Supplemental Digital Content is available in the text.
Keywords: advanced breast cancer, concentration–QTc modeling, ECG, palbociclib, QT interval
Abstract
The aim of this study was to assess the potential effects of palbociclib in combination with letrozole on QTc. PALOMA-2, a phase 3, randomized, double-blind, placebo-controlled trial, compared palbociclib plus letrozole with placebo plus letrozole in postmenopausal women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. The study included a QTc evaluation substudy carried out as a definitive QT interval prolongation assessment for palbociclib. Time-matched triplicate ECGs were performed at 0, 2, 4, 6, and 8 h at baseline (Day 0) and on Cycle 1 Day 14. Additional ECGs were collected from all patients for safety monitoring. The QT interval was corrected for heart rate using Fridericia’s correction (QTcF), Bazett’s correction (QTcB), and a study-specific correction factor (QTcS). In total, 666 patients were randomized 2 : 1 to palbociclib plus letrozole or placebo plus letrozole. Of these, 125 patients were enrolled in the QTc evaluation substudy. No patients in the palbociclib plus letrozole arm of the substudy (N=77) had a maximum postbaseline QTcS or QTcF value of ≥ 480 ms, or a maximum increase from clock time-matched baseline for QTcS or QTcF values of ≥ 60 ms. The upper bounds of the one-sided 95% confidence interval for the mean change from time-matched baseline for QTcS, QTcF, and QTcB at all time points and at steady-state Cmax following repeated administration of 125 mg palbociclib were less than 10 ms. Palbociclib, when administered with letrozole at the recommended therapeutic dosing regimen, did not prolong the QT interval to a clinically relevant extent.
Introduction
Certain drugs are known to cause a delay in cardiac repolarization, which can be measured as prolongation of the QT interval on an ECG 1,2. Delay in cardiac repolarization is considered undesirable because it increases the risk of cardiac arrhythmias, most notably torsades de pointes (TdP), which can lead to sudden cardiac death 3. Drugs in clinical development are recommended by the International Conference on Harmonization (ICH) guidance to be evaluated rigorously in a well-controlled, thorough QT/corrected QT (QTc) clinical study (i.e. TQT study) for their potential to prolong the QT interval/QTc to evaluate the risk–benefit ratio of cardiac arrhythmias during prolonged use 4.
A TQT study is typically carried out in healthy individuals and includes a placebo control, a positive control, and at least one dose level higher than those administered clinically to achieve supratherapeutic concentrations. However, the potential toxicity profiles associated with anticancer drugs often preclude their administration to healthy individuals, thus presenting unique challenges in the implementation of a TQT study for most anticancer drugs. When evaluating a drug effect on QTc prolongation in patients, it is often not feasible to include a placebo control to rule out the nondrug effects or a positive control to establish the sensitivity of the study 5. Furthermore, the potential toxicity of anticancer drugs may preclude evaluating their effects on QTc prolongation at a supratherapeutic dose. However, in cases where a dedicated TQT study in healthy individuals cannot be carried out, evaluation of the drug effect on QTc in the target patient population with a reduced study design at the therapeutic dose accompanied by exposure–response analysis of the concentration–QTc data can be considered an alternative approach to assess the potential drug effect on cardiac repolarization 6.
Palbociclib is a highly selective oral inhibitor of cyclin-dependent kinase 4/6 (CDK4/6) 7. Cyclin D1 and CDK4/6 are downstream of signaling pathways that lead to cellular proliferation 8. In vitro, palbociclib reduced cellular proliferation of estrogen receptor-positive breast cancer cell lines by blocking progression of the cell from G1 into the S phase of the cell cycle 9. Palbociclib is currently approved for the treatment of patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced or metastatic breast cancer in combination with an aromatase inhibitor as initial endocrine-based therapy in postmenopausal women or fulvestrant in women with disease progression following endocrine therapy 10,11 and is under clinical investigation in numerous other oncologic settings 12.
The effects of palbociclib on cardiac conduction were characterized in an in-vitro human Ether-a-Go-Go assay (hERG) and in telemetrized dog studies. The results of these assessments indicated a potential for QT prolongation at unbound palbociclib concentrations of ≥ four-fold the unbound steady-state Cmax associated with the therapeutic dose of 125 mg once daily 13. During early clinical development in phase 1 and 2 trials, the effect of palbociclib on QTc prolongation was investigated 14. An exposure–response analysis was carried out to evaluate the relationship between palbociclib concentrations and QTc changes using ECG data and blood samples for palbociclib plasma exposure concentrations obtained immediately after each ECG assessment. These blood samples were collected from 184 patients with advanced cancer who were receiving palbociclib doses ranging from 25 to 225 mg once daily in three early clinical studies. The predicted upper bound of the one-sided 95% confidence interval (CI) for the increase in QTc at the mean maximal steady-state palbociclib concentrations at 125 mg once daily was less than 10 ms, indicating a lack of clinically relevant effect on QTc prolongation 14. However, time-matched baseline ECGs were not collected in those studies and the ECG data were not assessed by a central laboratory. Hence, a more rigorous evaluation of the potential effect of palbociclib on QTc was carried out in a well-controlled substudy in the target population of patients with advanced breast cancer (ABC) at 125 mg once daily.
