A bioinorganic hybrid system based on bacterial surface display and biomimetic silicification for hydrogen production.
Abstract
Solar-to-chemical production by artificial and bioinspired photosynthetic systems is of tremendous interest to help solve current global energy and environmental problems. We developed a bioinorganic hybrid system for photocatalytic hydrogen production under aerobic conditions by combining light-harvesting semiconductors, hydrogenase catalysis, and self-aggregation of whole bacterial cells. We induced hydrogen production via self-photosynthesis in engineered Escherichia coli cells, which were originally designed for bioremediation, with in situ biosynthesis of biocompatible cadmium sulfide nanoparticles using a surface-display system. We also introduced a biomimetic silica encapsulation strategy into the engineered E. coli cells, enabling this hybrid system to continuously produce hydrogen for 96 hours, even under natural aerobic conditions. This biohybrid catalytic approach may serve as a general strategy for solar-to-chemical production.
INTRODUCTION
The immoderate consumption of fossil fuels has caused many serious environmental problems, such as the greenhouse effect, global climate change, acid rain, and ozone depletion, which may greatly restrict the economic and social development of humans. The utilization of renewable and clean energy resources is highly desirable to address these global problems. Solar energy is the most important renewable energy resource on Earth, but it is difficult to harness. Thus, strategies exploiting the efficiency of photosynthesis for solar energy capture to enable the sustainable production of chemicals, such as hydrogen or other fuels, are urgently needed (1, 2). Using H2 production as an example, several innovative inorganic-biological hybrid systems that combine the light-harvesting capabilities of photosystem I or a semiconductor and the catalytic power of the hydrogenase enzyme or a metal catalyst have been developed in the past two decades (3–5). Notably, King and colleagues (6–8) developed a series of highly efficient biohybrid hydrogen production systems by using CdTe nanocrystals/cadmium sulfide (CdS) nanorods and purified [Fe-Fe]-hydrogenase from Clostridium acetobutylicum. However, because of the oxygen-sensitive nature of these reactions, the low yield of the isolated hydrogenase, and the high cost of precious metals, the efficiency of photocatalytic H2 production by these bioinorganic hybrid systems requires further improvement.
Whole bacterial cells have been used as biohybrid catalysts for H2 production to overcome the limitations of both enzymes and synthetic catalysts in bioinorganic hybrid systems. Using this approach, several research groups have produced H2 by hybridizing a titanium dioxide (TiO2) semiconductor with wild-type bacterial cells (9). Recently, the significant breakthrough reported by Honda et al. (10) showed that recombinant Escherichia coli cells expressing both hydrogenase and maturase genes enabled whole-cell photocatalytic H2 production with TiO2. However, the low biocompatibility of semiconductors and the oxygen intolerance of hydrogenases limit the use of this whole-cell system for practical H2 production.
Researchers have been working to optimize hybrid systems to protect the catalytic activity from oxygen stress and thus overcome these limitations. Notably, Douglas et al. (11) reported a smart self-assembling biomolecular catalyst for H2 production using the bacteriophage P22 coat protein to encapsulate and protect the oxygen-tolerant [NiFe]-hydrogenase. Meanwhile, inspired by biomimetic mineralization, Tang et al. (12) developed a powerful silicification-induced green algae system for sustainable photobiological H2 production under aerobic conditions. Inspired by these pioneering studies, we propose the development of an ideal whole-cell photocatalytic hydrogen production system that incorporates the following components: (i) a biocompatible light-harvesting inorganic semiconductor, (ii) active engineered E. coli cells as a biocatalyst, and (iii) a reliable shell to protect the reaction from oxygen. Here, we aim to develop engineered E. coli cells that combine the biosynthetic capability of CdS nanoparticles with surface-displayed heavy metal–binding proteins and the biocatalysis of oxygen-tolerant [NiFe]-hydrogenase. Combined with the biomimetic silicon encapsulation of whole E. coli cells, the bioinorganic hybrid system achieves photocatalytic hydrogen production under aerobic conditions (Fig. 1).
