Supplemental Digital Content is available in the text.
Keywords: acute kidney injury, biomarker, cell-cycle arrest markers, exposures, nephrotoxicity
Objectives:
Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor binding protein 7 predict the development of acute kidney injury following renal insults of varied aetiology. To aid clinical interpretation, we describe the kinetics of biomarker elevations around an exposure.
Design:
In an ancillary analysis of the multicenter SAPPHIRE study, we examined the kinetics of the urinary [tissue inhibitor of metalloproteinase-2]•[insulin-like growth factor binding protein 7] in association with exposure to common renal insults (major surgery, IV radiocontrast, vancomycin, nonsteroidal anti-inflammatory drugs, and piperacillin/tazobactam).
Setting:
Thirty-five sites in North America and Europe between September 2010 and June 2012.
Patients:
Seven hundred twenty-three critically ill adult patients admitted to the ICU.
Interventions:
None.
Measurements and Main Results:
We compared the urinary [tissue metalloproteinase-2]•[insulin growth factor binding protein 7] kinetics from the day prior to exposure up to 5 days after exposure in patients developing acute kidney injury stage 2–3, stage 1, or no acute kidney injury by Kidney Disease Improving Global Outcome criteria. Among the 723 patients, 679 (94%) had at least one, 70% had more than one, and 35% had three or more exposures to a known renal insult. There was a significant association between cumulative number of exposures up to study day 3 and risk of acute kidney injury (p = 0.02) but no association between the specific type of exposure and acute kidney injury (p = 0.22). With the exception of radiocontrast, patients who developed acute kidney injury stage 2–3 after one of the five exposures, had a clear rise and fall of urinary [tissue inhibitor of metalloproteinase-2]•[insulin-like growth factor binding protein 7] from the day of exposure to 24–48 hours later. In patients without acute kidney injury, there was no significant elevation in urinary [tissue inhibitor of metalloproteinase-2]•[insulin-like growth factor binding protein 7].
Conclusions:
Exposure to potential renal insults is common. In patients developing acute kidney injury stage 2–3, the kinetics of urinary [tissue inhibitor of metalloproteinase-2]•[insulin-like growth factor binding protein 7] matched the exposure except in the case of radiocontrast.
Acute kidney injury (AKI) is a common complication of critical illness, affecting 50–60% of patients admitted to the ICU (1–5). Both, identification of high risk patients and the early diagnosis of AKI are essential in order to implement renoprotective measures in a timely manner and to prevent harm (6). We have previously shown that the urinary biomarkers tissue inhibition of metalloproteinase-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) predict the development of AKI secondary to a variety of renal insults, including surgery, sepsis, and medications (7–12). However, in order to better interpret biomarker results, the kinetics of biomarker elevations around an exposure are necessary.
The risk of AKI results from the interaction between susceptibility and exposures. During critical illness, patients are exposed to a large number of insults that are potentially harmful to renal function, often simultaneously and/or in succession. For instance, approximately 20% of the drugs prescribed in the ICU are nephrotoxic (13). The adverse impact of drug-induced AKI on patient outcomes can be severe with hospital mortality rates reported between 18% and 50% (14, 15). Exposure to iodinated contrast is also common. A prospective study in three teaching hospitals showed that more than 25% of all ICU patients had at least one CT scan and more than 80% were performed with iodinated contrast (16). The risk of dialysis following contrast is particularly increased in patients with a baseline estimated glomerular filtration rate less than or equal to 45 ml/min (17).
The detection of AKI following a nephrotoxic insult is usually delayed until a rise in serum creatinine is seen. As such, opportunities to intervene and prevent progression are missed. Numerous potential biomarkers for AKI have been identified (18). Their value lies in the ability to provide information beyond traditional tests, in particular in patients without “classical” signs of impaired renal function. In common with many diagnostic tests, the appearance of these biomarkers in serum or urine following a potentially nephrotoxic insult is dynamic and usually temporary.
In the multicentre SAPPHIRE study, we previously showed that TIMP-2 and IGFBP7 predicted the development of AKI stage 2–3 within 12 hours and before a rise in serum creatinine (7). In this ancillary analysis, we report the prevalence of five common renal insults during critical illness (19), that is, major surgery, administration of nonsteroidal anti-inflammatory drugs (NSAIDs), vancomycin, piperacillin/tazobactam, and IV iodinated radiocontrast and describe the kinetics of urinary [TIMP-2]·[IGFBP7] in association with exposure to one of these insults in patients developing AKI 2–3, AKI 1, or no AKI.
MATERIALS AND METHODS
Study Design
The Sapphire study has been described in detail elsewhere (7). In summary, it was a prospective multicenter study in which TIMP-2 and IGFBP7 were identified as biomarkers of AKI and subsequently validated in an independent validation cohort and compared with existing markers of AKI. Between September 2010 and June 2012, 744 critically ill adults (≥ 21 yr) were recruited at 35 sites in North America and Europe. Subjects were enrolled within 24 hours of admission to the ICU, had cardiovascular or respiratory dysfunction, and were expected to stay in the ICU for at least 48 hours. AKI was defined according to the Kidney Disease Improving Global Outcome (KDIGO) criteria (20). Subjects were excluded if they had AKI stage 2 or 3 at enrolment. The study showed that urinary [TIMP-2]•[IGFBP7] predicted the development of AKI 2–3 within 12 hours and before a serum creatinine rise. In this follow-up study, we describe the kinetics of urinary [TIMP-2]•[IGFBP7] around the exposure to five common insults and compare patients with and without AKI.