PALOMA-2 is a phase 3, international, multicenter, randomized, double-blind, placebo-controlled trial designed to demonstrate that the combination of palbociclib with letrozole is superior to placebo plus letrozole in prolonging progression-free survival in postmenopausal women with estrogen receptor-positive/human epidermal growth factor receptor 2-negative ABC who had not received any previous systemic therapy for their advanced/metastatic disease 15. Because of the aforementioned challenges in carrying out a typical TQT study with oncology drugs and in accordance with the ICH E14 guidelines on the alternative approaches to investigate the potential for QTc prolongation 4, this study included a QTc evaluation substudy where intensive ECG and pharmacokinetic (PK) data were collected to characterize the effect of palbociclib on QTc.
Patients and methods
Study design, patients, and treatments
Full details of the PALOMA-2 study design, patient population, key exclusion criteria, and study assessments have been published previously 15 (see Supplementary Section 1.1 in Supplemental digital content 1, http://links.lww.com/ACD/A235, which shows additional details on the study population and key exclusion criteria). Briefly, 666 patients were randomized 2 : 1 to the palbociclib plus letrozole arm or to the placebo plus letrozole arm. A subset of investigational sites in PALOMA-2 participated in the QTc evaluation substudy.
Patients assigned to the palbociclib plus letrozole arm received palbociclib 125 mg orally once daily from Day 1 to Day 21 of every 28-day cycle, followed by 7 days off treatment in combination with letrozole 2.5 mg orally once daily continuously. Those in the placebo plus letrozole arm received placebo orally from Day 1 to Day 21 of every 28-day cycle, followed by 7 days off treatment in combination with letrozole 2.5 mg orally once daily continuously.
PALOMA-2 was conducted in compliance with the ethical principles of Declaration of Helsinki and ICH Good Clinical Practice guidelines. The final protocol, any amendments, and informed consent documentation were approved by the institutional review board(s) and/or the independent ethics committee(s) at each investigational center participating in the study. All patients provided written informed consent.
ECG assessments
A centralized ECG collection system provided by Biomedical Systems (St Louis, Missouri, USA) was utilized in this study. Standardized ECG machines (Mortara ELI 150c; Mortara Instrument Inc., Milwaukee, Wisconsin, USA) with consistent software were supplied by Biomedical Systems to the study sites. All ECGs were performed using a 12-lead (with a 10-s rhythm strip) tracing.
For intensive ECG assessment, triplicate (recorded ∼2 min apart, but within 10 min) ECGs were obtained at 0, 2, 4, 6, and 8 h on the day preceding the start of blinded study treatment (Day 0, baseline) from the patients participating in the QTc evaluation substudy. On Cycle 1 Day 14 (C1D14±2 days), when palbociclib concentrations would have achieved steady state, triplicate ECGs were collected at time points clock time-matched to the corresponding baseline ECG assessments on Day 0 (±35 min). Dosing of blinded study treatments (palbociclib/placebo) with letrozole was to occur following the collection of time ‘0’ triplicate ECGs and the associated PK sample on C1D14.
To assess the safety of palbociclib, triplicate ECGs were obtained at 0 h (predose) on Day 1 of Cycle 1 and Day 14 of Cycles 1 and 2, then on Day 1 of Cycles 4, 7, and 10 from all patients in the study. ECGs beyond Cycle 10 were performed as indicated clinically.
All ECGs had to be obtained after a fast of at least 1 h and when scheduled at the same time as PK blood draws, the ECGs had to be performed immediately before the respective sampling times. All ECG tracings were sent to a central laboratory for blinded manual adjudication (e.g. computer-assisted, with manual over-read when appropriate), and the resulting measurements were used as the data inputs for the planned QTc evaluations. The ECG measurements included the PR interval, the QT interval, the RR interval, and the QRS complex.
Pharmacokinetic assessments
Blood samples were collected from all participating patients in the QTc evaluation substudy for PK assessments of palbociclib on C1D14 at predose (0 h) and at 2, 4, 6, and 8 h postdose. All PK samples were to be collected immediately after triplicate ECGs were obtained. Plasma concentrations of palbociclib were determined using high-performance liquid chromatography tandem mass spectrometry as described previously 16. The linearity of the calibration curve for palbociclib was in the range of 1–250 ng/ml and the lower limit of quantification for the palbociclib assay was 1.00 ng/ml.
Statistical analysis
Sample size determination
Sample size determination for the QTc evaluation substudy was based on a noninferiority hypothesis testing framework. To establish noninferiority between postbaseline and baseline (Day 0) (∆QTc) at all five QTc time points on C1D14 with 90% power, ∼60 patients were to be included for QTc evaluation to ensure 40 evaluable patients in the palbociclib plus letrozole treatment arm (2 : 1 randomization) of the QTc evaluation substudy. The test was based on a one-sided t-test for the paired ∆QTc mean difference with a significance level of 0.05. The difference in means between ∆QTc under the alternative hypothesis was 10 ms, assuming a noninferiority margin of 20 ms and the standard deviation of the paired differences equal to 16 ms on the basis of QTc data from PALOMA-1 17. If the upper bounds of one-sided 95% CI of ∆QTc for all five QTc postbaseline time points were less than 20 ms, the postbaseline QTc’s were to be considered noninferior to baseline, and the effect of the treatment of palbociclib plus letrozole on QTc was to be deemed not clinically relevant.
