Abstract
Background
Environmental chemical exposures have been implicated in pediatric kidney disease. No appraisal of the available evidence has been conducted on this topic.
Methods
We performed a systematic review of the epidemiologic studies that assessed association of environmental exposures with measures of kidney function and disease in pediatric populations. The search period went through July 2016.
Results
We found 50 studies that met the search criteria and were included in this systematic review. Environmental exposures reviewed herein included lead, cadmium, mercury, arsenic, fluoride, aflatoxin, melamine, environmental tobacco, bisphenol A, dental procedures, phthalates, ferfluorooctanoic acid, triclosan, and thallium/uranium. Most studies assessed environmental chemical exposure via biomarkers but four studies assessed exposure via proximity to emission source. There was mixed evidence of association between metal exposures, and other non-metal environmental exposures and pediatric kidney disease and other kidney disease biomarkers. The evaluation of causality is hampered by the small numbers of studies for each type of environmental exposure, as well as lack of study quality and limited prospective evidence.
Conclusion
There is a need for well-designed epidemiologic studies of environmental chemical exposures and kidney disease outcomes.
Keywords: Epidemiology, Kidney disease, Environmental exposures, Children, Metals
1. Introduction
Environmental chemical exposure remains a major global public health problem. The United States Environmental Protection Agency (EPA) estimates that thousands of chemicals are in use, but only a fraction have detailed toxicological data. Over 200 environmental chemicals can be detected in the blood and urine of the general population of the United States (Centers for Disease Control and Prevention, 2017; Buser and Ingber, 2017). It is estimated that nearly 25–33% of the global disease burden can be attributed to environmental risk factors.(Smith et al, 1999) Globally, the World Health Organization calculated that pollution is responsible for nearly 7 million deaths per year (World Health Organization, 2016). Patterns of environmental exposure differ across countries geographically and by per capita income levels, but environmental exposure remains a major cause of morbidity and mortality worldwide (Landrigan et al, 2016).
Children are particularly susceptible to environmental exposures compared to adults (Firestone and Amler, 2003). Firstly, children consume more food, water, and air per pound of body weight compared to adults and therefore are proportionally exposed to more environmental toxicants (Au, 2002, Firestone and Amler, 2003, Sly and Flack, 2008). Moreover, an individual’s susceptibiliy to environmental chemical exposure is greatest during “:windows of vulnerability,” the specific time periods when complex organs, pathways, and connections are being established (i.e. prenatal and early life development) (Selevan et al., 2000). Exposure to environmental chemicals during these periods can impact cell signaling and alter development. Due to physiological differences between children and adults, children more easily absorb toxicants such as lead, and have decreased elimination abilities. The blood-brain barrier is not fully developed for the first 36 months of life, leaving young children more susceptible to neruotoxicants (Sly and Flack, 2008). Taken together, these facts suggest that environmental chemical exposure in childhood poses substantial public health concern.
There is evidence to suggest that environmental exposures play a role in the development of Chronic Kidney Disease (CKD), a disease defined by reduced glomerular filtration rate, increased urinary albumin excretion, or both (Jha et al., 2013). CKD poses a significant and worldwide public health problem, therefore identification of preventable risk factors for CKD, including childhood environmental exposures, could contribute to a decrease in worldwide CKD incidence. CKD has a global prevalence of approximately 10.4% in men and 11.8% in women (Mills et al., 2015). Currently, CKD mortality is increasing worldwide.(Rhee and Kovesdy, 2015; GBD 2013 Mortality and Causes of Death Collaborators, 2015). As of 2005–2010, the prevalence of CKD in the United States is 13.1% (U.S. Renal Data System, 2013). Various studies have estimated a CKD prevalence of 15–74.7 cases per million children (Wong et al., 2012; Warady and Chadha, 2007). Children with CKD are at risk for increased morbidity and mortality, and poorer quality of life (Wong et al., 2012). The prevalence of End Stage Renal Disease (ESRD), a stage of chronic irreversible renal failure, in children (aged 19 and younger) has increased by 10.1% since 2000 (U.S. Renal Data System, 2013). ESRD in childhood carries a significant physical and emotional impact, and all-cause hospitalizations in this population is increasing, as is ESRD mortality among younger children (U.S. Renal Data System, 2013). The life expectancy for children with ESRD with dialysis treatment is poor, and mortality rates are 30–150 times higher than the general pediatric population (Warady and Chadha, 2007; McDonald et al., 2004). Kidney transplantation is the treatment of choice for children with ESRD.
Several environmental exposures, including metals such as lead and cadmium are known nephrotoxicants with clear associations with kidney disease (Soderland et al., 2010). Some metals such as cadmium and lead are associated with increased levels of biomarkers of poor kidney function in adults (Navas-Acien et al., 2009; Buser et al., 2016). Early studies demonstrated an association between increased levels of metals such as mercury in the kidneys of stillborn children (Lutz et al., 1996). Environmental arsenic exposure is associated with kidney disease outcomes in adults (Zheng et al., 2014) as well as in adolescents and young adults (Weidemann et al., 2015a). A study from Bangladesh found that maternal arsenic exposure was associated with a decrease in infant kidney function, although this was not statistically significant (Hawkesworth et al., 2013). A study from Chile found that adults with in utero and childhood exposure had a higher risk of chronic renal disease (Smith et al., 2012). Studies in the United States have found that higher blood lead levels are associated with a decrease in kidney function in children and teenagers (Fadrowski et al., 2010).
However, there remains limited research regarding environmental exposures in children, as most of the research into environmental risk factors for CKD has been conducted in adult populations (Weidemann et al., 2015b). Several narrative reviews of environmental exposures and pediatric renal disease (Chadha and Warady, 2005, Warady and Chadha, 2007, Soderland et al., 2010, Weidemann et al., 2015b, Kataria et al., 2015b) have been recently published, but not yet as a systematic review.
To evaluate the potential relationship between environmental exposures and kidney function outcomes in children, such as estimated glomerular filtration rate (GFR), proteinuria, albuminuria, and urinary biomarkers of tubular damage such as β-2-microglobulin and N-acetyl-β-D-glucosaminidase (NAG), we conducted a review of epidemiologic studies that have previously investigated the association between any environmental exposure via geography, environment, or biomarkers, and CKD endpoints. We also considered studies featuring urinary markers of kidney damage, such as β-2-microglobulin (β2MG), N-acetyl-β-D-glucosaminidase (NAG), α-1-microglobulin (A1M) and retinol binding protein (RBP), as well as many other biomarkers.
2. Materials and methods
We searched the PubMed (http://www.ncbi.nlm.nih.gov/pubmed) database to find published observational studies that evaluated the relationship between an environmental exposure and CKD status and/or kidney function markers. We used free text as well as Medical Subject Heading (MeSH) terms (Fig. 1). The search period was through July 2016 with no language restrictions. Eleven studies were identified manually (Gossner et al., 2009, Guan et al., 2009, Hawkesworth et al., 2013, Hu, 1991, Kooijman et al., 2015, Loghman-Adham, 1998, Moel and Sachs, 1992, Noonan et al., 2002, Ramirez-Rubio et al., 2015, Weidemann et al., 2015a). Two investigators independently reviewed each publication identified through the search and applied the study selection criteria. Epidemiologic studies with individual level or community level data on environmental exposures were included. Reviews, case series and case reports, animal and experimental studies, studies without an environmental exposure or a kidney disease outcome, and studies in non-children populations were excluded.
Fig. 1.

Search query in PubMed.
The two investigators (LZ and APS) independently abstracted the study data, including design, location, age and gender distribution, sample size, biomarker assessment, outcome ascertainment, exposure levels, study results, and adjustment factors. Studies were classified as those conducted with metals or “other” as the environmental exposure (s) of interest. For studies with multiple levels of adjustment, we abstracted and reported the measure of association adjusted for the most covariates. Study quality was evaluated using criteria reported by Longnecker et al. (1988) as performed in previous systematic reviews (Zheng et al., 2014). We confirmed our criteria with the PRISMA checklist for completeness of systematic review findings (Moher et al., 2010).
The authors concluded that the studies were of limited quality and too diverse in outcome measures, exposure levels, and too diverse in exposures to allow for meaningful meta-analysis of all studies (Pittler, 2002). Data were abstracted for summary tables and study quality evaluation tables and categorized by exposure types.
3. Results
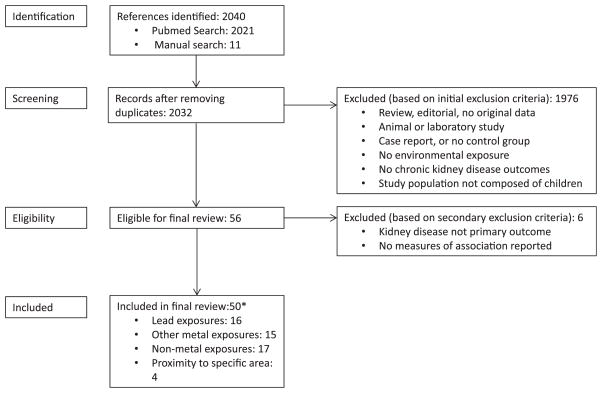
There were a total of 50 studies published between 1979 and 2016 that met our inclusion criteria (Fig. 2). Most were published in English, four (4) were published in Russian and two (2) were published in Chinese. These publications were translated by Russian or Chinese speakers before inclusion. Some publications were considered together (Bellinger et al., 2006; Geier et al, 2013; Hawkesworth et al., 2013; Barregard et al., 2008; DeRouen et al., 2006; Skroder et al., 2015; Woods et al., 2008; Kooijman et al., 2015; Taal et al., 2011), as they used the same study population and provided complementary information. Most studies were from non-occupationally-exposed populations, one included children of occupationally exposed parents (Khan et al., 2010). The studies were conducted in the United States, Canada, Germany, Czech Republic, Poland, Kosovo, Pakistan, Senegal, Belgium, Morocco, France, Portugal, Pakistan, China, Bangladesh, Mexico, Thailand, Egypt, and Netherlands. The study designs included 32 cross-sectional, 6 prospective cohort, 2 retrospective cohort, 4 case-control, and 2 randomized clinical trials (representing 6 publications) (Tables 1–4).
Fig. 2. Summary of search and screening process.
*Number may not sum to total since some studies examined multiple exposures
Table 1.
Epidemiological studies of lead exposures and kidney function biomarkers.