Sample and Data Collection
Following informed consent, urine and blood samples were taken at enrolment and at 12-hour intervals for 4 days and daily for another 3 days (7). Serum and urine supernatants were frozen within approximately 1 hour of collection, stored at –70°C or lower, and thawed immediately before analysis.
All clinical data, including patient demographics, reason for ICU admission, comorbidities, Acute Physiology and Chronic Health Evaluation (APACHE) III score, serum creatinine, and hourly urine output, were collected and stored in a password-protected dataset residing on servers at independent sites (Medidata Solutions, New York, NY).
Biomarker Assays
Urinary TIMP-2 and IGFBP7 concentrations were measured by technicians who were blinded to the clinical data using a clinical immunoassay (NephroCheck Test and Astute140 Meter; Astute Medical, San Diego, CA). The Astute140 Meter automatically multiplies the concentrations of both biomarkers and divides the product by 1,000 to report a single numeric result in (ng/ml)2/1,000. Creatinine was measured in study-specific serum samples at a central laboratory (LabCorp, San Diego, CA).
Exposure and Biomarker Analysis
We identified and described the proportion of patients who were exposed to at least one dose of vancomycin, NSAIDs, piperacillin/tazobactam, or IV iodinated radiocontrast or underwent major surgery within 5 days prior to enrollment through 7 days after enrolment. Major surgery was defined as in-patient surgery under general anesthesia involving opening of a major body cavity (21). These exposures were chosen because they are frequent, definable, potentially modifiable, and have a well-defined onset. We evaluated serial urinary [TIMP-2]•[IGFBP7] concentrations from the day prior to exposure up to 5 days later and compared the biomarker kinetics of patients according to their maximum AKI stage (no AKI, stage 1, stage 2–3) within 3 days following exposure.
Serum creatinine data from 6 months prior to enrolment through hospital discharge (truncated at 30 days after enrolment) and urine output data from the day prior to enrollment through 7 days after enrollment were collected to determine maximum AKI stage (20).
Ethics
According to the Declaration of Helsinki, the Sapphire study was approved by the Western Institutional Review Board (Olympia, WA) and individual investigational review boards/research ethics committees of each participating institution. All subjects or their legal representatives provided written informed consent prior to enrolment.
Statistical Analysis
We investigated the effect of cumulative exposures by counting the number of exposures each subject received up to each study day and calculating the maximum AKI stage according to KDIGO criteria from enrollment up to each study day. We examined if there was an association between the cumulative number of exposures up to each day and the maximum AKI stage up to that day using test for linear-by-linear association. We also examined the association between specific types of exposure and the development of any AKI or AKI stage 2–3. For this analysis, we only included patients who 1) did not have AKI stage 2–3 prior to the exposure; 2) had biomarker data between –1 and 5 days from the start of exposure; and 3) had serum creatinine and/or urine output data available within 3 days of exposure. Supplemental Table S1 (Supplemental Digital Content 1, http://links.lww.com/CCM/D36) lists the exclusions for each exposure. A generalized linear mixed model analysis was performed to test the significance of the association.
In patients who did not have AKI prior to any of the five selected exposures, we studied the kinetics of urinary [TIMP-2]•[IGFBP7] concentrations relative to each of the exposures separately. The null hypothesis for each exposure was that on each day up to 5 days after the first exposure, the median urinary [TIMP-2]•[IGFBP7] value within each AKI group (stage 0, 1, and 2–3) was less than 0.3 (ng/ml)2/1,000 (i.e., low risk for progression). For an individual subject, multiple [TIMP-2]•[IGFBP7] values may be determined within the same time interval (such as within 12, 24, or 36 hours from exposure, or on a calendar day following exposure). To account for repeated measurements of [TIMP-2]•[IGFBP7] results from the same subject within a time interval, the estimates of median and its interquartile range (IQR) were obtained by stratified bootstrap (stratified by AKI groups) (22). The p values for the tests of the null hypothesis that the median [TIMP-2]•[IGFBP7] values were below 0.3 (ng/ml)2/1,000, versus the alternative hypothesis of median above 0.3, were calculated using bootstrap stratified by AKI status.
We analyzed the urinary [TIMP-2]•[IGFBP7] kinetics in association with a specific exposure without taking into account other concomitant or sequential exposures. We identified the earliest time a patient was exposed to each insult and defined the maximum AKI stage from the earliest time to 3 days after the first exposure.
Statistical analyses were performed using SAS 9.3 and R3.0 (23). Two-sided p-values less than 0.05 and one-sided p-values less than 0.025 were considered statistically significant.
RESULTS
Patient Population
The Sapphire cohort consisted of 723 patients (Fig. 1); their median age was 64 years (Table 1). During the study period, 679 patients (94%) had at least one of the selected exposures (vancomycin, NSAIDs, piperacillin/tazobactam, IV contrast or major surgery), either alone or in combination. More than one exposure occurred in 503 patients (70%), three or more exposures in 250 (35%), and 74 patients (10%) were exposed to four. Six patients (0.8%) were actually exposed to all five renal insults.
