BACKGROUND
Hyaluronic acid (HA) fillers may differ in terms of gel characteristics and ease of use and it is of interest whether this might affect safety and duration of effect.
OBJECTIVE
To compare the long-term safety and effect of 2 HA fillers produced by 2 different technologies for lip enhancement.
MATERIALS AND METHODS
Subjects with very thin to moderately thick lips were randomized and treated with HA-RK (N = 31) or HA-JV (N = 29) to improve lip fullness by ≥ 1 grade on a 5-point scale, using a maximum of 3 mL of product.
RESULTS
A smaller volume of HA-RK compared with HA-JV was required to improve lip fullness by ≥ 1 grade (mean: 1.54 mL vs 1.94 mL, p < .001). Despite the smaller volume, lip fullness and global aesthetic improvement were comparably sustained in both groups. At 6 months, 60.0% versus 57.7% of subjects (HA-RK vs HA-JV) had improved lip fullness. At 12 months, 71.4% versus 76.0% had aesthetic improvement (blinded evaluations) and 85.7% versus 86.2% felt more attractive. Both products were well tolerated.
CONCLUSION
Both products achieved durable improvement in lip fullness and aesthetic appearance. A significantly smaller amount of HA-RK was required compared with HA-JV to achieve optimal treatment effect.
Lips become thinner and less well-defined with aging because of gradual loss of collagen and elastin.1 Historically, this effect of aging was addressed by surgical lifting procedures, but today, injection of hyaluronic acid (HA) filler products is becoming increasingly popular as a minimally invasive option.2 Hyaluronic acid dermal fillers are biodegradable fillers and may differ in duration of effect, HA concentration, adverse event profiles, and ease of use. This study was performed to compare the long-term effect and safety of 2 HA gels developed for lip enhancement: Restylane Kysse with lidocaine (HA-RK, named Emervel Lips Lidocaine at the time of the study) and Juvéderm Volbella with lidocaine (HA-JV).
Materials and Methods
Subjects and Study Design
This was a 12-month, randomized, evaluator-blinded study (ClinicalTrials.gov Identifier: NCT01916278), performed at 2 centers in Germany and 1 center in Sweden, Sep 2013–May 2015. Sixty subjects with very thin to moderately thick lips (Lip Fullness Grading Scale [LFGS]3 scores 0–2) were randomized 1:1 to treatment with HA-RK or HA-JV. Randomization was stratified by baseline LFGS score. The study was approved by independent ethics committees in accordance with the 1975 Declaration of Helsinki (and later revisions) and conducted in compliance with good clinical practice. All subjects gave written informed consent to participate. Key exclusion criteria included: previous surgery/tattoo to the lips, presence of any abnormal lip structure, active skin disease, history of angioedema, permanent lip implant, and lip enhancement or laser therapy performed within the preceding 12 months.
Treatments
Subjects were treated on Day 0 with either HA-RK (20 mg/mL HA, Galderma SA, Lausanne, Switzerland) or HA-JV (15 mg/mL HA, Allergan, Pringy, France). Both are biodegradable, HA gels of nonanimal origin, cross-linked with 1,4-butanediol diglycidyl ether but manufactured using different technologies. The study required injection of each subject with a sufficient amount of product to reach ≥1 grade increase in LFGS score in both lips. Touch-up treatment could be administered after 2 weeks. Study product was slowly administered by submucosal injection in the lips, using an injection technique chosen by the treating investigator (the same predominant injection technique was used for all subjects at each site). Up to 3 mL (1.5 mL in each lip) could be injected at the initial and touch-up treatment combined. Both study products contained lidocaine hydrochloride (3 mg/mL), but additional local anesthesia could be used. The injection technique, volumes injected, and ease of injection and molding were documented for each lip. Optional retreatment was offered at 12 months, and a maximum total volume of 3 mL was allowed.
Effect Assessments
Global Aesthetic Improvement Scale (GAIS) score was assessed by treating investigators and subjects, and by blinded evaluators after study end, using photographs taken with standardized settings.
Lip Fullness Grading Scale score (0 = very thin; 1 = thin; 2 = moderately thick; 3 = thick; 4 = full) was assessed by the treating investigator and by blinded evaluators (using photographs). Improvement in lip fullness was defined as a ≥1-grade in LFGS increase from baseline. A subject satisfaction questionnaire was completed.