Fig. 1. Proposed surface-display biohybrid approach to light-driven hydrogen production in air.
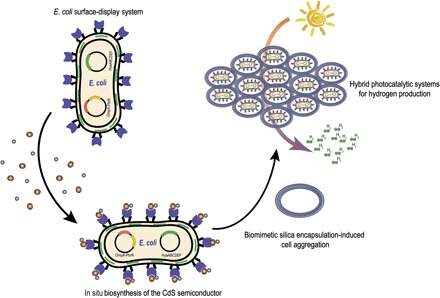
RESULTS
Biosynthesis of CdS nanoparticles
We began by exploring the biosynthesis of CdS nanoparticles by engineered E. coli cells. CdS is a well-studied semiconductor, and its photocatalytic activity has been thoroughly validated in bioinorganic hybrid systems (7, 13, 14). However, the synthesis of this semiconductor requires a complicated process and expensive reagents. The biocompatibility of the added exogenous metal materials also influences the efficiency of the biotic reactor (15). Yang et al. (16) achieved pivotal progress by pioneering a hybrid strategy for the photosynthesis of acetic acid from carbon dioxide using the nonphotosynthetic bacterium Moorella thermoacetica, which exhibits a unique metabolic pathway for biologically precipitated CdS nanoparticles. Inspired by the study of Yang and colleagues (17, 18), we realized that our previously engineered E. coli cells, which display a lead-specific binding protein PbrR on their surface, may perform a similar function as M. thermoacetica. The PbrR protein contains three conserved cysteine residues that coordinate the Pb(II) metal center through a unique hemi-directed geometry. The display of the PbrR protein on the E. coli cell surface permits the selective adsorption of both lead and cadmium ions and formed PbS and CdS nanoparticles on the outer membrane of the cells (19). Moreover, as the most important microorganism used for genetic engineering, E. coli has been well-studied in terms of its genomic and metabolic mechanisms and has become the most important cellular chassis in synthetic biology. Thus, the application of photosynthetic biohybrid systems to E. coli cells is highly desirable, thus expanding the scope of their applications.
Subsequently, we studied E. coli cells displaying PbrR on their surfaces for use in the biological precipitation of CdS nanoparticles. The fusion protein expression plasmid including E. coli outermembrane protein A (OmpA) and the PbrR protein, which was constructed as described in a previous study (19), was transformed into E. coli strain BL21 (Fig. 2A). The expression of the surface-displayed PbrR protein was induced with arabinose, and the biological precipitation of CdS nanoparticles was trigged by adding Cd2+ to the culture medium. We used transmission electron microscopy with energy-dispersive x-ray spectroscopy (TEM-EDX) to observe the precipitation of the CdS nanoparticles. As shown in the TEM images, cadmium ions accumulated on the cell surface and formed uniform nanoparticle clusters <50 nm in size (Fig. 2B), which was also observed in the study of the M. thermoacetica–CdS hybrid system by Yang et al. (16). Furthermore, the EDX analysis of a randomly chosen area encompassing a portion of the CdS nanoparticles confirmed the elemental composition (Fig. 2C). The amount of biosynthesized CdS nanoparticles on the PbrR-overexpressing E. coli cell surface was measured using inductively coupled plasma mass spectrometry (ICP-MS) and reached 8.09 ± 0.70 μg/108 cells after 24 hours (fig. S1), a value that was much higher than that on cells that were not displaying PbrR (19). These data confirmed the surface-displayed PbrR-mediated biological precipitation of CdS nanoparticles on the outer membranes of the cells.
Fig. 2. Hydrogen photosynthesis by the E. coli cell–CdS hybrid system.