Figure 1.
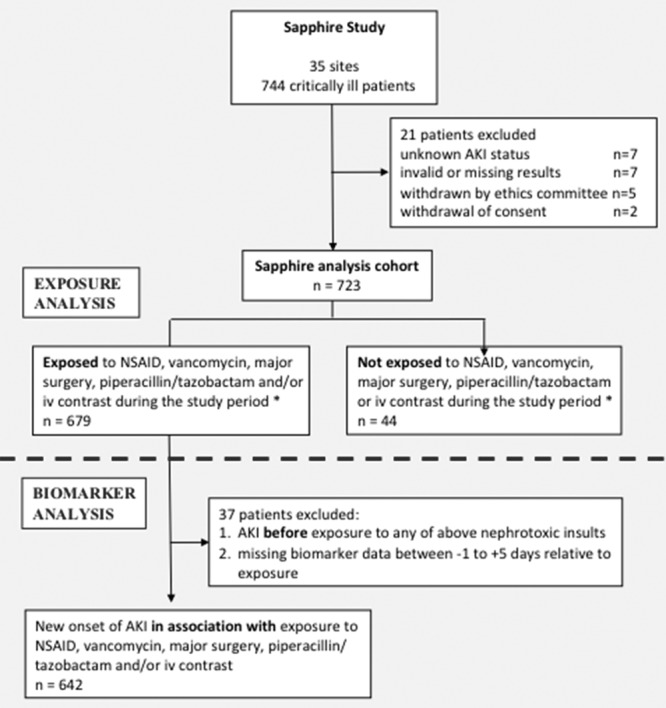
Patient flow diagram. AKI = acute kidney injury, NSAID = nonsteroidal anti-inflammatory drug. *Exposures occurred in hospital between 5 days prior to enrollment through 7 days after enrollment.
TABLE 1.
Baseline Demographics of Patients With or Without At least One of Five Selected Exposures
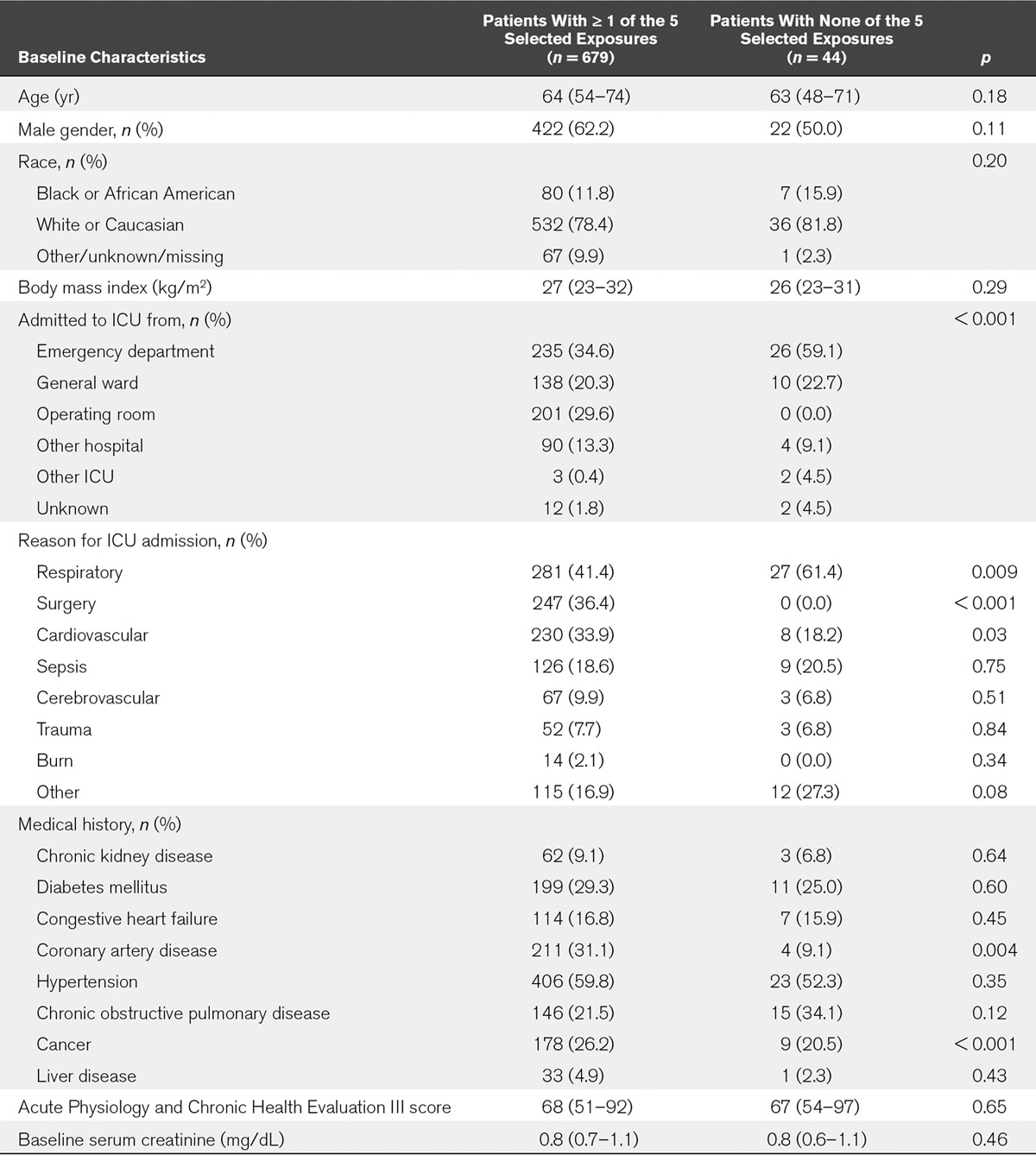
Forty-four patients were not exposed to any of the five insults of interest (Fig. 1 and Table 1). They were nonsurgical patients who were predominantly admitted from the Emergency Department or general ward. Their primary reason for admission to the ICU was respiratory failure (61%). Overall, patients with or without exposures had similar baseline characteristics (Table 1).
