BACKGROUND
Several formulations of Botulinum toxin serotype A (BoNT-A) for aesthetic indications are available, with numbers likely to increase. Preparations are not interchangeable, based on dose unit comparisons.
OBJECTIVE
Numerous myths and misconceptions regarding the use of BoNT-A for aesthetic indications have arisen, which this review aims to lay to rest.
MATERIALS AND METHODS
This review assesses evidence for and against each of the most common myths regarding BoNT use in aesthetics.
RESULTS
BoNT-A neurotoxin/protein complexes are irrelevant to the toxin's therapeutic/aesthetic indications. BoNT-A neurotoxin/protein complexes do not influence movement from injection site or immunogenicity. Any relationship between neutralizing antibody formation and clinical response is complex and clinicians should consider other factors that may induce an apparent loss of clinical response. Diffusion appears predominately, perhaps exclusively, dose dependent. Careful placement and correct dosing optimizes likelihood of good outcomes. Manufacturers recommend reconstitution of products with sterile nonpreserved saline. However, compelling evidence suggests that reconstitution using preserved saline dramatically improves patient comfort without compromising efficacy. Several post-treatment instructions/restrictions are widely used despite the lack of evidence, but muscle activity after injection may be beneficial. Cooling the treatment area might hinder BoNT-A translocation and should probably be abandoned.
CONCLUSION
The existing evidence suggests that experienced users should achieve equivalent results regardless of BoNT-A formulation, but additional, well-designed, adequately powered, controlled randomized studies should be performed.
The use of botulinum toxin A (BoNT-A) in aesthetic medicine has increased markedly since the first applications in this setting during the mid-1980s.1,2 Current aesthetic uses of BoNT-A include treating glabellar lines, forehead wrinkles, periorbital and perioral lines, platysmal bands, horizontal neck lines, and the masseter, among many other applications.3,4 Accurate figures for the extent of use of BoNT-A in aesthetics (as opposed to therapeutic indications) do not, to the authors' knowledge, exist. Nevertheless, the net revenue of onabotulinumtoxinA (BotoxCosmetic) reached $199.4 million in the fourth quarter of 2016 for aesthetic indications.5 Indeed, according to the American Society of Plastic Surgeons, 15.4 million minimally invasive aesthetic procedures were performed in the United States during 2016, and BoNT-A procedures, at 7 million, were the most common of these.6 Of almost 10 million treatments performed by members of the American Society for Dermatologic Surgery in 2015, 1.8 million of these were BoNT-A procedures.7
Three BoNT-A formulations are available in the United States for aesthetic uses, namely abobotulinumtoxinA (Dysport), Botox, and incobotulinumtoxinA (Xeomin). In addition, there are aesthetic product versions specifically available in the European Union (Azzalure—Speywood unit product; Vistabel/Vistabex—Botox unit product; Bocouture—Xeomin unit product). The number of products is likely to increase with the introduction of other BoNT-A formulations, especially from companies based in Asia. BoNT-B is also available as rimabotulinumtoxinB (Myobloc/Neurobloc), although this is not currently approved for aesthetic indications.4,8,9
The dose units of BoNT-A are specific to each product family and are not interchangeable.4 Partly because of inappropriate dose comparisons between formulations and heavy marketing campaigns to establish product differentiation, numerous myths and misconceptions about BoNT-A use for aesthetic indications have arisen. This review addresses the most important of these myths and misconceptions, and makes suggestions for further research.
Myth 1: Different Products Yield Different Results
Relatively few well-designed, suitably powered and controlled, randomized studies compare BoNT-A products in “routine” clinical settings. Many clinical trials of BoNT were performed in “artificial” settings, e.g., using fixed doses at fixed intervals in fixed positions on the face. These studies do not reflect normal clinical use, which varies widely depending on patient-related factors. Comparative studies are potentially compromised by the lack of consensus on any conversion ratio10 and the need to address a plethora of potentially confounding variables, including dilution/concentration, placement,11 and patient selection. Current claims of dose equivalence are based on preclinical and clinical data. However, there are no randomized controlled human clinical studies in which the different preparations are titrated to the same effect and tolerability.12 To date, the small number of methodologically weak studies report mixed results as exemplified by comparisons of Botox and Dysport for glabellar lines.
Some studies, for example, suggest Botox is superior to Dysport. In a pilot study, Botox 20 units provided better and more prolonged efficacy than Dysport 50 units for glabellar lines, assessed by a blinded investigator evaluating photographs. Nevertheless, the authors comment that differences in diffusion and electrophysiological characteristics preclude the proposal of a single dose conversion ratio.13 In another study, patients receiving Botox 20 units were more likely to show a 1-grade or better improvement in glabellar line severity than those treated with Dysport 50 units: 77% versus 59% at week 12; and 53% versus 28% at Week 16.14 However, this study has been criticized for being underpowered and inadequately randomized with respect to age (younger patients often have stronger muscles and require higher doses). In addition, the effect of Botox apparently increases over time in the study, which is not seen in any other study or in clinical practice.15
Certain studies suggest the converse, that Dysport is superior to Botox. Lowe and colleagues11 reported that Dysport (256 units total) was significantly more effective than Botox (64 units total) (4:1 dose unit ratio) for upper face lines. There was a trend toward greater efficacy on crow's feet with Dysport with a 3:1 dose unit ratio. In another study, Dysport produced a longer duration of effect on electromyographic activity and forehead wrinkles than Botox at a dose unit conversion ratio of 3:1.16 These differences may reflect a higher Dysport dose than in the studies that suggested Botox is superior to Dysport.
A third possibility is that there is no difference between Botox and Dysport. Lowe and colleagues11 found that Botox 30 units and Dysport 75 units (a 2:1 dose ratio) showed similar efficacy on glabellar lines. A further prospective study in which 53 patients received Dysport (62.5 units) on one side of the upper face and Botox (25 units; a 2.5:1 Dysport to Botox dose unit ratio) on the other showed no statistically significant differences using multiple methods of observation and measurement. Muscle height returned to near-baseline values after 150 days. However, visual grading systems suggested continued efficacy beyond Day 150.17
In conclusion, there is no compelling evidence of a superior efficacy of Botox compared with Dysport.11,13,14,16,17 However, when the doses are not equivalent (dose unit ratios higher than 2 or 2.5:1), the results are not equivalent for clear and obvious reasons: higher quantities of BoNT were injected.
