Supplemental Digital Content is available in the text.
Keywords: aging, atherosclerosis, biopsy, leukocytes, muscle, telomere
Rationale:
Short telomere length (TL) in leukocytes is associated with atherosclerotic cardiovascular disease (ASCVD). It is unknown whether this relationship stems from having inherently short leukocyte TL (LTL) at birth or a faster LTL attrition thereafter. LTL represents TL in the highly proliferative hematopoietic system, whereas TL in skeletal muscle represents a minimally replicative tissue.
Objective:
We measured LTL and muscle TL (MTL) in the same individuals with a view to obtain comparative metrics for lifelong LTL attrition and learn about the temporal association of LTL with ASCVD.
Methods and Results:
Our Discovery Cohort comprised 259 individuals aged 63±14 years (mean±SD), undergoing surgery with (n=131) or without (n=128) clinical manifestation of ASCVD. In all subjects, MTL adjusted for muscle biopsy site (MTLA) was longer than LTL and the LTL-MTLA gap similarly widened with age in ASCVD patients and controls. Age- and sex-adjusted LTL (P=0.005), but not MTLA (P=0.90), was shorter in patients with ASCVD than controls. The TL gap between leukocytes and muscle (LTL-MTLA) was wider (P=0.0003), and the TL ratio between leukocytes and muscle (LTL/MTLA) was smaller (P=0.0001) in ASCVD than in controls. Findings were replicated in a cohort comprising 143 individuals.
Conclusions:
This first study to apply the blood-and-muscle TL model shows more pronounced LTL attrition in ASCVD patients than controls. The difference in LTL attrition was not associated with age during adulthood suggesting that increased attrition in early life is more likely to be a major explanation of the shorter LTL in ASCVD patients.
Clinical Trial Registration:
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02176941.
Although early studies focusing on the relation between telomere length (TL) and atherosclerotic cardiovascular disease (ASCVD) have yielded mixed findings, more recent research, summarized in 2 meta-analyses, has confirmed that short TL, as expressed in leukocyte TL (LTL), is associated with ASCVD and its risks.1,2 However, the biological meaning of this association remains elusive. The prevailing explanation is that indolent inflammation and oxidative stress are the twin culprits, increasing the pace of atherosclerosis in tandem with age-dependent LTL shortening in adults.3,4 Alternatively, short LTL may precede the development of ASCVD and perhaps be a determinant in the development of the disease later in life, that is, TL plays a role in causal pathways rather than being solely a risk marker. Mendelian randomization of LTL genetic risk scores supports this supposition.5–7
Editorial, see p 546
Meet the First Author, see p 534
The question then is whether the association between a shorter LTL and ASCVD in adults reflects one or more of the following scenarios: (1) adults with ASCVD have inherently short LTL, established at birth; (2) individuals who go on to develop ASCVD later in life have a higher rate of LTL shortening before adulthood; and (3) LTL attrition is faster in adults with ASCVD.
A longitudinal study of LTL dynamics (LTL at birth and its age-dependent attrition thereafter) in which individuals are followed throughout their life course from birth onward could help resolve the uncertainty about the timing in the development of the LTL gap between subjects who go on to develop ASCVD versus those who do not. However, unless biobanked samples collected over the life course are available, outcomes of a new undertaking to resolve the timing of this LTL gap would not be known for many decades and the cost would be formidable.
We have proposed, instead, a blood-and-muscle model of TL dynamics to assess the potential role of inherently short TL versus a faster age-dependent LTL attrition in the development of ASCVD in adults.8,9 The model is based on the following considerations: an individual is born with similar TLs in different tissues and cells,10–12 whereas a wide variation in TL is observed across individuals from birth onward.8,10,13–15 During extrauterine life, age-dependent TL shortening within the individual varies in proportion to the replicative activities of somatic tissues, such that TL in skeletal muscle, a minimally replicating somatic tissue, is longer than TL in leukocytes, which represent the highly proliferative hematopoietic system. Thus, a faster attrition of LTL than muscle TL (MTL) during the first 2 decades of life is the main contributor to the LTL-MTL gap.8 During adulthood, this gap may further expand. Still, if MTL serves as a proxy of LTL early in life, the gap between LTL and MTL would yield an estimate of LTL attrition up to the sampling age. We applied this blood-and-muscle model to gain further insight into the relation between TL and ASCVD.
Methods
The data of this study can be available from the corresponding author on request from a third party.
Discovery Cohort
The overall goal of the TELARTA study (Telomere in Arterial Aging) is to examine the role of telomere dynamics in arterial aging and the development of atherosclerosis. This arm of our study has focused on measuring TL in skeletal muscle and leukocytes of patients with ASCVD and controls. To this end, we enrolled men and women (older than 20 years), who were admitted for various surgical procedures and for pacemaker/defibrillator implantation to university hospitals in Nancy (n=215) and Marseille (n=44), France (details of the enrolled subjects are given in Online Figure I). Muscle biopsies were obtained from six different sites in different patients (Online Table I). All participants provided written informed consent approved by the Ethics Committee (Comité de Protection des Personnes) of Nancy, France.
Patients with ASCVD included individuals with a history of 3 types of clinically evident atherosclerosis in (1) the coronary arteries (n=62), (2) carotid and cerebral arteries (n=43), and (3) iliac, femoral, and popliteal arteries (n=74). Among the 131 ASCVD patients, 84 patients had clinical ASCVD in 1 of these 3 sites, 46 patients in 2 sites, and 1 patient in all 3 sites. The control group comprised 128 participants with no clinical manifestations of ASCVD. We also matched 79 pairs by age (±2 years) and sex, such that one member of the pair displayed ASCVD and the other did not.
We excluded subjects with a body mass index (BMI) >40 kg/m2, a glomerular filtration rate under 30 mL min−1 1.73 m−2,16 active malignancy, or history of chemotherapy/radiotherapy for cancer. We excluded 11 subjects with aortic aneurism because the atherosclerotic nature of this arterial disease is debatable.