Safety Assessments
Intensity of local tolerability symptoms (bruising, redness, pain, tenderness, itching, and swelling) was recorded by subjects in a 14-day diary after initial treatment. Treating investigators recorded adverse events throughout the study after each treatment. Palpability was assessed by treating investigators and treatment pain by the subjects.
Statistics
Effect and safety analyses were based on the intention-to-treat (ITT) and safety populations, respectively. Injected filler volume was compared between products using Student's t test. Proportions of subjects with improvement in GAIS and LFGS were compared between products using Fisher exact test. The significance level was 0.05. The subject satisfaction questionnaire and safety variables were analyzed descriptively.
Results
Demographics and Subject Disposition
The demographic and baseline parameters were comparable between groups, but more subjects in the HA-RK group had had previous aesthetic procedures (Table 1). Three subjects in the HA-RK group and 4 in the HA-JV group withdrew from the study. No withdrawals were because of treatment-related adverse events, and no subjects who were lost to follow-up had severe adverse events (Figure 1). All 60 treated subjects were included in the ITT and safety populations.
TABLE 1.
Demographic and Baseline Parameters, ITT Population
Figure 1.
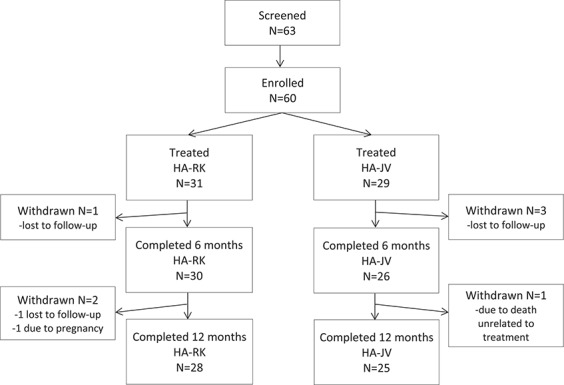
Disposition of subjects.
Treatments
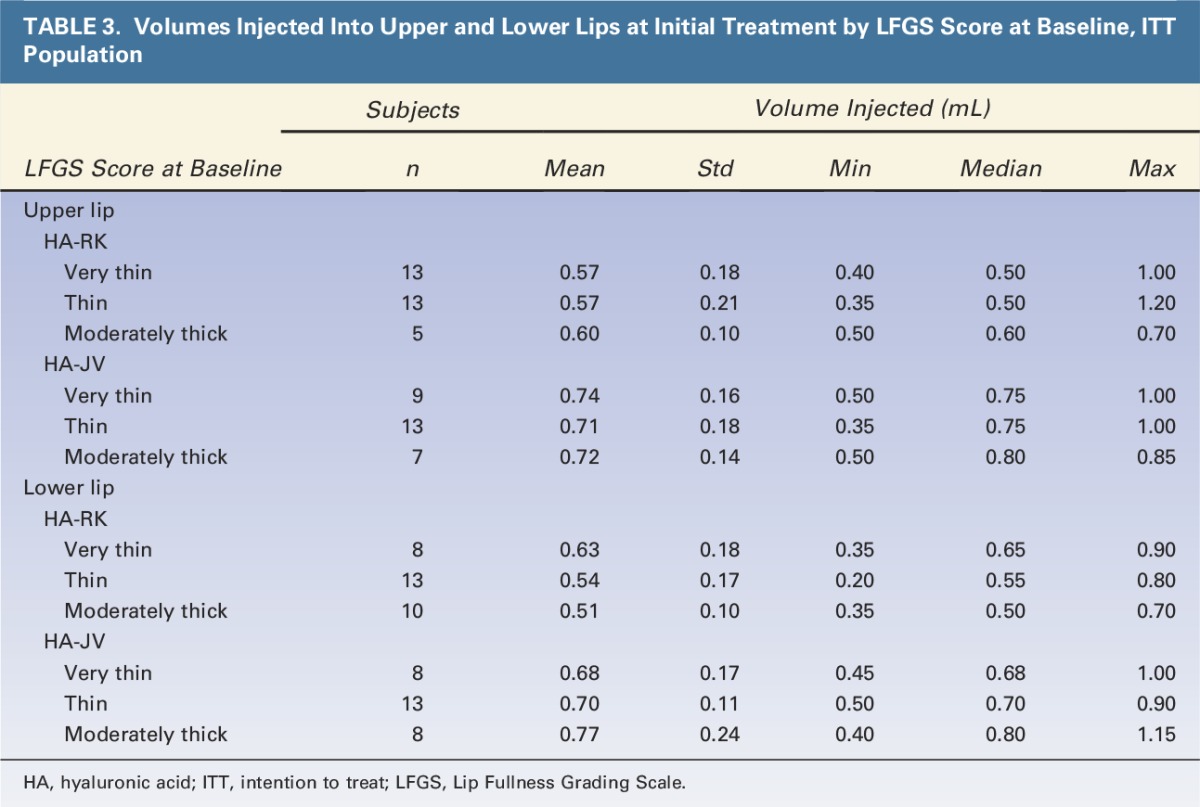
The total volumes injected at initial treatment and touch-up and the volumes injected at retreatment are described in Table 2. Volumes injected per grade of lip fullness at baseline are shown in Table 3. The mean product volume injected to achieve ≥1 grade improvement in lip fullness was significantly smaller in the HA-RK group than in the HA-JV group: 1.54 mL versus 1.94 mL, respectively (p < .001) (Table 2). The trend was the same for each grade of lip fullness at baseline (Table 3). At 12 months, for optional retreatment, a smaller volume was also used for HA-RK than for HA-JV, Table 2.
TABLE 2.
Volumes Injected at Initial and Touch-Up Treatment and Optional Retreatment, ITT Population
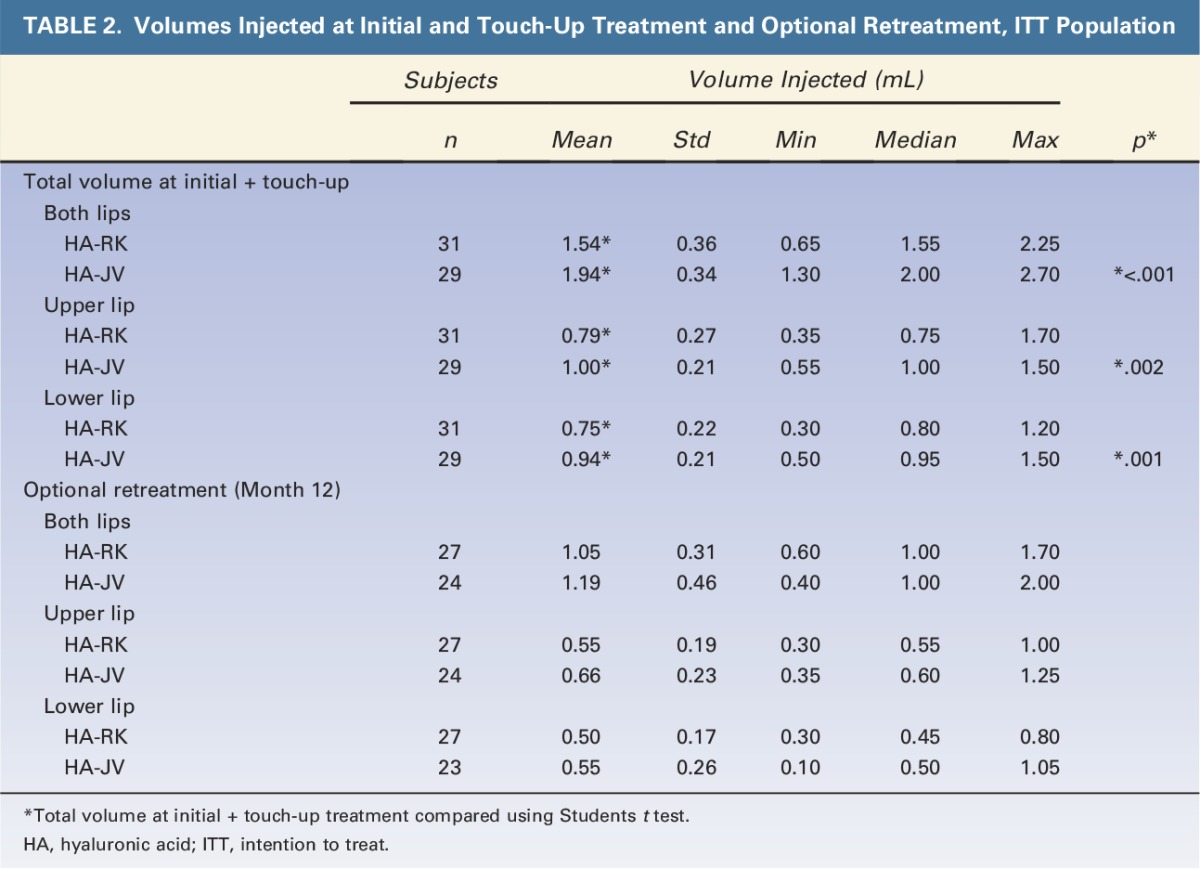
TABLE 3.