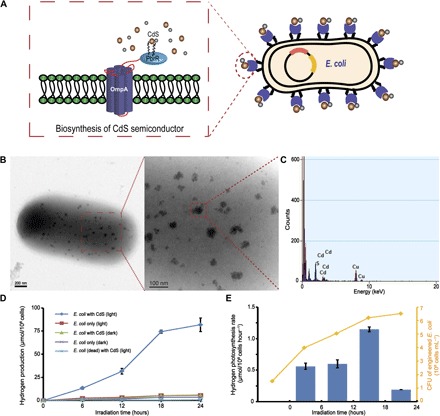
(A) Detailed diagram of an engineered E. coli cell. (B) TEM images of biosynthesized CdS nanoparticles on the surface of an engineered E. coli cell. (C) EDX confirmation of randomly chosen CdS nanoparticle. (D) H2 production by the hybrid system under anaerobic conditions. (E) Changes in the rate of H2 production by the hybrid system during irradiation. SDs represent the averages of three independent experiments. CFU, colony-forming units.
Hydrogen photosynthesis by the E. coli cell–CdS hybrid system
We performed ultraviolet-visible (UV-vis) spectral measurements to directly determine the optical band gap energy of these CdS nanoparticles and the photocatalytic capability for the biological precipitation of CdS nanoparticles on the outer membranes of the bacterial cells (7, 14, 16). The lowest-energy transition of the biosynthesized CdS nanoparticles was detected in the visible region of the solar spectrum (Eg = 2.92 eV, λabsorption = 424 nm; fig. S2), confirming the photocatalytic ability of the in situ biosynthesized CdS nanoparticles. The reaction solution also contained ascorbic acid and methylviologen (MV2+). Ascorbic acid is commonly used as the sacrificial electron donor to regenerate CdS nanoparticles (7, 10, 14, 20). The redox dye MV2+ is a well-established electron mediator. In combination with MV2+, the semiconductor/bacterial cell hybrid system easily served as a biocatalyst for H2 photosynthesis (fig. S3) (9, 10, 21). The concentrations of reduced MV in various experimental groups were measured under anaerobic conditions, confirming that the CdS nanoparticles precipitated on the engineered E. coli cells adsorb a photon of light and transfer an electron to MV2+ (fig. S4). The amount of H2 produced by this hybrid system under anaerobic conditions was detected using gas chromatography (GC). In the absence of light, trace amounts of H2 were produced by the engineered E. coli cells in the presence or absence of CdS due to the anaerobic fermentation mediated by the endogenously expressed hydrogenase (22, 23). However, under illumination with a 350-W Xe lamp, the engineered E. coli cells (1 × 108) carrying CdS nanoparticles formed nearly 13.40 ± 1.20 μmol H2 after 6 hours and up to 81.80 ± 7.39 μmol after 24 hours, a value that was significantly higher than the values of the other treatments (Fig. 2D). In addition, the rate of H2 photosynthesis in this hybrid system increased steadily from 0.56 to 1.15 μmol/108 cells per hour in the first 18 hours and then began to decrease due to bacterial death (Fig. 2E). On the basis of these results, H2 production by our engineered E. coli cell–CdS hybrid system under anaerobic conditions was directly related to the biosynthesized CdS semiconductors and the activity of the whole-cell biocatalyst.
Biomimetic silica encapsulation of the hybrid system
We next aimed to generate the semiconductor–E. coli cell hybrid system under aerobic conditions for more convenient applications. Some living organisms have developed specific mineral structures that provide extra protection and unique functions through biomimetic mineralization (24, 25). Recently, biomimetic silica encapsulation based on layer-by-layer (LbL) assembly was successfully used for the cell surface modifications of living organisms such as yeast, algae, and bacteria (12, 26–28). Inspired by these pioneering studies, we introduced this cell encapsulation strategy into our semiconductor–E. coli hybrid system for hydrogen production in air. Using the reported LbL self-assembly method, we coated E. coli cells with cationic polyelectrolyte poly(diallyldimethylammonium chloride) (PDADMAC) and anionic polyelectrolyte sodium polystyrene sulfonate (PSS). The biomimetic silica-encapsulated cells were formed by placing surface-coated cells into a medium containing 50 mM silicic acid (26). According to Tang et al. (12), the cell aggregation induced by silica encapsulation leads to a specific phenomenon termed spatial-functional differentiation (SFD). Upon SFD, cells in the shell and the mantle layer of the aggregate gradually consume oxygen through aerobic respiration, producing an anaerobic environment for the E. coli cells in the core. Consequently, the cells in the core are forced to undergo anaerobic metabolism, which protects the catalytic activity of the expressed hydrogenase (Fig. 3A). We used O2 microsensors to examine the interior environment and confirm the microstructural features of our encapsulated E. coli aggregates (Fig. 3B). In the 2000-μm aggregates, the O2 concentration decreased as the probe depth increased due to cell aggregation and aerobic metabolism. In the core of the aggregates, the O2 concentration was approximately zero, suggesting that overexpressed hydrogenase was activated to produce hydrogen (Fig. 3B). The scanning electron microscopy (SEM) images also show a visual representation of the encapsulated E. coli aggregates (Fig. 3, C and D). The encapsulation essentially involves the dehydration of silicic acid, which mineralizes into silicon dioxide and acts as a glue to aggregate single E. coli cells into microspheres.