| Reference and Country | Age, % male | Study Design | N | Outcome Ascertainment | Biomarker Assessment | Exposure Levels | Effect size (95% CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|---|
|
Fadrowski et al., 2013
United States and Canada |
Ages 1–19 years, 61% male | Prospective Cohort | 391 | Iohexol-based measured GFR | Blood lead (ICPMS) | Median (IQR) blood lead level of 1.2 (0.9, 1.8) μg/dL | Percent change (95% CI) per 1 μg/dL increase: −2.1 (−6.0, 1.8) | Age, sex, race, Hispanic ethnicity. BMI z-score, poverty status, CKD diagnosis, urine protein/creatinine ratio, blood cadmium |
|
Factor-Litvak et al, 1999
Kosovo autonomous region |
Measured at 5.5 years, % male unknown | Prospective cohort | 279 | Proteinuria via dipstick | Blood lead | Mean blood lead of 20 μg/dL in Kosovoka Mitrovica and 5 μg/dL in Pristina | Odds ratio for proteinuria comparing K. Mitrovica and Pristina of 3.5 (1.7, 7.2) | Not listed (assume age, gender. ethnic group) |
|
Moel et al, 1985
United States |
Mean age 16.1 years, 49.5% male | Case-control | 95 | Urine protein and creatinine clearance by Schwartz formula | Blood lead (AAS) | Mean (SD) blood lead levels in exposed: 14.5 (4.5) μg/dL Mean (SD) blood lead levels in controls: 11.6 (2.6) μg/dL |
Mean (SD) creatinine clearance in exposed: 127.8 (33.2) ml/min/1.73m2 Mean (SD) creatinine clearance in controls: 126.68 (27.4) ml/min/1.73m2 Mean (SD) urine protein/creatinine ratio in exposed: 0.02 (0.07) Mean (SD) urine protein/creatinine ratio in controls: 0.02 (0.08) |
None |
|
Scharer et al. 1991
Germany |
Median age of 9.8 years, 63.6% male in patients only | Case-control | 58 | Number of renal failure risk Actors | Non-carious deciduous teeth lead (AAS) | Mean(SD) of tooth lead Patients: 2.81 (0.87) μg/g Control 1:1.72 (0.33)μg/g Control 2: 1.45 (0.39)μg/g |
None | None |
|
Chan et al., 2012
China (Hong Kong)a |
Age range 1–21 years, 48.5% male | Cross-sectional | 2209 | Urine albumin levels | Blood and urine lead (ICPMS) | Range of urine lead: < 1.00–177 nmol/L Range of blood lead: 37.51–480.1 nmol/L |
Correlation coefficient between urine albumin and blood lead:−0.121 Correlation coefficient between urine albumin and urine lead: 0.151 |
None |
|
Filler et al., 2012
Canada |
Age range 1–20 years, % male unknown | Cross-sectional | 483 | Cystatic-c based eGFR | Blood lead (ICPMS) | Mean (SD) blood lead in CKD patients: 2.11 (1.58) μg/dL Median (IQR) blood lead in healthy controls: 0.635 (0.447, 0.871) μg/dL |
Regression coefficient between eGFR and levels: −0.5297, p value = 0.05 | None |
|
Bernard et al., 1995
Czech Republic |
Age range 12–15 years, 45.1% male | Cross-sectional | 195 | Urine β2MG and RBP levels | Blood lead and cadmium (AAS) | Mean (range) of blood lead (μg/L) in control area: Girls: 83.9 (42, 286) Boys: 87 (48, 146) |
Urine RBP was associated with blood lead with a regression coefficient of 0.302. Others were nonsignificant | None |
|
Fadrowski et al., 2010
United States |
Age range of 12–20 years, 50.4% male | Cross-sectional | 769 | Cystatin C and creatinine based eGFR | Blood lead (AAS) | Blood lead levels, μg/dL <1.0 1.0–1.5 1.6–2.9 >2.9 |
Mean difference (95% CI) in eGFR: 1.00 (reference) −1.4 (−7.4, 4.5) −2.6 (−7.3, 2.2) −6.6(−12.6, 0.7) |
Age, sex, race/ethnicity, urban vs rural residence, smoke exposure, obesity, income, familial education |
|
Chaumont et al., 2012 Belgium |
Median age of 15.4 years, 45.1% male | Cross-sectional | 736 | Urine albumin RBP, and β2MG levels | Blood and urine lead (ICPMS) | Median (IQR) of blood cadmium: 0.18 (0.14, 0.28) μg/L Median (IQR) of blood lead: 15.1 (11.6, 19.1) μg/L |
Mean difference in urine RBP level per increase in log-transformed urine cadmium: 0.16 (0.09, 0.24) Mean difference in urine β2MG levels per increase in log-transformed urine lead: 0.24 (0.14, 0.34) |
Sex, BMI, parental educationb (depends on model) |
|
Staessen et al., 2001 Belgium |
Mean age of 17.2 years in Peer and Hoboken, 17.8 years in Wilrijk, 40% male | Cross-sectional | 200 | Serum Cystatin C and urine β2MG levels | Blood lead, (others covered but only blood lead shown) | Mean (SD) of blood lead (nmol/L) Peer: 72.0 (65.0, 79.0) Wilrijk: 87.0 (75.0, 101) Hoboken: 132 (116, 149) |
% increase in serum cystatin C per unit increase in blood lead: 3.6 (1.5, 5.7) % increase urine β2MG per unit increase in blood lead: 16.0 (2.7, 31) |
Sex, smoking, mean atmospheric ozone, mean daily temperature week before blood samples |
|
Khan et al., 2010 Pakistan |
Median age of 4 years, 56% male | Cross-sectional (exposed vs. unexposed sites) | 246 | Serum urea and creatinine, total protein | Blood lead (ASV) | Median (range) of blood lead in exposed: 8.10 (1.0, 20.9) μg/dL Median (range) of blood lead in unexposed: 6.70 (1.4, 13.3) μg/dL |
Median (range) of serum urea in exposed: 4.5 (3.9, 5.5) mmol/L Median (range) of serum urea in unexposed: 4.3 (3.8, 4.8) mmol/L Median (range) of serum creatinine in exposed: 56 (51, 74) μmol/L Median (range) of serum creatinine in unexposed: 52 (49, 66) μmol/L |
None |
|
Laamech et al., 2014 Morocco |
Age range 6–12 years, 45.9% male | Cross-sectional (urban vs. rural) | 196 | Urine RBP and albumin | Urine cadmium, lead, and mercury (ICPMS) | Mean blood lead of 5.55 μg/dL Range: 0.75 – 23.11 μg/dL Mean blood cadmium of 0.221 μg/l Range: 0.067 – 0.676 μg/l |
No correlations found between blood lead and urine RBP and albumin (p = 0.589 and 0.064) | None |
|
Verberk et al., 1996 Romania |
Mean age of 4.6 years, 52% male | Cross-sectional | 151 | Urine albumin, A1M, RBP, AAP, and NAG levels | Blood lead (AAS), A1M, | Mean (SD) blood lead of 342(224) μg/L | % increase per 100 μg/L blood lead (90% CI) Albumin (mg/g): 2(−9, 15) A1M (mg/g): 8 (−4, 22) RBP (mg/g): −5 (−17, 8) AAP (U/g): 2 (−3, 7) NAG (U/g): 13 (7, 20) |
None |
|
Fels et al., 1998 Polandb |
Mean age of 9.9 years in unexposed, 10.6 years in exposed, 64.2% male | Cross-sectional | 112 | Urine total protein, albumin, NAG, RBP, and β2MG levels | Blood and urine lead and cadmium (AAS) | Mean (SD) of blood lead in unexposed: 39 (13) μg/L Mean (SD) of blood lead in exposed: 133 (62) μg/L Mean (SD) of urine lead in unexposed: 7.0 (3.7) μg/L Mean (SD) of urine lead in exposed: 16.4 (15.7) μg/L |
Median (Range) of urine NAG in unexposed: 2.1 (0.9, 4.8) U/g Median (Range) of urine NAG in exposed: 1.9 (0.6, 8.1) U/g Median (Range) of urine β2MG in unexposed: 37 (4, 764) μg/g Median (Range) of urine β2MG in exposed: 89 (5, 1145) μg/g Median (Range) of urine RBP in unexposed: 42 (11, 207) μg/g Median (Range) of urine RBP in exposed: 46 (7, 183) μg/g Median (Range) of urine total protein in unexposed: 34 (0, 127) mg/g Median (Range) of urine total protein in exposed: 34 (0, 212) mg/g Median (Range) of urine albumin in unexposed: 6 (0.1, 35) mg/g Median (Range) of urine albumin in exposed: 7 (0.3, 47) mg/g |
None |
|
Loghman-Adham 1998 United States |
Mean (SD) age 13.4 (1.9)% male NR | Cross-sectional | 134 | eGFR (Schwartz formula) | Blood lead (AAS) | Mean (SD) blood lead 18.6 (5.7) μg/dL | Mean (SD) of eGFR in lead-poisoned children: 115 (18) ml/min/1.73m2 Mean (SD) of eGFR in control: 117 (16) ml/min/1.73m2 |
None |
|
Cabral et al., 2012 Senegal |
Mean age of 8.3 years in unexposed, 8.9 years in exposed, 72% male | Cross-sectional (exposed vs. unexposed sites) | 58 | Urine RBP, albumin, and protein levels | Blood and urine lead (ICPMS) | Mean (SD) of blood lead in unexposed: 82.2 (31.6) μg/L Mean (SD) of blood lead in exposed: 149.7 (97.0) μg/L |
Mean (SD) of urine albumin in unexposed: 1.6 (1.3) g/L Mean (SD) of urine albumin in exposed: 3.0 (4.2) g/L Mean (SD) of urine protein in unexposed: 8.8 (2.8) g/L Mean (SD) of urine protein in exposed: 11.4 (4.1) g/L Correlation coefficient between blood lead and urine protein: 0.232 Correlation coefficient between urine lead and urine protein: 0.286 |
None |
RBP: retinol binding protein, eGFR: estimated glomerular filtration rate, GFR: glomerular filtration rate, β2MG: β-2-microglobulin, A1M: α-1-microglobulin, NAG: N-acetyl- β-D-glucosaminidase, ASV: Anodic Stripping Voltammetry, AAS: atomic absorption spectroscopy, ICPMS: inductively coupled plasma mass spectrometry; NR: Not reported
Chan et al., 2012: study also measures cadmium – see Table 2 for cadmium results.
Fels et al., 1998.,: study also measures cadmium – see Table 2 for cadmium results.
Table 4.
Epidemiological studies based on proximity to eiq)osure source and kidney function biomarkers.
| Reference and Country | Age, % male | Study Design | N | Outcome Ascertainment | Biomarker Assessment | Exposure Levels | Effect size (95% CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|---|
|
D’Andrea and Reddy, 2014 United States |
Mean age 13.46 years, 58.3% male | Retrospective cohort | 312 | Blood urea nitrogen (BUN) and serum creatinine levels | Exposed and unexposed area by proximity | None | Mean (SD) serum creatinine levels in exposed: 0.8 (0.2) mg/dL Unexposed: 0.6(0.2) Mean (SD) BUN in exposed: 12.1 (2.6) Unexposed: 12.5 (3.3) |
None |
|
Ignatova et al., 1996 Russia |
Age range of 3–15 years, 37.8% male in cases | Case-control | 95 | Hematuria status | Living in area with heavy metal contamination | None listed | Urine excretion of heavy metal salts of Cd, As, Cr, Sb were increased 2.5–5 times that of control. | None |
| Lagutina et al., 1979 | Age range of 1–15 years, 55.7% male | Cross-sectional | 1790 | Urine protein, blood, oxalates, urates, phosphates, caldum salts lUS, (isolated urinary syndrome) | Study area and proximity to industrial plants, rural area for controls | None listed | Prevalence of lUS (single abnormalities in urine) of 15.3% in Saratov City and 34.1% in Saratov region. | None |
| Former Soviet Union/currently Russia | Prevalence of IUS was increased in all rural groups compared to city region | |||||||
|
Rakhmanin et al., 2004 Russia |
Not listed | Cross-sectional | 486 | Urine protein and NAG levels | Study area and proximity to industrial sites and grain dust concentrations | Grain dust concentrations ranged from 0.10 to 0.24 mg/m3 | Elevated kidney damage biomarkers more frequently seen in children living closer to industrial plants | None |
3.1. Quality assessment
A majority of studies measured exposure at the individual level using blood or urine biomarkers. Four (4) studies did not measure the exposure at an individual level, these studies measured proximity to a high exposure source (Lagutina et al., 1979, Ignatova et al., 1996, D’Andrea and Reddy, 2014). Four (4) studies reported multiple exposures, such as lead and cadmium (Chan et al., 2012, Pels et al., 1998), or cadmium and mercury (Laamech et al., 2014, de Burbure et al., 2006). Outcome measure definitions were generally consistent. Most studies measured urine protein, urine albumin levels, or other biomarkers such as β2-microglobulin (β2MG), N-acetyl-β-D-glucosaminidase (NAG), α-1-microglobulin (A1M), and some other small low-molecular weight biomarkers. One study used a dipstick to measure proteinuria (Factor-Litvak et al., 1999). Among the studies that examined estimated glomerular filtration rate (eGFR), seven studies used creatinine based estimating equations (Fadrowski et al., 2010, Farzan et al., 2016, Garcia-Esquinas et al., 2013, Kataria et al., 2015a, Kooijman et al., 2015, Loghman-Adham, 1998, Watkins et al., 2013). Four studies used Cystatin C based estimating equations to determine eGFR (Fadrowski et al., 2010, Skroder et al., 2015, Hawkesworth et al., 2013, Filler et al., 2012). One study used an equation based on both creatinine and Cystatin C (Weaver et al., 2014). One study (Fadrowski et al., 2013) used measured GFR by plasma disappearance of iohexol. Not all studies adjusted for possible confounders such as age, gender, race/ethnicity, or BMI Z-scores (Tables 5–8). Overall, we found studies of high quality (with adjustment for possible confounders and standardized exposure and outcome assessment) as well as studies of low quality (lack of adjustment for potential confounders, no individual-level exposure assessment, and nonstandard outcome metrics).
Table 5.