Replication Cohort
This cohort was enrolled in 3 sites: the university hospitals of Nancy and Marseille (original sites of the TELARTA study); these sites enrolled 91 individuals. In addition, 52 individuals were enrolled at 3 Athens hospitals (Onassis Cardiac Surgery Center, Surgeon KP; Iaso General Hospital Surgeon MVG; Hippokration Hospital, Surgeon EM). The samples collected from these hospital have been collected at the Laboratory for Experimental Surgery and Surgical Research N S Christeas of the University of Athens. We relaxed the inclusion criteria for the replication cohort, including individuals that were excluded from the TELARTA, that is, BMI >40 kg/m2, glomerular filtration rate under 30 mL min−1 1.73 m−2, malignancy or history of chemotherapy/radiotherapy. Details of the enrolled subjects in the replication cohort are given in the Online Figure II. Participants enrolled in France provided written informed consent approved by the Ethics Committee (Comité de Protection des Personnes) of Nancy, France. Those enrolled in Athens provided written informed consent approved by the Ethics Committee of the University of Athens and Ethics Committee of each one of the 3 participating hospitals.
TL Measurements
TL was measured in DNA extracted by the phenol/chloroform method from peripheral blood leukocytes and skeletal muscle (≈50 mg) in the surgical field. Measurements were performed in duplicate by Southern blots of the terminal restriction fragments, as previously described.17 The measurement repeatability, as determined by the intraclass correlation coefficient, was 0.99 (95% confidence interval, 0.817–1.0) and 0.98 (95% confidence interval, 0.81–1.0) for LTL and MTL, respectively. The repeatability of the means of 2 duplicates (used in the analysis), known as the extrapolated repeatability, was 0.995 and 0.991 for LTL and MTL, respectively (equation 37 in ref 18).
Statistical Analysis
Continuous variables are presented as means±SD or mean±SE and discrete variables as frequencies or percentages. Pairwise comparisons were performed using the Mann–Whitney and χ2 tests, as appropriate. Telomere values are presented and compared with or without adjustment to age and sex. Statistical analyses were performed using the Number Crunching Statistical System (NCSS) 9 statistical software package (NCSS, Kaysville, UT) and JMP (v.9) software (Cary, NC). A paired t test was used for the intrapair comparison of matched subjects by age (±2 years) and sex. Bivariate relationships between continuous variables were determined using Pearson correlation coefficients. More complex analyses were performed using general linear models, assuming a normal error distribution for the analyses of variation in LTL and MTL and the derived telomere estimates below. Visual inspection of Q-Q plots of the residuals for LTL, MTL, and the derived telomere estimates supported the normality assumption. For the comparison of age effects between LTL and MTL, we used a general linear mixed model with donor identity introduced as a random effect to account for the statistical nonindependence of LTL and MTL within donors. ASCVD prevalence was analyzed using logistic regression (ie, accommodating the binomial error distributions). A P value <0.05 was considered significant.
In analyses with MTL as the dependent variable, we included the biopsy site as a factor, and when calculating TL odd ratios, we adjusted MTL for muscle site (ie, MTLA). The adjustment consisted of the addition of the site-specific difference between the weighted mean MTL (8.54 kb) and the site-specific least square mean MTL, estimated from a model that also included age and sex (Online Table II). Muscle site adjustment was made only for the discovery cohort. There was no effect of muscle site on MTL in the Replication Cohort.
Given that both TL measurements (LTL and MTL) may relate to ASCVD in differing ways, the following possibilities were tested: (1) only LTL is associated with ASCVD; (2) only MTLA is associated with ASCVD; (3) the absolute gap between LTL and MTLA (LTL-MTLA) is associated with ASCVD; and (4) the ratio between LTL and MTLA (LTL/MTLA) is associated with ASCVD. Models that included age and sex were fitted separately for each of these variables. Model selection was based on the Akaike Information Criterion with a correction (AICc).19 Values of P<0.05 and ΔAICc >2 were considered as statistically significant.
Results
Discovery Cohort
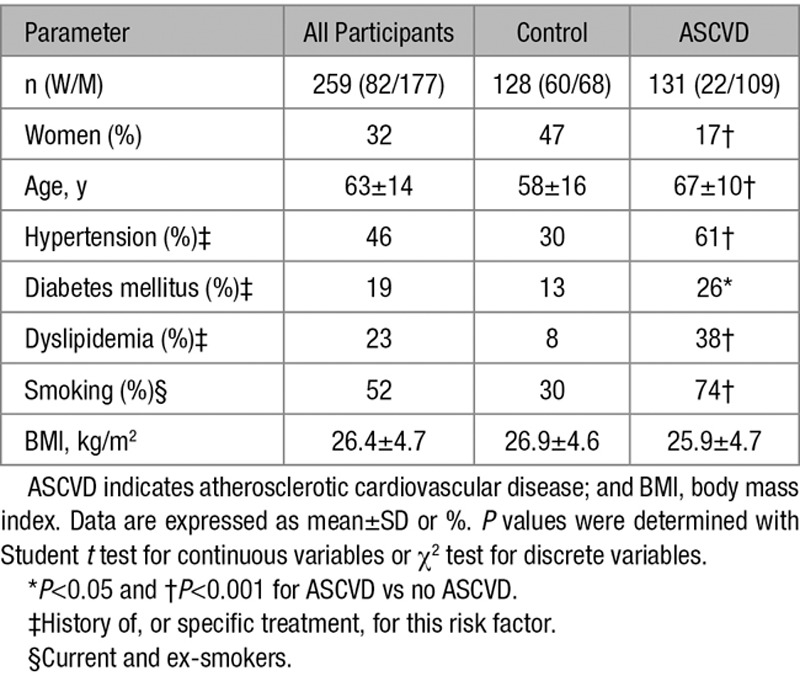
Characteristics of the cohort are summarized in Table 1. Participants with ASCVD were older, more likely to be males, and showed a higher prevalence of cardiovascular risk factors.
Table 1.