Pharmacokinetic analysis
For patients in the QTc evaluation substudy, PK parameters [maximum observed concentration (Cmax), time of Cmax (tmax), area under the curve from 0 to 24 h (AUC0–24), and oral clearance (CL/F)] were calculated from the PK samples collected on C1D14 using electronic noncompartmental analysis, version 2.2.4 (Pfizer Inc., Groton, Connecticut, USA). The predose plasma concentration was used as both the 0 and 24 h values for the calculation of AUC0–24. All PK parameters presented here were calculated using PK samples that fulfilled the dose-compliance criteria (see Supplementary Section 1.2 in Supplemental digital content 1, http://links.lww.com/ACD/A235, describing dose-compliance criteria).
ECG summaries
In this study, separate analyses were carried out to summarize the ECG data for patients in the QTc evaluation substudy (QTc evaluation population) and all treated patients with ECG data (safety-analysis population; see Supplementary Section 1.3 in Supplemental digital content 1, http://links.lww.com/ACD/A235, which defines the analysis populations). The average (arithmetic mean) of triplicate ECG measurements at each time point for each patient was used for all summary statistics, data presentations, and analyses. If one or two of the triplicate ECG measurements were missing, the average of the remaining two measurements or the single measurement was used in the analysis. To diminish the dependence of the QT interval on heart rate, three correction methods were evaluated including the Bazett’s [QT interval corrected for heart rate using Bazett’s formula (QTcB=QT/RR0.5)], Fridericia’s (QTcF=QT/RR0.33), and a study-specific correction method (QTcS=QT/RRS, where S is the slope of linear regression between unaveraged singlet values of the natural log of QT and RR intervals). QTcF was chosen prospectively as the primary endpoint for QTc analysis.
In the QTc evaluation population, for each patient at each time point (0, 2, 4, 6, and 8 h), the ΔQTc was calculated by subtraction of the time-matched baseline value (collected on Day 0) at a particular time point from the appropriately matched postbaseline value (collected on C1D14). Summary statistics of maximum postbaseline QTc (QTcF, QTcB, and QTcS) values and maximum change from baseline values for ECG parameters (QTcF, QTcB, QTcS, PR interval, and QRS complex) were calculated separately for each treatment arm. The categories used for the frequency distribution were <450, 450 to <480, 480 to <500, and ≥500 ms for QTc; <30, 30 to <60, and ≥60 ms for ΔQTc; ≥50% PR interval changes from baseline if the absolute baseline value was <200 ms and ≥25% PR interval changes from baseline if the absolute baseline value was ≥200 ms; ≥50% QRS complex changes from baseline if the absolute baseline value was less than 100 ms; and ≥ 25% QRS complex changes from baseline if the absolute baseline value was ≥ 100 ms. A random-effects model with the nominal time point (including visit and treatment group) as a fixed effect and the patient as a random effect was used to estimate the mean change in ECG data from clock time-matched baseline at each postbaseline nominal time point. For each ECG parameter, the point estimates of the least squares (LS) mean changes from baseline at all five time points and their two-sided 90% CI were summarized, and the resulting data for QTc parameters were tabulated and displayed graphically.
In the safety-analysis population, the most recent triplicate ECG assessment collected before the first dose of study medication was defined as the baseline. For each patient at each time point, the ΔQTc was calculated by subtraction of the baseline value from the postbaseline values. The same categories used for the QTc evaluation population were used to summarize the ECG data in this population.
Exposure–response analysis
The dataset used for assessing the concentration–QTc/RR relationships included the arithmetic mean of triplicate ECG measurements and the corresponding palbociclib concentrations from PK samples collected no more than 1 h apart at each time point in the QTc evaluation population. For baseline ECG measurements collected on Day 0, the corresponding palbociclib concentrations were set to 0 and included in the analysis. Observations with missing ECG or covariate data were not imputed.
A stepwise approach was used to assess the relationship between palbociclib concentrations and QTc. Because the QT interval is dependent on heart rate, the effect of palbociclib on the RR interval was evaluated in the QTc evaluation population before correcting the QT interval. If no correlation existed between the palbociclib concentration and the RR interval, a two-stage analysis was to be carried out where the appropriate correction method (QTcB, QTcF, or QTcS) that best removes the correlation between QT and RR intervals was first determined, followed by characterization of the relationship between the palbociclib concentration and the QTc. If a correlation existed between the palbociclib concentration and the RR interval, the relationship between drug concentration and the QT interval was to be characterized by a one-stage approach where the effect of drug on both RR and QT intervals was ascertained simultaneously.
A linear mixed-effects model was used to assess the relationships of the RR interval and QTc with the palbociclib concentration. The mean absolute values of triplicate RR and QTc intervals, rather than change from baseline, were used in the analyses. Nominal time was included as a factor variable on the intercept to remove the potential effect of circadian rhythm on the RR interval and QTc as shown in the following model equations:
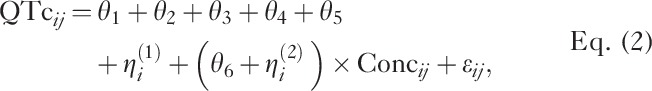 |
where j indexes the measurement time for the ith patient. The intercept parameters θ1, θ2, θ3, θ4, and θ5 represent the mean RR interval or QTc in the absence of drug (Conc=0 for baseline data) at nominal collection times 0, 2, 4, 6, and 8 h, respectively; θ6 represents the population mean slope; ηi(1) and ηi(2) represent patient-specific random effects, which were assumed to be normally distributed with mean 0 and variance–covariance matrix Ω and εij represents the residual random variable with mean 0 and variance σ2.