Quality assessment criteria for the evaluation of design and data analysis in epidemiologic studies of lead exposure and kidney function biomarkers.
| All Studies | Prospective | Case control, cross-sectional, ecological | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Study | Exposure assessed at individual level |
Exposure assessed using biomarker |
Standardized biomarkers used |
Internal comparisons presented |
Response rate ≥70% from source population |
Same exclusion criteria applied to all participants |
Control for relevant confounders |
Data collected in similar manner between exposed and unexposed |
Loss to follow-up independent of exposure |
Interviewer blinded with respect to case or exposure status |
Noncases would have been cases had they developed disease (CC only) |
Cases and controls/exposed interviewed over same time period |
| Fadrowski et al., 2013 | Yes | Yes | Yes | No | NR | Yes | Yes | Yes | - | - | - | Yes |
| Factor-Litvak et al., 1999 | Yes | Yes | No (dipstick) | Yes | No | No | Yes | Yes | NR | - | - | Yes |
| Moel et al., 1985 | Yes | Yes | Yes | Yes | NR | NR | No | NR | - | NR | - | NR |
| Scharer et al., 1991 | Yes | Yes | Yes | Yes | NR | NR | No | No | - | NR | Yes | NR |
| Chan et al., 2012 | Yes | Yes | Yes | Yes | NR | NR | No | NR | - | - | - | NR |
| Filler et al., 2012 | Yes | Yes | NR | Yes | NR | NR | No | Yes | NR | - | NR | |
| Bernard et al., 1995 | Yes | Yes | - | Yes | NR | - | Yes | NR | - | NR | No | NR |
| Fadrowski et al., 2010 | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | - | - | Yes | |
| Chaumont et al., 2012 | Yes | Yes | Yes | Yes | Yes | NR | Yes | NR | - | - | - | NR |
| Staessen et al., 2001 | Yes | Yes | Yes | Yes | NR | NR | Yes | NR | - | - | - | Yes |
| Khan et al., 2010 | Yes | Yes | Yes | Yes | NR | Yes | No | NR | - | - | - | NR |
| Laamech et al., 2014 | Yes | Yes | Yes | Yes | NR | NR | No | Yes/NR | - | - | - | NR |
| Verberk et al., 1996 | Yes | Yes | Yes | Yes | NR | NR | Yes | Yes | - | NR | - | NR |
| Fels et al., 1998 | Yes | Yes | Yes | Yes | - | NR | No | NR | - | NR | - | NR |
| Loghman-Adham, 1998 | Yes | Yes | Yes | Yes | NR | NR | No | NR | - | - | - | NR |
| Cabral et al., 2012 | Yes | Yes | Yes | Yes | NR | NR | No | NR | - | - | - | NR |
CC: case-control study, NR: not reported in the text
Standardized kidney biomarkers: use of albumin/creatinine ratio, GFR, eGFR, or biomarkers of kidney damage such as β2MG, NAG, AAP, A2M, or RBP
RBP: retinol binding protein, eGFR: estimated glomerular filtration rate, GFR: glomerular filtration rate, β2MG: β-2-microglobulin, A2M: α-2-microglobulin, NAG: N-acetyl-β-D-glucosaminidase,
Table 8.
Quality criteria for the evaluation of design and data analysis in epidemiologic studies of proximity to contaminated areas and kidney function biomarkers.
| All Studies | Prospective | Case control, cross-sectional, ecological | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Study | Exposure assessed at individual level |
Exposure assessed using biomarker |
Standardized biomarkers used |
Internal comparisons presented |
Response rate ≥ 70% from source population |
Same exclusion criteria applied to all participants |
Control for relevant confounders |
Data collected in similar manner between exposed and unexposed |
Loss to follow-up independent of exposure |
Interviewer blinded with respect to case or exposure status |
Noncases would have been cases had they developed disease (CC only) |
Cases and controls/exposed interviewed over same time period |
| D’Andrea et al., 2015 | No | No | Yes | Yes | NR | NR | No | Yes | - | - | - | NR |
| Lagutina et al., 1979 | No | No | Yes | Yes | NR | NR | No | NR | - | - | - | NR |
| Ignatova et al., 1996 | No | No | No | Yes | NR | NR | No | NR | - | NR | Yes | NR |
| Rakhmanin et al., 2004 | NR | No | Yes | Yes | NR | NR | No | NR | - | - | - | NR |
CC: case-control study, NR: not reported in the text of the study.
Standardized kidney biomarkers: use of albumin/creatinine ratio, GFR, eGFR, or biomarkers of kidney damage such as β2MG, NAG, AAP, A2M, or RBP.
RBP: retinol binding protein, eGFR: estimated glomerular filtration rate, GFR: glomerular filtration rate, β2MG: β-2-microglobulin, A2M: α-2-microglobulin, NAG: N-acetyl-β-D-glucosaminidase,
3.2. Lead exposure and kidney disease outcomes
We found 16 studies that examined children’s lead exposure and kidney health. The studies were published between 1985 and 2016 and were conducted in the United States, Canada, Czech Republic, Poland, Hong Kong Germany, Romania, Kosovo, Belgium, Pakistan, Senegal, and Morocco (Table 5). The study designs included 12 cross-sectional, 2 prospective cohort, and 2 case-control studies. The studies published before 1999 had small sample sizes and did not adjust for possible confounders. Nearly all studies of lead exposure used blood lead as a biomarker, although Scharer et al. (1991) used lead levels measured in deciduous teeth. Reported mean blood lead levels ranged from 2.9 μg/dL in a United States population (Fadrowski et al., 2010) to 34.2 μg/dL in Romania (Verberk et al., 1996).
Nine (9) studies reported significant associations between lead and kidney disease measures. In a cross-sectional study of 195 children in the Czech Republic, urine retinol binding protein (RBP) was associated with lead levels, but no other biomarkers (blood cadmium, zinc protoporphyrin) were associated with RBP levels (Bernard et al., 1995). Otherwise, no association was found between cadmium levels and RBP, B2MG, or albumin levels (Bernard et al., 1995). A cross-sectional study of 112 children in Poland reported a higher level of urine P2MG in exposed compared to unexposed participants but no other biomarkers were different by exposure status (Fels et al., 1998). A cross-sectional study of 58 children in Senegal found that children exposed to lead (via industrial exposure) had higher levels of urine albumin and protein than unexposed children (Cabral et al., 2012). A cross-sectional study of 2209 children from Hong Kong reported a positive correlation between urine lead levels and urine albumin, but observed a negative correlation between blood lead and urine albumin (Chan et al., 2012). A cross-sectional study from Canada reported a weak but statistically significant association between increased blood lead levels and decreased eGFR (Filler et al., 2012). A case-control study of 58 children in Germany found that levels of tooth lead were higher in children with renal disease with pre-existing CKD including some who were dialysis-dependent as compared to controls (Scharer et al., 1991). However, these studies did not include adjustment for possible confounders. A cross-sectional study of 279 children in Kosovo also reported an increased odds of proteinuria in the industrial town of Kosovoka Mitrovica, compared to the nonindustrial town of Pristina 25 miles away (Factor-Litvak et al., 1999). In this study, pregnant women were initially recruited at government clinics. Children were selected for follow-up based on umbilical cord lead, town of residence, and location. This study is one of the few with significant positive associations that adjusted for possible confounders. In a cross-sectional study of 200 children in Belgium, there was a 3.6 (95% CI: 1.5, 5.7) percent increase in serum cystatin C per unit increase in blood lead and a 16.0 (95% CI: 2.7, 31) percent increase in urine β2MG per unit increase in blood lead (Staessen et al., 2001). The findings were also adjusted for possible confounders. Another cross-sectional study of 736 children in Belgium also found an increase in urine β2MG levels with increased urine lead (Chaumont et al., 2012). However, the results were adjusted for many interaction variables in addition to possible confounders, making a direct comparison with other studies difficult.
Seven (7) studies of blood lead and kidney disease measures reported mostly null associations, or lacked statistically significant evidence of a relationship with kidney disease markers. A case-control study from the United States found no difference in creatinine clearance in children with lead poisoning (exposure levels 100–471 μg/dl) compared to sibling controls (Moel et al., 1985). A cross-sectional study of 196 children in Morocco found no significant correlations between blood lead and urine RBP and albumin levels (Laamech et al., 2014). A cross-sectional study of 246 children in Pakistan also found no significant difference in serum urea or serum creatinine levels between exposed (children of lead-exposed industrial workers) and unexposed (children of non-exposed workers) groups (Khan et al., 2010). A cross-sectional study of 151 children in Romania found no significant increases in biomarkers associated with blood lead, except for an increase in urine NAG (Verberk et al., 1996). A cross-sectional study of 134 children in the United States did not find differences between eGFR in unexposed or exposed (exposure levels 48–471 μg/dl) children (Loghman-Adham, 1998). However, these studies did not control for possible confounders. A cross-sectional study of 769 children in the United States found a dose-response decrease in eGFR with increasing blood lead levels, but their results were not statistically significant (Fadrowski et al., 2010). This was a study in the general United States population which used standardized definitions and controlled for possible confounders (Fadrowski et al., 2010). The same authors also reported a decrease in measured GFR in a prospective cohort study of 391 American and Canadian children with CKD but this was also not significant (Fadrowski et al., 2013). Both studies by Fadrowski et al. had larger study populations and adjusted for possible confounders, they are examples of high quality studies in this systematic review. Overall, there is some evidence that lead exposure is associated with various kidney disease outcomes.
3.3. Other metals and kidney disease outcomes
We found 15 publications representing 12 different studies on various other metals and kidney disease outcomes, particularly those with arsenic (Skroder et al., 2015; Hawkesworth et al., 2013), cadmium (Chan et al., 2012, de Burbure et al., 2006, Hossny et al., 2001, Swaddiwudhipong et al., 2015; Weaver et al., 2014; Skroder et al., 2015; Noonan et al., 2002). manganese (Nascimento et al., 2016), mercury (Barregard et al., 2008; de Burbure et al., 2006; Woods et al., 2008; Geier et al., 2013; Bellinger et al., 2006; DeRouen et al., 2006), selenium (Skroder et al., 2015), thallium (Weaver et al., 2014), or uranium (Weaver et al., 2014) (Table 2). These studies covered Egypt, France, Poland, Czech Republic, Portugal, China, Bangladesh, Mexico, Thailand, Brazil, and the United States. The study designs included 8 cross-sectional, 1 prospective cohort, and 2 randomized clinical trials (represented in 5 publications).
Table 2.
Epidemiological studies of exposure to metals (other than lead) and kidney function biomarkers.