Volumes Injected Into Upper and Lower Lips at Initial Treatment by LFGS Score at Baseline, ITT Population
The lip areas injected (vermilion mucosa or vermilion mucosa and border), injection techniques, and post-treatment care were comparable in the 2 groups. Local anesthesia was used at 2 of the sites only. The most common injection method was serial puncture, used in approximately 2-thirds of upper and lower lips in both groups. More than 1 injection technique could be used per lip.
The investigators rated the injections to be very or fairly easy to perform in >90% of subjects in both groups and molding to be very or fairly easy to perform in >95% of the subjects in both groups.
Effect of Treatment
GAIS
Aesthetic appearance (GAIS) was improved in both lips of all subjects in both groups at 1 month after treatment according to the treating investigators and subjects. This effect was sustained up to 6 months after treatment in ≥80% of subjects in both groups according to the treating investigators, subjects, and blinded evaluators, with no statistically significant difference between treatments (Fisher exact test). After 12 months, 81% of HA-RK–treated subjects and 74% of the HA-JV–treated subjects reported improvement (Figure 2).
Figure 2.
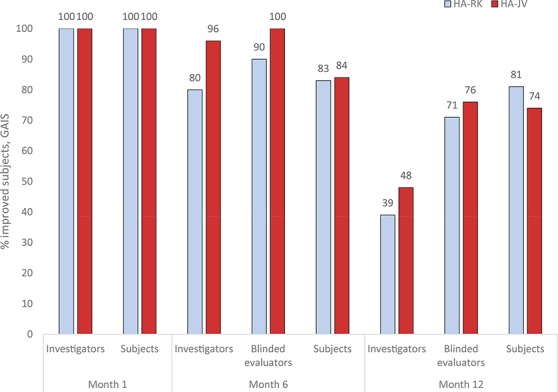
Proportions of subjects with improvement in Global Aesthetic Improvement Scale in both upper and lower lips assessed by treating investigators, blinded evaluators, and subjects, intention-to-treat population. No statistically significant differences between groups at any time point, Fisher exact test.
Lip Fullness Grading Scale
The baseline LFGS values, evaluated by the treating investigators, showed comparable lip fullness of the lower lips in the HA-RK and HA-JV groups but slightly thinner upper lips in the HA-RK group compared with the HA-JV group (Table 1).
Based on assessments by the treating investigators, a ≥1-grade increase from baseline was achieved in the majority of subjects in both groups at 1 month after treatment and sustained in more than half of the subjects in both groups up to 6 months after treatment. At 12 months after treatment, similar proportions of subjects in both groups had ≥1 grade improvement (Figure 3). There were no statistically significant differences between groups at any time point (Fisher exact test).
Figure 3.