Fig. 3. Light-driven hydrogen production by the encapsulated hybrid system in air.
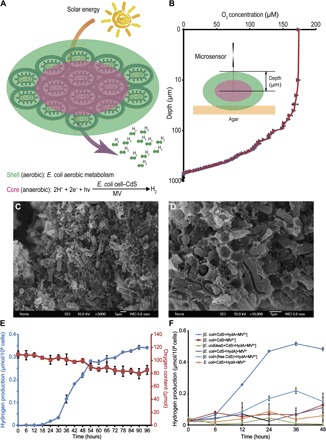
(A) SFD in the semiconductor–engineered E. coli hybrid system encapsulated by biomimetic polymers. (B) Microsensor-based measurement of O2 concentration in the various independent encapsulated cell aggregates (n = 3). (C and D) SEM images of encapsulated cell aggregates at different magnification. (E) Measurements of continuous hydrogen production in our biohybrid system. (F) Measurements of the amount of hydrogen produced by the various biohybrid systems under aerobic conditions ([] indicates silicification-induced aggregation). HydA, [NiFe]-hydrogenase HyaABCDEF.
Light-driven hydrogen production in air
Finally, we measured the amount of H2 produced by the encapsulated hybrid system under aerobic conditions. Because of the intrinsic oxygen sensitivity of E. coli hydrogenase, we transformed the [NiFe]-hydrogenase HyaABCDEF plasmid into the engineered E. coli. Protein expression was induced by adding isopropyl-β-d-thiogalactoside (IPTG) after encapsulation. Hydrogen production by the hybrid system containing E. coli/CdS/hydrogenase/MV2+ was monitored for 96 hours to test its ability to continuously produce hydrogen. We induced the expression of the surface-displayed PbrR protein and recombinant hydrogenase immediately after cell aggregation to extend the activities of the engineered E. coli cells. After the proteins were allowed to overexpress for 18 hours, we harvested the aggregates and resuspended them in a reaction solution containing 100 mM NaCl. Although 94.98 ± 5.27 μmol oxygen was present in the system, H2 production was significantly increased at 18 hours and steadily increased over the next 72 hours to 0.34 ± 0.01 μmol/108 cells (Fig. 3E).
The amount of H2 produced by different samples was detected using GC to examine the ability of encapsulation to protect the catalytic activity. As shown in Fig. 3F, H2 production was significantly increased 6 hours after hydrogenase overexpression in the encapsulated hybrid system under aerobic conditions. H2 production steadily increased and reached 0.52 ± 0.01 μmol/108 cells at 36 hours. In contrast, H2 production was not detected in the samples without silica encapsulation, indicating that encapsulation is required for H2 production under aerobic conditions. Meanwhile, the amount of H2 produced by hybrid samples in which E. coli cells were inactivated or hydrogenase expression was not induced was also analyzed. The catalytic activity of O2-intolerant hydrogenase experienced the greatest benefit from silicification-induced cell aggregation.