All model developments, diagnostics including graphical analysis, preprocessing and postprocessing of data were performed using R (version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria). The adequacy of the models developed was assessed by generating diagnostic and goodness-of-fit plots. A visual predictive check (VPC) for the final model was generated to evaluate whether the model provided an accurate description of the data (see Supplementary Section 1.4 in Supplemental digital content 1, http://links.lww.com/ACD/A235, showing the model diagnostics for exposure–response analysis).
Results
Patient disposition and demographics
A total of 666 patients were randomized in a 2 : 1 ratio to palbociclib plus letrozole (444 patients) or placebo plus letrozole (222 patients). The baseline demographic characteristics were comparable between the treatment groups (Table 1). The mean age of the participants was 62 years (range: 30–89 years) in the palbociclib plus letrozole group and 61 years (range: 28–88 years) in the placebo plus letrozole group. For safety analysis, ECGs were to be obtained from all patients during the study. The number of patients in the safety-analysis population who had both baseline and postbaseline ECGs were 443 in the palbociclib plus letrozole arm and 220 in the placebo plus letrozole arm.
Table 1.
Baseline demographic characteristics of the safety-analysis population and the QTc evaluation population
Due to the differences in the patient recruitment rate at various sites participating in the QTc evaluation substudy and the need to replace the patients who did not complete all PK collections and matched ECG assessments at both baseline and C1D14, more patients were enrolled than the initially planned 60 patients. A total of 125 patients were enrolled in the QTc evaluation substudy. Of these 125 patients, 77 were randomized to the palbociclib plus letrozole arm (76 provided postbaseline ECG data) and 48 were randomized to the placebo plus letrozole arm. The minimum number of clock time-matched baseline and C1D14 ECG pairs available at each time point in the palbociclib plus letrozole arm and placebo plus letrozole arm were 70 and 46, respectively. All 76 patients in the palbociclib plus letrozole arm who provided postbaseline assessments received daily 125 mg doses continuously up to the QTc assessment day. Baseline demographic characteristics were generally similar between the palbociclib plus letrozole group in the safety-analysis population and the QTc evaluation population, indicating that the QTc evaluation population is representative of the safety-analysis population (Table 1).
Plasma pharmacokinetics of palbociclib
Of the 77 patients randomized to the palbociclib plus letrozole arm in the QTc evaluation substudy, a total of 70 patients provided palbociclib concentration data and there were a minimum of 41 patients contributing to each of the PK parameters. Following oral doses of palbociclib 125 mg once daily with letrozole 2.5 mg once daily, palbociclib steady-state geometric mean AUC0–24 and Cmax were 1992 ng·h/ml and 110.4 ng/ml, respectively. The palbociclib geometric mean apparent CL/F at steady state was 62.71 L/h and the median tmax was 5.83 h (range: 1.87–8.18 h). The arithmetic mean and the median Cmax values (116.6 and 117 ng/ml, respectively) from this analysis were used to predict the mean drug-induced changes in QTc.
ECG analysis results
ECG substudy analysis (QTc evaluation population)
QTcS provided the best correction for the effect of heart rate on the QT interval for the QTc evaluation population, followed by QTcF and QTcB (see Supplementary Section 2.1 in Supplemental digital content 2, http://links.lww.com/ACD/A236, and Supplementary Fig. 1 in Supplemental digital content 3, http://links.lww.com/ACD/A237, which shows the relationship between QTc vs. RR intervals in the QTc evaluation population). QTcS, along with QTcF (the prespecified primary endpoint), were used for QTc analysis data interpretation and conclusion. The results of the QTcB analysis were included for completeness.
The results from the random-effects models used to estimate the mean change in ECG parameters from clock time-matched baseline at each postbaseline nominal time point are summarized in Table 2. The LS mean changes from time-matched baseline across ECG assessment time points are shown in Fig. 1. For treatment with palbociclib plus letrozole, LS mean changes from time-matched baseline for QTcS and QTcF ranged from 0.80 to 4.57 ms on C1D14, with the largest value reported at 6 h postdose for both parameters. This is consistent with the observed median tmax for palbociclib (5.83 h), indicating that there is no delay in the effect of palbociclib on the QTc, and thus there does not appear to be any evidence for hysteresis in the data. The upper bounds of the one-sided 95% CI for the LS mean change from time-matched baseline for QTcS and QTcF were less than 10 ms at all C1D14 time points (Fig. 1). For treatment with placebo plus letrozole, the LS mean changes from time-matched baseline in QTcS and QTcF ranged from 0.71 to 3.14 ms on C1D14, with the largest value reported at 8 h for QTcS and at 0 h for QTcF. The upper bounds of the one-sided 95% CIs for the LS mean change from time-matched baseline for QTcS and QTcF were less than 10 ms at all C1D14 time points monitored.
Table 2.