Myth 2: Diffusion Profiles Differ Between BoNT-A Formulations
There is no standardized nomenclature for discussing the “movement” of BoNT-A from the site of injection. This article follows the terminology proposed by one of the authors (A.P.).18 Spread refers to the relatively rapid physical movement of toxin from the original injection site, e.g., arising from the injection technique, including the volume, speed, and angle of injection. Alternatively, diffusion refers to the relatively slow kinetic dispersion of toxin beyond the original injection site, exemplified by the toxin's movement to receptors. Diffusion may be uneven and is highly dependent on receptor density in any given target area. Migration might refer to other mechanisms of movement, such as distal effects far from the injection point or “retrograde axonal transport.”18
Certain authors have suggested that protein load may influence diffusion. Aoki and colleagues12 claim that as Dysport contains protein with a lower molecular mass than Botox, the former will diffuse further from the injection site. They suggest that this raises the prospect that Dysport will diffuse from the site of injection into adjacent tissues or the systemic circulation, increasing the risk of adverse events. There is, however, no evidence from any robust clinical studies that supports this suggestion. The deficiencies and errors of the study by Aoki and colleagues have been described in detail elsewhere,18 but notably included product dilution errors and mistranslation relating to extrapolation of animal studies using BoNT-A into humans.
The diffusion of BoNT-A from the injection site depends in part on an active binding component that targets receptors, on nerve endings or on the surfaces of sebaceous and eccrine glands. As a result, the diffusion halo can vary in size and shape depending on the number of receptors. For example, in a study of 3 women with compensatory hyperhidrosis, the participants received 5-unit injections at 3 points on their backs at 3 depths (2, 3, and 4 mm). The field of effect was smallest in the midline, indicating a higher density of BoNT-A receptors in that area. Only a marginal effect of injection depth was observed. These findings suggest that the type of skin, anatomical location, number of BoNT receptors and amount of sweating (gland activity) may be more important in determining the field of effect than the depth of injection and concentration.19
In addition, there is little evidence of clinically relevant differences in diffusion (by inference, the field of effect) between BoNT-A formulations.10,20–23 These studies show that dose, not product, is the driver of the field of effect. For instance, a direct comparison of equal labeled doses (2 units) in the same injection volume (isovolumetric) found that the horizontal and vertical diameters and the areas of the fields of effect were significantly larger for Botox than those obtained for Dysport indicating an underdose of Dysport/more potent dose of Botox. This finding emphasizes that units of each product are specific to that product family and are not readily interchangeable. No significant differences emerged in the Wrinkle Severity Scale scores and evoked compound muscle action potentials.21 Therefore, these findings confirm that diffusion is dose dependent and the higher dose tested diffuses more.21
Carli and colleagues22 compared the diffusion of Botox (0.25 units), Dysport (1.0 unit), and Xeomin (0.25 units) using a mouse model of neural cell adhesion molecule expression. No significant difference emerged between the 3 products in terms of the diffusion results obtained and the BoNT-A formulations did not differ in the extent of their “spread” into adjacent muscles. Furthermore, a comparison of Dysport and Botox in 59 women with forehead wrinkles reported that a 2:1 dose ratio (Dysport:Botox units) was associated with statistically equivalent fields for muscle effects and anhidrosis. At a dose ratio of 2.5:1, Dysport showed greater area and larger horizontal diameter in the field of anhidrotic effect at both 28 and 112 days compared to Botox. These results further support the hypothesis that dose is the most important factor influencing the size of the field of effect.23
Against this background, careful placement of the correct dosing of BoNT-A offers the best chance of good patient outcomes. Further studies are warranted to characterize the relative importance of the numerous factors that influence comparative data on efficacy, diffusion, and spread. In the meantime, the lack of definitive data on the conversion rate between the various formulations of BoNT-A means that individual studies might have used suboptimal doses and counsels against drawing “too firm a conclusion from any 1 trial.”10 Moreover, different areas of the body may require different doses of BoNT-A for a clinical effect, reflecting differences in muscular structure and function.
Myth 3: Protein Load is Clinically Important
When BoNT-A is naturally produced by bacteria, the active molecule joins with neurotoxin-associated proteins (such as the several known hemagglutinins and the “nontoxic, nonhemagglutinin” protein), forming high-molecular weight “progenitor complexes” (BoNT-A neurotoxin/protein complexes).24 These complexes protect BoNT-A against destruction in the gastrointestinal tract and aid the toxin's absorption from the gut lumen; they are essential for BoNT-A's oral toxicity when ingested with food.24 Some authors suggest that complexing proteins, could, in theory, limit the spread and diffusion of BoNT-A from the target tissues.25 However, there is no evidence from robust clinical studies, relevant to the toxin's therapeutic or aesthetic uses, of any differences in the diffusion of the free or complexed form of BoNT-A products after injection into the muscle.25 Early speculation that the associated proteins may have a role in stabilization of the neurotoxin in the vial or in reducing diffusion after injection26 are incorrect. The advent of a complex protein-free product, IncobotulinumtoxinA (Xeomin), has demonstrated that the associated proteins are not required for product stability. In addition, this earlier speculation came before the work of Merz Pharmaceuticals GmbH demonstrating that the toxin progenitor complex dissociates on dilution of the products within the vials, not on injection.27
In 1928, researchers at the University of California reported chemically purifying BoNT-A to precipitate a stable, light brown, dry powder form of BoNT-A that was readily soluble in water.28 These earliest studies, however, did not identify that BoNT was produced as a toxin complex, which was not demonstrated until the 1950s.29 In 1977, Sugii and Sakaguchi reported the size of the complexes in culture supernatants produced in vegetable media without purification steps; different complexes of different sizes could be produced simultaneously.30 Indeed, despite almost 90 years of research, an accurate consensus estimate of the size of the BoNT-A complex has proved elusive.
