.022 | 0.052 | AA1 | |
| Methionine (μM) | 4.5 (4.3,4.8) | 4.6(4.29,5.1) | 4.7 (4.3,5.12) | 0.737 | 0.804 | AA2 | |
| Phenylalanine (μM) | 20.9 (19.4, 22.4)b | 24.48 (19.4,22.4)a,b | 25 (22.7, 27.6)a | 0.005 | 0.014 | AA1 | |
| Tyrosine (μM) | 23.2 (22, 24.4)b | 26.4 (24,29)a | 28 (26.6,31.2)a | <0.001 | <0.001 | AA2 | |
| Valine (μM) | 56.6 (53.8,59.5)b | 63.035 (12.9)a,b | 67.69 (16.2)a | 0.004 | 0.011 | AA1 | |
| Ornitine (μM) | 7.9 (7.4,8.6)b | 10.15 (8.7,11.8)a | 10.23(9.1,11.4)a | <0.001 | 0.001 | AA2 | |
| Proline (μM) | 61.2(57.1,65.7)a | 64.6(56.8,73.6)a,b | 74.5(67.19,82.79)a | 0.012 | 0.031 | AA2 | |
| Acylcarnitines | |||||||
| C0 (μM) | 12.4 (11.7,13.2)b | 13.9 (12.8,15.3)a | 13.8(12.6,15.2)a | 0.044 | 0.09 | AC3 | |
| C2 (μM) | 0.12 (0.11,0.13) | 0.13(0.10,0.15) | 0.13 (0.10,0.16) | 0.794 | 0.875 | AC1 | |
| C3 (μM) | 0.029 (0.026,0.031) | 0.033(0.028,0.04) | 0.034(0.029,0.040) | 0.075 | 0.37 | AC1 | |
| C4 (μM) | 0.035 (0.033,0.036) | 0.034(0.030,0.039) | 0.035(0.032,0.039) | 0.922 | 0.922 | AC1 | |
| C5 (μM) | 0.030 (0.027,0.032) | 0.035(0.031,0.039) | 0.035(0.028,0.039) | 0.078 | 0.39 | AC2 | |
| C6 (μM) | 0.016 (0.0148,0.173) | 0.017(0.015,0.02) | 0.017(0.015,0.02) | 0.243 | 0.36 | AC2 | |
| C8 (μM) | 0.036(0.033, 0.04) | 0.039(0.034,0.04) | 0.036(0.03,0.04) | 0.745 | 0.745 | AC2 | |
| C10 (μM) | 0.07 (0.06, 0.07) | 0.069(0.06, 0.07) | 0.06 (0.05, 0.07) | 0.11 | 0.22 | AC2 | |
| C10:1 (μM) | 0.105 (0.10,0.11) | 0.10 (0.0.11, 0.98) | 0.103 (0.09,0.11) | 0.571 | 0.6852 | AC2 | |
| C10:2 (μM) | 0.018 (0.0.017,0.019)b | 0.018(0.015,0.021)a,b | 0.021(0.019,0.023)a | 0.05 | 0.08 | AC2 | |
| C12 (μM) | 0.38 (0.036,0.041) | 0.037(0.034,0.042) | 0.036(0.032,0.040) | 0.353 | 0.45 | AC2 | |
| C12:1 (μM) | 0.049(0.047, 0.052) | 0.051(0.048,0.055) | 0.047(0.043,0.052) | 0.769 | 0.877 | AC2 | |
| C14(μM) | 0.014 (0.013,0.015) | 0.014 (0.012, 0.016) | 0.015(0.013, 0.017) | 0.887 | 0.877 | AC2 | |
| C14:1 (μM) | 0.037 (0.035,0.039) | 0.037(0.033,0.041) | 0.036(0.032,0.040) | 0.037 | 0.22 | AC2 | |
| C14:2 (μM) | 0.015 (0.014,0.016) | 0.015 (0.013,0.018) | 0.014(0.012,0.017) | 0.579 | 0.579 | AC2 | |
| C16 (μM) | 0.029 (0.028, 0.031) | 0.034(0.030, 0.038) | 0.031 (0.027, 0.034) | 0.859 | 0.859 | AC3 | |
| C16:1 (μM) | 0.009 (0.007, 0.011) | 0.008(0.004, 0.015) | 0.01 (0.009, 0.011) | 0.813 | 0.89 | AC3 | |
| C18:1OH (μM) | 0.0005(0.0002, 0.0012) | 0.0007 (0.0001,0.003) | 0.0008(0.0002, 0.002) | 0.83 | 0.859 | AC4 | |
| C18:2 (μM) | 0.015 (0.014, 0.016) | 0.018(0.016, 0.020) | 0.016(0.013, 0.018) | 0.12 | 0.24 | AC3 | |
| C18OH (μM) | 0.001 (0.0009,0.0004) | 0.0005(0.0001, 0.002) | 0.0001(0.00005, 0.0006) | 0.377 | 0.37 | AC4 | |
Contrast between groups by one-way ANOVA test. Fisher Post hoc test was done. Variable values were recovered using antilogarithms. FDR = False Discovery Rate. Only the acylcarnitines and amino acids used in SEM are showed in the table. Superscript letters show homogenous groups.