Characteristics of the Participants of the Discovery Cohort
LTL and MTLA were strongly correlated (slope±SE; 0.896±0.052; P<0.0001), but MTLA was longer (mean±SD; 8.54±0.72 kb) than LTL (6.66±0.88 kb) in all individuals (n=259) regardless of biopsy site (Figure 1). The mean difference LTL-MTLA was −1.88±0.61 kb, and the mean ratio LTL/MTLA was 77.9±7.0%.
Figure 1.
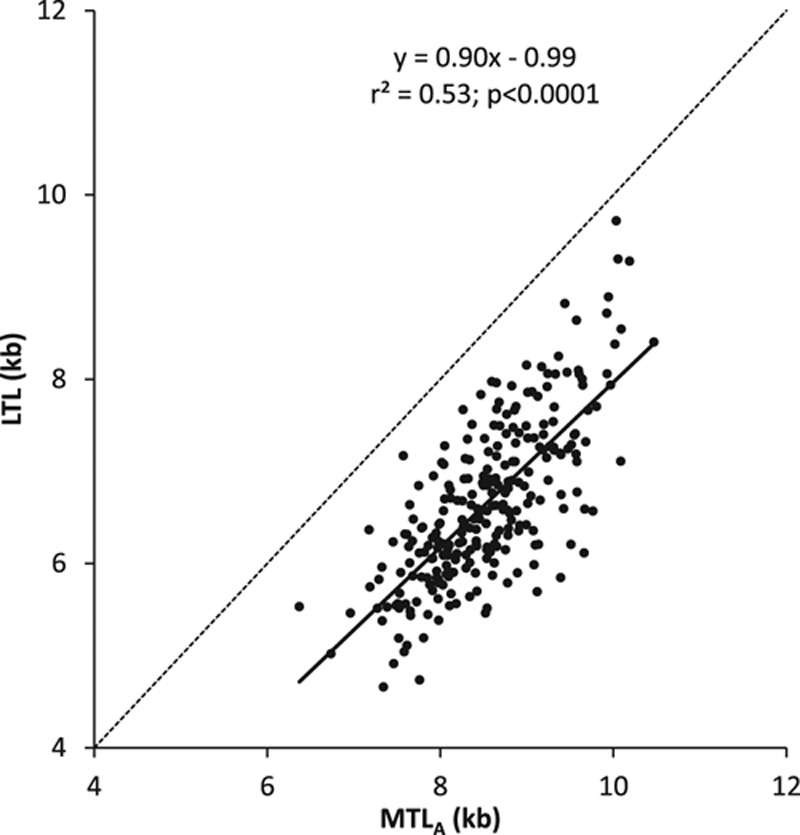
Leukocyte telomere length vs telomere length in the Discovery Cohort. Dotted line represents line of identity. LTL indicates leukocyte telomere length; MTL, muscle telomere length; and MTLA, site-adjusted muscle telomere length.
Effect of Age on TL
Both LTL and MTL shortened with age (Online Figure III). The rate of TL shortening was independent of sex in both tissues (age×sex interaction; P>0.49; Online Table II) and muscle biopsy site (age×muscle type; P>0.55). Adding age squared to the model did not significantly increase the explained variance in LTL, indicating that LTL attrition with age was approximately linear within the age range of this study. That said, there was a tendency for a slower MTL shortening with age (age-squared effect, P=0.1). The slope of the effect of age was considerably steeper for LTL than MTL (Online Table II; LTL=−30 bp/y, MTL=−12 bp/y; P<0.0001 for comparison of slopes).
Sex, Smoking, and BMI Associations With LTL and MTL
Both age-adjusted LTL and MTL were shorter in men than in women (least square means and SE from models in Online Table III: LTL, women=6.87±0.08 kb, men=6.56±0.06 kb, P=0.002; MTL, women=8.72±0.08 kb, men=8.41±0.06 kb, P=0.0008). No difference was observed in age- and sex-adjusted LTL and MTL between never-smokers versus smokers (LTL, never-smokers=6.73±0.07 kb and smokers=6.70±0.07 kb, P=0.70; MTL, never-smokers=8.53±0.07 kb and smokers=8.61±0.08 kb, P=0.30). No significant association was observed between age- and sex-adjusted BMI and LTL or MTL (BMI, kg/m2, regression slope coefficient −0.0121±0.010 kb, P=0.22, for LTL, and −0.01±0.010 kb, P=0.17 for MTL).
ASCVD and TL Dynamics
Individuals with ASCVD had 282±100 bp shorter age- and sex-adjusted LTL than those without ASCVD (t=2.83; df=267; P=0.005); this difference was independent of age and sex (interactions with ASCVD when added to the model, P>0.2). MTLA was not different in patients with versus those without ASCVD. As shown in Figure 2, the gap between LTL and MTL widened with age at a similar rate in patients with ASCVD and controls (LTL-MTLA: slope ± SE; −14.9±0.5 versus −14.6±0.3 bp/y, respectively; ASCVD×age interaction: P>0.9). Similarly, the LTL/MTLA ratio diminished at the same rate in patients with ASCVD and controls (slope %±SE; −0.19±0.05 versus −0.20±0.03%/y, respectively; ASCVD×age interaction: P>0.8). The LTL difference between individuals with and without ASCVD was significantly greater than that observed for MTLA (t=4.69; P<0.0001). Both LTL-MTLA and LTL/MTLA were significantly different in individuals with ASCVD compared with the controls (both t>3.7; P<0.001; controlling for age and sex; Figure 3).
Figure 2.