In 1988, Stell and colleagues31 raised the issues of dose standardization of Dysport and Botox because of differences in the total “protein load.” The protein load could, in theory at least, influence the development of neutralizing antibodies (NAbs; see Myth 4) and, potentially, the subsequent lack of clinical efficacy in certain patients. For clinical doses that are approximately equivalent, the protein content of Dysport is 40% lower than that of Botox. These results may explain why the frequency of NAbs has been reported to be higher for Botox than for Dysport.32
Nevertheless, Dysport and Botox are assayed by different methods and therefore no direct or absolute dose unit conversions can be made.33 In addition, the complex size has generally been measured after many purification steps, any one of which may influence the composition (and hence size) of a progenitor complex. Also, Lietzow and colleagues34 reported that the BoNT-A complex is 880 kDa based on size-exclusion high-performance liquid chromatography (HPLC), 934 ± 63 kDa determined by capillary electrophoresis, and 925 ± 45 kDa when measured using multi-angle laser light-scattering HPLC, a potential range of 870 to 970 kDa. The size of the complex therefore depends on the measurement technique used. Nevertheless, the molecular composition and, therefore, the size of the BoNT-A complex is specific to the producing strain and can be influenced by the method of growth and purification.33 Despite those authors being employed by Allergan, it is not clear if the data definitely relate to Botox.33,34
Complexing proteins undoubtedly contribute to protein load. The original Botox formulation used before 1998 contained 25 ng protein per 100 units.35 A reformulation reduced this to 5 ng protein per 100 units.35 Xeomin is reported to be free from complexing proteins (although the manufacturer, Merz, has not published suitable data to substantiate this claim).36 However, 2 protein loads for Xeomin have been published: 0.637 and 0.44 ng per 100-unit vial,38 an interesting difference that has been criticized.39
Currently, Dysport is the only BoNT-A product supported by long-term data on protein load across many batches of Drug Substance (the active BoNT added to the formulation) over many years: the mean content in 6 batches was 4.57 ± 0.47 ng toxin protein per 500-unit vial, which was similar to a previous analysis of 8 batches (4.40 ± 1.3 ng).40 Data from these 14 batches were added to another 20 taken at various times since Dysport's approval in Europe for specific dystonias in December 1990, which provided a mean toxin protein content of 4.35 ng per 500-unit vial.40
The significance, if any, of the differences in complex size and protein load in the treatment of aesthetic indications is unclear. The reconstitution of Botox and Dysport results in complete dissociation of 900 kDa complexes, with at least 85% of the Botox BoNT-A, and 100% of Dysport BoNT-A, present as free neurotoxins.27 As a result, the complex will not impede or contribute to BoNT-A diffusion after reconstitution.27 Against this background, whether the lack of complexing proteins in Xeomin confers a therapeutic advantage is not yet established, and long-term comparative studies are still needed, despite the product being available for a decade.25,36 However, based on the lack of marked differences in either immunogenicity (discussed below) or the field of effect,10,20–23,41,42 the discordance in protein load between contemporary BoNT formulations seems clinically irrelevant in aesthetic applications, where very low doses of the products are used.
Myth 4: Neutralizing Antibodies Are an Important Determinant of Treatment Failure in Aesthetic Indications
Initially, some researchers expressed concern that the protein load would increase the risk of inducing NAbs,25 which could undermine the effectiveness of treatment. Only antibodies that neutralize the effects of the active molecule (sometimes called “blocking antibodies”) are relevant. For example, Jankovic and colleagues35 reported that 4 of 42 (9.5%) patients with cervical dystonia treated with the original Botox formulation developed NAbs. In contrast, none of the 119 patients treated exclusively with the replacement Botox formulation developed NAbs. This offers circumstantial evidence that the protein load was at least partly responsible for the enhanced immunogenicity of the original Botox formulation.
Development of NAbs seems relatively uncommon with contemporary formulations of BoNT-A. These formulations of BoNT-A are associated with a very low rate of clinically detectable levels of antibodies when compared with other approved biologic products, especially when used at low doses for aesthetic indications.4,41,42 For instance, a meta-analysis of 16 clinical studies encompassing 2,240 patients assessed subjects who received between 1 and 15 treatments (mean 3.8 treatments) with Botox across a range of indications. The doses per treatment varied from 10 or 20 units in glabellar lines to 20 to 500 units in cervical dystonia. The proportion of patients who developed NAbs ranged between 1.28% and 0% depending on indication. Only 3 of 11 patients (27%) across the various indications became clinically unresponsive to Botox after testing positive for NAbs.43
Rates of NAb formation reported with Dysport are broadly comparable, ranging from 0% in glabellar lines to less than 3% in cervical dystonia.41 The rate of NAb formation with Xeomin was 1.1% in their overall development program.41 However, there are no direct head-to-head trials assessing NAb formation with the different products when used at either therapeutic or aesthetic doses. The data suggest that the immunogenicity of BoNT-A generally, and at the doses used in aesthetic indications in particular, do not seem to result in clinically significant rates of NAb formation.
Interestingly, Myobloc/NeuroBloc seems to be more immunogenic than contemporary formulations of BoNT-A. Myobloc/Neurobloc is associated with NAb rates of 10% to 44% in cervical dystonia, for example.42 The reason for the apparent difference in immunogenicity (e.g., variations in the epitopes responsible) between BoNT-A and BoNT-B requires further investigation. The difference is most likely because of the 20- to 40-fold higher doses of BoNT-B required to gain therapeutic effect compared with BoNT-A.44 Such high protein doses are likely to trigger immune responses in individuals who would not otherwise be sensitive to BoNT-A.44
A recent study explains why such high doses of BoNT-B are needed. Humans and chimpanzees express BoNT-B synaptotagmin-II receptors that are much less efficient than those in other species.45 This again emphasizes the species-specificity of the BoNT molecules. The relationship of NAb formation to clinical response is not clear: some patients express NAbs without being nonresponsive.46 Many patients who are clinically nonresponsive to BoNT-A do not have detectable NAb expression.42 Patients may also show detectable NAbs that are below the titer required to reduce the clinical response.46
Secondary nonresponse refers to patients who initially show a positive response to BoNT-A but their response declines (or is absent) with subsequent treatments.47 Against this background, Lange and colleagues47 took serum samples from 503 patients treated with BoNT-A for one of several indications who developed secondary nonresponse to BoNT-A. Of these, 44.5% were positive for NAbs. Therefore, NAb formation does not account for the lack of efficacy in more than half of secondary nonresponders.
Although the likelihood of developing NAbs did not seem to depend on the product, the risks rose with increasing dose. Lange and colleagues47 reported that 68.8% of patients with secondary nonresponse received Dysport, 13.5% received Botox, and 6.8% received both products. Of these, 42.9% who received <6,000 total cumulative Dysport units developed NAbs compared with 63.7% of those who received ≥6,000 units. In addition, 7.6% and 5.5%, respectively, showed threshold values for NAbs. Overall, 44.5% of serum samples across all BoNT-A formulations showed NAbs, with a further 13.1% showing threshold values.