Time-matched change from baseline of ECG parameters by time point on Cycle 1 Day 14 for the QTc evaluation population
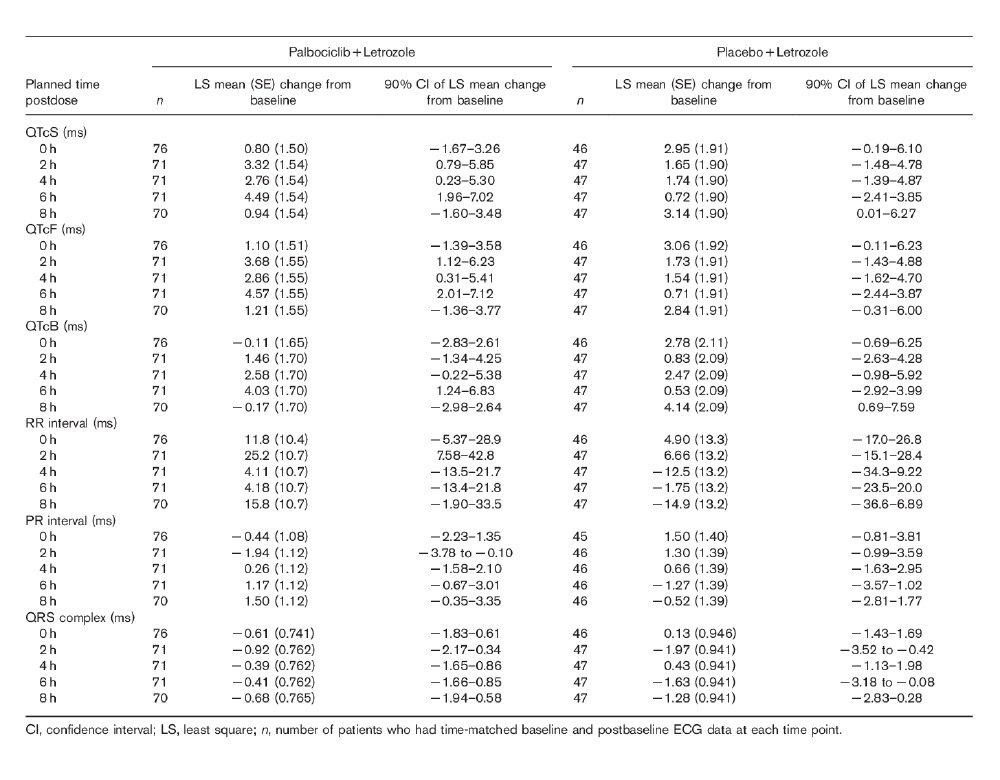
Fig. 1.
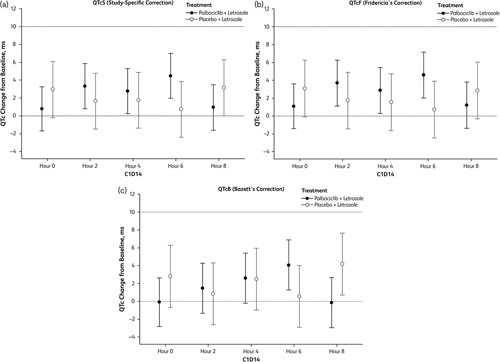
Least-squares mean (90% confidence interval) change from the time-matched baseline in QTc on C1D14 for the QTc evaluation population. (a) QTcS. (b) QTcF. (c) QTcB.
Categorical summaries of maximum postbaseline and maximum change from clock time-matched baseline ECG parameters for the QTc evaluation population are summarized in Table 3 to describe population outliers. In the QTc evaluation population, no patients in the palbociclib plus letrozole arm had a maximum postbaseline QTcS or QTcF value of ≥ 480 ms or a maximum increase from clock time-matched baseline for QTcS or QTcF values of ≥ 60 ms. In the placebo plus letrozole arm, two (4.2%) patients had a QTcS value between 480 and less than 500 ms, one (2.1%) patient had a QTcF value between 480 and less than 500 ms, and no patient had a maximum postbaseline QTcS or QTcF value of ≥ 500 ms. No patient had a maximum increase from time-matched baseline in QTcS or QTcF values of ≥ 60 ms in the placebo plus letrozole arm.
Table 3.
Categorical summary of maximum postbaseline and maximum increase from baseline of ECG parameters for the QTc evaluation population
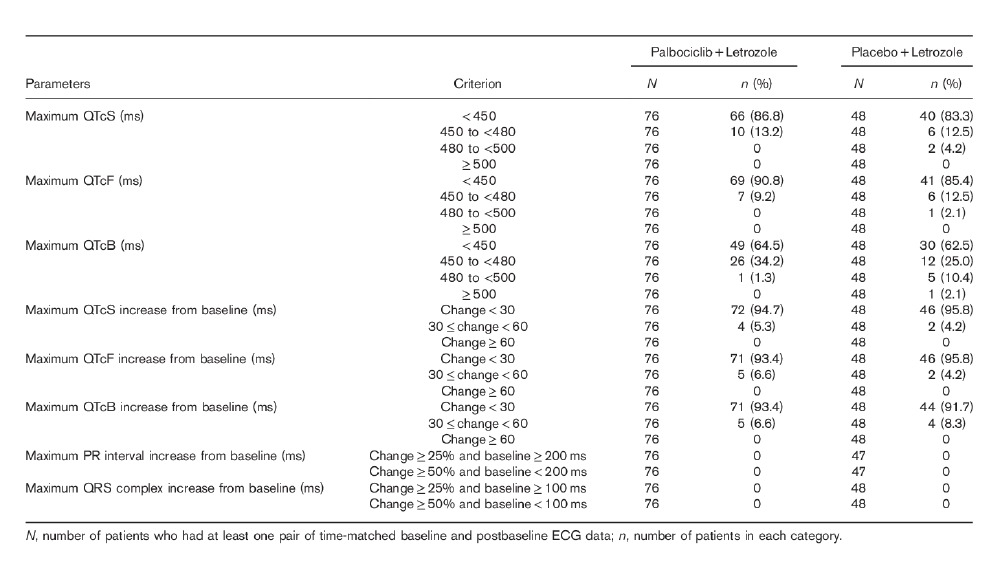
As shown in Tables 2 and 3, there were no clinically meaningful changes from clock time-matched baseline values for the PR interval, the QRS complex, or the RR interval in the QTc evaluation population on C1D14.