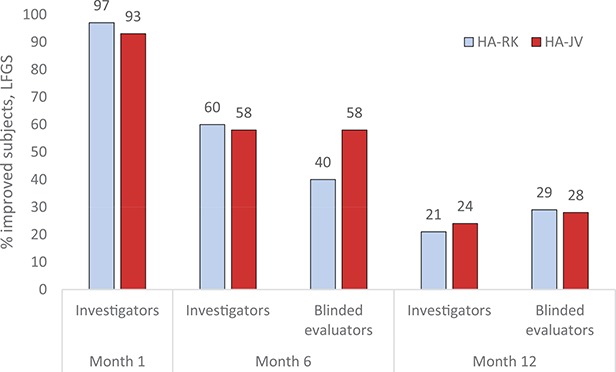
Proportions of subjects with ≥1 grade improvement in Lip Fullness Grading Scale in both lips by treating investigators and blinded evaluators, intention-to-treat population. No statistically significant differences between groups at any time point, Fisher exact test.
Subject Questionnaire
Treatment satisfaction was high and comparable in the 2 groups throughout the study; 1 month after treatment, ≥96% of subjects were somewhat or very satisfied with lip fullness, ≥86% somewhat or fully agreed to feeling more attractive, ≥96% somewhat or fully agreed that their lips had a natural look, and ≥86% somewhat or fully agreed that the treatment had added balance to their facial features (Figure 4). At 12 months after treatment, ≥64% of subjects were still satisfied with lip fullness and agreed that the treatment added balance to their facial features, and ≥96% of subjects agreed that their lips had a natural look. Figure 5 shows a representative subject before and 12 months after treatment with HA-RK. Almost all subjects in both groups agreed that they would do the treatment again (HA-RK 96% vs HA-JV 91% at 12 months) and that they would recommend the treatment to a friend (HA-RK 100% vs HA-JV 95% at 12 months). Recovery time after treatment was considered acceptable by most subjects at 2 weeks after treatment in both groups (HA-RK 97% vs HA-JV 81%).
Figure 4.
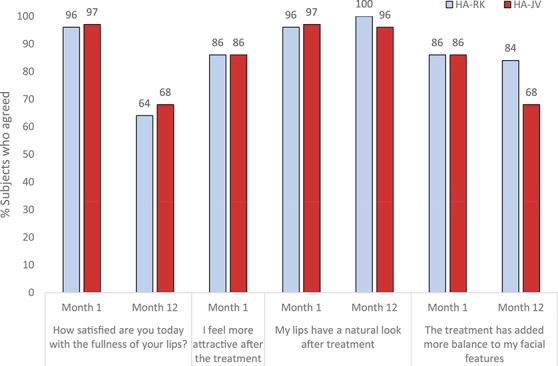
Responses to the subject satisfaction questionnaire on satisfaction, attractiveness, natural looking lips, and adding balance to facial features, intention-to-treat population. Total number of subjects responding to the questionnaire in the HA-RK/HA-JV groups at Month 1: N = 28/N = 29; Month 12: N = 25/N = 22. HA, hyaluronic acid.
Figure 5.
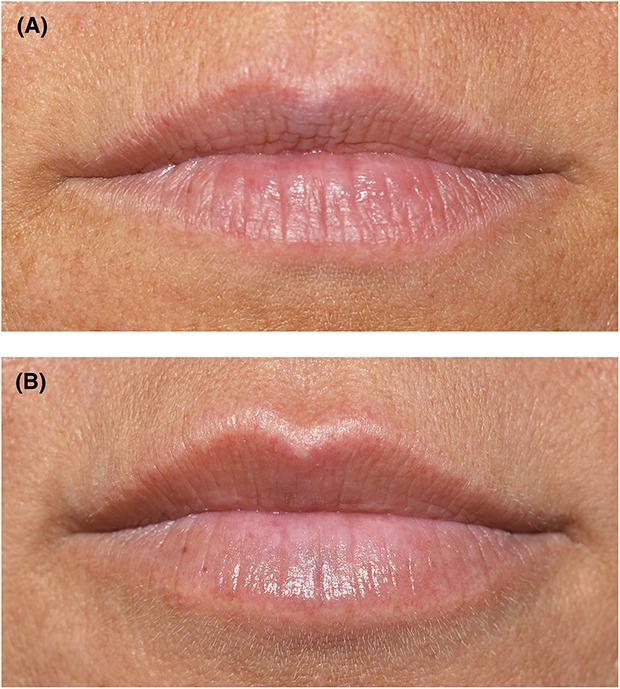
Photographs of lips from 1 subject treated with HA-RK at baseline (A) and 12 months after treatment (B). HA, hyaluronic acid.