Researchers have proposed numerous factors that may influence NAb production, including: single administered and cumulative dose; timing of serum sampling; previous treatment with BoNT-A; product manufacturing processes; genetic predisposition; presence of complexing proteins; and frequency and duration of treatment (including the administration of so-called “booster doses” a short interval after the first injection).4,46 The consistently low rates in studies to date, however, suggest that a statistically significant difference in NAb formation would not be apparent.41 Any claims of differences between products in the propensity to induce NAb formation is speculation and statistically meaningful confirmation studies are now unlikely to be conducted.
To complicate matters further, differences in the analytical methodology means that rates of NAb formation in studies are not directly comparable. For example, enzyme-linked immunosorbent assays, Western blots, and radioimmunoprecipitation assays can quantitatively estimate the antibody titer against the core neurotoxin. However, these in vitro methods cannot distinguish nonneutralizing antibodies that are responsible for reduced efficacy. The results from such nonspecific assays will inevitably demonstrate a higher incidence of antibodies than are clinically relevant. Results from sensitive in vitro assays often correlate poorly with in vivo or clinical test results.42
Concurrently, clinicians must consider factors other than NAb formation that may induce an apparent loss of clinical response in patients receiving BoNT-A, including: changes in muscle activity (because of disease progression or adaptation); inadequate dosing; failure to accurately identify and inject the muscles; difficulty in targeting the intended muscle(s); and, very importantly, changes in patient expectations.41,42 Using the lowest effective doses of BoNT-A and the longest inter-injection interval, consistent with an acceptable aesthetic outcome, may limit NAb formation.42
The evidence clearly emphasizes that treatments need to be individualized. This may become more difficult as several new BoNT-A products will reach the market over the next few years.48 The increasing number of products raises concerns over immunogenicity and therapeutic equivalence: differences in the propensity to induce NAb could alter BoNT-A pharmacokinetics, pharmacodynamics, clinical efficacy, and tolerability.49 No standardized assay to assess BoNT-A immunogenicity has been internationally adopted,33 which, together with the low incidence of nonresponding patients to aesthetic treatments, hinders product comparisons.
Clinicians need to remain vigilant for immunogenicity and tolerability issues with any new BoNT-A product. For example, the oldest Asian BoNT-A product is BTXA, first licensed in 1997. BTXA uses dextran and gelatin as stabilizers instead of the standard Human Serum Albumin included in almost every other formulation of BoNT-A. The presence of gelatin may be unacceptable to some cultural and religious groups and, in addition, gelatin confers an increased risk of allergic reactions, including anaphylactic shock.9,48
Myth 5: Reconstitution Solution Matters
Most formulations of BoNT-A are stored as powders that are reconstituted as recommended by the manufacturers, with sterile, nonpreserved saline.44 However, compelling evidence now suggests that reconstitution using preserved saline dramatically improves patient comfort without compromising efficacy.50–54 In addition, aggressive reconstitution may reduce efficacy.44,55
In a prospective study, one side of the face was treated with BoNT-A reconstituted with preserved saline and the other side with BoNT-A reconstituted with preservative-free saline. All 15 patients reported less pain (mean difference: 54%) on the side treated with preserved saline. Neither investigators nor patients reported differences in efficacy between the sides.50 In the retrospective arm, 90% of 20 patients treated mainly for upper facial dynamic lines reported that injections of BoNT-A reconstituted with preserved saline were less painful than previous treatment with BoNT-A reconstituted with preservative-free saline.50
Other studies similarly confirm that BoNT-A reconstituted with preserved saline is less painful. For example, Allen and Goldenberg treated glabellar lines and crow's feet on one side of the face with Dysport reconstituted with preserved saline and the other side with preservative-free saline. Of 20 volunteers, 90% reported 60% less pain on the side injected with preserved saline.53
Sarifakioglu and Sarifakioglu51 used a 10-point visual analog scale to compare pain after administration of Dysport, reconstituted with preserved or preservative-free saline, into the upper face, neck, and axillary regions. The average pain score was 1.2 with preserved saline and 4.5 with preservative-free saline in the upper face and were 0.6 and 3.9 with preserved and preservative-free saline, respectively, in the neck. For axillary administration, the pain scores were 0.9 and 5.1 with preserved and preservative-free saline, respectively.51
Some studies suggest that vigorous reconstitution may undermine outcomes. Kazim and Black54 injected one side of the foreheads of 7 consecutive patients with BoNT-A that had been reconstituted vigorously and the other side with product gently reconstituted, as recommended by the manufacturers. There was no difference between the results obtained with the 2 reconstitution methods during the 6-month follow-up.54
However, another more recent study has compared aggressive and gentle reconstitution. In a mouse model, aggressive reconstitution increased the time to paralysis from 72.0 to 106.0 minutes, equivalent to a loss of potency of 42%.55
Therefore, compelling evidence confirms that reconstitution using preserved saline improves patient comfort without compromising efficacy.50–53 Based on the evidence regarding reconstitution, clinicians or pharmacists should reconstitute BoNT-A using large-diameter injection needles, and a limited number of injection-aspiration-injection cycles with, if necessary, a few gentle shakes to rinse the vial wall.55
Myth 6: The Volume of Injection Matters
Different physicians use different BoNT-A dilutions (different reconstitution volumes for the products): oculoplastic specialists typically use 1 mL per 100 units of Botox, whereas dermatologists and plastic surgeons generally use dilutions of 1 to 5 mL per 100 units.56 Therapeutic injections into the biceps of patients with spastic hemiparesis of a high volume (5 mL per 100-unit vial) were superior to low-volume injections (1 mL per 100-unit vial). The higher volume produced greater neuromuscular block of the elbow flexors, greater reduction of spasticity and co-contraction, and greater improvement in the range of extension.57 These findings probably reflect the very large area of muscle and the position of BoNT-A targets on those muscles compared with other body areas.
The same does not seem to apply to the use of BoNT-A in aesthetic indications. One study, for example, enrolled 20 individuals and used a within-subject paired comparison to evaluate injections into the lateral orbital area of 5 units of Botox, with a 5-fold difference in concentration. No statistically significant differences emerged between the 2 sides.58 In addition, in a study of 80 participants receiving Botox into the glabellar region at dilutions of 100, 33.3, 20, or 10 U/mL, no difference was identified in improvement in facial wrinkle score or the number of adverse events reported.59
Similarly, Hsu and colleagues60 injected the dynamic forehead lines of 10 volunteers with a single Botox injection approximately 2.5 cm above the orbital rim. One side of the forehead was injected with 2 units diluted in 0.1 mL and the other side with 2 units in 0.02 mL. In 9 subjects, the field of effect was 50% greater in the side with the larger compared with the smaller volume: averages of 6.05 and 4.12 cm2, respectively. The average width was greater than the average height, producing an oval field of effect. However, this small difference associated with the oval field of effect and, in turn, probably related to injection angle, is unlikely to be clinically significant.