Table 2. Family history of obesity and diabetes in 3 groups.
| G1 (%) | G2 (%) | G3 (%) | p-value | Variable names in SEM Model |
||
|---|---|---|---|---|---|---|
| number = 137 | number = 82 | number = 24 | number = 31 | |||
| Family History of Obesity (FHO) | 72 (53) | 38 (46) | 14 (58) | 20 (65) | 0.14 | |
| Direct FHO | 46 (34) | 23 (28) | 14 (58) | 9 (29) | 0.02 | |
| Mother FHO | 32 (23) | 14(17) | 13 (54) | 5 (16) | <0.001 | MomFHO |
| Father FHO | 24 (17) | 15 (60) | 6 (25) | 5 (16) | 0.57 | DadFHOB |
| Indirect FHO | 54 (39) | 30 (37) | 10 (41.7) | 14 (45) | 0.57 | IndFHOB |
Family history of obesity in G1, G2, G3. G1 = BMI<25, NAFLD negative; G2 = BMI>30, NAFLD negative; G3 = BMI>30+NAFLD. Chi square test.
Clinical, metabolomic and inflammatory differences between G1-G3
Clinical differences were observed among the three groups as expected: the patients with obesity with or without NAFLD (G2, G3) had higher BMI, percentage of fat and abdominal circumference (p<0.001) (Table 1). There were no differences in exercise activity between groups (p>0.05).
Even though none of the patients had diabetes or glucose intolerance, both G2 and G3 had higher glucose and insulin levels with lower Matsuda index (p<0.001). These groups also had higher cholesterol and triglyceride levels (p<0.001) (Table 1); and G3 only had high levels of CRP, arginine, alanine, leucine, phenylalanine, tyrosine, valine, ornithine, and proline (p<0.01).
Medium and long chain acylcarnitines such as C8:1, C10:2 and C18:1 had higher serum levels (p<0.05) in patients with obesity regardless of NAFLD compared to lean controls, though no differences were found between obese patients with and without NAFLD.
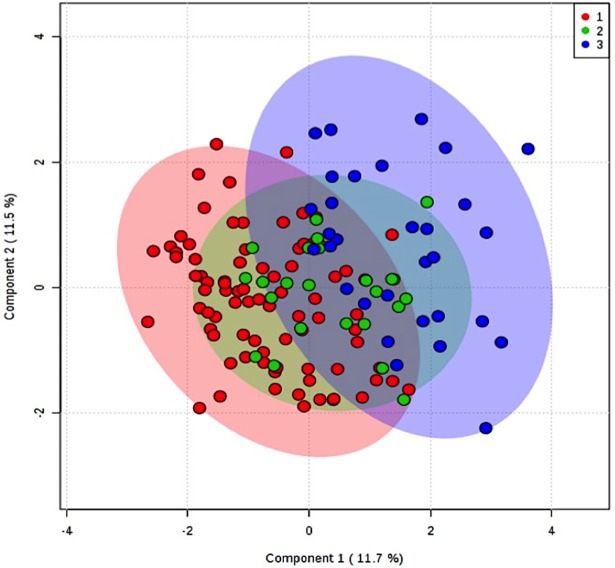
PLS-DA analysis demonstrated a mild separation between G1 and G3, while as expected G2, was over imposed between G1 and G3; three components explained 62% of the variance (accuracy 0.68, R2 0.6, Q2 0.39, p<0.001). VIP scores > 1.2 were: CRP, insulin, triglycerides, C:16: 1OH, C8:1, ornithine and tyrosine (Fig 1).
Fig 1. PLS-DA of metabolytes in 3 groups.
1 = BMI<25, 2 = BMI<30 and NAFLD negative, and 3 = BMI>30 and NAFLD positive.
Structural equation models
In order to test our hypothesis, we created two structural equation models and a third derived from the other two. An exploratory factor analysis clustered acylcarnitines into 4 factors and amino acids into two factors. These models are shown in Table 1. We only used TNFα and IL-6 (both grouped as “INFL”) and CRP in our models since they are the most common inflammatory makers reported in the literature.
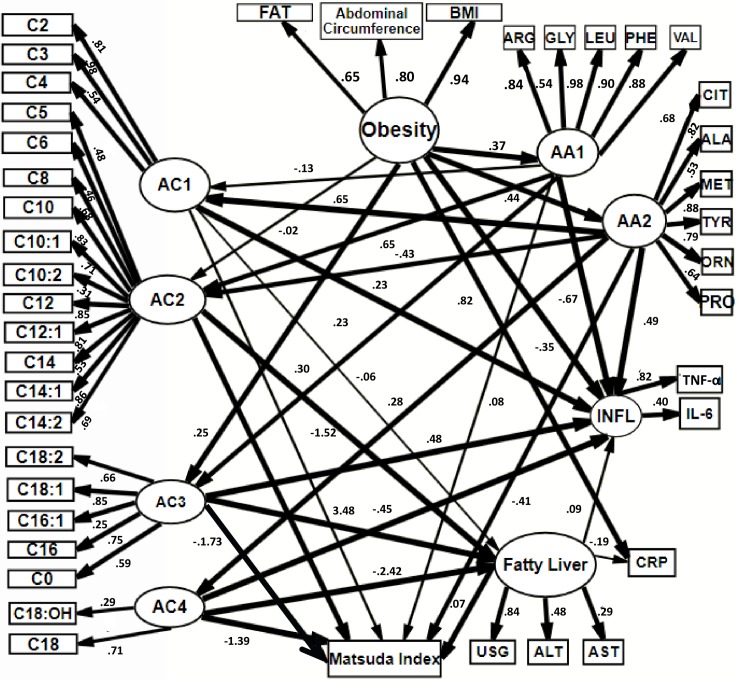
The first model (Fig 2, S1 Table) showed that obesity correlates with plasma amino acids, which contributes to increase of specific acylcarnitins and inflammatory markers. The excess free fatty acids were associated with NAFLD and also with inflammatory markers and insulin resistance in that order. The RMSEA was 0.078 (0.0.71, 0.084), and standardized β values > 0.2 (shown in parenthesis) supported obesity as predictor of endogenous variables such as amino acid concentration grouped in both factors AA1 and AA2 (0.37 and 0.44 respectively). Amino acids grouped in the factor AA1 predicted the blood concentration of medium, long and very long chain acylcarnitines grouped in AC2- AC4 (0.65 and 0.30). AA2 predicted AC1, AC2 and AC4 (0.65, -0.43 and 0.28). Short, long and very long chain acylcarnitines, AC1, AC3, and AC4, predicted inflammatory markers TNFα and IL-6 (INFL) (0.23, 0.48, -0.45), while amino acids grouped in AA1 predicted negatively INFL (-0.67), and amino acids grouped in AA2 predicted positively (0.49). AC2-AC4 was associated with NAFLD (-1.52, 3.48, -2.42). Finally, AC3, AC4, and AA2 predicted Matsuda index (1.39, 0.72, -0.41).