| Reference and Country | Age, % male | Study Design | N | Outcome Ascertainment | Biomarker Assessment | Exposure Levels | Effect size (95% CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|---|
| Studies of Cadmium exposures | ||||||||
|
Weaver et al., 2014 Mexico |
Mean age of 14.0 years, 51.2% male | Cross-sectional | 512 | eGFR using serum creatinine and cystatin C measurement | Urine Cadmium Thallium Uranium (ICPMS) |
Mean (SD) μg/L: Cadmium: 0.34 (0.37) Thallium: 0.35 (0.20) Uranium: 0.07 (0.14) |
Mean difference in creatinine eGFR per doubling of: Cadmium: 3.1 (1.4, 4.8) Thallium: 3.6 (1.8, 5.3) Uranium: 1.1 (−0.2, 2.3) |
Age, sex, BMI, maternal education, income, smoking, systolic BP, blood lead, urine creatinine |
|
Swaddiwudhipong et al., 2015 Thailand |
Mean age of 9.3 years, 48.3% male | Cross-sectional | 594 | Urine β2MG levels and total protein levels | Urine and blood cadmium (AAS) | Geometric mean: 0.54 μg/g creatinine | OR of β2MG ≥ 200 μg/g: Middle/low tertile of urine cadmium: 2.06 (1.15, 3.68) High/low tertile of urine cadmium: 2.28 (1.28, 4.04) OR of urine protein ≥ 100 μg/g: Middle/low tertile of urine cadmium: 1.25 (0.82, 1.92) High/low tertile of urine cadmium: 1.39 (0.91–2.13) |
Age, sex, blood lead |
|
De Burbure et al., 2006 France, Poland, Czech Republic |
Age range 8.5–12.3 years, 49.2% male | Cross-sectional | 804 | Urine RBP, β2MG, and NAG levels | Blood and urine cadmium and mercury (AAS) | Geometric mean (SD) of blood cadmium in Unexposed males in France: 0.46 (1.40) μg/L Exposed males in France: 0.52 (1.40) μg/L Unexposed females in France: 0.47 (1.38) μg/L Exposed females in France: 0.5 (1.25) μg/L Unexposed males in Poland: 0.07 (1.85) μg/L Exposed males in Poland: 0.19 (2.36) μg/L Unexposed females in Poland: 0.08 (2.25) μg/L Exposed females in Poland: 0.19 (2.58) μg/L Unexposed males in Czech Republic 0.20 (1.47) μg/L Exposed males in Czech Republic: 0.29 (1.73) μg/L Unexposed females in Czech Republic: 0.20 (1.43) μg/L Exposed females in Czech Republic: 0.24 (1.62) μg/L Geometric mean (SD) of urine mercury in of Unexposed males in France: 0.99 (4.93) μg/g Exposed males in France: 0.92 (6.60) μg/g Unexposed females in France: 0.89 (5.20) μg/g Exposed females in France: 1.19 (5.78) μg/g Unexposed males in Poland: 0.06 (1.51) μg/g Exposed males in Poland: 0.06 (1.61) μg/g Unexposed females in Poland: 0.05 (1.59) μg/g Exposed females in Poland: 0.06 (1.53) μg/g Unexposed males in Czech Republic 0.26 (2.81) μg/g Exposed males in Czech Republic: 0.13 (3.59) μg/g Unexposed females in Czech Republic 0.32 (3.14) μg/g Exposed females in Czech Republic: 0.18 (2.50) μg/g |
Mean difference (p-value) in urine RBP per unit increase in blood Cd: 0.06 (< 0.001) Mean difference in urine NAG per increase in blood Cd: 0.053 (0.004) Mean difference (p-value) in urine RBP per unit increase in urine Cd: 0.097 (< 0.001) Mean difference in urine NAG per increase in urine Cd: 0.017 (< 0.03) Mean difference (p-value) in serum B2MG per unit increase in urine Hg: −0.023 (0.02) Mean difference in urine NAG per increase in urine Hg: 0.215 (0.03) |
Models for cadmium: Urine creatinine, blood lead x urine mercury, urine mercury x blood cadmium, urine mercury Models for mercury: Urine creatinine, blood lead x urine mercury, urine mercury x blood cadmium, blood cadmium |
|
Chan et al., 2012 China (Hong Kong)a |
Age range 1–21 years, 48.5% male | Cross-sectional | 2209 | Urine albumin levels | Blood and urine lead and cadmium (ICPMS) | Range of urine cadmium: < 0.30–29.26 nmol/L Range of blood cadmium: Smokers: < 0.27–39.27 nmol/L Non-smokers: < 0.27–36.94 nmol/L |
Correlation coefficient between urine albumin and blood cadmium: −0.014 Correlation coefficient between urine albumin and urine cadmium: 0.119 |
None |
|
Hossny et al., 2001 Egypt |
Age range: 0–18 years 49.3% male |
Cross-sectional | 405 | Urine A1M | Blood cadmium (AAS) | Geometric mean (SD) of blood cadmium in Neonates: 0.92 (1.9) μg/L Infants: 1.33 (1.5) μg/L Preschool: 1.11 (1.6) μg/L Primary school: 1.34 (1.6) μg/L Adolescents: 1.24 (1.5) μg/L |
In primary school aged children and adolescents, those who were A1M positive had higher blood Cd than those without A1M (p > 0.05 in school aged children and p < 0.01 in adolescents) | Age |
|
Fels et al., 1998 Polandb |
Mean age of 9.9 years in unexposed, 10.6 years in exposed, 64.2% male | Cross-sectional | 112 | Urine total protein, albumin, NAG, RBP, and β2MG levels | Blood and urine lead and cadmium (AAS) | Mean (SD) of blood cadmium in unexposed: 0.55 (0.18) μg/L Mean (SD) of blood cadmium in exposed: 0.69 (0.23) μg/L Mean (SD) of urine cadmium in unexposed: 0.40 (0.24) μg/L Mean (SD) of urine cadmium in exposed: 0.49 (0.35) μg/L |
Median (Range) of urine NAG in unexposed: 2.1 (0.9, 4.8) U/g Median (Range) of urine NAG in exposed: 1.9 (0.6, 8.1) U/g Median (Range) of urine β2MG in unexposed: 37 (4, 764) μg/g Median (Range) of urine β2MG in exposed: 89 (5, 1145) μg/g Median (Range) of urine RBP in unexposed: 42 (11, 207) μg/g Median (Range) of urine RBP in exposed: 46 (7, 183) μg/g Median (Range) of urine total protein in unexposed: 34 (0, 127) mg/g Median (Range) of urine total protein in exposed: 34 (0, 212) mg/g Median (Range) of urine albumin in unexposed: 6 (0.1, 35) mg/g Median (Range) of urine albumin in exposed: 7 (0.3, 47) mg/g |
None |
|
Noonan et al., 2002c United States |
Age range of 6–17 years, 55.3% male | Cross-sectional | 159 | NAG, AAP, albumin, and β2MG levels | Urine cadmium (AAS) | Geometric mean (95% confidence interval) of urine cadmium in females: 0.08 (0.07, 0.10) μg/g creatinine Geometric mean (95% confidence interval) of urine cadmium in females: 0.07 (0.06, 0.08) μg/g creatinine |
Spearman correlation coefficients (95% confidence interval) between urine cadmium and NAG (U/L): 0.09 (−0.07, 0.24) Spearman correlation coefficients (95% confidence intervals) between urine cadmium and albumin (mg/L): 0.03 (−0.12, 0.19) Spearman correlation coefficients (95% confidence intervals) between urine cadmium and AAP (U/L): 0.15 (0.00, 0.30, 0.19) Spearman correlation coefficients (95% confidence intervals) between urine cadmium and β2MG(mg/L) −0.01 (−0.20, 0.19) |
Urine creatinine, age, sex |
| Studies of Mercury exposures | ||||||||
|
DeRouen et al., 2006 Woods et al., 2008 Geier et al., 2013 Portugal |
Age range 8–12 years at baseline, 54% male Mean (SD) age 10.09 (1.0) and 58% male |
Randomized trial (amalgam vs composite) | 507 Geier: 344/447d |
Urine albumin levels (Woods et al) Grier: urine GST-α and GST-π levels |
Urine Mercury (AAS) Geier et al. score derived from number of amalgams, BMI, age, gender |
Mean urine mercury of 1.8 μg/g at baseline, 3.2 μg/g after 2 years Mercury levels significantly higher in amalgam group Geier et al. mean (SD) urine mercury of 8.51 (9.5) |
No significant difference in creatinine adjusted urine albumin by treatment year (DeRouen et al., 2006) Mean difference in urine albumin per log-increase in mercury: 1.29 (1.15, 1.45) (Woods et a 2008) Grier: Mean difference (SE) in urine GST-α per increase in restorations: 0.0047 (0.002) Mean difference (SE) in urine GST-π per increase in restorations: 0.0042 (0.002) |
Age, gender, race, age at baseline, urine creatinine (Woods et al., 2008) Grier: urine mercury, porphyrin measures, gender, race, blood lead, and GST-α and GST-π levels |
|
Bellinger et al., 2006 Barregard et al., 2008 United States |
Mean (SD) age of 7.9 (1.3) years and 50.9% male in amalgam group Mean (SD) age of 7.9 (1.4) years and 41.6% male in amalgam group |
Randomized trial (amalgam vs composite) | 534 | Urine albumin levels (Bellinger et al., 2006) Urine albumin, γ-GT, A1M, NAG levels (Barregard et al., 2008) |
Urine and hair mercury (AAS) | Mean (SD) hair mercury of 0.4(0.5) μg/g in amalgam group at baseline Mean (SD) hair mercury of 0.4(0.5) μg/g in composite group at baseline |
Adjusted OR (95%CI) of albuminuria comparing amalgam to composite group: 1.8 (1.1, 2.9) (Barregard et al., 2008) | Randomization stratum, age, sex, race, socioeconomic status, basiline hair mercury, baseline blood lead, lean body mass, time of urine collection, creatinine concentration, storage time |
| Studies of Arsenic exposures | ||||||||
|
Hawkesworth et al., 2013 Skroder et al., 2015 Bangladesh |
Mother-child pairs 52.7% male Mean age mothers 26.7 y |
Cohort (children assessed at 4.5 years) Cross-sectional (Skroder et al) |
2012: 1334 2015: 1106 |
eGFR levels via Cystatin-C formulae | Urine Arsenic (Hydride generation atomic absorption) Urine Arsenic, Cadmium, Selenium (ICP-MS) |
Median (10th, 90th) maternal urine As (μg/l) 80 (24, 383) at 8 weeks of pregnancy Median (5th, 95th) of child urine arsenic: 51 (16, 364) μg/L urine cadmium: 0.22 (0.08, 0.63) μg/L urine selenium: 13(6.0–26) μg/L |
Mean difference in child’s eGFR −14.2 (−32.2, 3.7) per unit increase in maternal As; Mean difference in child’s eGFR −0.29 (−0.86, 0.28) per 100 μg/L increase in arsenic Mean difference in child’s eGFR −1.8 (−3.4, −0.071) per 0.5 μg/L increase in cadmium Mean difference in child’s eGFR 0.34 (−0.76, 1.4) per 10 μg/L increase in selenium |
Age, sex, parental wealth index, height at age 4.5, season of birth Age, sex, birth weight, season of birth, weight for age z-score, maternal BMI, parity, SES, arsenic, cadmium and selenium (depending on model) |
| Studies of Manganese exposures | ||||||||
|
Nascimento et al., 2016 Brazil |
Mean (SD) age 8.6 (0.3) in rural and 10.4 (0.3) in urban, 50.7% male | Cross-sectional (urban vs rural) | 63 | Urine albumin and NAG levels | Blood, hair, drinking water manganese (ICPMS) | Median (min, max) Manganese in rural children: blood: 32.0 (28.0, 44.0) μg/L hair 1.5 (0.19, 11.5) μg/g drinking water: 6.0 (1.0, 16.7) μg/L Urban children: blood: 19.0 (14.0, 31.0) μg/L hair 1.5 (0.09, 0.98) μg/g drinking water 1.0 (1.0, 3.0) μg/L |
Significant difference in mean (SE) in urine albumin levels between rural and urban children 11.3 (1.5)mg/g in rural children and 7.0 (1.4) mg/g in urban children P < 0.05 Significant difference in mean (SE) of urine NAG levels between rural and urban children 5.2 (0.4) mg/g in rural children and 3.4 (0.2) mg/g in urban children P < 0.05 |
None |
RBP: retinol binding protein, eGFR: estimated glomerular filtration rate, GFR: glomerular filtration rate, β2MG: β-2-microglobulin, A1M: α-1-microglobulin, NAG: N-acetyl-β-D-glucosaminidase, AAP: alanine aminopeptidase, ASV: Anodic Stripping Voltammetry, AAS: atomic absorption spectroscopy, ICPMS: inductively coupled plasma mass spectrometry
Footnote about Chan et al. (2012): study also measures lead – see Table 1 for lead results.
Footnote about Fels et al. (1998): study also measures lead – see Table 1 for lead results.
Noonan et al., results only reported for individuals aged 6–17 years.
N = 344 for GSTα, N = 447 for GSTπ.
Formula from Grubb et al., “Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children.” Clin Chem 2005 51:1420–31.
Seven (7) studies examined the renal effects of cadmium exposures. Studies of cadmium exposure reported both positive and mixed results. A cross-sectional study of 112 children in Poland reported a higher level of urine β2MG in exposed compared to unexposed participants but no other biomarkers were different by exposure status (Fels et al., 1998). A cross-sectional study of 405 children in Egypt found that adolescents who were urine AIM positive had higher blood cadmium levels than those who were not, but null associations were identified between A1M and blood cadmium levels in school aged children (Hossny et al., 2001). A cross-sectional study of 804 children from France, Poland, and the Czech Republic reported statistically significant increases in urine RBP and NAG with increased blood and urine cadmium. Their study population was large and the analysis used multiple linear regression models that selected covariates with stepwise regression (de Burbure et al., 2006). However, they adjusted for several interaction terms (such as with mercury levels), making comparison with other studies difficult. A cross-sectional study of 2209 children from Hong Kong reported a positive correlation between urine cadmium levels and urine albumin, but observed a negative correlation between blood cadmium and urine albumin, suggesting that blood levels of cadmium may not be suitable for estimating intrarenal exposure (Chan et al., 2012). A cross-sectional study of 159 children from the United States found positive correlations between urine cadmium and NAG, albumin, and alanine aminopeptidase (AAP) levels, although these were not statistically significant (Noonan et al., 2002). A cross-sectional study of 512 children in Mexico reported an increase in eGFR per doubling of urine cadmium (Weaver et al., 2014). A cross-sectional study of 594 children in Thailand reported significantly increased odds of elevated β2MG among individuals who experienced higher levels of urine and blood cadmium compared to those with lower levels (Swaddiwudhipong et al., 2015). Notably, these two studies were of high quality, and had a large number of study participants (> 500) and adjusted for possible confounders. Overall, there is suggestive evidence of an effect of early life cadmium exposure on renal health outcomes.
Six publications representing three separate studies assessed the effects of mercury exposure. These include two randomized clinical trials: the New England Children’s Amalgam Trial in the United States (Barregard et al., 2008; Bellinger et al., 2006), and the Casa Pia study in Portugal (DeRouen et al., 2006; Geier et al., 2013; Woods et al., 2008). The New England Children’s Amalgam Trial randomized children to either amalgam (containing mercury) or composite (no mercury) dental treatments (Bellinger et al, 2006). The Casa Pia study randomized children to either amalgam (containing mercury) or to resin (no mercury) dental treatments (DeRouen et al., 2006). There was also a cross-sectional study conducted in parts of France, Poland, and the Czech Republic (de Burbure et al., 2006). The Casa Pia study of 507 children reported no significant difference in urine albumin levels by treatment year (Derouen et al., 2006), but other investigators demonstrated an increase in urine albumin per log-increase in the children’s urine mercury levels (Woods et al., 2008). A secondary analysis reported a statistically significant increase in urine GST-α and GST-π levels per increase in tooth restorations (Geier et al., 2013). However, the methods and post-hoc design of this study are questionable for several reasons, including the use of an exploratory method that uses the data to suggest how to configure the model, as opposed to taking advantage of the Casa Pia’s randomized clinical trial design, and the use of GST-α and GST-π levels as a primary outcome (DeRouen et al., 2015). In the New England Children’s Amalgam Trial of 534 (Bellinger et al., 2006) children comparing amalgam (proxy for mercury exposure) versus composite dental treatments, the OR (95% CI) of albuminuria comparing amalgam versus composite was 1.8 (1.1, 2.9) (Barregard et al., 2008). This was a large randomized clinical trial design, and the study adjusted for potential confounders. A cross-sectional study of 804 children from France, Poland, and the Czech Republic reported statistically significant increases in serum β2MG and urine NAG with increased urine mercury. Their study population was large and the analysis used multiple linear regression models that selected covariates with stepwise regression (de Burbure et al., 2006). However, they adjusted for several interaction terms with other metals (such as cadmium), making comparison with other studies difficult.