Prevalence of Exposures With Renal Injury Potential
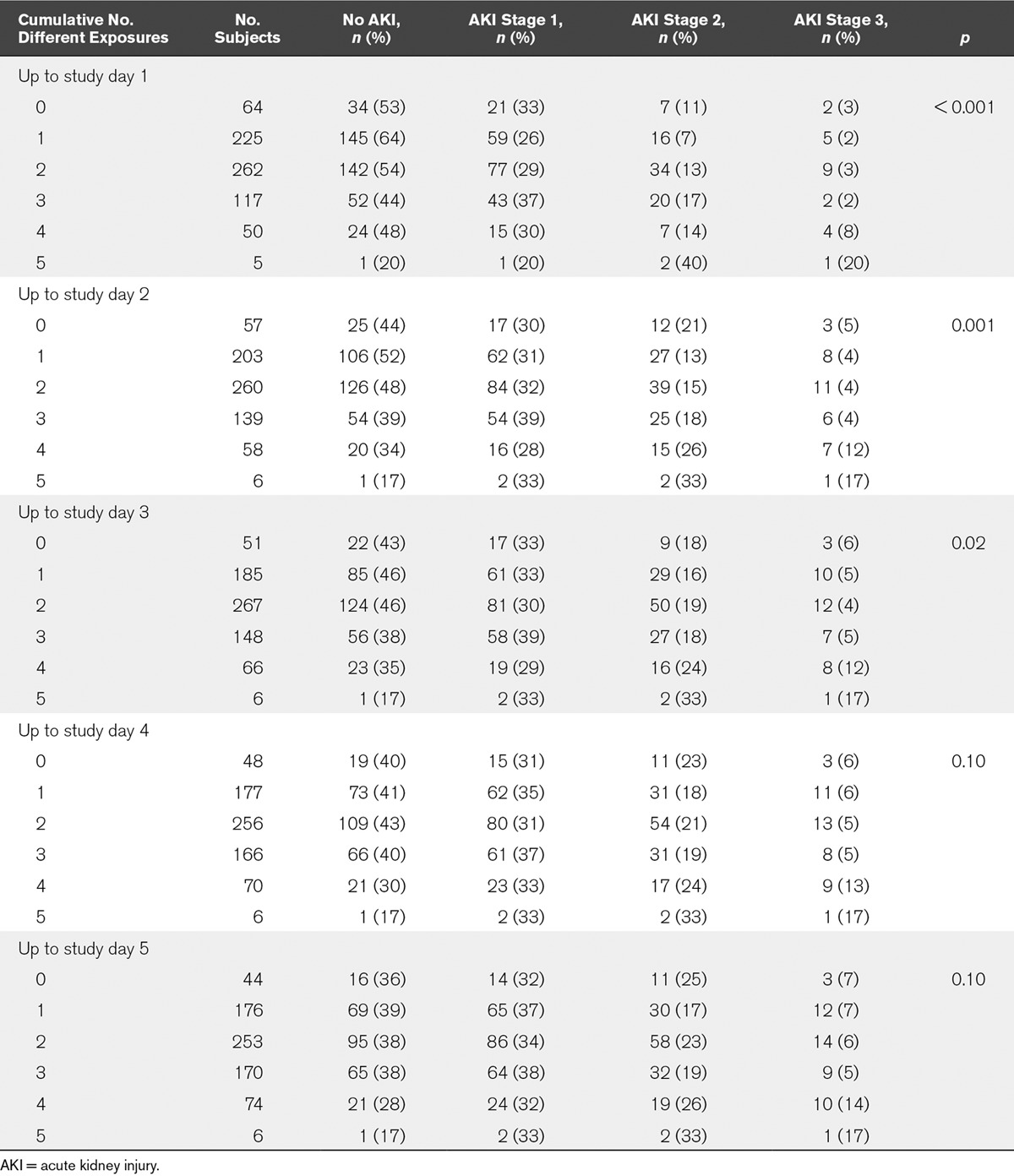
Exposure to substances or events that may be harmful to the kidney was very common (Tables 2 and 3). In total, 659 patients (91.1%) had at least one exposure up to the first study day, and 666 patients (92.1%) had one or more exposures up to study day 2 (Table 2). The median time of exposure relative to ICU admission was 0 days (IQR, –0.3 to 1.0). There was a significant association between cumulative number of exposures up to study day 3 and risk of AKI (p = 0.02) but not beyond day 3 (Table 2).