Fig 2. SEM hypothesis 1.
Obesity correlates with plasmatic amino acids, which contributes to increase of specific acylcarnitines and inflammatory markers. Obesity correlated directly with acylcarnitines. These were associated with NAFLD and then related to inflammatory markers and insulin resistance. Circles = latent variables, rectangles = observed variables, e = error term. Latent variable obesity is formed by: BMI = Body mass Index, Abd_circumf = abdominal circumference, FAT = % of Fat. AA1 and AA2 = latent variables that represent factor for amino acids AC1, AC2, AC3 and AC4 = latent variables that represent factors grouped for acylcarnitines. C:0,C2-C18:2. ALA = alanine, CIT = citrulline, Met = methionine, TYR = tyrosine, ORN = ornithine, PRO = proline, ARG = arginine, GLY = glycine, LEU = leucine, PHE = phenylalanine, VAL = valine. CRP = C reactive protein. TNFα = Tumor necrosis factor alpha, IL-6 = Interleukine-6, both form latent variable INFL = inflammatory markers. Latent variable NAFLD integrates USG = liver ultrasound. ALT = Alanine aminotransferase, AST = Aspartate aminotransferase. Lines in bold correspond to standardized β estimates > 0.2. Numbers in each line correspond to standardized β estimate. To simplify, error terms were not included in the figure.
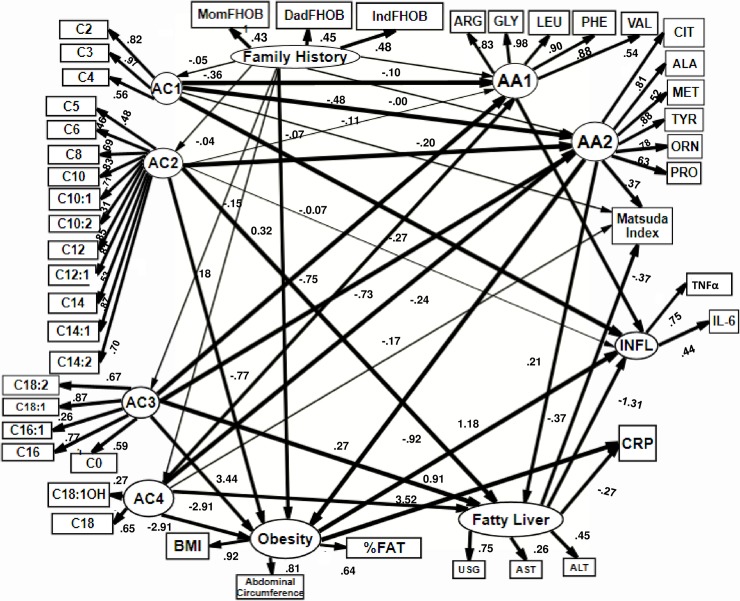
In the second model (Fig 3, S2 Table), a positive family history of obesity (exogen variable), was the major determinant of acylcarnitines and amino acids. This was associated with NAFLD, obesity, insulin resistance and pro-inflammatory process. In this model, we proposed obesity as a secondary, or a consequence of a previous alteration of fatty acid and amino acids metabolism. The RMSEA of this model was 0.075 (0.069, 0.081).
Fig 3. SEM hypothesis 2.
Positive family history of obesity was the mayor determinant of acylcarnitines and amino acids, This were associated with NAFLD, obesity, insulin resistance and pro-inflammatory process. In this model we proposed obesity as a secondary, or a consequence of a previous alteration of fatty acid and amino acids metabolism. Circles = latent variables, rectangles = observed variables, e = error term. Family History of obesity is formed by: IndFOB = Second degree family history of obesity. DadFHOB = Parental history of obesity. MomFHOB = Maternal family history of Obesity. Latent variable obesity is formed by: BMI = Body mass Index, Abd_circumf = abdominal circumference, FAT = % of Fat. AA1 and AA2 = latent variables that represent factor for amino acids AC1, AC2, AC3 and AC4 = latent variables that represent factors grouped for acylcarnitines. C:0,C2-C18:2. ALA = alanine, CIT = citrulline, Met = methionine, TYR = tyrosine, ORN = ornithine, PRO = proline, ARG = arginine, GLY = glycine, LEU = leucine, PHE = phenylalanine, VAL = valine. CRP = C reactive protein. TNFα = Tumor necrosis factor alpha, IL-6 = Interleukine-6, both form latent variable INFL = inflammatory markers. Latent variable NAFLD integrates USG = liver ultrasound. ALT = Alanine aminotransferase, AST = Aspartate aminotransferase,. Lines in bold correspond to standardized β estimates > 0.2. Numbers in each line corresponds to standardized β estimate Lines in bold correspond to standarized B estimates > 0.2. Numbers in line correspond to standardized β estimates. To simplify, error terms were not included in the figure.