Two publications representing one study assessed the effects of arsenic. A prospective cohort study of 1334 children in Bangladesh found that urine arsenic is associated with a decrease in children’s eGFR per unit increase in maternal urine arsenic, although this was not significant (Hawkesworth et al., 2013). There was also an association between the child’s urine cadmium and decreased eGFR (Skroder et al., 2015). No change in children’s eGFR was reported per unit increase in the child’s urine selenium or arsenic in a cross-sectional analysis of the cohort (Skroder et al., 2015). One study reported on the effects of thallium and uranium. A cross-sectional study of 512 children in Mexico reported an increase in eGFR per doubling of urine thallium but not for urine uranium (Weaver et al., 2014). One study reported on the effects of manganese. A cross-sectional study of 63 children in Brazil found a significant increase in urine albumin and urine NAG levels between urban and rural children, with rural children having higher levels of blood, hair, and drinking water manganese (Nascimento et al., 2016). However, this study was comprised of a small number of participants and did not control for potential confounders for kidney function biomarkers (Nascimento et al., 2016).
3.4. Non-metal environmental exposures
We identified 17 studies that examined non-metal environmental exposures. These include studies of fluoride levels(Xiong et al., 2007; Liu et al., 2005), aflatoxin (Hassan et al., 2006a) and ochratoxin (Hassan et al, 2006b), maternal smoking levels (Taal et al., 2011, Kooijman et al., 2015), secondhand smoking (Garcia-Esquinas et al., 2013), Bisphenol A, Triclosan, Benzophenone-3 (Trasande et al., 2013), PFOA (Geiger et al., 2013; Watkins et al., 2013; Kataria et al., 2015a), Bisphenol-A-glycidyl-dimethacrylate (bisGMA) (Trachtenberg et al., 2014), melamine exposure (Lam et al., 2008; Guan et al., 2009), polycyclic aromatic hydrocarbons (PAHs) (Farzan et al., 2016) and various phthalate compounds (Trasande et al., 2014, Tsai et al., 2016) (Table 3). The study designs included 12 cross-sectional, 1 case control, 2 prospective cohort, 1 retrospective cohort, and 1 randomized clinical trial.
Table 3.
Epidemiological studies of non-metal environmental exposures and kidney function biomarkers.
| Reference and Country | Age, % male | Study Design | N | Outcome Ascertainment | Biomarker Assessment | Exposure Levels | Effect size (95% CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|---|
| Studies of Fluoride exposures | ||||||||
|
Liu et al. 2005 China |
Age range 8–14 years old % male MR | Cross-sectional | 210 | Urine NAG and γ-GT levels | Categorized by exposure level to water fluoride and dental patient status | Controls: < 1.0 mg/L Group 1 (low fluoride area, no dental fluorosis): 1.0–2.0 mg/L Group 2 (low fluoride area, with dental fluorosis): 1.0–2.0 mg/L Group 3(medium fluoride area, no dental fluorosis): 2.0–3.0 ng/ml Group 4 (medium fluoride area, with dental fluorosis): 2.0–3.0 ng/ml Group 5(high fluoride area, no dental fluorosis): > 3.0 mg/L Group 6 (high fluoride area, with dental fluorosis):): > 3.0 mg/L |
Mean (SD) of urine NAG (U/mmol) Controls: 1.38 (0.36) Group 1: 1.37 (0.59) Group 2: 1.36 (0.60) Group 3: 1.42 (0.50) Group 4: 1.82 (1.00) Group 5: 1.83 (0.67) Group 6: 1.91 (0.88) Mean (SD) of urine γ-GT (U/mmol) Controls: 12.42 (3.81) Group 1: 14.00 (4.33) Group 2: 11.08 (2.37) Group 3: 13.40 (4.03) Group 4: 15.06 (3.35) Group 5: 24.81 (4.34) Group 6: 27.41 (8.00) |
None |
|
Xiong et al., 2007 China |
Age range of 10–12 years % male NR | Case-control | 210 | Urine NAG levels | Urine and serum fluoride (selective electrode) | Mean(SD) serum fluoride (mg/L) Controls: 0.16 (0.06) L1 (low fluoride area, no dental fluorosis):0.19 (0.06) L2(low fluoride area, with dental fluorosis):0.21 (0.03) M1 (median fluoride area, no dental fluorosis):0.22 (0.04) M2 (median fluoride area, with dental fluorosis):0.25 (0.04) H1 (high fluoride area, no dental fluorosis):0.25 (0.04) H2 (high fluoride area, with dental fluorosis):0.26 (0.03)5 |
Mean(SD) urine NAG (U/mmol creatinine) Controls: 1.38 (0.36) L1:1.40 (0.59) L2:1.41 (0.60) M1:1.42 (0.50) M2:1.82 (1.00) H1:1.83 (0.67) H2:1.91 (0.88) |
Matched on age, sex, nutrition status |
| Studies of Aflatoxin and Ochratoxin exposures | ||||||||
|
Hassan et al., 2006a Egypt |
Mean age of 5.4 months, 54% male | Cross-sectional | 50 | Urine albumin and β2MG levels | AFB1 levels | Mean (SD) of AFB1 in infant’s serum: 1.8 (0.9) ng/ml Mother’s serum: 8.9 (4.2) ng/ml |
Mean (SD) of urine β2MG levels in negative maternal exposure group: 172.3 (173.8) μg/ml Mean (SD) of urine β2MG levels in positive maternal exposure group: 215.1 (177.6) μg/ml Mean (SD) of urine albumin levels in negative maternal exposure group: 22.5 (14.2) mg/1 Mean (SD) of urine albumin levels in positive maternal exposure group: 26.5 (15.9) mg/l |
None |
|
Hassan et al., 2006b Egypt |
Mean age of 5.4 months, 54% male | Cross-sectional | 50 | Urine albumin and β2MG levels | Ochratoxin A levels (ICPMS) | Mean (SD) of ochratoxin A in infant serum: 1.26 (1.1) ng/ml Mother’s serum: 4.28 (3.97) ng/ml |
Mean (SD) of urine β2MG levels in negative maternal exposure group: 30.3 (7.9) μg/ml Mean (SD) of urine β2MG levels in positive maternal exposure group: 260.1 (162.5) μg/ml Mean (SD) of urine albumin levels in negative maternal exposure group:11.6 (7.7) mg/l Mean (SD) of urine albumin levels in positive maternal exposure group: 29.7 (13.9) mg/l |
Not listed |
| Studies of secondhand smoking exposures | ||||||||
|
Taal et al., 2011 Kooijman et al., 2015 Netherlands |
Measured at 2 years, 52.1% male Mean age 6.0 years, 50% male |
Prospective cohort subcohort Prospective cohort |
538 5622 |
Kidney size measured via ultrasound Urine albumin and GFR based on serum creatinine |
Maternal smoking levels via questionnaire | Smoking levels: None First trimester only Continued < 5 cigarettes/day 5–10/day > 10/day Smoking levels: None Stopped when known Continued < 5 cigarettes ≥5 cigarettes |
Mean difference in kidney volume: 0.00 (ref) 3.92 (0.24, 7.61) 0.18 (−3.08, 3.43) 0.49 (−3.81, 4.78) −0.48 (−5.66, 4.71) −2.33 (−8.65, 4.00) Mean difference in eGFR (ml/min/1.73m2): 0.00 (ref) −0.13 (−1.94, 1.69) −2.25 (−3.70, −0.79) −1.77 (−3.93, 0.39) −2.38 (−4.57, −0.18) Mean difference in ACR (mg/g) 0.00 (ref) 1.45 (1.05, 2.01) 1.08 (0.80, 1.44) 1.16 (0.76, 1.77) 1.21 (0.78, 1.88) |
Fetal sex, maternal height and weight before pregnancy, maternal weight gain during pregnancy, caloric intake, education, income, child’s age, weight, and height at follow-up visit Child sex, current age, maternal age, parity, educational level, ethnicity, prepregnancy body mass index, blood pressure, child’s gestational age at birth, birth weight, breastfeeding status, childhood body surface area. |
|
Garcia-Esquinas et al., 2013 United States |
Age range 12–17 years, 39% male | Cross-sectional | 7516 | eGFR based on serum creatinine | Serum cotinine and smoking questionnaire | Serum cotinine, ng/mL Secondhand smoke < 0.05 0.05–0.10 0.10–0.54 0.55–10 Active smoking 0.05–12 12–103 ≥ 104 |
Mean difference in eGFR (ml/min/1.73m2): 0.0 (ref) −0.4 (−1.9, 1.2) −0.9 (−2.7, 0.9) −2.2 (−4.0, −0.4) 0.2 (−2.2, 2.6) −1.9 (−3.8, 0.0) −2.6 (−4.6, −0.6) |
Age, gender, BMI, parental education, race/ethnicity, NHANES year |
| Studies of Bisphenol A exposures | ||||||||
|
Trachtenberg et al., 2014 United States |
Mean age of 7.4 years, 46% male | Randomized trial | 410 | Urine albumin and NAG levels | bisGMA via intervention type | Intervention types: Composite Copolymer(with 5–10% bisGMA) Preventive dental sealant |
OR of Albumin > 30 mg/g comparing highest to lowest surface-years: Composite: 0.30 (0.08, 1.19) Copolymer: 0.45 (0.17, 1.16) Sealant: 2.54 (0.29, 22.5) OR of NAG > 3 U/g comparing highest to lowest surface-years: Composite: 1.24 (0.24, 6.50) Copolymer 3.24 (0.51, 20.5) Sealant: 0.02 (0.00, 0.47) |
Randomization after stratification by geographic location and number of dental caries |
|
Trasande et al., 2013 United States |
Age range 6–19 years, 51.7% male | Cross-sectional | 667 | Urine albumin levels | Urine Bisphenol A, Triclosan, Benzophenone-3 (ICP-TMS) | Bisphenol A levels, ng/mL <1.1 1.1–2.1 2.1–4.3 >4.3 |
Change in albumin/creatinine ratio 0.00 (reference) 0.19 (−0.84, 1.45) 0.70 (−0.36, 1.98) 0.91 (0.28, 1.63) Increment per log-unit of BPA: 0.28 (0.03, 0.52) Odds of micro/macroalbuminuria 1.00 (reference) 0.66 (0.23, 1.85) 1.22 (0.54, 2.72) 1.12 (0.26, 4.81) |
Sex, poverty/income ratio, caregiver education, serum cotinine, urine creatinine, age, prehypertension, insulin resistance, body mass index, hypercholesterolemia, and race/ethnicity |
| Triclosan levels, ng/mL <2.7 2.7–9.7 9.7–44.75 > 44.75 |
Increment per log-unit of BPA: 1.11 (0.77, 1.61) Change ACR (mg/g): 0.00 (reference) 0.11 (−0.84, 0.75) 0.05 (0.61, 1.32) 0.01 (−0.69, 0.90) |
|||||||
| Benzophenone-3, ng/mL < 5.5 5.5–12.9 12.9 −41.1 > 41.1 |
Increment per log-unit of triclosan: 0.02 (−0.14, 0.19) Change in albumin/creatinine ratio 0.00 (reference) 0.22 (−0.91, 1.63) 0.27 (−0.91, 0.47) 0.25 (−0.56, 1.21) |
|||||||
| Increment per log-unit of Benzophenone-3: 0.03 (−0.14, 0.21) | ||||||||
| Studies of exposures Phthalate | ||||||||
|
Trasande et al., 2014 United States |
Age range 6–19 years, 51.7% male | Cross-sectional | 667 | Urine albumin levels | Urine phthalate compounds (HPLC-TMS) | Median IQR) LMW: 0.51 (0.27, 1.10) μM HMW: 0.40 (0.21, 0.72) μM DEHP: 0.20 (0.10, 0.36) μM |
Difference in ACR (mg/g) per log unit increase in phthalate (μM): LMW: 0.16(−0.29, 0.65) HMW: 0.52 (0.02, 1.05) DEHP: 0.55 (0.11, 1.01) DIDP: 0.24 (−0.33, 0.86) DINP: 0.18 (−0.18, 0.56) |
Sex, poverty/income ratio, caregiver education, serum cotinine, urine creatinine, age, prehypertension, insulin resistance, body mass index, hypercholesterolemia, and race/ethnicity |
|
Tsai et al., 2016 Taiwan |
Mean (SD) age of 4.8 (2.5), 4.5 (2.0), 5.2 (2.0), 6.5 (2.3) years in unexposed, low, medium, and high exposed groups. 58.5% male | Cross-sectional | 195 | Urine albumin levels, urine β2MG levels, urine NAG levels | Urine DEHP (LC-ESI-TMS) | DEHP intake Unexposed Low: < 0.02 mg/kg/day Middle: 0.02 −0.05 mg/g/day High: > 0.05 mg/kg/day |
Mean difference in log-transformed ACR (mg/g) levels 1.00 (ref) 0.19 (−0.09, 0.47) 0.22 (−0.05, 0.50) 0.44 (0.17, 0.71) Prevalence of microalbuminuria: High(> 0.05 mg/kg/day): 12.9% Middle(0.02 −0.05 mg/kg/day): 8.2% Low(< 0.02 mg/kg/day): 2.1% Unexposed: 0% No association between DEHP and urine NAG levels |
Age, gender, BMI, MAP, insulin resistance, cholesterol, uric acid, MMP, MEP, MnBP, MBzP, MEHP, MEHHP, MEOHP, MiBP |
| Studies of PFOA exposures | ||||||||
|
Watkins et al., 2013 United States |