Abbasi and colleagues61 treated 10 patients with 2 concentrations of Dysport (6 units diluted in 0.1 mL or 0.3 mL) injected into the first prominent horizontal crease between 2.5 and 3.0 cm above the orbital rim. The more concentrated solution produced a mean wrinkle reduction of 476.6 mm2 compared with 794.1 mm2 using the higher volume. However, the concentration seemed to exert less influence on the field of effect than the marked interpatient variation, which highlights the importance of other factors, including bulk of muscle, dynamic movement of muscles and dose, emphasizing the individual patient needs.
Rzany and colleagues,62 reviewing the recommendations for the optimal use of the Speywood unit products, indicated that the range of injection volumes normally used for BoNT-A products (0.05–0.1 mL) is unlikely to be associated with a difference in effect in general clinical practice. Indeed, a recent randomized, comparative study evaluating the efficacy and safety of injection volumes of 0.05 or 0.1 mL per injection point (to deliver 10 units of Azzalure) for the treatment of glabellar lines found no significant differences; both injection volumes resulted in comparable onset and duration of effect, frequency and severity of adverse events and similar patient satisfaction.63
Myth 7: Post-Treatment Protocols Are Well Supported by Clinical Evidence
A number of instructions and restrictions related to postaesthetic treatment protocols for patients are widely used, despite a lack of evidence that they influence efficacy, adverse event frequency, or severity. Many recommendations are highly anecdotal and speculative. Some physicians suggest that patients should keep their head elevated for 6 hours after the injection to reduce the chance of unwanted spread of the toxin.64 This advice may reflect concerns that ptosis can be an adverse event, however there is no evidence that changing to a horizontal position or lowering the head influences lid ptosis or diffusion.64 Indeed, most BoNT-A is inside the synaptic vesicles in muscles 5 or 10 minutes after binding.65
Many clinicians suggest moving or massaging muscles to increase the uptake of toxin, smooth post-injection “bumps” (that occur with the often-used subdermal injection technique) and to aid physical spread. However, some authors counsel caution when massaging areas where diffusion can be a problem, such as the procerus and bunny lines.62 No studies have assessed the effect of the massage directly to the authors' knowledge.
However, indirect evidence emerged from a study conducted by Wei and colleagues,66 who injected 98 patients with masseter muscle hypertrophy with 35 units of BoNT-A per side. Half the patients increased their masticatory effort for more than 2 hours everyday before the masseter muscles reached their maximum level of reduction, monitored using real-time objective and quantitative ultrasound every month. Control patients were not given this instruction. The duration of the masseter muscle atrophy was significantly prolonged in those who strengthened their masticatory effort. The thickness and the volume of the other masticatory muscles also significantly increased (Figure 1). Control patients showed no or a slight decrease in the structure and function of masticatory muscles. Masseter muscle reductions in the active and control groups were 25.3% and 19.3%, respectively, 6 months after injection and 18.0% and 7.8%, respectively, after 12 months, indicating that a longer study duration must be used in such cases to clearly establish the true effects of BoNT treatment.66 This study provides the first clinical evidence that muscle activity by a patient after aesthetic treatment can be beneficial, although further studies are needed to confirm this finding and ascertain the relevance to the aesthetic setting. The study also showed that, in this setting, a single injection series can produce a duration of effect that persists for 12 months. Other studies have confirmed this finding.67
Figure 1.
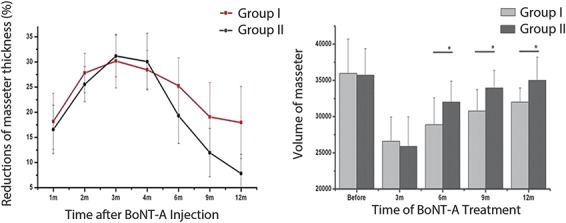
Line chart and histogram demonstrating the percentage of the masseter muscle reduction in thickness and volume observed in Groups I (who strengthened their masticatory movements) and II (control group). The maximal reduction in masseter muscle was observed 3 to 4 months after injection, and there were no statistically significant differences in the 2 groups before this stage. However, the duration of the masseter muscles' decrease was significantly longer in Group I than in Group II. Republished with permission of American Society for Dermatologic Surgery, from prolonging the duration of masseter muscle reduction by adjusting the masticatory movements after the treatment of masseter muscle hypertrophy with botulinum toxin type A injection, Wei and colleagues, 41, S1, 2015; permission conveyed through Copyright Clearance Center, Inc.
Ice or cooling is commonly used for comfort and to prevent bruising after treatment. For example, Beer and colleagues56 suggest applying ice to injection sites before and after treatment. The resulting vasoconstriction, they argue, may decrease the pain of injection and reduce the risk of swelling, oozing and bruising, and is especially useful when treating crow's feet and the infraorbital areas. Using preserved saline may also reduce pain on injection as discussed earlier.50–53
Whether cooling an area affects the uptake of the BoNT-A remains unclear. However, Pirazzini and colleagues found that translocation of the light chain of botulinum neurotoxins Type C and D across the plasma membrane of cerebellar granular neurons takes just minutes at 37°C, but the light chain does not enter neurons at 20°C.68 In fact, this temperature effect has been known about for more than 15 years in the literature, but seems to have been forgotten!69 This suggests that cooling the area might undermine BoNT efficacy and should probably be abandoned pending further investigation. Similarly, the other post-treatment instructions and restrictions that are widely used, despite a lack of evidence that they influence either the efficacy or adverse events of BoNT-A, should probably not be used routinely.
Conclusions
BoNT-A is a well-established aesthetic treatment for a number of areas, notably of the face.3,4 Several BoNT-A formulations are available and the number of preparations is likely to increase.4,8,9 These are not, however, interchangeable based on dose units or, potentially, immunogenicity.4 Partly because of inappropriate dose comparisons between the various formulations and also marketing campaigns, numerous myths and misconceptions about the use of BoNT-A for aesthetic indications have arisen that are not supported by the evidence.