TABLE 2.
Development of Acute Kidney Injury by Cumulative Number of Different Exposures (n = 723)
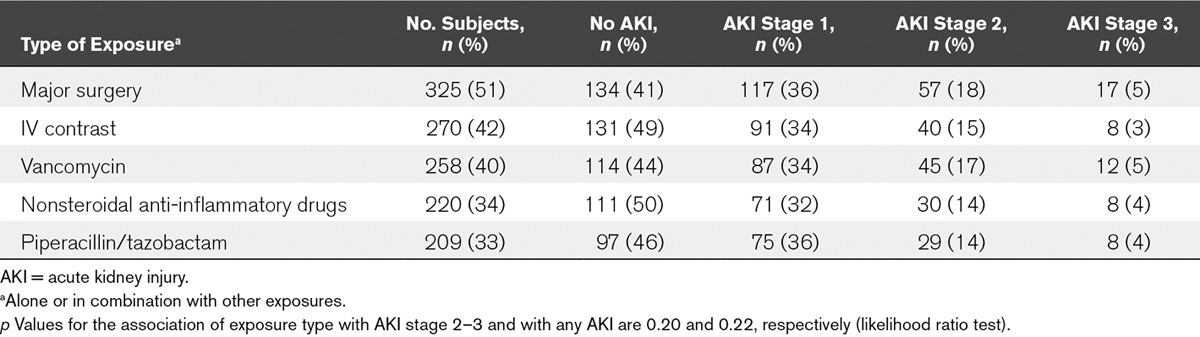
After exclusion of patients who had AKI before any of the five exposures, we found that 325 (51%) had had major surgery, 270 (42%) had received IV radiocontrast, 258 (40%) had vancomycin, 220 (34%) had received at least one dose of a NSAID, and 209 patients (33%) had had piperacillin/tazobactam (Table 3). There was no statistically significant association between type of exposure and AKI stage 2–3 (p = 0.20) or any stage of AKI (p = 0.22).
TABLE 3.
Development of New Acute Kidney Injury in Association With Different Types of Exposures
Biomarker Kinetics
Analysis of biomarker kinetics was performed in 642 patients together with corresponding serum creatinine kinetics (Figs. 1 and 2A-E; and Supplemental Fig. S1a-e, Supplemental Digital Content 1, http://links.lww.com/CCM/D36).
Biomarker Kinetics Postmajor Surgery
Three hundred twenty-five patients had major surgery (Fig. 2A; and Supplemental Table S2a, Supplemental Digital Content 1, http://links.lww.com/CCM/D36). Urinary [TIMP-2]•[IGFBP7] in those who developed AKI stage 2–3 (n = 74 [23%]) were significantly elevated from the day of surgery to 48 hours later. The median serum creatinine results during this period were between 1.0–1.1 mg/dL (Supplemental Fig. S1a, Supplemental Digital Content 1, http://links.lww.com/CCM/D36). In addition, for patients with AKI stage 1, urinary [TIMP-2]•[IGFBP7] was significantly elevated at 24–48 hours postoperatively (Fig. 2A). By contrast, there was no significant elevation in urinary [TIMP-2]•[IGFBP7] for patients who did not develop AKI.
Figure 2.
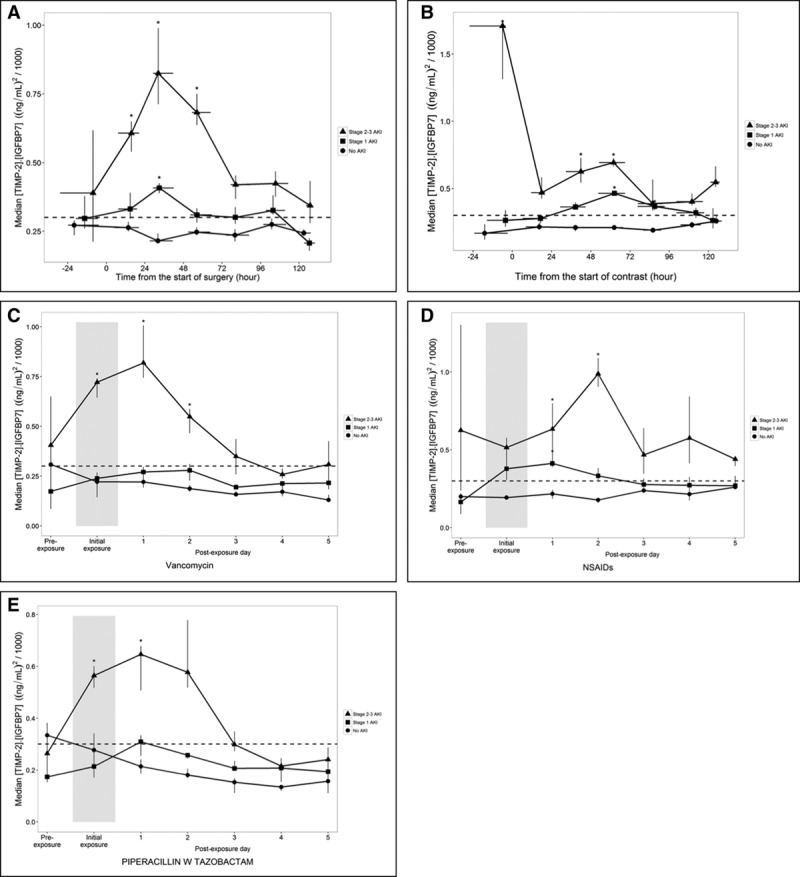
Biomarker kinetics in association with specific exposures. Time course of urinary tissue inhibition of metalloproteinase [TIMP-2]•insulin-like growth factor binding protein [IGFBP7] concentrations relative to the time or day of exposure by acute kidney injury (AKI) stage for patients exposed to major surgery (A), IV contrast (B), vancomycin (C), NSAIDs (D), or piperacillin/tazobactam (E). Symbols show median urinary [TIMP-2]•[IGFBP7] concentrations for patients who had no AKI (circles), AKI stage 1 (squares), and AKI stage 2–3 (triangles) within 3 days postexposure. Vertical and horizontal lines through the symbols show the interquartile range of bootstrap medians for the [TIMP-2]•[IGFBP7] concentrations and the time from exposure, respectively. Median urinary [TIMP-2]•[IGFBP7] concentrations are shown by day for drug exposures because only the day and not the time of exposure was recorded. The width of the shaded area indicates the day of the first dose of each drug.
Biomarker Kinetics Following IV Radiocontrast Exposure