Safety and Tolerability
Subject Diary
The most common local reactions recorded in the 14-day subject diary were swelling, bruising, and tenderness. All local reactions, except redness, occurred in similar subject proportions in both groups. Redness was more common in the HA-RK group but most additional reports of redness in the HA-RK group compared with the HA-JV group were of mild intensity and only 1 subject had redness after Day 14, which continued for 32 days.
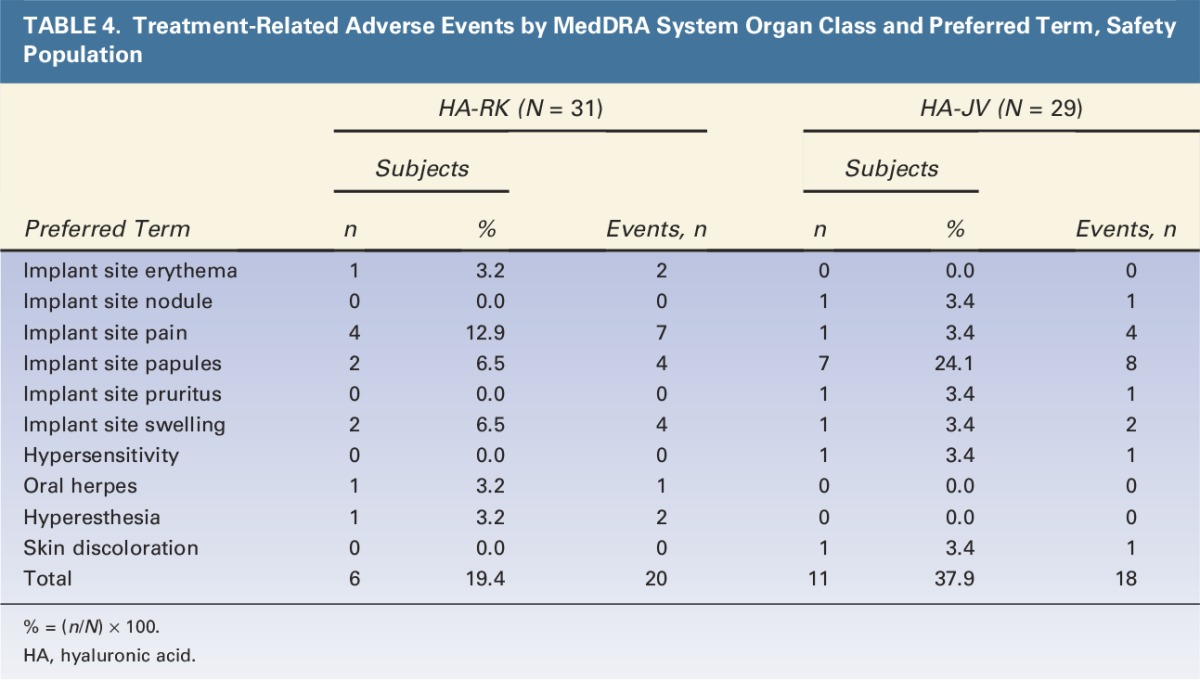
Most local reactions were mild or moderate in intensity and had resolved within 14 days after initial treatment. Any reactions still ongoing after Day 14 were reported as adverse events; these included tenderness (4 subjects in the HA-RK group and 1 subject in the HA-JV group), swelling (2 subjects in the HA-RK group and 1 subject in the HA-JV group), and redness, pain, and itching reported by single subjects in either group (Table 4). These adverse events resolved within approximately 1 month.
TABLE 4.
Treatment-Related Adverse Events by MedDRA System Organ Class and Preferred Term, Safety Population
Treatment Pain
Pain during initial treatment occurred at similar frequency and intensity in both groups. One subject in each group reported severe pain. At touch-up treatment, pain was reported by similar subject proportions as at the initial treatment but the pain intensity tended to be lower, mostly mild (data not shown).
Palpability
There were overall few abnormal palpability findings in both groups up to 2 weeks after each treatment occasion (up to 10% per group) and no apparent difference between groups (data not shown).