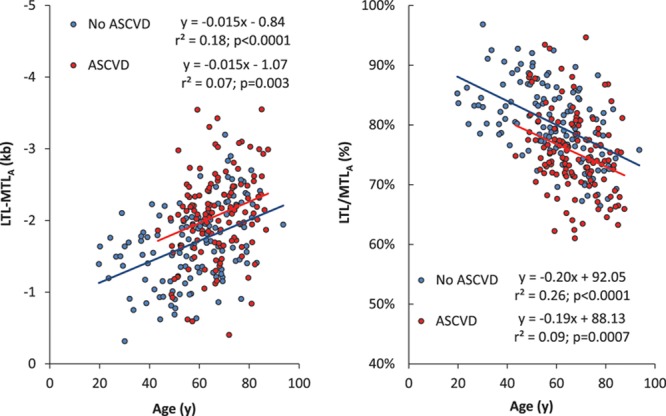
Telomere length models vs age in the Discovery Cohort. Difference between leukocyte telomere length (LTL) and site-adjusted muscle telomere length (MTLA) (left); ratio of leukocyte telomere length and site-adjusted muscle telomere length (right). ASCVD indicates atherosclerotic cardiovascular disease.
Figure 3.
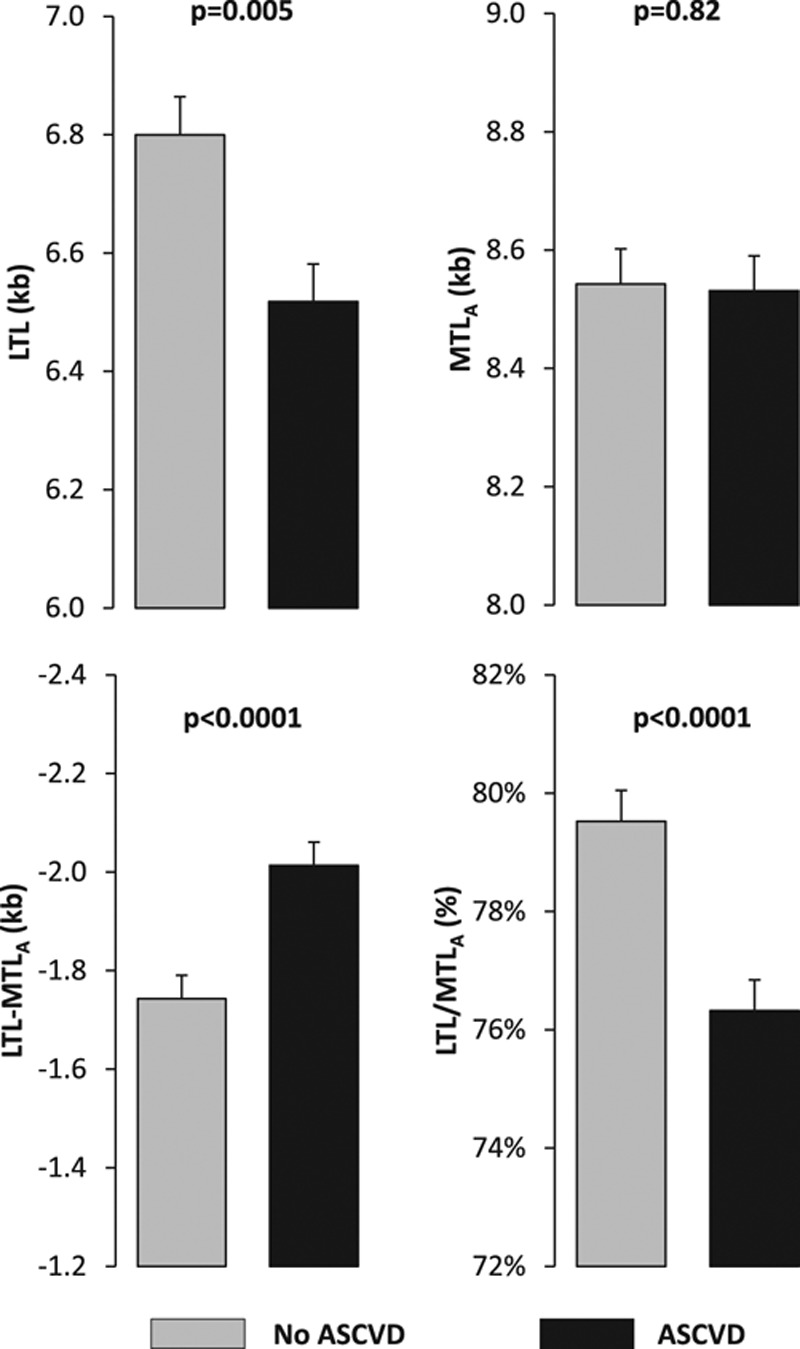
Four telomere length models in subjects of the Discovery Cohort. Values are adjusted for age and sex. ASCVD indicates atherosclerotic cardiovascular disease; LTL, leukocyte telomere length; and MTLA, site-adjusted muscle telomere length.
Best Fitting Blood-and-Muscle Model
Having examined the age- and sex-adjusted LTL-MTLA and LTL/MTLA models in individuals with and without ASCVD, we next compared the ability of the 4 TL models (LTL, MTLA, LTL-MTLA, and LTL/MTLA) to capture the association of TL with ASCVD (Table 2). In addition to age and sex, age squared was also included in the models because preliminary analysis revealed that the association between age and ASCVD prevalence in our sample plateaued after 60 years.
Table 2.
ORs for Atherosclerotic Cardiovascular Disease, Age, and Sex in the 4 TL Models in the Discovery Cohort
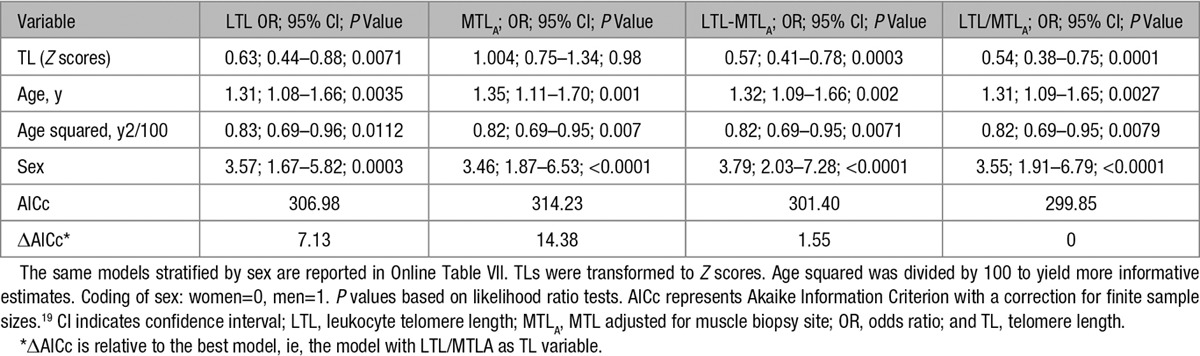
When TL was added to this model (Table 2; Z-transformed for comparability), the odds ratio (OR) was lower, that is, stronger, for LTL compared with MTLA (0.63 versus 1.00), and the AICc was significantly lower (ΔAICc=−7.25). LTL-MTLA and LTL/MTLA yielded ORs of 0.57 and 0.54, respectively, and substantially better fitting models (Table 2). Moreover, the LTL/MTLA model yielded a slightly better fitting model than LTL-MTLA model (ΔAICc=1.55; Table 2).