ECG analysis (safety-analysis population)
Results from the analysis of safety-analysis population indicate that there was no evidence of clinically significant effects of palbociclib plus letrozole on QTc, the PR interval, or the QRS complex (see Supplementary Section 2.2 in Supplemental digital content 2, http://links.lww.com/ACD/A236, showing ECG analysis for the safety-analysis population; Supplementary Fig. 2 in Supplemental digital content 3, http://links.lww.com/ACD/A237, illustrating the relationship between QTc vs. RR intervals in the safety-analysis population; and Supplementary Table 1 in Supplemental digital content 4, http://links.lww.com/ACD/A238, showing categorical summary of the maximum postbaseline and maximum increase from the baseline of ECG parameters for the safety-analysis population). Further, there was no trend in the mean changes from baseline across ECG assessment days for the treatment duration in either treatment arm for QTcS or QTcF (see Supplementary Fig. 3 in Supplemental digital content 3, http://links.lww.com/ACD/A237, showing changes from baseline in QTcS and QTcF over time in the palbociclib plus letrozole arm in the safety-analysis population).
Exposure–response analysis of ECG data
Data for the concentration–QTc/RR population included a total of 320 matched PK-ECG pairs from 70 patients in the palbociclib plus letrozole treatment arm. The average age and baseline body weight of patients in the analysis dataset providing matched PK-ECG pairs was 61.2 years (range: 36–86 years) and 75.2 kg (range: 48.1–157 kg), respectively. The average QT interval, RR interval, QTcF, and QTcS were in the range of 381–387, 795–824, 412–414, and 413–415 ms, respectively, across the nominal time points at baseline.
The relationship between the palbociclib concentration and the RR interval was analyzed using a linear mixed-effects model with nominal time as a factor variable on the intercept as shown in Eq. (1). The results indicated that palbociclib had no effect on the heart rate because the slope (95% CI) estimate cannot be ruled out from 0 [0.0442 (−0.265–0.354) ms/ng/ml]. Because no correlation was observed between the RR interval and the drug concentration, fixed correction methods were used. On the basis of visual inspection of the QTc versus RR interval plots and the slope values generated for the three correction methods, QTcS provided the best correction by decreasing the correlation between QT and RR intervals (see Supplementary Section 2.3 in Supplemental digital content 2, http://links.lww.com/ACD/A236, showing the evaluation of correction factors in exposure–response analysis). Therefore, QTcS was selected as the primary endpoint for the exposure–response analysis. Analyses results with QTcF (prespecified primary endpoint for the QTc substudy) and QTcB were also included for the purpose of comparison.
The linear mixed-effects model, with random effects on slope and intercept as well as nominal time as a factor variable on intercept, was adequate to describe the data (see Supplementary Table 2 in Supplemental digital content 4, http://links.lww.com/ACD/A238, showing the parameter estimates for the final models). The slope (95% CI) estimates of the final models were 0.0347 (0.00776–0.0616) and 0.0355 (0.00826–0.0628) ms/ng/ml for QTcS–drug and QTcF–drug concentration, respectively, suggesting a weak correlation between QTc (QTcS and QTcF) and palbociclib concentration. Diagnostic plots (not shown) and VPC plots (see Supplementary Fig. 4 in Supplemental digital content 3, http://links.lww.com/ACD/A237, which shows the VPC plot for the final concentration–QTcS model and concentration–QTcF model) for each model showed no apparent model misspecifications, indicating that the models were adequate to describe the observed data.
Prediction of drug effects on QTc
The final models were used to predict the mean and two-sided 90% CI change from baseline in QTc at the observed steady-state mean and median palbociclib Cmax of 116.6 and 117.0 ng/ml, respectively. As shown in Table 4, the upper bounds of the two-sided 90% CI (equivalent to the upper bound of the one-sided 95% CI) change from baseline in QTc (QTcS and QTcF) at the mean and median of steady-state palbociclib Cmax were all less than 10 ms, indicating that palbociclib had no clinically relevant effect on QTc prolongation at the recommended therapeutic dosing regimen.
Table 4.
Estimated effect of palbociclib on QTc at steady-state Cmax following repeated administration of 125 mg palbociclib once daily

Discussion
The QTc evaluation substudy in PALOMA-2 was carried out as the definitive study to investigate the potential effects of palbociclib on cardiac repolarization at the recommended therapeutic dose of 125 mg once daily in the target patient population. The results of QTc analysis as well as concentration–QTc modeling showed that palbociclib, when coadministered with letrozole, did not prolong QTc to a clinically relevant extent in patients with ABC. Findings from the safety assessment in the phase 3 study and the QTc substudy analysis confirmed that there were no clinically relevant changes in ECG parameters. Because letrozole is not associated with delays in cardiac repolarization, its coadministration with palbociclib did not confound the results observed in this study.