For example, BoNT-A neurotoxin/protein progenitor complexes do not seem to be relevant to the toxin's therapeutic or aesthetic indications when BoNT-A is administered by injection. Therefore, the often quoted “one complex is larger than another and so safer, not moving from the injection site” is quite simply wrong. Similarly, there is no evidence of clinically significant differences in either immunogenicity or the field of effect with contemporary formulations of BoNT-A, when appropriate and correct data-driven comparisons are made. Indeed, the development of NAbs after aesthetic treatments seems to be relatively uncommon with contemporary formulations of BoNT-A, which are associated with a very low rate of clinically detectable levels of NAb compared with other biologics.4,41–43,47 Moreover, any relationship of NAb to clinical response is not clear and seems to be influenced by numerous factors.4,42,46 Therefore, clinicians need to consider factors other than NAb formation that may induce an apparent loss of clinical response.41,42
No evidence supports the speculation that “protein load” produces clinically relevant differences in diffusion (and, by inference, the field of effect) between BoNT-A formulations.10,12,19–23 Diffusion seems to be predominately, perhaps exclusively, dose dependent.21,23 Careful placement and correct dosing optimizes the likelihood of a good outcome.10
Different specialties use different BoNT-A dilution/reconstitution volumes, which may influence outcomes in treatment of large muscles.56,57 However, within a certain range, the dilution seems to have no clinically significant effect on outcomes in aesthetic indications.58–61 Other factors seem to be more influential.61
All companies recommend reconstituting their products with sterile nonpreserved saline. However, compelling evidence now suggests that reconstitution using preserved saline improves patient comfort without compromising efficacy.50–53 Moreover, relatively few, well designed, controlled randomized studies compare the BoNT-A products. Indeed, comparative studies are potentially compromised by the lack of consensus on the dose unit conversion ratio10 and the need to address a plethora of potentially confounding variables.11 The small number of methodologically weak studies published to date reported inconsistent results.11,13–17 Moreover, further studies need to definitively characterize the “shelf-life” of reconstituted BoNT-A: in other words, how long the reconstituted product remains active.
A number of post-treatment instructions and restrictions are widely used, despite a lack of evidence that they influence either the efficacy or adverse events of BoNT-A.56,64 Very preliminary evidence suggests that muscle activity by a patient after injection may be beneficial.66 Further studies are needed to confirm this finding and any relevance to the aesthetic setting. Temperature seems to influence BoNT translocation.68 This suggests that cooling the area might undermine BoNT efficacy and should probably be abandoned, pending further investigation.
The existing evidence suggests that experienced users should achieve equivalent results regardless of BoNT-A formulation, but additional, well-designed, adequately powered, controlled randomized studies should be performed.
Acknowledgments
The authors acknowledge the editorial support of MedSense Ltd. (High Wycombe, United Kingdom), funded by Galderma.
Footnotes
Supported by Galderma Laboratories LP, which funded the development of this manuscript. Editorial assistance was funded by Galderma Laboratories LP and provided by M. Greener and MedSense Ltd (High Wycombe, United Kingdom).
J.S. Dover has received clinical research grants from Allergan, Alphaeon, Galderma, Merz, and Revance. He also serves as a consultant for Allergan, Galderma, and Revance. G. Monheit has received clinical research grants from Galderma, Allergan, Revance, Alphaeon, and CROMA. He also serves as a consultant for Galderma, Allergan, Revance, and Merz. A. Pickett is Director and Founder of Toxin Science Limited, Wrexham, United Kingdom, Adjunct Professor at the Botulinum Research Center, Institute of Advanced Sciences, Dartmouth, MA, and Senior Program Leader & Scientific Expert, Neurotoxins in Galderma Aesthetic and Corrective Global Business Unit. The opinions and views expressed herein are those of the author and Toxin Science Limited only. The remaining author has indicated no significant interest with commercial supporters.
References
- 1.Clark RP, Berris CE. Botulinum toxin: a treatment for facial asymmetry caused by facial nerve paralysis. Plast Reconstr Surg 1989;84:353–5. [PubMed] [Google Scholar]
- 2.Carruthers A, Carruthers J. History of the cosmetic use of Botulinum A exotoxin. Dermatol Surg 1998;24:1168–70. [DOI] [PubMed] [Google Scholar]
- 3.Blitzer A, Brin MF, Keen MS, Aviv JE. Botulinum toxin for the treatment of hyperfunctional lines of the face. Arch Otolaryngol Head Neck Surg 1993;119:1018–22. [DOI] [PubMed] [Google Scholar]
- 4.Dorizas A, Krueger N, Sadick NS. Aesthetic uses of the botulinum toxin. Dermatol Clin 2014;32:23–36. [DOI] [PubMed] [Google Scholar]
- 5. Allergan. Press release. Available from: https://www.allergan.com/investors/events-presentations/events/q4-2016-allergan-plc-earnings-conference-call. Accessed March 23, 2017.
- 6. American Society of Plastic Surgeons. American society of plastic surgeons 2016 National Clearinghouse of plastic Surgery statistics. Available from: https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/2016-plastic-surgery-statistics-report.pdf. Accessed March 23, 2017.
- 7. American Society for Dermatologic Surgery. ASDS survey: nearly 10 million treatments performed in 2015. Available from: https://www.asds.net/_Media.aspx?id=9449. Accessed February 1, 2017.