Adverse Events
The adverse events collected during the 12-month study period did not indicate any safety concerns for either HA product or any major differences in the safety profiles of the 2 products (Tables 4 and 5). There were no treatment-related serious adverse events.
TABLE 5.
Summary of Adverse Events, Safety Population
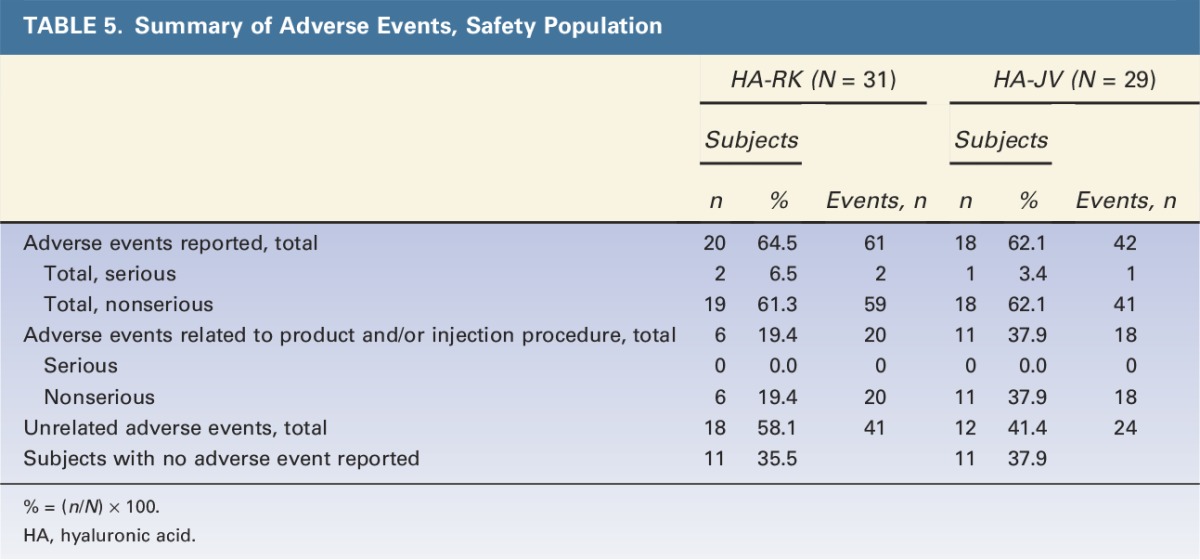
Adverse events related to the study product and/or injection procedure occurred in a smaller proportion of subjects in the HA-RK group (6 subjects; 19.4%) than in the HA-JV group (11 subjects; 37.9%). The most common treatment-related adverse events were nonserious, local site reactions: implant site papules, implant site pain, and implant site swelling. Most treatment-related adverse events were of mild or moderate intensity, except for 3 events in the HA-JV group. Treatment-related adverse events of severe intensity (nonserious) occurred only in the HA-JV group: implant site swelling (both lips) in 1 subject and hypersensitivity (facial numbness and swelling, and shortness of breath) in 1 subject.
All treatment-related events started within 19 days after initial or touch-up treatment and had a duration of 32 days or less in the HA-RK group and 51 days or less in the HA-JV group except 2 events, both in the HA-JV group. These events affected 2 subjects (6.9%): skin discoloration (1 subject; onset: 57 days after touch-up; duration: 287 days) and implant site papules (1 subject; onset: 78 days after touch-up).
Discussion
The study results showed that significantly less HA-RK was required to achieve the same effect, a ≥1-grade improvement in lip fullness, compared with HA-JV (1.54 mL HA-RK vs 1.94 mL HA-JV, p < .001). For each grade of lip fullness, consistently less HA-RK was injected to achieve optimal aesthetic result. Both groups were also treated using the same injection techniques and post-treatment care (massage and cooling).
In general, the groups had comparable demographics, but, despite stratification by LFGS at randomization, the upper lips were on average slightly thinner in the HA-RK group. However, this cannot explain the difference in filler volume required, as smaller volumes were injected to achieve ≥1 grade improvement also in the lower lips of the HA-RK group, which had similar lip fullness at baseline as the HA-JV group.