Additional adjustment of LTL/MTLA, the best fitting model, for diabetes mellitus status, ever smoking, dyslipidemia, BMI, and hypertension had little effect on the association (OR, 0.55; compared with OR=0.54 without these covariates in the model; see Online Table III for details).
Association of ASCVD Sites With TL Parameters
Table 3 shows the age- and sex-adjusted LTL, MTLA, LTL-MTLA, and LTL/MTLA in participants with atherosclerosis in each of the 3 ASCVD sites (coronary arteries, carotid and cerebral arteries, and IFPA [iliac, femoral, or popliteal arteries]) compared with the 128 controls. In all ASCVD groups, LTL was shorter, LTL-MTLA wider, and LTL/MTLA smaller in ASCVD patients than in controls. MTLA did not differ between ASCVD patients and controls. We also examined the TL models according to the number of ASCVD sites. This analysis showed that a higher number of ASCVD sites was associated with a shorter LTL (P<0.003), a wider LTL-MTLA, and a smaller LTL/MTLA (both P<0.001; Figure 4).
Table 3.
Four TL Models in the Discovery Cohort According to the Presence or Not of Clinical Manifestations of Atherosclerosis in Different Arteries
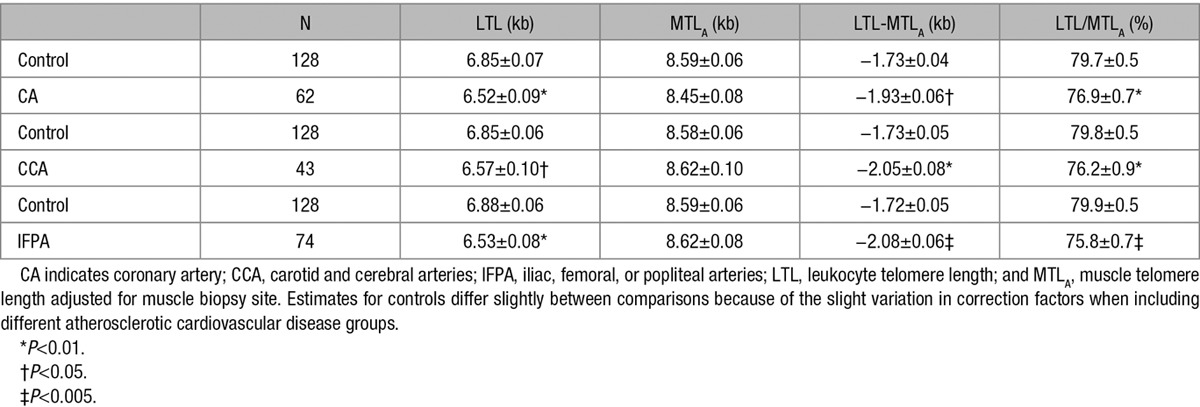
Figure 4.
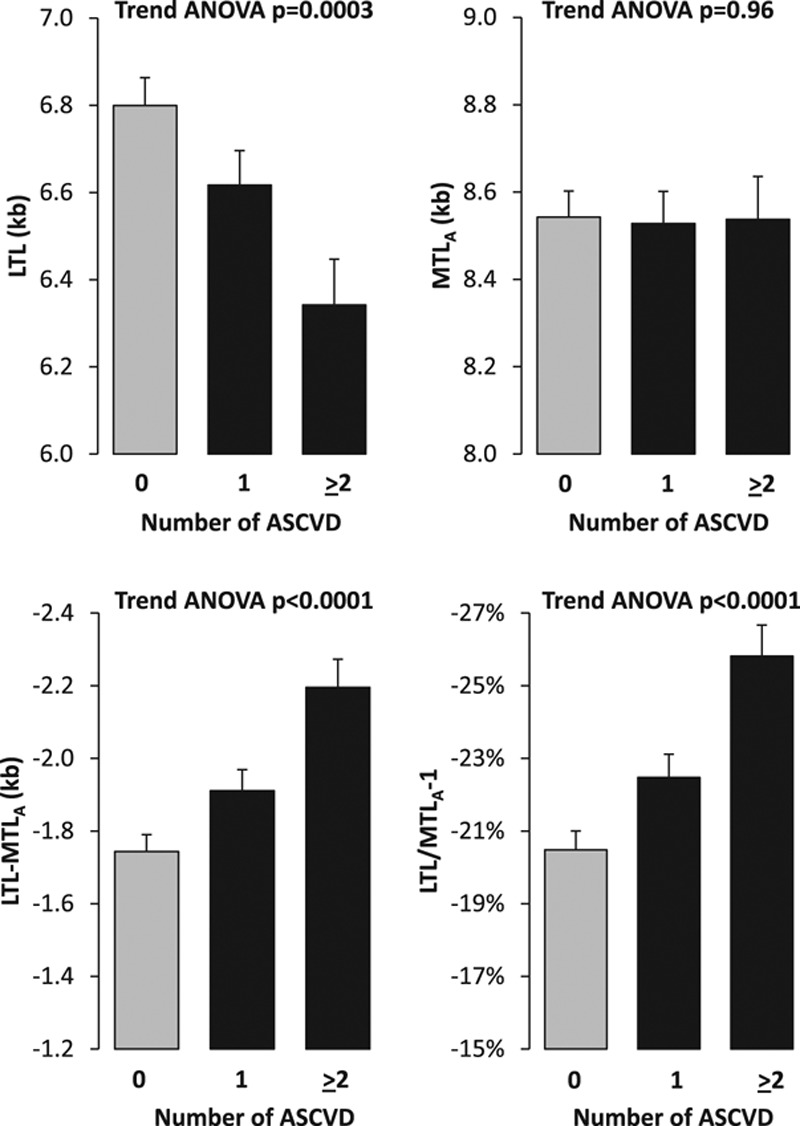
Four telomere length models vs the number of atherosclerotic sites in the Discovery Cohort. 0=Control; 1, one site; ≥2, two or more sites. Values are adjusted for age and sex. ASCVD indicates atherosclerotic cardiovascular disease; LTL, leukocyte telomere length; and MTLA, site-adjusted muscle telomere length.