We measured hydrogen production in hybrid systems supplemented with MV2+ after cell encapsulation under aerobic conditions to probe whether the reduced MV is protected from O2 in the aggregates. If MV2+ was added after cell encapsulation and exposed to aerobic conditions during the reaction, then the hybrid system produced almost no hydrogen (Fig. 3F). Thus, both hydrogenase and MV2+ were protected from oxygen in the aggregates.
We added the same amount of free CdS nanoparticles to the reaction mixture, instead of the in situ biosynthesized CdS nanoparticles, under aerobic conditions to determine the photocatalytic activity of the free CdS nanoparticles in our biohybrid system. The system containing free CdS nanoparticles produced 0.22 ± 0.02 μmol H2/108 cells, implying a lower catalytic efficiency than in situ biosynthesized CdS nanoparticles.
DISCUSSION
Efficient solar-to-chemical conversion strategies are greatly needed to address global energy and environmental problems. One important challenge is the development of simple and practical photosynthetic systems for the production of hydrogen or other fuels by exploring the fundamental chemistry of photosynthesis. Here, we developed a photocatalytic hydrogen biosynthesis strategy based on a semiconductor–engineered E. coli biohybrid system encapsulated with biomimetic polymers that function under aerobic conditions. Unlike reported microorganisms that induce nanoparticle precipitation through natural metabolic pathways, the surface-displaying bacterial system applied here facilitates the controllable biosynthesis of biocompatible CdS semiconductors by the model organism E. coli under mild conditions. In addition, our results further verified the light-harvesting capability of biosynthesized CdS semiconductors. This strategy could be applied to another well-established biological chassis, such as Bacillus or yeast, to expand its applications. The self-aggregated E. coli cells protect the activity of an oxygen-intolerant enzyme, ensuring that the enzyme performs efficient biological whole-cell catalysis under simple and mild conditions in air. Together, this bioinorganic hybrid system based on bacterial surface display and biomimetic silica encapsulation technologies will likely become an alternative approach for the convenient utilization of solar energy.
MATERIALS AND METHODS
In vivo expression of hydrogenase
E. coli BL21 transformed with the expression plasmid pET28a/HyaABCDEF was cultured in LB medium supplemented with kanamycin (50 μg/ml) at 37°C. When the optical density at 600 nm (OD600) reached 0.6, cultures were transferred to a Coy anaerobic chamber (Coy Laboratory Products Inc.) with 95% N2 and 5% H2 gas. During anaerobic growth, cultures were supplemented with a sterile solution containing 1 mM IPTG, 1 mM (NH4)2Fe(SO4)2, and 1 mM NiCl2 in LB medium (all v/v, 1:1000). Cultures were incubated at room temperature under anaerobic conditions for 12 hours, with stirring.
Surface display of the PbrR protein and in situ synthesis of CdS nanoparticles
The construction of the OmpA expression plasmid and methods used to overexpress OmpA-PbrR fusion proteins were previously described by Zhao et al. (19). For the in situ synthesis of CdS nanoparticles, 100 μM cadmium ions were added to LB medium during the induction of the expression of OmpA-PbrR fusion proteins. Then, sample cells were harvested from LB medium every 6 hours by centrifugation (4000 rpm for 10 min) and washed with a 100 mM NaCl solution at least three times before analysis. The number of cells in each sample was determined by monitoring the OD600. The CdS-adsorbed cells were lyophilized and subjected to wet ashing. All samples were further analyzed using ICP-MS to measure the amount of biosynthesized CdS nanoparticles on the engineered E. coli cell surface. E. coli cells containing biosynthesized CdS nanoparticles were harvested after 12 hours of induction for the subsequent analysis of biohydrogen production to maintain the best activity.
TEM-EDX analysis of CdS nanoparticles
All TEM images were recorded using a JEOL JEM-2100 electron microscope at an accelerating bias voltage of 200 kV. After cadmium ion adsorption, the engineered cells were dispersed in double-distilled H2O. A thick carbon film (20 to 30 nm) on a copper grid was dipped into the solution for 1 s, dried under atmospheric conditions, and then examined using TEM. The elemental analysis was performed with the EDX system (EDAX, AMETEK) attached to the microscope.