Despite the smaller volume injected in the HA-RK group, the duration of lip fullness and aesthetic improvement over 12 months were comparable in the 2 groups. At 6 months after treatment, approximately 60% of subjects in both groups had lip fullness improvement, and ≥80% of subjects were improved according to GAIS. At 12 months after treatment, approximately 25% of subjects in both groups still had lip fullness improvement, and ≥39% of subjects were improved according to GAIS. A decline in lip fullness improvement after 6 months is to be expected with HA fillers.4 Effect of treatment with HA-JV has also been studied previously, and the results from this study are consistent with results from published studies.5,6
In both groups, subject satisfaction was high during the whole study, and >95% of subjects agreed that they would recommend the treatment to a friend at 12 months after treatment. The high aesthetic improvement rates and high degree of subject satisfaction in this study are in agreement with a previous study with HA-RK, showing similar results for at least 6 months.7
The frequency and types of treatment-related adverse events in the study were as expected for HA-based products (according to the Instructions for Use for each product). Treatment-related adverse events occurred in 19.4% versus 37.9% of subjects in the HA-RK and HA-JV groups, and reactions starting later than 19 days (57 and 78 days) after touch-up treatment were reported for 2 subjects (6.9%), both in the HA-JV group. There were no serious treatment-related adverse events, and most adverse events were of mild or moderate intensity. Treatment-related adverse events of severe intensity (nonserious) were reported in the HA-JV group: implant site swelling (both lips) in 1 subject and hypersensitivity (facial numbness and swelling, and shortness of breath) in 1 subject.
Conclusion
In summary, this study shows that a smaller volume of HA-RK filler was required to achieve optimal effect. The 2 fillers were comparable in ease of use, effect, and duration of effect. Both products were well tolerated and achieved high rates of lip fullness improvement, aesthetic improvement, and satisfied subjects.
Footnotes
Presented as posters at the following conferences: 1) International Master Course on Aging Skin (IMCAS), Paris, January 2016—12-month data. 2) Aesthetic & Anti-Aging Medicine World Congress (AMWC), Monaco, March 26, 2015–March 28, 2015—6-month data.
Supported by Galderma. S. Hilton is a consultant for Allergan, Galderma, Q-Med, S&V Technologies, Cosmetique, L'Oreal, Sanofi-Aventis, and HAL. G. Sattler has lectured for and serves on the advisory board for Allergan, Galderma, and Merz. U. Samuelson has lectured for Allergan, Galderma, and Novus Scientific and serves on the advisory board for Eternogen. C. Wong is an employee for Galderma. Medical Writing Assistance was provided by PCG Clinical Services. The remaining author has indicated no significant interest with commercial supporters.
References
- 1.Penna V, Stark GB, Eisenhardt SU, Bannasch H, et al. The aging lip: a comparative histological analysis of age-related changes in the upper lip complex. Plast Reconstr Surg 2009;124:624–8. [DOI] [PubMed] [Google Scholar]
- 2.Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol 2006;19:141–50. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers A, Carruthers J, Hardas B, Kaur M, et al. A validated lip fullness grading scale. Dermatol Surg 2008;34(Suppl 2):S161–6. [DOI] [PubMed] [Google Scholar]
- 4.Dayan S, Bruce S, Kilmer S, Dover JS, et al. Safety and effectiveness of the hyaluronic acid filler, HYC-24L, for lip and perioral augmentation. Dermatol Surg 2015;41(Suppl 1):S293–301. [DOI] [PubMed] [Google Scholar]
- 5.Raspaldo H, Chantrey J, Belhaouari L, Saleh R, et al. ; for the Juvéderm Volbella Study Group. Juvéderm Volbella with lidocaine for lip and perioral enhancement: a prospective, randomized, controlled trial. Plast Reconstr Surg Glob Open 2015;3:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philipp-Dormston WG, Hilton S, Nathan M. A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol 2014;13:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartier H, Trevidic P, Rzany B, Sattler G, et al. Perioral rejuvenation with a range of customized hyaluronic acid fillers: efficacy and safety over 6 months with a specific focus on the lips. J Drugs Dermatol 2012;11(Suppl 1):17–26. [PubMed] [Google Scholar]