Results for Matched Pairs
Because participants with ASCVD were older, more likely to be males, we also analyzed data for matched pairs. Online Table IV shows the results of the t paired analyses for the 79 age- and sex-matched pairs. Individuals with ASCVD had shorter LTL (P=0.023), wider LTL-MTLA (P=0.022), and lower LTL/MTLA (P=0.0097). MTL was not different in the 2 groups (P=0.59). Additional analyses separately in men and women of the Discovery Cohort showed similar impact of ASCVD in both sexes (Online Table V; Online Figures IV and V).
Replication Cohort
Characteristics of participants are shown in the Online Table VI. LTL and MTL were strongly correlated (slope±SE; 0.76±0.07; P<0.0001), but MTL was longer (mean±SD; 8.62±0.69 kb) than LTL (6.80±0.75 kb) in all individuals (P<0.0001), as evidenced in the Discovery Cohort. The mean difference LTL-MTL was −1.81±0.57 kb, and the ratio LTL/MTL was 79.0±6.5%.
Effect of Age on TL
Both LTL and MTL shortened with age. The rate of decline was independent of sex in both tissues (age×sex interaction, P>0.6). Adding age squared to the model did not significantly increase the explained variance in LTL or MTL (both P>0.19). The slope of the effect of age was considerably steeper for LTL than MTL (slope±SE: LTL=−29.3±3.0 bp/y, P<0.0001; MTL=−15.0±3.3 bp/y, P<0.0001; interaction tissue type×age: P<0.0001).
ASCVD and TL Dynamics
In the Replication Cohort, the difference in the TL dynamics between ASCVD and controls was closely comparable to those observed in the Discovery Cohort. Individuals with ASCVD had 189.3±113.3 bp shorter age- and sex-adjusted LTL than those without ASCVD (t=1.67; df=139; P<0.1; and this effect was independent of population: interaction ASCVD×country of recruitment: P>0.9). MTL did not differ significantly between subjects with versus without ASCVD (P=0.9). Both LTL-MTL and LTL/MTL were significantly different in individuals with ASCVD versus controls (both t>2.0; P<0.05, controlling for age and sex; Online Figure VI, corresponding to Figure 3 for the Discovery Cohort). The gap between LTL and MTL widened with age at a similar rate in patients with ASCVD and controls (LTL-MTL: slope±SE; −19.4±6.0 versus −9.4±3.3 bp/y, respectively; ASCVD×age interaction: P=0.18). Similarly, the LTL/MTL ratio diminished at a similar rate in patients with ASCVD and controls (slope %±SE; −0.26±0.06 versus −0.14±0.03%/yr, respectively; ASCVD×age interaction: P=0.1).
When TL (Z-transformed for comparability) was added to a model with age, age squared, and sex (Online Table VII, corresponding to Table 2 for the Discovery Cohort), the OR was lower, that is, stronger, for LTL compared with MTL (0.58 versus1.03), and the AICc was significantly lower (ΔAICc=−5.64). LTL-MTL and LTL/MTL yielded ORs of 0.60 and 0.56, respectively, and better fitting models (Online Table VII). Moreover, the LTL/MTLA model yielded a slightly better fitting model than LTL-MTLA model (ΔAICc=1.35; Online Table VII). Adjusting all these analyses for the presence of cancer, morbid obesity, and severe renal failure did not affect these associations.
Discussion
The central findings of this study are that patients with ASCVD display a shorter LTL, a wider gap between LTL and MTLA, and a smaller ratio of LTL/MTLA than controls. These differences between patients with ASCVD and controls were consistent across the age range of the studied population (Figure 2). Moreover, the severity of ASCVD, as defined by the number of sites with atherosclerotic plaques, scaled with these 3 TL models (Figure 4), lending further confidence in the validity of the findings. MTL (adjusted for muscle site for the Discovery Cohort) was not significantly different between patients with ASCVD and controls, suggesting that LTL attrition is the main explanation for the shorter LTL in patients with ASCVD.
The LTL-MTL gap in the participants (mean age 63 years for the Discovery Cohort) in this study was 1.87 kb. In our previous study, comprising younger adults (mean age, 44 years), the LTL-MTLA gap was 1.55 kb.8 Moreover, the rate of LTL attrition in the present study was found to be faster compared with MTL, in contrast with our previous findings in a younger cohort.8 This is attributed to the older age of the Discovery Cohort, a finding supported by a longitudinal study.20 Although LTL attrition across the cohort was best described by a linear function, analysis of TL attrition in individuals <60 years in the Discovery Cohort, mean age 45 years, without ASCVD showed similar slopes for LTL/age (−30.53±8.42bp/y) and MTL/age (27.01±7.34 bp/y), both being close to findings in the previous study8 (not shown).
In a recent study, examining the blood-and-muscle model in fetuses and children, we generated data showing that TL is largely determined very early in life and that at least 1 kb of the LTL-MTLA gap is established before adulthood.21 As the pace of widening the LTL-MTLA gap in the present study is similar in participants with ASCVD versus controls, we infer that the wider LTL-MTLA gap and smaller LTL/MTLA in ASCVD patients than controls were principally established before adulthood.