Among 270 patients exposed to IV radiocontrast, 48 patients (18%) developed AKI stage 2–3 (Table 3). They had significantly higher urinary [TIMP-2]•[IGFBP7] from the day of contrast administration up to 72 hours later (Fig. 2B; and Supplemental Table S2b, Supplemental Digital Content 1, http://links.lww.com/CCM/D36). Unlike the biomarker kinetics described following the four other exposures, there was no typical rise and fall of urinary [TIMP-2]•[IGFBP7] around radiocontrast exposure. Median serum creatinine results were not significantly elevated from their baseline values (Supplemental Fig. S1b, Supplemental Digital Content 1, http://links.lww.com/CCM/D36). In patients who did not develop AKI, there was no significant elevation in urinary [TIMP-2]•[IGFBP7].
Biomarker Kinetics in Association With Exposure to Vancomycin
Urinary [TIMP-2]•[IGFBP7] for the 57 of 258 patients (22%) who developed AKI stage 2–3 were significantly elevated on the day of first dose of vancomycin and the following 2 days with a peak on day 1 (Fig. 2C; and Supplemental Table S2c, Supplemental Digital Content 1, http://links.lww.com/CCM/D36). There was no significant elevation in urinary [TIMP-2]•[IGFBP7] results in patients without AKI or those with AKI stage 1 (Fig. 2C).
Biomarker Kinetics in Association With Exposure to NSAIDs
Among 220 patients who received at least one dose of NSAID, urinary [TIMP-2]•[IGFBP7] concentrations were significantly elevated on day 1 and 2 around the first dose in those who developed AKI stage 2–3 (n = 38 [17%]) (Fig. 2D; and Supplemental Table S2d, Supplemental Digital Content 1, http://links.lww.com/CCM/D36). On both days, the median serum creatinine results were not significantly different from their baseline values (Supplemental Fig. S1d, Supplemental Digital Content 1, http://links.lww.com/CCM/D36). In patients with AKI stage 1, urinary [TIMP-2]•[IGFBP7] were also significantly elevated around NSAID exposure but only on day 1 (Fig. 2D). There was no significant elevation in urinary [TIMP-2]•[IGFBP7] in patients who did not develop AKI.
Biomarker Kinetics in Association With Piperacillin/Tazobactam Administration
Of 209 patients who received at least one dose of piperacillin/tazobactam, 37 patients (18%) developed AKI stage 2–3. They had significantly elevated urinary [TIMP-2]•[IGFBP7] on the first day of exposure and the following day (Fig. 2E; and Supplemental Table S2e, Supplemental Digital Content 1, http://links.lww.com/CCM/D36). There was no significant elevation in [TIMP-2]•[IGFBP7] in patients with AKI stage 1 or no AKI.
Association Between Biomarker Rise and Severity of Illness on the Day of Exposure
In patients who developed AKI 2–3, urinary [TIMP-2]•[IGFBP7] concentration was already elevated on the day of exposure to vancomycin, piperacillin/tazobactam, and contrast. However, the exact time of vancomycin and piperacillin/tazobactam administration was not recorded, and therefore, it was not possible to determine whether the increase in urinary [TIMP-2]•[IGFBP7] occurred after the exposure or was already present at time of exposure.