Characterization of biologically precipitated CdS nanoparticles
The photocatalytic MV2+ reduction assay was performed using a 10-mm quartz cuvette with a cap and a light source (350-W Xe lamp). E. coli cells containing biosynthesized CdS nanoparticles were harvested from LB medium by centrifugation (4000 rpm for 10 min). The reaction system consisted of the same amounts of different semiconductors [TiO2 anatase (10) and synthesized free CdS nanoparticles (29)] and 3 ml of 100 mM tris-HCl (pH 7), 150 mM NaCl, 5% glycerol, 100 mM ascorbic acid, and 5 mM MV2+ in the quartz cuvette. O2 was removed by bubbling N2 into the solution for 30 min. The reaction was initiated by light irradiation and stopped by centrifugation and separation of E. coli–CdS nanoparticles from the MV buffer. The absorption spectra were immediately measured after centrifugation (1000g for 1 min). The amount of reduced MV2+ (MV+) that formed was calculated by monitoring the OD605 using the molar conversion coefficient ε = 1.3 × 104 M−1 cm−1. The UV-vis spectra of the E. coli–CdS hybrids in the solution were also obtained. The conduction band energy of the photoexcited CdS was determined using the Nernst equation.
Measurements of photocatalytic H2 production
Photocatalytic H2 production was measured using an external light source (350-W Xe lamp). Under anaerobic conditions in an anaerobic chamber (Coy Laboratory Products Inc.), 20 ml of LB medium containing 1 mM (NH4)2Fe(SO4)2, 1 mM NiCl2, 100 mM ascorbic acid, and 5 mM MV2+, and engineered E. coli cells were prepared in 100-ml serum bottles. The system was evacuated to remove the N2 and H2 from the glove box atmosphere. The in situ synthesis of CdS nanoparticles and overexpression of hydrogenase were successively induced by adding IPTG after the reaction was initiated. The amount of H2 produced was analyzed by GC after direct injection onto the gas sampler using a microsyringe. Samples were then injected into a gas chromatograph (Kejie GC5890C, with silica gel column and thermal conductivity detector sensor) equipped with a thermal conductivity detector for the simultaneous determination of the concentrations of H2, O2, and N2. For measurements under aerobic conditions, the reaction system prepared under anaerobic conditions was subjected to biomimetic silica encapsulation.
Biomimetic silica encapsulation
The engineered E. coli cells containing CdS nanoparticles were collected by centrifugation and then subjected to the biomimetic silica encapsulation protocol described by Choi et al. (26). The engineered cells were alternately immersed in the PDADMAC solution and the PSS solution for 5 min per step. After the LbL process, multilayer-coated cells were placed in the 50 mM silicic acid solution. The encapsulated biohybrid aggregates were finally harvested by centrifugation and uniformly dispersed into 2000-μm particles (fig. S5).
Microsensor measurements
The O2 microsensor was an OX25 microsensor (Unisense) with a 25-μm tip diameter. The O2 concentrations in the aggregated E. coli were detected by piercing the aggregates with the O2 microelectrodes. The freshly prepared aggregated E. coli cells were used for measurements of hydrogen production for at least 12 hours under aerobic conditions. Then, the aggregates were harvested from the reaction system. A single layer of aggregates was fixed on the agar substrate (1 weight % agar), and the step size of the micromanipulator was 1 μm when the tips of the microsensors pierced the aggregates.
Supplementary Material
Acknowledgments
Funding: Financial support was provided by the National Science Foundation of China (nos. 21622103, 21571098, and 21671099), the Natural Science Foundation of Jiangsu Province (no. BK20160022), and the Fundamental Research Funds for the Central Universities (nos. 020514380117 and 020814380002). Author contributions: W.W. and P.S. designed the experiments. W.W., P.S., Z.L., K.S., W.S., and B.W. performed the experiments. W.W., P.S., Z.L., Y.L., and J.Z. wrote the manuscript, and J.Z. and W.W. supervised the research. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/2/eaap9253/DC1
fig. S1. The amount of biosynthesized CdS nanoparticles on the engineered E. coli cell surface was measured by ICP-MS.
fig. S2. Characterization of biologically precipitated CdS nanoparticles on the outer membranes of E. coli cells.
fig. S3. Photon transfer by in situ biosynthesized CdS nanoparticles.
fig. S4. Quantitative comparison of the photoelectrical capacity of an in situ biosynthesized CdS nanoparticle.
fig. S5. Image of encapsulated hybrid aggregates.