Based on the the ICH E14 guideline, the threshold level of regulatory concern for QTc prolongation is that the upper bound of the one-sided 95% CI around the largest time-matched mean effect on QTc is less than 10 ms 4, whereas the threshold level of less than 20 ms is widely accepted for oncology drugs. The random-effects analysis of the QTc data in the QTc evaluation population showed that the upper bounds of the one-sided 95% CI for the mean time-matched change from baseline for QTcF, QTcS, and QTcB were less than 10 ms at all five time points in the QTc assessment period. In the safety-analysis population, less than 1% of patients receiving palbociclib plus letrozole had a postbaseline absolute mean maximum QTcF or QTcS of ≥ 500 ms or a maximum increase from baseline of ≥ 60 ms during the entire study period, which are the commonly used thresholds for drug interruption or dose modification in a given individual. Collectively, these results clearly indicate a lack of a clinically relevant effect of palbociclib on QTc when added to letrozole.
Further evidence of the lack of QT prolongation effect of palbociclib was demonstrated by the exposure–response (concentration-QTc) modeling. The linear mixed-effects analysis was adequate to describe the relationship between palbociclib concentrations and QTc or RR interval. Incorporating nominal time as a factor variable on the intercept in the model removes the potential effect of the circadian rhythm on the QT interval. Such models with time as a factor variable provide similar accuracy of the slope estimates compared with complex biological models with circadian functions, which require more extensive ECG sampling for a precise estimation of the model parameters 18. The results from this analysis showed that palbociclib did not appear to have a concentration-dependent effect on the heart rate and the predicted upper bound of the one-sided 95% CI for the increase in QTc at the mean or median maximal steady-state palbociclib concentrations at the therapeutic dose was less than 10 ms. Taken together, these findings indicate the lack of a clinically relevant effect of palbociclib on QTc.
The incidence of breast cancer increases with age and hormone receptor-positive disease is more common in postmenopausal women (median age in PALOMA-2, 62 years) 19. Increasing age is associated with an increase in cardiac risk factors and older patients are more likely to be receiving concomitant medications that may have an effect on the QTc 19–21. When considering treatment options for these patients, it is important to consider medications that could impact QTc. Among the CDK4/6 inhibitors currently approved for treatment, it has been shown that ribociclib prolongs the QT interval in a concentration-dependent manner 22. Following the administration of ribociclib at the recommended therapeutic dose (600 mg once daily), the estimated mean increase in QTc at the mean steady-state Cmax was 22.9 ms (90% CI: 21.6–24.1). As a result, the use of ribociclib should be avoided in patients who already have or who are at a significant risk of developing QTc prolongation and with drugs known to prolong QTc 22. Palbociclib did not pose a clinical risk for QT prolongation at the recommended therapeutic dosing regimen, supporting the current prescribing information with no contraindications on concurrent cardiovascular disease and no requirement for routine ECG monitoring. These data suggest that QTc changes are not a class-effect of CDK4/6 inhibitors, but rather molecule specific.
One limitation of the current study is the lack of higher doses to achieve supratherapeutic concentrations in the patient population. As discussed, dose-limiting toxicity profiles associated with many oncology drugs preclude the administration of doses higher than the maximum tolerated dose (for palbociclib, 125 mg once daily 3 weeks on, 1 week off) in cancer patients. However, previous exposure–response analysis carried out using data from early clinical trials included doses up to 225 mg once daily administered in a 2 weeks on 1 week off schedule, which provides sufficient coverage to assess the effect of palbociclib at supratherapeutic concentrations 14. This earlier analysis also showed that the upper bound of the one-sided 95% CI for the increase in QTcS did not exceed 10 ms.
Another limitation of the study is the lack of a positive control (e.g. moxifloxacin), which is generally included in TQT studies to establish the sensitivity of an assay to detect the threshold of QTc changes of regulatory concern. However, in a double-blinded, randomized phase 3 study, it was not feasible to administer a positive control in a cross-over design to patients. Similar study designs without a positive control have been implemented in various oncology trials 23,24. Moreover, the use of a positive control, which is known to prolong the mean QTc interval, may not be tolerated in patients with advanced cancer who are already at an increased risk for cardiac-related conditions because of their poor health and age 19–21. Nevertheless, it should be emphasized that the current analysis was based on robust data collected in a randomized, double-blind study with strictly controlled conditions and standardized methods for ECG and PK data collection, which ascertains the validity of the results.
It is known that women have 10–20 ms longer QTc than men 25,26 and are at a higher risk for developing proarrhythmias caused by drugs that further prolong the QTc 27. Although the current analysis included data from only female patients, a previous exposure–response analysis carried out using data from both male and female patients indicated that sex had no effect on the palbociclib concentration–QTc relationship 14.
Conclusion
The analyses presented in this article collectively demonstrate that palbociclib, when coadministered with letrozole, does not prolong QTc to a clinically meaningful extent and QTc prolongation is not a safety concern for palbociclib at the recommended therapeutic dosing regimen.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.anti-cancerdrugs.com.
Acknowledgements
The authors thank the patients who participated in this study, their families, the study coordinators, and the support staff at the clinical sites.
This study was sponsored by Pfizer Inc. Editorial support was provided by Lauren D’Angelo, PhD, of Complete Healthcare Communications, LLC (West Chester, Pennsylvania), a CHC Group Company, and was funded by Pfizer Inc.