To further investigate any potential causes of [TIMP-2]•[IGFBP7] elevations prior to exposures, as seen with IV contrast, we compared the frequency of concomitant exposures and severity of illness by AKI stage. There were no statistically significant differences in concomitant exposures or APACHE III scores between patients with different stages of AKI. However, although not reaching statistical significance (p = 0.06), higher APACHE III scores were observed in patients with AKI 2–3 in the context of IV contrast administration (median APACHE III score, 95.5 [IQR, 56.5–108.2]) compared with those with AKI stage 1 (median 51 [IQR, 46.5–65.5]) and patients without AKI (median 60 [IQR, 46.25–73.7]), which may have contributed to the release of biomarkers.
DISCUSSION
Our analysis confirms that critically ill patients are frequently exposed to insults that may be harmful to the kidney. More than 90% of patients had at least one potential renal insult immediately before admission or up to the first study day. Cumulative exposures posed a significant additive risk for AKI. In patients who developed AKI stage 2–3, urinary [TIMP-2]•[IGFBP7] concentrations increased on the day of exposure and exhibited a characteristic rise and fall around most exposures. The exception appeared to be radiocontrast where the characteristic biomarker rise was not seen but instead high levels were already present on the day of exposure in those with AKI. Importantly, in patients who did not develop AKI, there was no significant biomarker rise.
Drug-induced nephrotoxicity is a major problem. Drugs contribute to 20% of community-acquired AKI episodes that result in hospitalization (13, 15, 19, 24). In the ICU, drug-associated AKI affects approximately 15–25% of patients (1, 14, 25). The consequences are potentially serious, with reports of dialysis dependence and/or mortality similar to other types of AKI (40–50%) (13–15).
It has been suggested that the decision to initiate a potentially nephrotoxic compound should be guided by personalized clinical decision making and close monitoring of renal function (20, 26, 27). However, serum creatinine as a marker of glomerular function is not well suited for this purpose, especially since potentially nephrotoxic drugs often exert considerable tubular damage before a significant serum creatinine rise is observed (28, 29). Novel biomarkers of renal tubular injury have been shown to predict both short- and long-term outcomes after AKI (29, 30). The U.S. Food and Drug Administration approved the use of urinary [TIMP-2]•[IGFBP7] to assess the risk for AKI in clinical practice. Our results clearly show that AKI biomarkers are dynamic and change in association with exposures to potentially nephrotoxic insults. Understanding the kinetics of AKI biomarkers is important for interpreting their changing levels over time. Our analysis represents the first such information on the kinetics of urinary [TIMP-2]•[IGFBP7]. Although not directly examined, we hypothesize that the early use of appropriate biomarkers could potentially help clinicians to identify patients with renal stress who may benefit from early interventions to prevent progression (31). The molecules IGFBP7 and TIMP-2 are known to be associated with mechanisms implicated in the pathogenesis of AKI (7, 32, 33). It has been shown that renal tubular cells enter a short period of G1 cell cycle arrest during the early phases of cell injury. It is believed that this prevents them from dividing until the damage is repaired. Markers of cell cycle arrest, such as IGFBP7 and TIMP-2, may signal that the renal epithelium has been stressed and has shut down function (7). Emlet et al (32) showed that TIMP-2 was both expressed and secreted preferentially by cells of distal tubule origin while IGFBP7 was preferentially secreted by cells of proximal tubule origin. Despite significant progress in this area, much remains unknown and work is ongoing.
Our study has certain limitations as expected from retrospective analyses. First, we evaluated only five selected insults while acknowledging that a proportion of patients may have been exposed to many other nephrotoxins. Second, we did not analyze the biomarker kinetics of patients without any of the five chosen exposures, mainly because the majority was exposed to other less frequently used nephrotoxins and therefore could not be considered as true controls. Third, we only knew the day that vancomycin, NSAIDs, and piperacillin/tazobactam were first administered but not the precise time. Our results describe the biomarker kinetics around administration of the first dose. This reflects clinical practice where patients are often exposed to different potentially nephrotoxic insults simultaneously without the exact timing of all exposures being known. Fourth, we included “major surgery” as a separate potentially injurious insult but did not differentiate between different types of surgery and specific perioperative factors. Finally, we analyzed different exposures as binary variables and did not evaluate their intensity, number of doses, mode of administration, and duration.
In conclusion, exposure to multiple nephrotoxic insults is common during critical illness and associated with an increased risk of AKI. Urinary [TIMP-2]•[IGFBP7] exhibit a characteristic rise and fall around various exposures but importantly, only in patients who ultimately develop AKI. By contrast, serum creatinine is not altered during the early hours after exposure to a renal insult. Further studies are necessary to explore whether serial biomarker measurement has a role in selecting and monitoring medications and preventing drug-induced AKI.
ACKNOWLEDGMENT
We wish to thank all investigators and research coordinators who contributed to the original SAPPHIRE study (for participating sites, see the supplemental data, Supplemental Digital Content 1, http://links.lww.com/CCM/D36).
Supplementary Material
Footnotes
For a full list of the SAPPHIRE Investigators, see the supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/D36).