REFERENCES AND NOTES
- 1.Blankenship R. E., Tiede D. M., Barber J., Brudvig G. W., Fleming G., Ghirardi M., Gunner M. R., Junge W., Kramer D. M., Melis A., Moore T. A., Moser C. C., Nocera D. G., Nozik A. J., Ort D. R., Parson W. W., Prince R. C., Sayre R. T., Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Larkum A. W. D., Limitations and prospects of natural photosynthesis for bioenergy production. Curr. Opin. Biotechnol. 21, 271–276 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Ihara M., Nishihara H., Yoon K.-S., Lenz O., Friedrich B., Nakamoto H., Kojima K., Honma D., Kamachi T., Okura I., Light-driven hydrogen production by a hybrid complex of a [NiFe]-hydrogenase and the cyanobacterial photosystem I. Photochem. Photobiol. 82, 676–682 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Reisner E., Powell D. J., Cavazza C., Fontecilla-Camps J. C., Armstrong F. A., Visible light-driven H2 production by hydrogenases attached to dye-sensitized TiO2 nanoparticles. J. Am. Chem. Soc. 131, 18457–18466 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Utschig L. M., Dimitrijevic N. M., Poluektov O. G., Chemerisov S. D., Mulfort K. L., Tiede D. M., Photocatalytic hydrogen production from noncovalent biohybrid photosystem I/Pt nanoparticle complexes. J. Phys. Chem. Lett. 2, 236–241 (2011). [Google Scholar]
- 6.Brown K. A., Dayal S., Ai X., Rumbles G., King P. W., Controlled assembly of hydrogenase-CdTe nanocrystal hybrids for solar hydrogen production. J. Am. Chem. Soc. 132, 9672–9680 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Brown K. A., Wilker M. B., Boehm M., Dukovic G., King P. W., Characterization of photochemical processes for H2 production by CdS nanorod–[FeFe] hydrogenase complexes. J. Am. Chem. Soc. 134, 5627–5636 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Wilker M. B., Shinopoulos K. E., Brown K. A., Mulder D. W., King P. W., Dukovic G., Electron transfer kinetics in CdS nanorod–[FeFe]-hydrogenase complexes and implications for photochemical H2 generation. J. Am. Chem. Soc. 136, 4316–4324 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Gurunathan K., Photobiocatalytic production of hydrogen using sensitized TiO2–MV2+ system coupled Rhodopseudomonas capsulata. J. Mol. Catal. A Chem. 156, 59–67 (2000). [Google Scholar]
- 10.Honda Y., Hagiwara H., Ida H., Ishihara T., Application to photocatalytic H2 production of a whole-cell reaction by recombinant Escherichia coli cells expressing [FeFe]-hydrogenase and maturases genes. Angew. Chem. Int. Ed. 55, 8045–8048 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Jordan P. C., Patterson D. P., Saboda K. N., Edwards E. J., Miettinen H. M., Basu G., Thielges M. C., Douglas T., Self-assembling biomolecular catalysts for hydrogen production. Nat. Chem. 8, 179–185 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Xiong W., Zhao X., Zhu G., Shao C., Li Y., Ma W., Xu X., Tang R., Silicification-induced cell aggregation for the sustainable production of H2 under aerobic conditions. Angew. Chem. Int. Ed. 54, 11961–11965 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Vogel R., Hoyer P., Weller H., Quantum-sized PbS, CdS, Ag2S, Sb2S3, and Bi2S3 particles as sensitizers for various nanoporous wide-bandgap semiconductors. J. Phys. Chem. 98, 3183–3188 (1994). [Google Scholar]
- 14.Brown K. A., Harris D. F., Wilker M. B., Rasmussen A., Khadka N., Hamby H., Keable S., Dukovic G., Peters J. W., Seefeldt L. C., King P. W., Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid. Science 352, 448–450 (2016). [DOI] [PubMed] [Google Scholar]