Standardized β estimates for family history of obesity to predict acylcarnitines and amino acids were < 0.2. However, the β estimate for family history of obesity to predict obesity was 0.32 (see Fig 3).
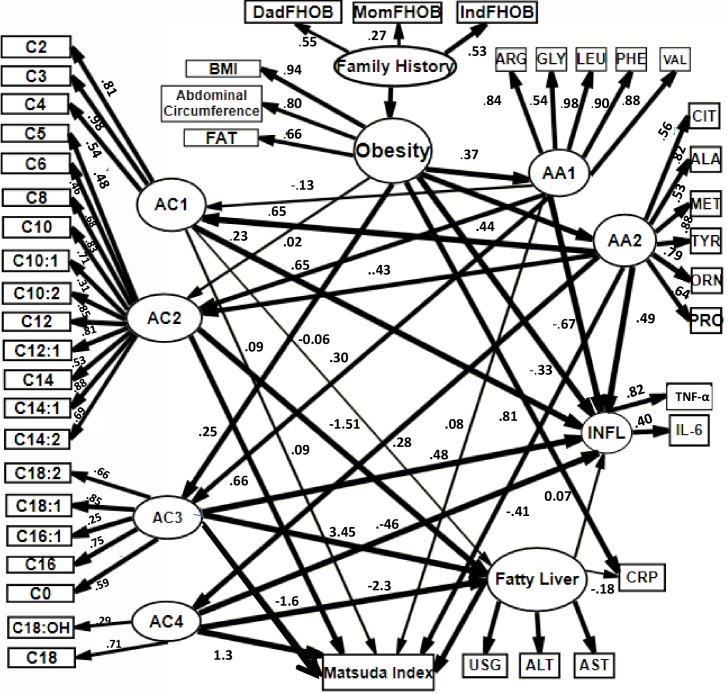
Based on the results of the previous models we created a third model were family history predicted obesity. This correlated with plasma amino acids that contributed to an increase of specific acylcarnitins and inflammatory markers. The excess of free fatty acids related to obesity was associated with NAFLD and then related to inflammatory markers and insulin resistance. The RMSEA of the model was 0.075 (0.069, 0.081) (Fig 4, S3 Table).
Fig 4. SEM model 3.
Family history predicted obesity. This correlates with plasmatic amino acids that contribute to an increase of specific acylcarnitins and inflammatory markers. This was associated with NAFLD and then related to inflammatory markers and insulin resistance. Circles = latent variables, rectangles = observed variables, e = error term. Family History of obesity is formed by: IndFOB = Second degree family history of obesity. DadFHOB = Parental history of obesity. MomFHOB = Maternal family history of Obesity. Latent variable obesity is formed by: BMI = Body mass Index, Abd_circumf = abdominal circumference, FAT = % of Fat. AA1 and AA2 = latent variables that represent factor for amino acids AC1, AC2, AC3 and AC4 = latent variables that represent factors grouped for acylcarnitines. C:0,C2-C18:2. ALA = alanine, CIT = citrulline, Met = methionine, TYR = tyrosine, ORN = ornithine, PRO = proline, ARG = arginine, GLY = glycine, LEU = leucine, PHE = phenylalanine, VAL = valine. CRP = C reactive protein. TNFα = Tumor necrosis factor alpha, IL-6 = Interleukine-6, both form latent variable INFL = inflammatory markers. Latent variable NAFLD integrates USG = liver ultrasound. ALT = Alanine aminotransferase, AST = Aspartate aminotransferase. Lines in bold correspond to standardized β estimates > 0.2. Numbers in each line corresponds to standardized β estimate. To simplify, error terms were not included in the figure.
Discussion
The presented SEM analysis evaluated hypothetical causal relationships of phenotypic, metabolomics, inflammatory markers and family history of obesity in an integrated model and found that the family history strongly correlates with a subject’s obesity.
Family history of obesity serves as a proxy for an individual’s genetic, epigenetic and fetal development background and obesity results in severe disruption of regulation of key metabolic enzymes and pathways as indicated by acylcarnitines and amino acids, and both metabolites predict inflammation, insulin resistance, obesity and NAFLD.
Traditional statistical methods such as ANOVA, Chi-square and PLS-DA demonstrated demographic, clinic and metabolomics showed differences among G1-G3 groups, but provided insufficient information about their relationship, also multiple ANOVA increase the risk of type 1 error. Our use of SEMs overcame such drawbacks and provided the following advantages: (1) good cohesion between variables; (2) small error values that led to clean sample selection; (3) good effect size (4) biological support, and (5) detection of previously undetected correlations.
The first model we proposed obesity results from imbalance in positive energy intake, giving adipose tissue expansion. There is an increase of free fatty acids and a disruption in mitochondrial β oxidation that results in an increase of acylcarnitines. An increase of amino acids also predicts increased alfa-ketoacids with subsequent increase in acylcarnitins, specifically short chain. This metabolic disruption predicts a inflammatory process, NAFLD and insulin resistance.
It has been previously published that branched-chain amino acid–derived C3- and C5-carnitine, with fatty acids derived C6 and C8 acylcarnitines have high plasma concentration in patients with obesity and T2D compared with lean controls [19].