Further support for this inference comes from the following findings: first, in a 9-year longitudinal study, we observed the same rate of LTL attrition in individuals with carotid atherosclerosis and controls.22 Second, given that LTL is highly heritable and LTL attrition is also heritable to some extent,23,24 long or short LTL might be largely inherent. Third, genetic analyses, including Mendelian randomization, show that single-nucleotide polymorphisms associated with LTL are also associated with ASCVD.5–7 Some of these single-nucleotide polymorphisms are in loci that harbor telomere maintenance genes, for example, TERC, TERT, CTC1, and OBFC1.5–7 Such findings largely exclude reverse causality, that is, ASCVD causing TL shortening. Together, these findings suggest that the wider LTL-MTL gap in ASCVD patients than controls is probably because of higher early-life LTL attrition in these subjects, which could be influenced by both genetic and environmental factors.
We acknowledge several limitations of the study. First, the sample size is modest, although this drawback was offset in part by the high reproducibility of the Southern blot measurements of TL. Moreover, we replicated the findings in another cohort. Second, our cohort comprised participants who underwent various surgical procedures for a host of conditions, rather than individuals who were subjected to elective muscle biopsies. Thus, although statistically adjusted for the different sources of muscle biopsies, residual confounding cannot be excluded. In addition, although the muscle site influenced TL in the Discovery Cohort, it had no apparent effect in the Replication Cohort. Third, subjects with ASCVD were older and comprised more males than controls, although age and sex were adjusted in the statistical analyses. Moreover, paired comparisons of subjects with ASCVD with age- and sex-matched controls confirmed the findings. Last, several control subjects had cardiovascular risk factors and might have subclinical ASCVD, which would tend to underestimate the association.
This is the first study applying the blood-and-muscle TL model in the context of ASCVD. Our findings suggest that a higher attrition rate in early life might be a major explanation of the shorter LTL in ASCVD patients than controls. Thus, although age (aging) is the principle determinant of ASCVD risk, LTL may modify this risk and the timing of developing the clinical manifestations of ASCVD (Figure 5). Our findings underscore the importance of understanding TL dynamics before the clinical manifestations of ASCVD. Such life-course information is crucial for gaining a better understanding of the role of telomeres in human health. We have relied on skeletal muscle as a reference for deriving such information. Whether other minimally dividing tissues, for example, subcutaneous fat,8 may serve this purpose is unknown at present. Finally, it is yet to be determined whether the blood-and-muscle TL model might reveal insight into the role of telomere biology in other human diseases and in ethnic groups other than individuals of European ancestry.
Figure 5.
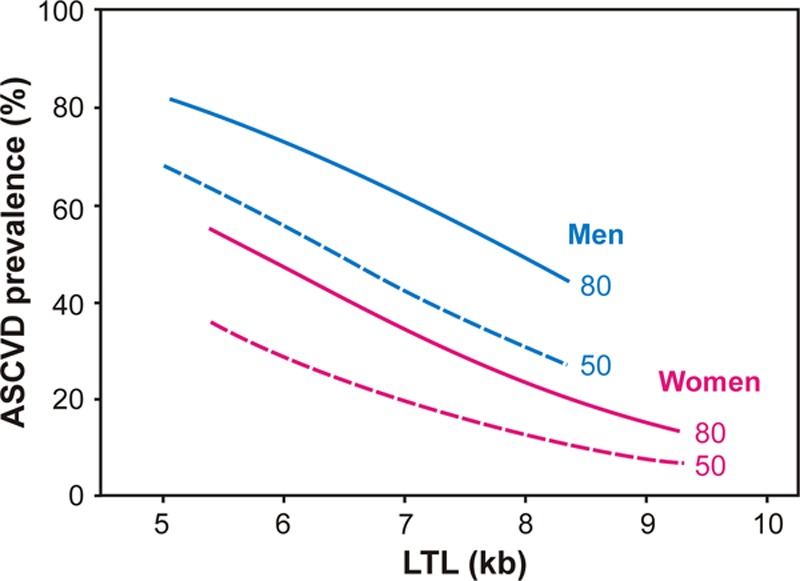
Atherosclerotic cardiovascular disease (ASCVD) prevalence in the sample as a function of telomere length and sex for ages 50 and 80 years. Lines were computed for leukocyte telomere length (LTL) and LTL/site-adjusted muscle telomere length (MTLA) using the coefficients in Table 2. Lines drawn over 95% TL range in the data for the 2 sexes. Note that ages 50 (dashed lines) and 80 (solid lines) were chosen for illustration purpose only; age was entered as continuous variable in the analyses.
Acknowledgments
We thank Cecile Lakomy for her technical assistance. We also thank Nikolaos Katsilambros for his valuable advices and help.
Sources of Funding
This study has been supported by the French National Research Agency (ANR), Translationnelle: N°ID RCB: 2014-A00298-39: 2014 to 2017 and the Investments for the Future program under grant agreement No. ANR-15-RHU-0004. A. Aviv research is supported by National Institutes of Health grants R01HD071180, R01HL116446, and R01HL13840.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AICc
- akaike information criterion with a correction
- ASCVD
- atherosclerotic cardiovascular disease
- BMI
- body mass index
- LTL
- leukocyte telomere length
- MTL
- skeletal muscle telomere length
- MTLA
- adjusted MTL for muscle site
- OR
- odds ratio
- TELARTA
- Telomere in Arterial Aging
- TL
- telomere length
In November 2017, the average time from submission to first decision for all original research papers submitted to Circulation Research was 11.99 days.
S. Verhulst and A. Aviv contributed equally to this study.
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.117.311751/-/DC1.
Novelty and Significance
What Is Known?
Telomere length (TL) is largely determined at birth and undergoes age-dependent shortening, which is inversely related to the replicative history of somatic tissues.