Mean age of 12.4 years, 52% male | Retrospective cohort | 9660 | eGFR based on serum creatinine | Blood PFOA and historical estimates | Median serum PFOA of 28.3 ng/mL | Change in eGFR(ml/min/1.73 m2) comparing 75th to 25th percentile in: PFOA: −0.73 (−1.38, −0.08) PFOS:−1.34 (−1.91, −0.77) PFNA: −0.88 (−1.41, −0.36) PFHxS: −1.02 (−1.64, −0.40) |
age, sex, race, smoking, household income, regular exercise, and BMI z-scores |
|
Geiger et al., 2013 United States |
Mean (SD) age of 15.0 (0.1) years, 51.9% male | Cross-sectional | 1772 | Serum uric add levels | Serum PFOA and PFOS (HPLC-TMS) | Serum PFOA levels < 2.9 μg/L 2.9–4.0 μg/L 3.1–5.4 μg/L > 5.4 μg/L Serum PFOS levels < 10.7 μg/L 10.7–16.5 μg/L 16.6–25.5 μg/L > 25.5 μg/L |
odds ratio of hyperuricemia: 1.00 (ref) 0.94 (0.58, 1.63) 1.01 (0.62, 1.63) 1.62 (1.10, 2.37) 1.00 (ref) 1.17 (0.80, 1.72) 1.18 (0.74, 1.87) 1.65 (1.10, 2.49) |
Age, sex, race/ethnicity, BMI, household income, activity, serum cholesterol, serum cotinine |
|
Kataria et al., 2015 United States |
Mean (SD) age of 15.5 (2.3) years, 54.3% male | Cross-sectional | 1960 | eGFR based on serum creatinine | Serum PFAAs, PFNA, and PFHxS (HPLC-TMS) | PFOA: < 2.5 ng/mL 2.5–3.5 ng/mL 3.5–4.7 ng/mL ≥ 4.7 ng/mL PFOS: < 7.9 ng/mL 7.9–12.8 ng/mL 12.8 19.4 ng/mL ≥ 19.4 ng/mL PFNA: < 0.7 ng/mL 0.7–1.0 ng/mL 1.0–1.5 ng/mL ≥ 1.5 ng/mL PFHxS < 1 ng/mL 1–2 ng/mL 2–3.95 ng/mL ≥ 4 ng/mL |
Mean difference in eGFR (ml/min/1.73m2): 1.00 (ref) −2.63 (−7.07, 1.81) −5.42 (−11.46, 0.61) −6.61 (−11.39, −1.83) 1.00(ref) −5.24 (−9.75, −0.73) −7.21 (−12.21, −2.21) −9.47 (−14.68, −4.25) 1.00 (ref)1.24 (−4.29, 6.78) 2.76 (−1.68, 7.20) −1.06 (−5.49, 3.37) 1.00 (ref) 1.37 (−3.59, 6.34) 1.85 (−3.36, 7.05) −0.32 (−4.44, 3.81) |
Sex, poverty/income ratio, caregiver education, serum cotinine, urine creatinine, age, prehypertension, insulin resistance, body mass index, hypercholesterolemia, and race/ethnicity |
| Studies of Melamine exposures | ||||||||
|
Lam et al., 2008 China |
Mean age of 6.4 years, 55.1% male | Cross-sectional | 3170 | Proteinuria via test trip | Consumption of melamine tainted milk products | Milk consumption ranged from 250ml to 1.5L per day | 1.86% prevalence of proteinuria | None |
|
Guan et al., 2009 China |
Age 36 months or younger, 57.9% male | Cross-sectional | 589 | Urine albumin, α2M levels, urine NAG levels | Consumption of melamine tainted milk products | Melamine content in formula None Moderate High |
Odds ratio (95% CI) for urinary stones by melamine levels: 1.00 (ref) 2.0 (0.6, 6.2) 7.0 (2.1, 23.00) Odds ratio (95% CI) for suspected urinary stones by melamine levels: 1.00 (ref) 1.7 (0.9, 3.2) 2.6 (1.2, 5.4) |
Age, sex, birth type, melamine content in formula, use of formula alone or in combination with breast milk |
| Studies of PAH exposures | ||||||||
|
Farzan et al., 2016 United States |
Mean (SE) age of 15.5 (0.2) years, 51.3% male | Cross-sectional | 660 | eGFR based on serum creatinine and urine albumin levels | Urine polycyclic aromatic (PAHs) compounds (GC-HRMS) | Mean (SE) of PAH compounds 1-Hydroxynaphthalene 5439.1 (747.7) ng/L 2-Hydroxynaphthalene 5712.0 (580.0) ng/L 3-Hydroxyfluorene 245.0 (22.7) ng/L 2-Hydroxyfluorene 513.0 (37.5) ng/L 3-Hydroxyphenanthrene 186.7 (14.8) ng/L 1-Hydroxyphenanthrene 238.3 (12.5) ng/L 2-Hydroxyphenanthrene 93.0 (7.2) ng/L 1-Hydroxypyrene 208.6 (12.7) ng/L 9-Hydroxyfluorene 469.3 (48.5) ng/L 4-Hydroxyphenanthrene 38.6 (3.0) ng/L. Σ hydroxy-PAHs 13,143.2 (1262.0) ng/L |
Mean difference (95% CI) in log(eGFR) (ml/min/1.73m2):for 1-unit increase in PAH level: −0.613 (−2.240, 1.014) −0.535 (−2.751, 1.680) −0.519 (−2.678, 1.640) −0.117 (−2.448, 2.215) −2.265 (−4.479, −0.331) −1.658 (−4.970, 1.654) −0.602 (−3.582, 2.378) −0.228 (−2.868, 2.411) −1.923 (−5.056, 1.210) −0.977 (−4.23, 2.277) −0.641 (−2.905, 1.623) Mean difference (95% CI) in log (ACR) (mg/g) 1.680 (−2.459, 5.818) 1.223 (−4.456, 6.902) 1.366 (−5.728, 8.459) 5.671 (−1.975, 13.316) −3.181 (−12.216, 5.853) −3.363 (−12.286, 5.561) −0.222 (−9.197, 8.754) −1.445 (−8.735, 5.844) −2.114 (−9.629, 5.402) −1.225 (−10.983, 8.533) −0.409 (−3.515, 2.698) |
Age, gender, race/ethnicity, caregiver education level, poverty-income ratio, BMI z-score, high blood pressure, insulin resistance, serum cotinine, urine creatinine |
RBP: retinol binding protein, eGFR: estimated glomerular filtration rate, GFR: glomerular filtration rate, β2MG: β-2-microglobulin, A2M: α-2-microglobulin, NAG: N-acetyl-β-D-glucosaminidase, bisGMA: Bisphenol-A-glycidyl-dimethacrylate, AFB1: Aflatoxin B1, AAS: atomic absorption spectroscopy, ICPMS: inductively coupled plasma mass spectrometry, HPLC-TMS: high performance liquid chromatography tandem mass spectrometry, LC-ESI-TMS: liquid chromatography/electrospray ionization tandem mass spectrometry, GC-HRMS: capillary gaschromatography combined with high-resolution mass spectrometry, ACR: albumin/creatinine ratio, PFAA: perfluoroalkyl acid, PFOA: Perfluorooctanoic acid, PFOS: Perfluorooctane sulfonic acid, PFNA: Perfluorononanoic Acid, PFHxS: Perfluorohexane Sulfonic Acid, DEHP: di-2-ethylhexyl phthalate, LMW: low molecular weight phthalates, MBzP:mono-benzyl phthalate; MEHHP:mono (2-ethyl-5-hydroxyhexyl) phthalate; MEHP: mono-(2-ethylhexyl) phthalate; MEOHP: mono-(2-ethyl-5-oxohexyl) phthalate; MEP: mono-ethyl phthalate; MMP: mono-methyl phthalate; MiBP: mono-isobutyl phthalate; MnBP: mono-n-butyl phthalate, HMW: high molecular weight phthalates, PAHs: polycyclic aromatic compounds, NR: Not reported
Two studies investigated the association of fluoride exposure, including 1 cross-sectional and 1 case-control. In a cross-sectional study of fluoride exposure in 210 children in China, the investigators found increases of urine NAG and urine γ-GT levels (Liu et al., 2005). In case-control study of fluoride levels in 210 children in China, slightly higher urine NAG levels were found in participants with higher serum fluoride levels (Xiong et al., 2007). While both studies were from China, the first study did not offer adjustment for confounders while the second study matched on age, sex, and nutritional status. One cross-sectional study investigated the effects of Aflatoxin and Ochratoxin exposure. A cross-sectional study of 50 mother-child pairs in Egypt found no statistically significant differences in children’s urine β2MG or urine albumin levels between individuals with negative and positive maternal Aflatoxin exposure.(Hassan et al., 2006a). However, they found higher levels of urine β2MG in children with maternal Ochratoxin A levels (Hassan et al., 2006b).
Three publications representing two studies, a cross-sectional and a prospective cohort, investigated smoking exposures assessed via questionnaire. A prospective cohort study of 538 children in the Netherlands found that maternal smoking was associated with decreased kidney volume at age 2, although this was not statistically significant (Taal et al., 2011). The same prospective study followed the children to age 6 and found that maternal smoking was associated with both decreased child eGFR and increased albumin-creatinine ratio (ACR) when the mother continued to smoke ≥ 5 cigarettes per day during preganancy (Kooijman et al., 2015). This study also found an association between paternal smoking and smaller combined kidney volume at age 6, although there was not association with eGFR and ACR (Kooijman et al., 2015).
A cross-sectional study of 7516 children in the United States found that serum cotinine levels (a biomarker of smoking exposure) were associated with a decrease in eGFR. This was present at the highest levels of cotinine indicate of secondhand smoke as well as the higher levels of smoke exposure associated with active smoking (Garcia-Esquinas et al., 2013). This study was conducted in he general US population and controlled for possible confounders.
Two studies, a randomized trial and a cross-sectional study examined the exposure of Bisphenol A. In a randomized trial of 410 children in the New England Children’s Amalgam Trial in the United States, of dental interventions (as a proxy for bisphenol-A-glycidyl-dimethacrylate (bisGMA) compounds), the use of preventive dental sealant was associated with an increased odds ratio of microalbuminuria (urine albumin > 30 mg/g) and that composite and copolymer treatments were associated with an increased OR of high urine NAG levels (Trachtenberg et al., 2014), however, the results were not significant. A cross-sectional study of 667 children in the United States found a slight increase in albumin/creatinine ratio but no increased odds of micro/macroalbuminuria by Bisphenol A levels (Trasande et al., 2013). This study also reported that triclosan and benzophenone-3 were not associated with albumin/creatinine ratio (Trasande et al., 2013).
Two studies, both cross-sectional, examined phthalate exposure. A cross-sectional study of 667 children in the United States found that urine high molecular weight (HMW) phthalates and the HMW phthalate di-2-ethylhexylphthalate (DEHP) were associated with increases in urine albumin/creatinine ratio (Trasande et al., 2014). This study was also conducted in general US populations and controlled for possible confounders. Meanwhile, a cross-sectional study of 195 children in Taiwan found an association between the phthalate metabolite DEHP and increased urine albumin/creatinine ratio as well as in increased prevalence of microalbuminuria in those exposed to higher levels of DEHP. However, they did not find a significant difference in urine NAG levels (Tsai et al., 2016). This study also controlled for possible confounders (Tsai et al., 2016).
Three studies, two cross-sectional and a retrospective cohort, investigated perfluorooctanoic acid (PFOA) exposure. A cross-sectional study of 1772 children in the United States found an association with serum PFOA and perfluorooctane sulfonate (PFOS) levels and odds of hyperuricemia only at the highest levels of exposure (Geiger et al., 2013). Similarly, another cross-sectional study of 1960 children in the United States found that serum PFOA was only associated with decreased eGFR in the highest quartile of exposure and serum PFOS was associated with a decrease in eGFR in all quartiles of exposure (Kataria et al., 2015a). The investigators did not find an association between perfluorononanoic acid (PFNA) and perfluorohexane sulfonate (PFHxS) and decreased eGFR (Kataria et al., 2015a). Meanwhile, a retrospective cohort study of 9660 children in the United States found that blood PFOA, PFOS, PFNA, PFHxS were associated with a decrease in eGFR (Watkins et al., 2013). All of these studies utilized a large study population and controlled for possible confounders. Taken together, these studies suggest that there is a possible association between PFOA compounds and decreased kidney function in children.