- 8.Maas C, Kane MAC, Bucay VW, Allen S, et al. Current aesthetic use of abobotulinumtoxinA in clinical practice: an evidence-based consensus review. Aesthet Surg J 2012;32(1 Suppl):8S–29S. [DOI] [PubMed] [Google Scholar]
- 9.Kamin W, Staubach P, Klär-Hlawatsch B, Erdnüss F, et al. Anaphylaxis after vaccination due to hypersensitivity to gelatin. Klin Padiatr 2006;218:92–4. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky MA, Swope DM, Grimes D. Diffusion of botulinum toxins. Tremor Other Hyperkinet Mov (N Y) 2012;2:tre-02-85-417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe NJ, Shah A, Lowe PL, Patnaik R. Dosing, efficacy and safety plus the use of computerized photography for botulinum toxins type A for upper facial lines. J Cosmet Laser Ther 2010;12:106–11. [DOI] [PubMed] [Google Scholar]
- 12.Aoki KR, Ranoux D, Wissel J. Using translational medicine to understand clinical differences between botulinum toxin formulations. Eur J Neurol 2006;13(Suppl 4):10–9. [DOI] [PubMed] [Google Scholar]
- 13.Lowe PL, Patnaik R, Lowe NJ. A comparison of two botulinum type a toxin preparations for the treatment of glabellar lines: double-blind, randomized, pilot study. Dermatol Surg 2005;31:1651–4. [DOI] [PubMed] [Google Scholar]
- 14.Lowe P, Patnaik R, Lowe N. Comparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: a double-blind, randomized study. J Am Acad Dermatol 2006;55:975–80. [DOI] [PubMed] [Google Scholar]
- 15.Rzany B, Nast A. Head-to-head studies of botulinum toxin A in aesthetic medicine: which evidence is good enough? J Am Acad Dermatol 2007;56:1066–7. [DOI] [PubMed] [Google Scholar]
- 16.Karsai S, Adrian R, Hammes S, Thimm J, et al. A randomized double-blind study of the effect of Botox and Dysport/Reloxin on forehead wrinkles and electromyographic activity. Arch Dermatol 2007;143:1447–9. [DOI] [PubMed] [Google Scholar]
- 17.Michaels BM, Csank GA, Ryb GE, Eko FN, et al. Prospective randomized comparison of onabotulinumtoxinA (Botox) and abobotulinumtoxinA (Dysport) in the treatment of forehead, glabellar, and periorbital wrinkles. Aesthet Surg J 2012;32:96–102. [DOI] [PubMed] [Google Scholar]
- 18.Pickett A. Dysport: pharmacological properties and factors that influence toxin action. Toxicon 2009;54:683–9. [DOI] [PubMed] [Google Scholar]
- 19.Hexsel DM, Soirefmann M, Rodrigues TC, do Prado DZ. Increasing the field effects of similar doses of Clostridium botulinum type A toxin-hemagglutinin complex in the treatment of compensatory hyperhidrosis. Arch Dermatol 2009;145:837–40. [DOI] [PubMed] [Google Scholar]
- 20.Cliff SH, Judodihardjo H, Eltringham E. Different formulations of botulinum toxin type A have different migration characteristics: a double-blind, randomized study. J Cosmet Dermatol 2008;7:50–4. [DOI] [PubMed] [Google Scholar]
- 21.Hexsel D, Hexsel C, Siega C, Schilling-Souza J, et al. Fields of effects of 2 commercial preparations of botulinum toxin type A at equal labeled unit doses: a double-blind randomized trial. JAMA Dermatol 2013;149:1386–91. [DOI] [PubMed] [Google Scholar]
- 22.Carli L, Montecucco C, Rossetto O. Assay of diffusion of different botulinum neurotoxin type a formulations injected in the mouse leg. Muscle Nerve 2009;40:374–80. [DOI] [PubMed] [Google Scholar]
- 23.Hexsel D, Brum C, do Prado DZ, Soirefmann M, et al. Field effect of two commercial preparations of botulinum toxin type A: a prospective, double-blind, randomized clinical trial. J Am Acad Dermatol 2012;67:226–32. [DOI] [PubMed] [Google Scholar]
- 24.Benefield DA, Dessain SK, Shine N, Ohi MD, et al. Molecular assembly of botulinum neurotoxin progenitor complexes. Proc Natl Acad Sci USA 2013;110:5630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frevert J, Dressler D. Complexing proteins in botulinum toxin type A drugs: a help or a hindrance? Biologics 2010;4:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson EA, Bradshaw M. Clostridium botulinum and its neurotoxins: a metabolic and cellular perspective. Toxicon 2001;39:1703–22. [DOI] [PubMed] [Google Scholar]
- 27.Eisele KH, Fink K, Vey M, Taylor HV. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon 2011;57:555–65. [DOI] [PubMed] [Google Scholar]
- 28.Sommer EW, Sommer H, Meyer KF. The purification of botulinum toxin. J Infect Dis 1926;39:345–50. [Google Scholar]
- 29.Wagman J, Bateman JB. Botulinum type A toxin: properties of a toxic dissociation product. Arch Biochem Biophys 1953;45:375–83. [DOI] [PubMed] [Google Scholar]
- 30.Sugii S, Sakaguchi G. Botulogenic properties of vegetables with special reference to the molecular size of the toxin in them. J Food Saf 1977;1:53–65. [Google Scholar]
- 31.Stell R, Coleman R, Thompson P, Marsden CD. Botulinum toxin treatment of spasmodic torticollis. BMJ 1988;297:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panjwani N, Pickett A, O'Keeffe R. Botulinum type A toxins in clinical use: a comparison of specific potency and protein content. Poster presented at the International Conference 2005, Basic and Therapeutic Aspects of Botulinum and Tetanus Toxins, Denver, CO, USA, June 23–25, 2005.