TL is highly variable across individuals, but similar within somatic tissues of the individual, such that a person with relatively short or long telomeres in 1 somatic tissue displays short or long telomeres in other somatic tissues.
TL, as expressed in leukocyte TL, is shorter in individuals with atherosclerotic cardiovascular disease, but it is uncertain whether this phenomenon relates to leukocyte TL dynamics (leukocyte TL and its age-dependent rate of shortening) before adulthood or thereafter.
What New Information Does This Article Contribute?
This work uses TL in skeletal muscle, a minimally proliferative somatic tissue, as reference of early-life TL in leukocytes, which reflect the highly proliferative hematopoietic system.
The blood (leukocyte)-and-muscle telomere model suggests that the comparatively short leukocyte TL in individuals with atherosclerotic cardiovascular disease is largely determined before the clinical manifestations of the disease and very likely before adulthood.
A body of work, principally based on cross-sectional studies, indicates that leukocyte TL is shorter in individuals with atherosclerotic cardiovascular disease than in their peers. The mechanistic underpinning of this finding is not fully understood. To gain insight, we need to answer a core question: does short leukocyte TL in individuals with atherosclerosis reflect leukocyte TL dynamics when the clinical manifestations of atherosclerosis unfold, or does it reflect factors in the individual’s earlier life? The findings of the present study, using a blood-and-muscle model of TL dynamics, support the tenet that a comparatively short leukocyte TL exist before the clinical manifestations of atherosclerosis, reflecting mainly higher leukocyte telomere attrition rates during the first years of life. Focusing on mechanisms that define leukocyte TL dynamics in the first 2 decades of life and learning how short telomeres might compromise repair capacity of the arterial wall are essential for understanding the role of telomere biology in the pathogenesis of atherosclerosis.
References
- 1.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, Paré G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet. 2015;8:82–90. doi: 10.1161/CIRCGENETICS.113.000485. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- 3.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res. 2012;730:68–74. doi: 10.1016/j.mrfmmm.2011.05.001. doi: 10.1016/j.mrfmmm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10:274–283. doi: 10.1038/nrcardio.2013.30. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- 5.Codd V, Nelson CP, Albrecht E, et al. CARDIoGRAM; Consortium. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427, 427e1–2. doi: 10.1038/ng.2528. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheller Madrid A, Rode L, Nordestgaard BG, Bojesen SE. Short telomere length and ischemic heart disease: observational and genetic studies in 290 022 individuals. Clin Chem. 2016;62:1140–1149. doi: 10.1373/clinchem.2016.258566. doi: 10.1373/clinchem.2016.258566. [DOI] [PubMed] [Google Scholar]
- 7.Haycock PC, Burgess S, Nounu A, et al. Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian Randomization Study. JAMA Oncol. 2017;3:636–651. doi: 10.1001/jamaoncol.2016.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benetos A, Kimura M, Labat C, Buchoff GM, Huber S, Labat L, Lu X, Aviv A. A model of canine leukocyte telomere dynamics. Aging Cell. 2011;10:991–995. doi: 10.1111/j.1474-9726.2011.00744.x. doi: 10.1111/j.1474-9726.2011.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youngren K, Jeanclos E, Aviv H, Kimura M, Stock J, Hanna M, Skurnick J, Bardeguez A, Aviv A. Synchrony in telomere length of the human fetus. Hum Genet. 1998;102:640–643. doi: 10.1007/s004390050755. [DOI] [PubMed] [Google Scholar]
- 11.Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, Aviv A. Synchrony of telomere length among hematopoietic cells. Exp Hematol. 2010;38:854–859. doi: 10.1016/j.exphem.2010.06.010. doi: 10.1016/j.exphem.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Factor-Litvak P, Susser E, Kezios K, McKeague I, Kark JD, Hoffman M, Kimura M, Wapner R, Aviv A. Leukocyte telomere length in newborns: implications for the role of telomeres in human disease. Pediatrics. 2016;137:e20153927. doi: 10.1542/peds.2015-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, Steenstrup T, Christensen K, Herbig U, von Bornemann Hjelmborg J, Srinivasan SR, Berenson GS, Labat C, Aviv A. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12:615–621. doi: 10.1111/acel.12086. doi: 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8:e1002696. doi: 10.1371/journal.pgen.1002696. doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 17.Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, Harley CB, Aviv A. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5:1596–1607. doi: 10.1038/nprot.2010.124. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa S, Schielzeth H. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev Camb Philos Soc. 2010;85:935–956. doi: 10.1111/j.1469-185X.2010.00141.x. doi: 10.1111/j.1469-185X.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- 19.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer Science & Business Media; 2003. [Google Scholar]
- 20.Berglund K, Reynolds CA, Ploner A, Gerritsen L, Hovatta I, Pedersen NL, Hägg S. Longitudinal decline of leukocyte telomere length in old age and the association with sex and genetic risk. Aging (Albany NY) 2016;8:1398–1415. doi: 10.18632/aging.100995. doi: 10.18632/aging.100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabharwal S, Verhulst S, Guirguis G, Kark JD, Labat C, Roche NE, Martimucci K, Patel K, Heller DS, Kimura M, Chuang D, Chuang A, Benetos A, Aviv A. Telomere length dynamics in early life: the blood-and-muscle model. FASEB J. 2018;32:529–534. doi: 10.1096/fj.201700630R. doi: 10.1096/fj.201700630R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toupance S, Labat C, Temmar M, Rossignol P, Kimura M, Aviv A, Benetos A. Short telomeres, but not telomere attrition rates, are associated with carotid atherosclerosis. Hypertension. 2017;70:420–425. doi: 10.1161/HYPERTENSIONAHA.117.09354. doi: 10.1161/HYPERTENSIONAHA.117.09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hjelmborg JB, Dalgård C, Möller S, Steenstrup T, Kimura M, Christensen K, Kyvik KO, Aviv A. The heritability of leucocyte telomere length dynamics. J Med Genet. 2015;52:297–302. doi: 10.1136/jmedgenet-2014-102736. doi: 10.1136/jmedgenet-2014-102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]