Conflicts of interest
C.D., A.R.-G., E.R.G., X.H., D.R.L., J.T.H., J.Z., K.D.W., and D.D.W. are employees of Pfizer Inc. H.S.R. reports research funding to the Regents of the University of California from Plexxikon, Macrogenics, OBI Pharma, Eisai, Pfizer, Novartis, Lilly, GlaxoSmithKline, Genentech, Celsion, Merck, Clovis Oncology. R.S.F. reports honoraria from Bayer, Pfizer, Bristol-Myers Squibb, Novartis, Eisai, consulting or advisory role for Pfizer, Bayer, Novartis, Bristol-Myers Squibb, Merck, and research funding from Pfizer. A.A.J. reports consulting or advisory for Pfizer, Novartis, Roche, Eli Lilly, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim. J.E. reports honoraria from Pfizer, Novartis Pharma KK, Roche-Peru, consulting or advisory role for Pfizer, Novartis Pharma KK, and serving on a speakers bureau for Pfizer, Novartis Pharma KK, Roche KK.
References
- 1.Shah RR. Cardiac repolarisation and drug regulation: assessing cardiac safety 10 years after the CPMP guidance. Drug Saf 2007; 30:1093–1110. [DOI] [PubMed] [Google Scholar]
- 2.Shah RR, Morganroth J, Shah DR. Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf 2013; 36:295–316. [DOI] [PubMed] [Google Scholar]
- 3.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart 2003; 89:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ICH Harmonised Tripartite Guideline. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. [Accessed 31 January 2017.
- 5.Liu QMR, Booth GB. Experience in QT evaluation of oncology drug products since ICH E14 guidance [abstract]. J Clin Oncol 2008; 26:2554. [Google Scholar]
- 6.Darpo B, Sarapa N, Garnett C, Benson C, Dota C, Ferber G, et al. The IQ-CSRC prospective clinical phase 1 study: ‘can early QT assessment using exposure response analysis replace the thorough QT study?’. Ann Noninvasive Electrocardiol 2014; 19:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004; 3:1427–1438. [PubMed] [Google Scholar]
- 8.Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 2016; 18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn R, Dering J, Conklin D, Kalous O, Chohen D, Desai A, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker AJ, Wedam S, Amiri-Kordestani L, Bloomquist E, Tang S, Sridhara R, et al. FDA approval of palbociclib in combination with fulvestrant for the treatment of hormone receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 2016; 22:4968–4972. [DOI] [PubMed] [Google Scholar]
- 11. Pfizer Inc. IBRANCE® (palbociclib): highlights of prescribing information. New York, NY: Pfizer Inc.; 2017. [Google Scholar]
- 12.Dhillon S. Palbociclib: first global approval. Drugs 2015; 75:543–551. [DOI] [PubMed] [Google Scholar]
- 13. Pfizer Inc. Data on file. New York, NY: Pfizer Inc.; 2017. [Google Scholar]
- 14.Zheng J, Amantea M, Wang D. Effect of palbociclib concentration on heart rate-corrected QT interval in patients with cancer [abstarct]. In: Proceedings of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium; 9–13 December 2014; San Antonio, TX. Philadelphia, PA: American Association for Cancer Research; 2015; 75(Suppl):Abstract nr P5-19-16.
- 15.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Mukai H, Naito Y, Yonemori K, Kodaira M, Tanabe Y, et al. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci 2016; 107:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16:25–35. [DOI] [PubMed] [Google Scholar]
- 18.Huh Y, Hutmacher MM. Evaluating the use of linear mixed-effect models for inference of the concentration-QTc slope estimate as a surrogate for a biological QTc model. CPT Pharmacometrics Syst Pharmacol 2015; 4:e00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Cancer Institute. Breast cancer risk in American women. Available at: https://www.cancer.gov/types/breast/risk-fact-sheet. [Accessed 21 March 2017].
- 20.Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil 2005; 12:363–368. [DOI] [PubMed] [Google Scholar]
- 21.Straus SM, Kors JA, de Bruin ML, van der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 2006; 47:362–367. [DOI] [PubMed] [Google Scholar]
- 22. Novartis Pharmaceuticals Corporation. Kisqali® (ribociclib): full prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- 23.Agrawal S, Waxman I, Lambert A, Roy A, Darbenzio R. Evaluation of the potential for QTc prolongation in patients with solid tumors receiving nivolumab. Cancer Chemother Pharmacol 2016; 77:635–641. [DOI] [PubMed] [Google Scholar]
- 24.Munster PN, Rubin EH, van Belle S, Friedman E, Patterson JK, van Dyck K, et al. A single supratherapeutic dose of vorinostat does not prolong the QTc interval in patients with advanced cancer. Clin Cancer Res 2009; 15:7077–7084. [DOI] [PubMed] [Google Scholar]
- 25.Burke JH, Ehlert FA, Kruse JT, Parker MA, Goldberger JJ, Kadish AH. Gender-specific differences in the QT interval and the effect of autonomic tone and menstrual cycle in healthy adults. Am J Cardiol 1997; 79:178–181. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg RJ, Bengtson J, Chen ZY, Anderson KM, Locati E, Levy D. Duration of the QT interval and total and cardiovascular mortality in healthy persons (The Framingham Heart Study experience). Am J Cardiol 1991; 67:55–58. [DOI] [PubMed] [Google Scholar]
- 27.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 1993; 270:2590–2597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.anti-cancerdrugs.com.