- 33.Pickett A, O'Keeffe R, Panjwani N. The protein load of therapeutic botulinum toxins. Eur J Neurol 2007;14:e11. [DOI] [PubMed] [Google Scholar]
- 34.Lietzow MA, Gielow ET, Le D, Zhang J, et al. Subunit stoichiometry of the Clostridium botulinum type A neurotoxin complex determined using denaturing capillary electrophoresis. Protein J 2008;27:420–5. [DOI] [PubMed] [Google Scholar]
- 35.Jankovic J, Vuong KD, Ahsan J. Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology 2003;60:1186–8. [DOI] [PubMed] [Google Scholar]
- 36.Jost WH, Benecke R, Hauschke D, Jankovic J, et al. Clinical and pharmacological properties of incobotulinumtoxinA and its use in neurological disorders. Drug Des Devel Ther 2015;9:1913–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jost WH, Blümel J, Grafe S. Botulinum neurotoxin type A free of complexing proteins (XEOMIN) in focal dystonia. Drugs 2007;67:669–83. [DOI] [PubMed] [Google Scholar]
- 38.Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D 2010;10:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickett A. Consistent biochemical data are essential for comparability of botulinum toxin type A products. Drugs R D 2011;11:97–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panjwani N, O'Keeffe R, Pickett A. Biochemical, functional and potency characteristics of type A botulinum toxin in clinical use. Botulinum J 2008;1:153–66. [Google Scholar]
- 41.Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics 2014;8:227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naumann M, Boo LM, Ackerman AH, Gallagher CJ. Immunogenicity of botulinum toxins. J Neural Transm (Vienna) 2013;120:275–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naumann M, Carruthers A, Carruthers J, Aurora SK, et al. Meta-analysis of neutralizing antibody conversion with onabotulinumtoxinA (BOTOX®) across multiple indications. Mov Disord 2010;25:2211–8. [DOI] [PubMed] [Google Scholar]
- 44.Dressler D, Eleopra R. Clinical use of non-A botulinum toxins: botulinum toxin type B. Neurotox Res 2006;9:121–5. [DOI] [PubMed] [Google Scholar]
- 45.Strotmeier J, Willjes G, Binz T, Rummel A. Human synaptotagmin-II is not a high affinity receptor for botulinum neurotoxin B and G: increased therapeutic dosage and immunogenicity. FEBS Lett 2012;586:310–3. [DOI] [PubMed] [Google Scholar]
- 46.Benecke R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs 2012;26:e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lange O, Bigalke H, Dengler R, Wegner F, et al. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol 2009;32:213–8. [DOI] [PubMed] [Google Scholar]
- 48.Pickett A. The different botulinum toxins from around the world available for clinical use. In: Benedetto A, ed. Botulinum Toxins in Clinical Aesthetic Practice, 3rd ed, New York Publisher: Informa Healthcare. In press. [Google Scholar]
- 49.Wadhwa M, Knezevic I, Kang HN, Thorpe R. Immunogenicity assessment of biotherapeutic products: an overview of assays and their utility. Biologicals 2015;43:298–306. [DOI] [PubMed] [Google Scholar]
- 50.Alam M, Dover JS, Arndt KA. Pain associated with injection of botulinum A exotoxin reconstituted using isotonic sodium chloride with and without preservative: a double-blind, randomized controlled trial. Arch Dermatol 2002;138:510–4. [DOI] [PubMed] [Google Scholar]
- 51.Sarifakioglu N, Sarifakioglu E. Evaluating effects of preservative-containing saline solution on pain perception during botulinum toxin type-a injections at different locations: a prospective, single-blinded, randomized controlled trial. Aesthet Plast Surg 2005;29:113–5. [DOI] [PubMed] [Google Scholar]
- 52.Kwiat DM, Bersani TA, Bersani A. Increased patient comfort utilizing botulinum toxin type a reconstituted with preserved versus nonpreserved saline. Ophthal Plast Reconstr Surg 2004;20:186–9. [DOI] [PubMed] [Google Scholar]
- 53.Allen SB, Goldenberg NA. Pain difference associated with injection of abobotulinumtoxinA reconstituted with preserved saline and preservative-free saline: a prospective, randomized, side-by-side, double-blind study. Dermatol Surg 2012;38:867–70. [DOI] [PubMed] [Google Scholar]
- 54.Kazim NA, Black EH. Botox: shaken, not stirred. Ophthal Plast Reconstr Surg 2008;24:10–2. [DOI] [PubMed] [Google Scholar]
- 55.Dressler D, Bigalke H. Reconstituting botulinum toxin drugs: shaking, stirring or what? J Neural Transm (Vienna) 2016;123:523–5. [DOI] [PubMed] [Google Scholar]
- 56.Beer K, Cohen J, Carruthers A. Cosmetic uses of botulinum toxin A. In: Ward A, Barnes M, editors. Clinical Uses of Botulinum Toxins. Cambridge, United Kingdom: Cambridge University Press; 2007; pp. 328–48. [Google Scholar]
- 57.Gracies JM, Lugassy M, Weisz DJ, Vecchio M, et al. Botulinum toxin dilution and endplate targeting in spasticity: a double-blind controlled study. Arch Phys Med Rehabil 2009;90:9–16.e2. [DOI] [PubMed] [Google Scholar]
- 58.Carruthers A, Bogle M, Carruthers JDA, et al. A randomized, evaluator-blinded, two-center study of the safety and effect of volume on the diffusion and efficacy of botulinum toxin type A in the treatment of lateral orbital rhytides. Dermatol Surg 2007;33:567–71. [DOI] [PubMed] [Google Scholar]
- 59.Carruthers A, Carruthers J, Cohen J. Dilution volume of botulinum toxin type A for the treatment of glabellar rhytides: does it matter? Dermatol Surg 2007;33:S97–104. [DOI] [PubMed] [Google Scholar]
- 60.Hsu TS, Dover JS, Arndt KA. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch Dermatol 2004;140:1351–4. [DOI] [PubMed] [Google Scholar]
- 61.Abbasi NR, Durfee MA, Petrell K, Dover JS, et al. A small study of the relationship between abobotulinum toxin A concentration and forehead wrinkle reduction. Arch Dermatol 2012;148:119–21. [DOI] [PubMed] [Google Scholar]
- 62.Rzany B, Fratila AA, Fischer TC, Hilton S, et al. Recommendations for the best possible use of botulinum neurotoxin type a (Speywood units) for aesthetic applications. J Drugs Dermatol 2013;12:80–4. [PubMed] [Google Scholar]
- 63.Punga AR, Alimohammadi M, Fagrell D, Nyberg F, et al. A randomized, comparative study to evaluate efficacy and safety of two injection volumes of abobotulinumtoxina in treatment of glabellar lines. Dermatol Surg 2016;42:967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Sa Earp AP, Marmur ES. The five D's of botulinum toxin: doses, dilution, diffusion, duration and dogma. J Cosmet Laser Ther 2008;10:93–102. [DOI] [PubMed] [Google Scholar]
- 65.Colasante C, Rossetto O, Morbiato L, Pirazzini M, et al. Botulinum neurotoxin type A is internalized and translocated from small synaptic vesicles at the neuromuscular junction. Mol Neurobiol 2013;48:120–7. [DOI] [PubMed] [Google Scholar]
- 66.Wei J, Xu H, Dong J, Li Q, et al. Prolonging the duration of masseter muscle reduction by adjusting the masticatory movements after the treatment of masseter muscle hypertrophy with botulinum toxin type a injection. Dermatol Surg 2015;41(Suppl 1):S101–9. [DOI] [PubMed] [Google Scholar]
- 67.Bhattacharjee K, Singh M, Bhattacharjee H. Extended effect after a single dose of type A botulinum toxin for asymmetric masseter muscle hypertrophy. Indian J Plast Surg 2015;48:196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pirazzini M, Rossetto O, Bertasio C, Bordin F, et al. Time course and temperature dependence of the membrane translocation of tetanus and botulinum neurotoxins C and D in neurons. Biochem Biophys Res Commun 2013;430:38–42. [DOI] [PubMed] [Google Scholar]
- 69.Simpson LL. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther 1980;212:16–21. [PubMed] [Google Scholar]