Our models support every amino acids biochemical structure is related to obesity and not only branched chained amino acids (BCAA); but only amino acids grouped in Factor AA2 predicted Matsuda index. A study conducted in India and China showed a correlation of HOMA with alanine, proline, valine, and leucine [34]. It has been previously reported that the increased concentrations of BCAA associated with IR is related to chronic phosphorylation of the mammalian target of rapamycin, c-jun N-terminal kinase and insulin receptor substrate 1[19]. Also, there are decreased insulin-stimulated tyrosine phosphorylation of IRS-1 and IRS-2; decreased binding of grb2 and the p85 subunit of phosphatidylinositol 3-kinase to IRS-1 and IRS-2, and a marked inhibition of insulin-stimulated phosphatidylinositol 3-kinase[35]. However, it still remains unclear the mechanisms underlying the non-BCAA association with obesity and IR.
Niu et al. has previously studied the relation between amino acids and pro-inflammatory response. They reported histidine and arginine were negatively associated to IL-6 and CRP in obese women [36]. We found similar findings in our model. In another study, C57BL/6J mice were fed with high fat diet and compared with lean controls, mRNA levels in down regulation genes associated to branched-chain amino acid pathways in visceral adipose showed a decrease in the metabolism of BCAA/TCA cycle related to increased concentrations of TNFα, IL-6, IL-1β, and IFNγ [37]. Our models supported a clear interrelationship between all analyzed amino acid residues with IL-6, TNFα and CRP.
Obesity is associated with high concentration of short chain acylcarnitines, which may reflect higher lipid fluxes, mitochondrial and β-oxidation overload, incomplete channeling of fatty acids (FA) to complete oxidation, or the oxidation rate of amino acids. There is a lack of clarity if short chain acylcarnitines have a negative effect on insulin signaling processes and if this effect is rather indirect. Our models did not support significant relationship (standardized β values < 0.2) between short chain acylcarnitines and Matsuda index [38].
Schooneman et al., reported patients with obesity have lower carnitine palmitoyltransferase 1 (CPT1) and citrate synthase content that promote lower fatty acid oxidation and an increase in long chain acylcarnitines [39]. The latter have been associated with insulin resistance, making a role for long-chain acylcarnitines conceivable in this mechanism [40]. Our models defined a clear correlation between long chain acylcarnitines, obesity and Matsuda index.
It has been reported previously that patients with NAFLD have higher levels of free carnitine, and short chain acylcarnitines such as C3-C5 [23] and long chain acylcarnitines such as C18, C18:2, C16. [24]. A mouse model reported an association of C:10 acylcarnitine and NAFLD [17]. In our study, free carnitine was higher in patients with NAFLD and medium and long acylcarnitines concentrations negatively predicted presence of NAFLD. The normal AST and ALT reference values in Mexican population are 23.7 ± 6.3 IU/L and 20.3 ± 7.6 IU/L respectively. [41] The G3 had ALT levels discreetly higher in our group. High ALT serum concentration correlate with esteatohepatitis. The difference in metabolomics of acylcarnitins and amino acids in esteatohepatitis vs steatosis has been explored previosuly by Satish C. Kalhan et al.[23] they obtained biopsies from patients with steatosis and steatohepatitis. They did not find any difference between groups. In an other study conducted by J Barr et al. (39), there were differences in acylcarnitines in obese class III, but not in lean subjects, neither obese classes I and II, suggesting that the differences were related to obesity not by inflammatory process of the liver.
Studies have suggested that acylcarnitines may be involved in inflammation. A study using mouse bone marrow derived macrophages treated with L-C12-carnitine polarized towards the M1 pro-inflammatory phenotype, down regulating AMPK and secreted pro-inflammatory cytokines such as IL-3, IL-6, IL-12, IL-11, IL-16, IL-23 y TNFα [42].
In a study using a murine macrophage RAW 264.7 cell line, C12 and C14 acylcarnitines significantly stimulated nuclear factor kappa-B activity (up to 200% of controls) in RAW264.7 cells [43,44].
Our model shows for the first time that short chain and long chain acylcarnitines may correlate with IL-6 and TNF-α, experimental models are need to prove if short chains C2 to C4 acylcarnitines predict inflammatory markers directly, or they have an indirect effect threw amino acids pathways. New models must be done to study the relation between inflammation, acylcarnitines and NAFLD which relations seem not necessary proportional to BMI.
Our second model defined family history explains different capability for the metabolism of amino acids, and mitochondrial β oxidation leading to increase the circulating levels who could lead to the development of obesity, IR and NAFLD. However, the B estimates from this model have very low effect. Other type of study design must be made in the future to test this hypothesis; whole genomic sequencing could be the starting point.
Finally, the novelty of the results shows that family history of obesity does predict obesity phenotype, with a standardized β estimate of 0.3. Once obesity phenotype has established acylcarnitne and aminoacide disruption was supported.
We did not included a group of BMI<25 with NAFLD since their physiopathology seems to be independent of obesity and insulin resistance. This group deserve a more complete study in the future. Some mechanisms proposed are related to genetic alteration in transportation of triglycerides and cholesterol in the body, such as mutations in cholesteryl ester transfer protein, sterol regulatory element binding protein or apolipoprotein 3. The widely studied PNPLA3 gene, encoding for adiponutrin, is an example of a genetic mutation independent of obesity and insulin resistance case. The most relevant polymorphism, I148M is associated with a decreased lipolytic activity. It is also associated with higher aminotransferases levels, but not with insulin resistance. In a previous published paper we had a group of patients with BMI<25 and NAFLD which showed the higher inflammatory process. So if we had included this fourth group in our present models we probably would found higher alteration in β oxidation and inflammatory markers[45–47].
Our study has certain limitations. We evaluated family history as a nominal (dichotomous) variable, which could produce mild instability in the precision of our coefficients, but we concluded this effect was minimal and methodologically tolerable. Our study sample had more women than men, which is in accordance with attendance to the local hospital. In addition, ultrasonography is not considered as the gold standard for NAFLD diagnosis, however this is a screening method with high sensitivity/specificity accuracy.