- 15.King P. W., Designing interfaces of hydrogenase–nanomaterial hybrids for efficient solar conversion. Biochim. Biophys. Acta 1827, 949–957 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Sakimoto K. K., Wong A. B., Yang P., Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 351, 74–77 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Chen P. R., Wasinger E. C., Zhao J., van der Lelie D., Chen L. X., He C., Spectroscopic insights into lead(II) coordination by the selective lead(II)-binding protein PbrR691. J. Am. Chem. Soc. 129, 12350–12351 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Huang S., Liu X., Wang D., Chen W., Hu Q., Wei T., Zhou W., Gan J., Chen H., Structural basis for the selective Pb(II) recognition of metalloregulatory protein PbrR691. Inorg. Chem. 55, 12516–12519 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Wei W., Liu X., Sun P., Wang X., Zhu H., Hong M., Mao Z.-W., Zhao J., Simple whole-cell biodetection and bioremediation of heavy metals based on an engineered lead-specific operon. Environ. Sci. Technol. 48, 3363–3371 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Borsook H., Keighley G., Oxidation-reduction potential of ascorbic acid (vitamin C). Proc. Natl. Acad. Sci. U.S.A. 19, 875–878 (1933). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demuez M., Cournac L., Guerrini O., Soucaille P., Girbal L., Complete activity profile of Clostridium acetobutylicum [FeFe]-hydrogenase and kinetic parameters for endogenous redox partners. FEMS Microbiol. Lett. 275, 113–121 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Nandi R., Sengupta S., Microbial production of hydrogen: An overview. Crit. Rev. Microbiol. 24, 61–84 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Trchounian A., Mechanisms for hydrogen production by different bacteria during mixed-acid and photo-fermentation and perspectives of hydrogen production biotechnology. Crit. Rev. Biotechnol. 35, 103–113 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Hamm C. E., Merkel R., Springer O., Jurkojc P., Maier C., Prechtel K., Smetacek V., Architecture and material properties of diatom shells provide effective mechanical protection. Nature 421, 841–843 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Nudelman F., Sommerdijk N. A. J. M., Biomineralization as an inspiration for materials chemistry. Angew. Chem. Int. Ed. 51, 6582–6596 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Yang S. H., Lee K.-B., Kong B., Kim J.-H., Kim H.-S., Choi I. S., Biomimetic encapsulation of individual cells with silica. Angew. Chem. Int. Ed. 48, 9160–9163 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Chen W., Wang G., Tang R., Nanomodification of living organisms by biomimetic mineralization. Nano Res. 7, 1404–1428 (2014). [Google Scholar]
- 28.Xiong W., Yang Z., Zhai H., Wang G., Xu X., Ma W., Tang R., Alleviation of high light-induced photoinhibition in cyanobacteria by artificially conferred biosilica shells. Chem. Commun. 49, 7525–7527 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Yan H., Yang J., Ma G., Wu G., Zong X., Lei Z., Shi J., Li C., Visible-light-driven hydrogen production with extremely high quantum efficiency on Pt–PdS/CdS photocatalyst. J. Catal. 266, 165–168 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/2/eaap9253/DC1
fig. S1. The amount of biosynthesized CdS nanoparticles on the engineered E. coli cell surface was measured by ICP-MS.
fig. S2. Characterization of biologically precipitated CdS nanoparticles on the outer membranes of E. coli cells.
fig. S3. Photon transfer by in situ biosynthesized CdS nanoparticles.
fig. S4. Quantitative comparison of the photoelectrical capacity of an in situ biosynthesized CdS nanoparticle.
fig. S5. Image of encapsulated hybrid aggregates.