Supported by Astute Medical, San Diego, CA.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Ostermann disclosed that the original Sapphire study was funded by Astute Medical. She received grant support from Fresenius Medical Care. Dr. Forni received funding from Astute Medical and Orthodox Clinical Diagnostic (lecture fees). Dr. Bagshaw received funding from Baxter Healthcare. He was also supported by a Canada Research Chair in Critical Care Nephrology. Dr. Joannidis received speaker´s fees from Astute Medical. Dr. Shi received funding from Astute Medical and disclosed that her husband also consults for Astute Medical. Dr. Kashani’s institution received funding from Astute Medical. Dr. Honore’s institution received funding from Baxter. Dr. Chawla disclosed that he is an employee of La Jolla Pharmaceutical. He also disclosed that he and his institution received funding from Astute Medical. Dr. Kellum and his institution received funding from Astute Medical. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 2015; 41:1411–1423. [DOI] [PubMed] [Google Scholar]
- 2.Lewington AJ, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int 2013; 84:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta RL, Cerdá J, Burdmann EA, et al. International Society of Nephrology’s 0 by 25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015; 385:2616–2643. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 2012; 380:756–766. [DOI] [PubMed] [Google Scholar]
- 5.Kerr M, Bedford M, Matthews B, et al. The economic impact of acute kidney injury in England. Nephrol Dial Transplant 2014; 29:1362–1368. [DOI] [PubMed] [Google Scholar]
- 6.Meersch M, Schmidt C, Hoffmeier A, et al. Erratum to: Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med 2017; 43:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013; 17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honore PM, Nguyen HB, Gong M, et al. ; Sapphire and Topaz Investigators: Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med 2016; 44:1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunnerson KJ, Shaw AD, Chawla LS, et al. ; Sapphire Topaz investigators: TIMP2•IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J Trauma Acute Care Surg 2016; 80:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoste EA, McCullough PA, Kashani K, et al. ; Sapphire Investigators: Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant 2014; 29:2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014; 189:932–939. [DOI] [PubMed] [Google Scholar]
- 12.Jia HM, Huang LF, Zheng Y, Li WX. Prognostic value of cell cycle arrest biomarkers in patients at high risk for acute kidney injury: A systematic review and meta-analysis. Nephrology (Carlton) 201722:831–837. [DOI] [PubMed] [Google Scholar]
- 13.Kane-Gill SL, Goldstein SL. Drug-induced acute kidney injury: A focus on risk assessment for prevention. Crit Care Clin 2015; 31:675–684. [DOI] [PubMed] [Google Scholar]
- 14.Mehta RL, Pascual MT, Soroko S, et al. ; Program to Improve Care in Acute Renal Disease: Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int 2004; 66:1613–1621. [DOI] [PubMed] [Google Scholar]
- 15.Wu TY, Jen MH, Bottle A, et al. Ten-year trends in hospital admissions for adverse drug reactions in England 1999-2009. J R Soc Med 2010; 103:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aliaga M, Forel JM, De Bourmont S, et al. Diagnostic yield and safety of CT scans in ICU. Intensive Care Med 2015; 41:436–443. [DOI] [PubMed] [Google Scholar]
- 17.McDonald JS, McDonald RJ, Williamson EE, et al. Erratum to: Post-contrast acute kidney injury in intensive care unit patients: A propensity score-adjusted study. Intensive Care Med 2017; 43:956. [DOI] [PubMed] [Google Scholar]
- 18.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant 2014; 29:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein SL, Mottes T, Simpson K, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 2016; 90:212–221. [DOI] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; 2:1–138. [Google Scholar]
- 21. Available at: http://www.merckmanuals.com/home/special-subjects/surgery/surgery#v830537. Accessed September 17, 2017.
- 22.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. 1993New York, Chapman & Hall/CRC Monographs on Statistics & Applied Probability. [Google Scholar]
- 23. R Development Core Team. R: A Language and Environment for Statistical Computing. 2008Vienna, Austria: The R Foundation for Statistical Computing. [Google Scholar]
- 24.Kaufman J, Dhakal M, Patel B, et al. Community-acquired acute renal failure. Am J Kidney Dis 1991; 17:191–198. [DOI] [PubMed] [Google Scholar]
- 25.Uchino S, Kellum JA, Bellomo R, et al. ; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005; 294:813–818. [DOI] [PubMed] [Google Scholar]
- 26. National Clinical Guideline Centre. Acute kidney injury: Prevention, detection and management of acute kidney injury up to the point of renal replacement therapy. Clinical guidelines, CG169. Available at: http://guidance.nice.org.uk/CG169. Accessed May 05, 2017
- 27.Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: Update 2017: Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med 2017; 43:730–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 2009; 4:1275–1283. [DOI] [PubMed] [Google Scholar]
- 29.Bonventre JV, Vaidya VS, Schmouder R, et al. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 2010; 28:436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murrary PT, Mehta RL, Shaw A, et al. Potential use of biomarkers in acute kidney injury: Report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 2014; 85:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waring WS, Moonie A. Earlier recognition of nephrotoxicity using novel biomarkers of acute kidney injury. Clin Toxicol (Phila) 2011; 49:720–728. [DOI] [PubMed] [Google Scholar]
- 32.Emlet DR, Pastor-Soler N, Marciszyn A, et al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: Differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol 2017; 312:F284–F296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng ZY, Zhou F, Kellum JA. Cross-species validation of cell cycle arrest markers for acute kidney injury in the rat during sepsis. Intensive Care Med Exp 2016; 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]