Two studies examined melamine exposure. One cross-sectional study of 3170 children in China examined the consumption of mela-mine-contaminated milk products. They found that there was a 1.86% prevalence of proteinuria (Lam et al., 2008). However, this study did not adjust for potential confounders. Another study of 589 children in China found a strong association between kidney stones and melamine content in formula, with adjusted odds ratios of 2.0 (0.6, 3.2) and 7.0 (2.1, 23.00) with comparing moderate and high melamine content in formula to children with no melamine in their formula (Guan et al., 2009). For children with suspected stones, they reported an adjusted odds ratio of 1.7 (0.9, 3.2) and 2.6 (1.2, 5.4) comparing moderate and high melamine content in formula to children with no melamine in their formula (Guan et al., 2009).
One cross-sectional study examined PAH exposure. A cross-sectional study of 660 children in the United States found that out of 10 PAH compounds, only 3-Hydroxyphenanthrene was associated with a statistically significant decrease in eGFR in children (Farzan et al., 2016). All other PAH metabolites were not significantly associated with a decrease in eGFR or an increase in albumin/creatinine ratio (Farzan et al., 2016).
3.5. Proximity studies
There were four studies that examined kidney outcomes with respect to proximity to specific area. Three of these studies are from Russia (and former Soviet Union) (Lagutina et al., 1979, Ignatova et al., 1996, Rakhmain lu et al., 2004). The study designs included 2 cross-sectional, 1 retrospective cohort, and 1 case-control. A cross-sectional study of 1790 children in the former Soviet Union found that the prevalence of kidney and other related abnormalities was higher in polluted areas than non-polluted areas (Lagutina et al., 1979). A cross-sectional study of 486 children in Russia found elevated kidney damage biomarkers (proteins, NAG) were more frequently seen in children living closer to industrial plants (Rakhmanin et al., 2004). A case-control study of 95 children in Russia found that urine heavy metal salt excretion increased in areas with heavy metal contamination compared to control areas (Ignatova et al., 1996). On the other side of the world, a retrospective cohort study of 312 children in the United States found similar levels of blood urea nitrogen (BUN) and serum creatinine comparing children from areas with and without industrial contamination (D’Andrea and Reddy, 2014) However, none of these studies included adjustment for possible confounders.
4. Dscussion
CKD is a growing public health problem worldwide and its incidence is increasing (Jha et al., 2013). Evidence of kidney effects of some environmental chemicals has been investigated in other systematic analyses for both arsenic (Zheng et al., 2014) and cadmium (Byber et al., 2016) in adult populations. The systematic review herein evaluated the current body of literature regarding environmental chemical exposures and pediatric renal outcomes. Some of the studies in this review provide evidence of an association with environmental exposures and impaired children’s renal function. Since environmental exposures may to some extent represent a modifiable risk factor for pediatric kidney disease, of which few currently exist, the design and implementation of epidemiological studies may ultimately contribute to the prevention of CKD progression. A systematic appraisal of the literature showed mixed evidence of an association between lead exposure and pediatric kidney disease markers, whether defined as eGFR, urine protein, urine albumin, or other kidney damage biomarkers. We found nine studies that reported a positive association of blood lead levels with kidney damage biomarkers such as REP, β2MG, and proteinuria status. However, seven additional studies did not report an association of blood lead with renal biomarkers. Current evidence is supportive of lead exposure contributing to an increased risk of kidney disease; however, the lack of consistent findings suggests potential limitations among study designs and the magnitude of this association is unclear.
Six of six studies reviewed reported an association between cadmium levels and A1M, RBP, NAG, β2MG, urine albumin, urine protein, and eGFR. Overall, there is suggestive evidence of an effect of early life cadmium exposure measured in urine or blood on a variety of renal health outcomes.
Four of six studies reviewed reported an association between mercury levels and albumin, β2MG, NAG, GST-α, and GST-π levels. This evidence comes from randomized trials such as the Casa Pia study and the New England Children’s Amalgam Trial. Another study found an association between urine mercury and β2MG and NAG levels. Overall, there is evidence suggestive of an effect of mercury exposure on renal health outcomes.
Three out of three studies reported an association of PFOA compounds with eGFR or serum uric acid levels. All three studies were conducted in the United States and controlled for possible confounders. This suggests that there may be an association between PFOA and kidney function. However, the literature on PFOA and kidney function remains fairly limited, and may be influenced by publication bias.
We identified two studies that showed a positive association between fluoride levels and urine NAG levels. This suggests that there is a possible association between fluoride and kidney function. We identified 2 studies that showed an association between Bisphenol A compounds and urine albumin levels. However, the literature on both fluoride and Bisphenol A and kidney function remains limited. We identified two studies that investigated the health effects of melamine exposure. Both found incidences of impaired kidney function and one found an association between melamine consumption and kidney stones. However, one of these studies came from a widely publicized case of melamine contamination of baby formula where the exposures were orders of magnitude higher than the other study. In this case, the link between the melamine exposure and the kidney stones was very clear as the stones were atypical in size and composition (composed of powdered melamine) compared to other kidney stones. This acute exposure to melamine was very likely the cause of these kidney stones.
We identified three publications representing two studies that investigated the health effects of smoking exposure. One study found no difference in kidney volume by maternal smoking levels, but found an association between maternal smoking and child eGFR levels. Another study found that high levels of serum cotinine were associated with a decrease in eGFR. This suggests a possible association between smoking exposures and kidney function, however the literature remains limited.
Both studies of arsenic exposure found no association between urine arsenic and eGFR. Additional environmental chemicals of concern have only been examined in single studies to date. These include metals such as uranium, thallium, selenium, and manganese, as well as non-metal compounds such as aflatoxin and ochratoxin, triclosan, and Benzophenone-3. However, the literature is limited and more research with need to be conducted.
5. Gaps and limitations of existing research
Our review identified several gaps in scientific knowledge. These include a critical need for additional studies of environment chemicals exposures and renal health, including but not limited to lead, arsenic, manganese, cadmium, fluoride, Bisphenol A, PFOA, and PAH compounds. There is also a need for research on other environmental exposure combinations such as urbanization. A study in China found an association between urbanized and reduced eGFR in adults (Inoue et al., 2017). However, few studies have examined this exposure and this may be a potential risk factor for children. Additionally, it is clear that studies on children’s renal health suffer from a lack of unified outcome measures. Diverse outcomes, including albuminuria, eGFR, NAG, β2MG, RBP, GST-α, GST-π, serum creatinine, serum urea, kidney size, urine protein, and blood urea nitrogen levels are currently used as markers of renal health. These markers themselves are imprecise, limiting analysis to larger effect sizes or requiring very large cohorts. Appropriate exposure assessment and appropriate use of adjustment for urine dilution remains controversial (adjustment for urinary concentration in statistical modeling versus simple ratios, urine osmolality, urine flow rates are all used in exposure assessment and have yet to be standardized). The pediatric setting, particularly the use of different definitions/equations for estimated GFR in children remains challenging to compare studies which define eGFR with different formulas. There is also complexity in using urinary concentration of toxins as measures of exposure in that the renal effects may impact urinary concentrating ability and other renal tubular functions (Weaver et al., 2016; Yeh et al., 2015; Weaver et al., 2014). In addition, the presence of so many different outcome measures precluded the possibility of meta-analysis. The need for improved and validated biomarkers (or combinations of biomarkers) is a priority of the NIDDK, and future studies should strive to standardize renal function measures that will enable cross-study comparison.
This systematic review found limitations in the design of current epidemiologic studies of environmental exposures and renal health. These limitations include a limited number of prospective studies and randomized clinical trials, relatively small study populations, lack of unified outcome measures, and a lack of adjustment for possible confounders. Other limitations included poor exposure assessment (some studies used proxies such as dental procedures or questionnaire, and others examined geographic areas as a proxy for exposure), and lack of precision of exposure timing (few studies collected longitudinal exposure data). No studies examined critical window methods as most exposures were assessed at baseline study visit(s).
This review also found several strengths in the current epidemiologic literature. A strength of the studies we reviewed was that most exposures were measured at the individual level using validated biomarkers. As human exposure to environmental chemicals is ubiquitous, understanding the effects on kidney disease and kidney function in children would contribute to potentially preventable disease worldwide. Another strength was that most studies used biomarkers to assess renal function, despite a lack of uniform outcome measures. Several of the studies were conducted in the general US population, therefore allowing results to be easily generalizable in the United States. Overall, due to the limitations of the existing literature, such as small sample size, cross-sectional design, and lack of adjustment for potential confounders, we were unable to make definitive inferences on these environmental chemicals.
6. Future perspectives
Due to the gaps and limitations of the scientific literature on environmental chemical exposure and childhood kidney disease and dysfunction, we propose that investigators should conduct additional research in this area and suggest guidelines for future studies: (1) conduct prospective studies, (2) collect longitudinal biomarkers to assess exposure, (3) standardize renal biomarkers (e.g. albuminuria, eGFR, NAG, or β2MG) to facilitate comparisons across study populations. The addition of studies meeting these guidelines will help to further the scientific knowledge of environmental exposures and pediatric renal health.
Table 6.
Quality assessment criteria for the evaluation of design and data analysis in epidemiologic studies of metals other than lead and kidney function biomarkers.
| All Studies | Prospective | Case control, cross-sectional, ecological | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Study | Exposure assessed at individual level |
Exposure assessed using biomarker |
Standardized biomarkers used |
Internal comparisons presented |
Response rate ≥ 70% from source population |
Same exclusion criteria applied to all participants |
Control for relevant confounders |
Data collected in similar manner between exposed and unexposed |
Loss to follow- up independent of exposure |
Interviewer blinded with respect to case or exposure status |
Noncases would have been cases had they developed disease (CC only) |
Cases and controls/exposed interviewed over same time period |
| Cadmium | ||||||||||||
| Weaver et al., 2014 | Yes | Yes | Yes | Yes | NR | NR | Yes | NR | - | - | - | NR |
| Swaddiwudhipong et al., 2015 | Yes | Yes | Yes | Yes | NR | NR | Yes | Yes | - | - | - | NR |
| De Burbure et al., 2006 | Yes | Yes | Yes | Yes | NR | NR | Yes but unusual | No | - | - | - | NR |
| Chan et al., 2012 | Yes | Yes | Yes | Yes | NR | NR | No | NR | - | - | - | NR |
| Hossny et al., 2001 | Yes | Yes | Yes | Yes | NR | Yes | Yes | NR | - | - | - | Yes |
| Fels et al., 1998 | Yes | Yes | Yes | Yes | - | NR | No | NR | - | NR | - | NR |
| Noonan et al., 2002 | Yes | Yes | Yes | Yes | NR | NR | Yes | NR | - | NR | - | NR |
| Mercury | ||||||||||||
| DeRouen et al., 2006 | Yes | Yes | Yes | Yes | Yes if baseline assessment | Yes | Yes | Yes | NR | - | - | NR |
| Woods et al., 2008 | Yes | Yes | Yes | Yes | Yes if baseline assessment | Yes | Yes | Yes | NR | - | - | NR |
| Geier et al., 2013 | Yes | Yes | Yes | Yes | Yes if baseline assessment | Yes | Yes | Yes | NR | - | - | NR |
| Bellinger et al., 2006 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | NR | - | - | Yes |
| Barregard et al., 2008 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | NR | - | - | Yes |
| Arsenic | ||||||||||||
| Hawkesworth et al., 2013 | Yes | Yes | Yes | Yes | No | NR | Yes | NR | NR | - | - | - |
| Skroder et al., 2015 | Yes | Yes | Yes | Yes | No | NR | Yes | NR | NR | - | - | - |
| Manganese | ||||||||||||
| Nascimento et al., 2016 | Yes | Yes | Yes | Yes | Yes | NR | No for kidney outcomes | Yes | - | - | - | NR |
CC: case-control study, NR: not reported
Standardized kidney biomarkers: use of albumin/creatinine ratio, GFR, eGFR, or biomarkers of kidney damage such as β2MG, NAG, AAP, A2M, or RBP
RBP: retinol binding protein, eGFR: estimated glomerular filtration rate, GFR: glomerular filtration rate, β2MG: β-2-microglobulin, A2M: α-2-microglobulin, NAG: N-acetyl-β-D-glucosaminidase,
Table 7.