Another limitation is that we cannot conduct biopsies in this population for ethical reasons. In addition, due to the study design, we were not able to obtain longitudinal data metabolomic profile. However, SEM does let us evaluate dose–response of the variables interrelationships.
It would be interesting to apply SEM based approaches on larger scale studies with other family history approach, and omics area for example proteomics, epigenomics transcriptomics, etc.
Conclusion
Our study provides SEM that support that family History of obesity, correlates with patients obesity which results in several disruption in the regulation of key metabolic enzymes and pathways that predicts metabolomics (acylcarnitines and amino acids) and this predicts inflammation, insulin resistance, obesity and NAFLD.
Supporting information
Standardized and not standardized β values (β = not standardized estimate, Std β = standardized estimate). BMI = Body Mass Index. Abd_circumf = abdominal circumference, FAT = % of Fat. AC1, AC2, AC3 and AC4 = factors grouped for acylcarnitines C2-C18:2. AA1 and AA2 factors grouped for amino acids. ALA = alanine, CIT = citrulline, Met = methionine, TYR = tyrosine, ORN = ornithine, PRO = proline, ARG = arginine, GLY = glycine, LEU = leucine, PHE = phenylalanine, VAL = valine. CRP = C reactive protein. INFL = inflammatory markers, TNF-a = Tumor necrosis factor alpha, IL-6 = Interleukine 6. USG: liver ultrasound. ALT = Alanine aminotransferase, AST = Aspartate aminotransferase.
(PDF)
Standardized and not standardized β values (β = not standardized estimate, Std β = standardized estimate). AC1, AC2, AC3 and C4 = factors grouped for acylcarnitines C2-C18:2. AA1 and AA2 factors grouped for amino acids. ALA = alanine, CIT = citrulline, Met = methionine, TYR = tyrosine, ORN = ornithine, PRO = proline, ARG = arginine, GLY = glycine, LEU = leucine, PHE = phenylalanine, VAL = valine. BMI = Body mass Index. Abd_circumf = abdominal circumference, FAT = % of Fat. CRP = C reactive protein. INFL = inflammatory markers, TNF-a = Tumor necrosis factor alpha, IL-6 = Interleukine 6. USG: liver ultrasound. ALT = Alanine aminotransferase, AST = Aspartate aminotransferase. DadFHOB = Parental family History of obesity. MomFHOB = Maternal family History of obesity. IndFHOB: Second degree family history of obesity.
(PDF)
Standardized and not standardized β values (β = not standardized estimate, Std β = standardized estimate). BMI = Body mass Index. Abd_circumf = abdominal circumference, FAT = % of Fat. AC1, AC2, AC3 and AC4 = factors grouped for acylcarnitines C2-C18:2. AA1 and AA2 factors grouped for aminoacids. ALA = alanine, CIT = citrulline, Met = methionine, TYR = tyrosine, ORN = ornithine, PRO = proline, ARG = arginine, GLY = glycine, LEU = leucine, PHE = phenylalanine, VAL = valine. CRP = C reactive protein. INFL = inflammatory markers, TNF-a = Tumor necrosis factor alpha, IL-6 = Interleukine 6. USG: liver ultrasound. ALT = Alanine aminotransferase, AST = Aspartate aminotransferase. IndFOB = Second degree family history of obesity. DadFHOB = Parental history of obesity. MomFHOB = Maternal family hitory of Obesity.
(PDF)
Data Availability
All data are all contained within the paper.
Funding Statement
This study was supported by internal funds of Instituto Nacional de Medicina Genomica and Hospital General de Mexico. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO | Obesity and overweight. In: WHO [Internet]. [cited 6 Nov 2016]. Available: http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA. 2014;311: 806–814. doi: 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OECD. Health at a Glance 2017 [Internet]. OECD Publishing; 2017. doi: 10.1787/health_glance-2017-en [Google Scholar]
- 4.La Barquera S, Campos-Nonato I (segundo), Hernández-Barrier L (tercero), Pedroza-Tobias TO (cuarto), Rivera-Dommarco JA (quinto). Prevalencia de obesidad en adultos mexicanos, ENSANUT 2012. Salud Pública Méx. 2013;55: 151–160. [PubMed] [Google Scholar]
- 5.Shamah-Levy T, Cuevas-Nasu L, Rivera-Dommarco J, Hernández-Ávila M. Encuesta Nacional de Nutrición y Salud de Medio Camino 2016 (ENSANUT MC 2016). Informe final de resultados. Recuperado de https://www.insp.mx/ensanut/medio-camino-16.html/[Links]; 2016. [Google Scholar]
- 6.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY COMPREHENSIVE CLINICAL PRACTICE GUIDELINES FOR MEDICAL CARE OF PATIENTS WITH OBESITY: EXECUTIVE SUMMARY Complete Guidelines available at https://www.aace.com/publications/guidelines. Endocr Pract. 2016;22: 842–884. doi: 10.4158/EP161356.ESGL [DOI] [PubMed] [Google Scholar]
- 7.Vázquez-Vela MEF, Torres N, Tovar AR. White Adipose Tissue as Endocrine Organ and Its Role in Obesity. Arch Med Res. 2008;39: 715–728. doi: 10.1016/j.arcmed.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 8.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121: 2111–2117. doi: 10.1172/JCI57132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung U, Choi M-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int J Mol Sci. 2014;15: 6184–6223. doi: 10.3390/ijms15046184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pigeyre M, Yazdi FT, Kaur Y, Meyre D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci. 2016;130: 943–986. doi: 10.1042/CS20160136 [DOI] [PubMed] [Google Scholar]
- 11.Fesinmeyer MD, North KE, Ritchie MD, Lim U, Franceschini N, Wilkens LR, et al. Genetic risk factors for BMI and obesity in an ethnically diverse population: Results from the population architecture using genomics and epidemiology (PAGE) study. Obesity. 2013;21: 835–846. doi: 10.1002/oby.20268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graff M, Scott RA, Justice AE, Young KL, Feitosa MF, Barata L, et al. Genome-wide physical activity interactions in adiposity—A meta-analysis of 200,452 adults. PLoS Genet. 2017;13: e1006528 doi: 10.1371/journal.pgen.1006528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97: 1019–1026. doi: 10.1136/archdischild-2012-302263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cederberg H, Stančáková A, Kuusisto J, Laakso M, Smith U. Family history of type 2 diabetes increases the risk of both obesity and its complications: is type 2 diabetes a disease of inappropriate lipid storage? J Intern Med. 2015;277: 540–551. doi: 10.1111/joim.12289 [DOI] [PubMed] [Google Scholar]
- 15.Fiehn O. Metabolomics—the link between genotypes and phenotypes. Plant Mol Biol. 2002;48: 155–171. [PubMed] [Google Scholar]
- 16.Holmes E, Wilson ID, Nicholson JK. Metabolic Phenotyping in Health and Disease. Cell. 2008;134: 714–717. doi: 10.1016/j.cell.2008.08.026 [DOI] [PubMed] [Google Scholar]
- 17.Kim H-J, Kim JH, Noh S, Hur HJ, Sung MJ, Hwang J-T, et al. Metabolomic Analysis of Livers and Serum from High-Fat Diet Induced Obese Mice. J Proteome Res. 2011;10: 722–731. doi: 10.1021/pr100892r [DOI] [PubMed] [Google Scholar]
- 18.Oberbach A, Blüher M, Wirth H, Till H, Kovacs P, Kullnick Y, et al. Combined Proteomic and Metabolomic Profiling of Serum Reveals Association of the Complement System with Obesity and Identifies Novel Markers of Body Fat Mass Changes. J Proteome Res. 2011;10: 4769–4788. doi: 10.1021/pr2005555 [DOI] [PubMed] [Google Scholar]
- 19.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009;9: 311–326. doi: 10.1016/j.cmet.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen MD, Haymond MW. Protein metabolism in obesity: effects of body fat distribution and hyperinsulinemia on leucine turnover. Am J Clin Nutr. 1991;53: 172–176. [DOI] [PubMed] [Google Scholar]
- 21.Li Y-C, Li C-L, Qi J-Y, Huang L-N, Shi D, Du S-S, et al. Relationships of Dietary Histidine and Obesity in Northern Chinese Adults, an Internet-Based Cross-Sectional Study. Nutrients. 2016;8: 420 doi: 10.3390/nu8070420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luzi L, Castellino P, DeFronzo RA. Insulin and hyperaminoacidemia regulate by a different mechanism leucine turnover and oxidation in obesity. Am J Physiol. 1996;270: E273–281. [DOI] [PubMed] [Google Scholar]
- 23.Kalhan SC, Guo L, Edmison J, Dasarathy S, McCullough AJ, Hanson RW, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60: 404–413. doi: 10.1016/j.metabol.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lake AD, Novak P, Shipkova P, Aranibar N, Robertson DG, Reily MD, et al. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids. 2015;47: 603–615. doi: 10.1007/s00726-014-1894-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17: 448–453. doi: 10.1038/nm.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xi B, Gu H, Baniasadi H, Raftery D. Statistical Analysis and Modeling of Mass Spectrometry-Based Metabolomics Data In: Raftery D, editor. Mass Spectrometry in Metabolomics. New York, NY: Springer New York; 2014. pp. 333–353. doi: 10.1007/978-1-4939-1258-2_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosa GJ, Valente BD, de los Campos G, Wu X- L, Gianola D, Silva MA. Inferring causal phenotype networks using structural equation models. Genet Sel Evol. 2011;43: 6 doi: 10.1186/1297-9686-43-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, de la Fuente A, Hoeschele I. Gene Network Inference via Structural Equation Modeling in Genetical Genomics Experiments. Genetics. 2008;178: 1763–1776. doi: 10.1534/genetics.107.080069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995; 289–300. [Google Scholar]
- 30.Browne MW, Cudeck R. Alternative Ways of Assessing Model Fit. Sociol Methods Res. 1992;21: 230–258. doi: 10.1177/0049124192021002005 [Google Scholar]
- 31.McDonald RP, Ho M-HR. Principles and practice in reporting structural equation analyses. Psychol Methods. 2002;7: 64–82. [DOI] [PubMed] [Google Scholar]
- 32.Valente BD, Rosa GJM, Gianola D, Wu X-L, Weigel K. Is Structural Equation Modeling Advantageous for the Genetic Improvement of Multiple Traits? Genetics. 2013;194: 561–572. doi: 10.1534/genetics.113.151209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 2015;43: W251–W257. doi: 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tai ES, Tan MLS, Stevens RD, Low YL, Muehlbauer MJ, Goh DLM, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53: 757–767. doi: 10.1007/s00125-009-1637-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101: 1519–1529. doi: 10.1172/JCI1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu Y-C, Feng R-N, Hou Y, Li K, Kang Z, Wang J, et al. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br J Nutr. 2012;108: 57–61. doi: 10.1017/S0007114511005289 [DOI] [PubMed] [Google Scholar]
- 37.Burrill JS, Long EK, Reilly B, Deng Y, Armitage IM, Scherer PE, et al. Inflammation and ER stress regulate branched-chain amino acid uptake and metabolism in adipocytes. Mol Endocrinol Baltim Md. 2015;29: 411–420. doi: 10.1210/me.2014-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morino K, Petersen KF, Shulman GI. Molecular Mechanisms of Insulin Resistance in Humans and Their Potential Links With Mitochondrial Dysfunction. Diabetes. 2006;55: S9–S15. doi: 10.2337/db06-S002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: Reflecting or Inflicting Insulin Resistance? Diabetes. 2013;62: 1–8. doi: 10.2337/db12-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FGS, et al. Increased Levels of Plasma Acylcarnitines in Obesity and Type 2 Diabetes and Identification of a Marker of Glucolipotoxicity. Obesity. 2010;18: 1695–1700. doi: 10.1038/oby.2009.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavarría-Arciniega S, López-Alvarenga JC, Uribe-Uribe NO, Herrera-Hernández M, González-Barranco J. [Relationship between morphological diagnosis of NASH (non-alcoholic steatohepatitis) and liver function tests in a group of patients with morbid obesity]. Rev Investig Clin Organo Hosp Enfermedades Nutr. 2005;57: 505–512. [PubMed] [Google Scholar]
- 42.Sampey BP, Freemerman AJ, Zhang J, Kuan P- F, Galanko JA, O’Connell TM, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PloS One. 2012;7: e38812 doi: 10.1371/journal.pone.0038812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutkowsky JM, Knotts TA, Ono-Moore KD, McCoin CS, Huang S, Schneider D, et al. Acylcarnitines activate proinflammatory signaling pathways. AJP Endocrinol Metab. 2014;306: E1378–E1387. doi: 10.1152/ajpendo.00656.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCoin CS, Knotts TA, Adams SH. Acylcarnitines—old actors auditioning for new roles in metabolic physiology. Nat Rev Endocrinol. 2015;11: 617–625. doi: 10.1038/nrendo.2015.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verdelho Machado M, Cortez-Pinto H. Fatty liver in lean patients: is it a different disease? Ann Gastroenterol. 2012;25: 1–2. [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar R, Mohan S. Non-alcoholic Fatty Liver Disease in Lean Subjects: Characteristics and Implications. J Clin Transl Hepatol. 2017;XX: 1–8. doi: 10.14218/JCTH.2016.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero-Ibarguengoitia ME, Herrera-Rosas A, Domínguez-Mota AA, Camas-Benitez JT, Serratos-Canales MF, León-Hernández M, et al. Nonalcoholic fatty liver disease can be predicted by retinal vascular changes in patients with obesity without hypertension or diabetes. Eur J Gastroenterol Hepatol. 2017;29: 962–967. doi: 10.1097/MEG.0000000000000900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standardized and not standardized β values (β = not standardized estimate, Std β = standardized estimate). BMI = Body Mass Index. Abd_circumf = abdominal circumference, FAT = % of Fat. AC1, AC2, AC3 and AC4 = factors grouped for acylcarnitines C2-C18:2. AA1 and AA2 factors grouped for amino acids. ALA = alanine, CIT = citrulline, Met = methionine, TYR = tyrosine, ORN = ornithine, PRO = proline, ARG = arginine, GLY = glycine, LEU = leucine, PHE = phenylalanine, VAL = valine. CRP = C reactive protein. INFL = inflammatory markers, TNF-a = Tumor necrosis factor alpha, IL-6 = Interleukine 6. USG: liver ultrasound. ALT = Alanine aminotransferase, AST = Aspartate aminotransferase.
(PDF)
Standardized and not standardized β values (β = not standardized estimate, Std β = standardized estimate). AC1, AC2, AC3 and C4 = factors grouped for acylcarnitines C2-C18:2. AA1 and AA2 factors grouped for amino acids. ALA = alanine, CIT = citrulline, Met = methionine, TYR = tyrosine, ORN = ornithine, PRO = proline, ARG = arginine, GLY = glycine, LEU = leucine, PHE = phenylalanine, VAL = valine. BMI = Body mass Index. Abd_circumf = abdominal circumference, FAT = % of Fat. CRP = C reactive protein. INFL = inflammatory markers, TNF-a = Tumor necrosis factor alpha, IL-6 = Interleukine 6. USG: liver ultrasound. ALT = Alanine aminotransferase, AST = Aspartate aminotransferase. DadFHOB = Parental family History of obesity. MomFHOB = Maternal family History of obesity. IndFHOB: Second degree family history of obesity.
(PDF)
Standardized and not standardized β values (β = not standardized estimate, Std β = standardized estimate). BMI = Body mass Index. Abd_circumf = abdominal circumference, FAT = % of Fat. AC1, AC2, AC3 and AC4 = factors grouped for acylcarnitines C2-C18:2. AA1 and AA2 factors grouped for aminoacids. ALA = alanine, CIT = citrulline, Met = methionine, TYR = tyrosine, ORN = ornithine, PRO = proline, ARG = arginine, GLY = glycine, LEU = leucine, PHE = phenylalanine, VAL = valine. CRP = C reactive protein. INFL = inflammatory markers, TNF-a = Tumor necrosis factor alpha, IL-6 = Interleukine 6. USG: liver ultrasound. ALT = Alanine aminotransferase, AST = Aspartate aminotransferase. IndFOB = Second degree family history of obesity. DadFHOB = Parental history of obesity. MomFHOB = Maternal family hitory of Obesity.
(PDF)
Data Availability Statement
All data are all contained within the paper.