Quality criteria for the evaluation of design and data analysis in epidemiologic studies of non-metal environmental exposures and kidney function biomarkers.
| All Studies | Prospective | Case control, cross-sectional, ecological | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Study | Exposure assessed at individual level |
Exposure assessed using biomarker |
Standardized biomarkers used |
Internal comparisons presented |
Response rate ≥ 70% from source population |
Same exclusion criteria applied to all participants |
Control for relevant confounders |
Data collected in similar manner between exposed and unexposed |
Loss to follow-up independent of exposure |
Interviewer blinded with respect to case or exposure status |
Noncases would have been cases had they developed disease (CC only) |
Cases and controls/exposed interviewed over same time period |
| Fluoride | ||||||||||||
| Liu et al., 2005 | Uncertain | Uncertain | Yes | Yes | NR | NR | No | NR | - | - | - | NR |
| Xiong et al., 2007 | Yes | Yes | Yes | Yes | NR | NR | Yes | NR | - | NR | Yes | NR |
| Aflatoxin and Ochratoxin | ||||||||||||
| Hassan et al., 2006 #1 | Yes | Yes | Yes | Yes | NR | NR | No | Yes | - | - | - | NR |
| Hassan et al., 2006 #2 | Yes | Yes | Yes | Yes | NR | NR | Yes but not listed | Yes | - | - | - | NR |
| Secondhand smoking | ||||||||||||
| Taal et al., 2011 | Yes | No | No | Yes | Yes | NR | Yes | Yes | Yes | - | - | Yes |
| Kooijman et al., 2015 | Yes | No | No | Yes | Yes | NR | Yes | Yes | Yes | - | - | Yes |
| Gaicia-Esquinas et al., 2013 | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | - | - | - | Yes |
| Bisphenol A | ||||||||||||
| Trachtenberg et al., 2014 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | NR | - | - | Yes |
| Trasande et al., 2013 | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | - | - | - | Yes |
| Pthalates | ||||||||||||
| Trasande et al., 2014 | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | - | - | - | Yes |
| Tsai et al., 2016 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | - | - | - | Yes |
| PFOAs | ||||||||||||
| Watkins et al., 2013 | Yes | Yes | Yes | Yes | NR | Yes | Yes | NR | NR | - | - | - |
| Geiger et al., 2013 | Yes | Yes | No | Yes | NR | Yes | Yes | Yes | - | - | - | Yes |
| Kataria et al., 2015 | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | - | - | - | Yes |
| Melamine | ||||||||||||
| Lam et al., 2008 | Yes | No | No | Yes | NR | NR | No | Yes | - | - | - | NR |
| Guan et al., 2009 | Yes | No | Yes | Yes | NR | NR | No | Yes | - | - | - | NR |
| PAHs | ||||||||||||
| Farzan et al., 2016 | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | - | - | - | Yes |
CC: case-control study, NR: not reported
Standardized kidney biomarkers: use of albumin/creatinine ratio, GFR, eGFR, or biomarkers of kidney damage such as β2MG, NAG, AAP, A2M, or RBP
RBP: retinol binding protein, eGFR: estimated glomerular filtration rate, GFR: glomerular filtration rate, β2MG: β-2-microglobulin, A2M: α-2-microglobulin, NAG: N-acetyl-β-D-glucosaminidase,
Acknowledgments
Funding sources
This work was supported in part by funding from the NIH: T32HD049311, P30ES23515, R01ES013744, R01ES020268, R01ES021357, R01ES026033, DP2ES025453, and UG3OD023337.
Laura Zheng performed the systematic review and wrote the paper. Alison Sanders provided vital writing assistance and source material inclusion. Manish Arora, Jeffrey Saland, and Robert Wright provided input and suggestions for revision. Manish Arora supervised the research. Yula Ma, Leon Hsu, Mathilda Chiu, Vasily Aushev, and Tudor Mustata provided article translation assistance.
Footnotes
Declaration of interest
Laura Zheng, Alison Sanders, Jeffrey Saland, Manish Arora, and Robert Wright report no conflicts of interest.
References
- Au WW. Susceptibility of children to environmental toxic substances. Int J Hyg Environ Health. 2002;205:501–503. doi: 10.1078/1438-4639-00179. [DOI] [PubMed] [Google Scholar]
- Barregard L, Trachtenberg F, Mckinlay S. Renal effects of dental amalgam in children: the New England children’s amalgam trial. Environ Health Perspect. 2008;116:394–399. doi: 10.1289/ehp.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Trachtenberg F, Barregard L, Tavares M, Cernichiari E, Daniel D, Mckinlay S. Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295:1775–1783. doi: 10.1001/jama.295.15.1775. [DOI] [PubMed] [Google Scholar]
- Bernard AM, Vyskocil A, Roels H, Kriz J, Kodl M, Lauwerys R. Renal effects in children living in the vicinity of a lead smelter. Environ Res. 1995;68:91–95. doi: 10.1006/enrs.1995.1012. [DOI] [PubMed] [Google Scholar]
- Buser MC, Ingber SZ Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. U. S. Department of Health and Human Services: Centers for Disease Control and Prevention; Atlanta, GA: Jan, 2017. [Google Scholar]
- Buser MC, Ingber SZ, Raines N, Fowler DA, Scinicariello F. Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012. Int J Hyg Environ Health. 2016;219:261–267. doi: 10.1016/j.ijheh.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byber K, Lison D, Verougstraete V, Dressel H, Hotz P. Cadmium or cadmium compounds and chronic kidney disease in workers and the general population: a systematic review. Crit Rev Toxicol. 2016;46:191–240. doi: 10.3109/10408444.2015.1076375. [DOI] [PubMed] [Google Scholar]
- Cabral M, Dieme D, Verdin A, Garcon G, Fall M, Bouhsina S, Dewaele D, Cazier F, Tall-Dia A, Diouf A, Shirali P. Low-level environmental exposure to lead and renal adverse effects: a cross-sectional study in the population of children bordering the Mbeubeuss landfill near Dakar, Senegal. Hum Exp Toxicol. 2012;31:1280–1291. doi: 10.1177/0960327112446815. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. U.S. Department of Health and Human Services: Centers for Disease Control and Prevention; Atlanta, GA: Jan, 2017. [Google Scholar]
- Chadha V, Warady BA. Epidemiology of pediatric chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:343–352. doi: 10.1053/j.ackd.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Chan IH, Kong AP, Leung TF, Tsui TK, Cheung RC, Osaki R, Ho CS, Wong GW, Wong CK, Lam CW, Chan JC, Chan MH. Cadmium and lead in Hong Kong school children. Pathology. 2012;44:626–631. doi: 10.1097/PAT.0b013e328359cfe7. [DOI] [PubMed] [Google Scholar]
- Chaumont A, Nickmilder M, Dumont X, Lundh T, Skerfving S, Bernard A. Association between proteins and heavy metals in urine at low environmental exposures: evidence of reverse causality. Toxicol Lett. 2012;210:345–352. doi: 10.1016/j.toxlet.2012.02.005. [DOI] [PubMed] [Google Scholar]
- D’andrea MA, Reddy GK. Health effects of benzene exposure among children following a flaring incident at the British Petroleum Refinery in Texas City. Pediatr Hematol Oncol. 2014;31:1–10. doi: 10.3109/08880018.2013.831511. [DOI] [PubMed] [Google Scholar]
- De Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, Smerhovsky Z, Cikrt M, Trzcinka-Ochocka M, Razniewska G, Jakubowski M, Bernard A. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect. 2006;114:584–590. doi: 10.1289/ehp.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouen TA, Martin MD, Leroux BG, Townes BD, Woods JS, Leitao J, Castro-Caldas A, Luis H, Bernardo M, Rosenbaum G, Martins IP. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295:1784–1792. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- Derouen TA, Woods JS, Leroux BG, Martin MD. Critique of reanalysis of Casa Pia data on associations of porphyrins and glutathione-S-transferases with dental amalgam exposure. Hum Exp Toxicol. 2015;34:330–332. doi: 10.1177/0960327114542885. [DOI] [PubMed] [Google Scholar]
- Factor-Litvak P, Wasserman G, Kline JK, Graziano J. The Yugoslavia prospective study of environmental lead exposure. Environ Health Perspect. 1999;107:9–15. doi: 10.1289/ehp.991079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadrowski JJ, Abraham AG, Navas-Acien A, Guallar E, Weaver VM, Furth SL. Blood lead level and measured glomerular filtration rate in children with chronic kidney disease. Environ Health Perspect. 2013;121:965–970. doi: 10.1289/ehp.1205164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, Guallar E, Weaver VM, Furth SL. Blood lead level and kidney function in US adolescents: the third national health and nutrition examination survey. Arch Intern Med. 2010;170:75–82. doi: 10.1001/archinternmed.2009.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Chen Y, Trachtman H, Trasande L. Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003–2008. Environ Res. 2016;144:149–157. doi: 10.1016/j.envres.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fels LM, Wunsch M, Baranowski J, Noska-Borowka I, Price RG, Taylor SA, Patel S, DE Broe M, Elsevier MM, Lauwerys R, Roels H, Bernard A, Mutti A, Gelpi E, Rosello J, Stolte H. Adverse effects of chronic low level lead exposure on kidney function–a risk group study in children. Nephrol Dial Transplant. 1998;13:2248–2256. doi: 10.1093/ndt/13.9.2248. [DOI] [PubMed] [Google Scholar]
- Filler G, Roach E, Yasin A, Shama AP, Blake PG, Yang L. High prevalence of elevated lead levels in pediatric dialysis patients. Pediatr Nephrol. 2012;27:1551–1556. doi: 10.1007/s00467-012-2150-8. [DOI] [PubMed] [Google Scholar]
- Firestone MP, Amler RW. Children’s environmental health–an international perspective. Int J Hyg Environ Health. 2003;206:395–400. doi: 10.1078/1438-4639-00236. [DOI] [PubMed] [Google Scholar]
- Garcia-Esquinas E, Loeffler LF, Weaver VM, Fadrowski JJ, Navas-Acien A. Kidney function and tobacco smoke exposure in US adolescents. Pediatrics. 2013;131:el415–el423. doi: 10.1542/peds.2012-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Carmody T, Kern JK, King PG, Geier MR. A significant dose-dependent relationship between mercury exposure from dental amalgams and kidney integrity biomarkers: a further assessment of the Casa Pia children’s dental amalgam trial. Hum Exp Toxicol. 2013;32:434–440. doi: 10.1177/0960327112455671. [DOI] [PubMed] [Google Scholar]
- Geiger SD, Xiao J, Shankar A. Positive association between perfluoroalkyl chemicals and hyperuricemia in children. Am J Epidemiol. 2013;177:1255–1262. doi: 10.1093/aje/kws392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossner CM, Schlundt J, BEN Embarek P, Hird S, Lo-Fo-Wong D, Beltran JJ, Teoh KN, Tritscher A. The melamine incident implications for international food and feed safety. Environ Health Perspect. 2009;117:1803–1808. doi: 10.1289/ehp.0900949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan N, Fan Q, Ding J, Zhao Y, Lu J, Ai Y, Xu G, Zhu S, Yao C, Jiang L, Miao J, Zhang H, Zhao D, Liu X, Yao Y. Melamine-contaminated powdered formula and urolithiasis in young children. N Engl J Med. 2009;360:1067–1074. doi: 10.1056/NEJMoa0809550. [DOI] [PubMed] [Google Scholar]
- Hassan AM, Sheashaa HA, Abdel Fatah MF, Ibrahim AZ, Gaber OA. Does aflatoxin as an environmental mycotoxin adversely affect the renal and hepatic functions of Egyptian lactating mothers and their infants? A preliminary report. Int Urol Nephrol. 2006a;38:339–342. doi: 10.1007/s11255-006-0056-8. [DOI] [PubMed] [Google Scholar]
- Hassan AM, Sheashaa HA, Fattah MF, Ibrahim AZ, Gaber OA, Sobh MA. Study of ochratoxin A as an environmental risk that causes renal injury in breast-fed Egyptian infants. Pediatr Nephrol. 2006b;21:102–105. doi: 10.1007/s00467-005-2033-3. [DOI] [PubMed] [Google Scholar]
- Hawkesworth S, Wagatsuma Y, Kippler M, Fulford AJ, Arifeen SE, Persson LA, Moore SE, Vahter M. Early exposure to toxic metals has a limited effect on blood pressure or kidney function in later childhood, rural Bangladesh. Int J Epidemiol. 2013;42:176–185. doi: 10.1093/ije/dys215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossny E, Mokhtar G, El-Awady M, Ali I, Morsy M, Dawood A. Environmental exposure of the pediatric age groups in Cairo City and its suburbs to cadmium pollution. Sci Total Environ. 2001;273:135–146. doi: 10.1016/s0048-9697(00)00848-2. [DOI] [PubMed] [Google Scholar]
- Hu H. A 50-year follow-up of childhood plumbism. Hypertension, renal function, and hemoglobin levels among survivors. Am J Dis Child. 1991;145:681–687. doi: 10.1001/archpedi.1991.02160060099029. [DOI] [PubMed] [Google Scholar]
- Ignatova MS, Kharina EA, Dlin VV, Trukhina ON, Iur’eva EA, Osmanov MI, Balkarov IV. Nephropathies in a region contaminated by heavy metal salts and the possibilities for therapeutic and prophylactic measures. Ter Arkh. 1996;68:31–35. [PubMed] [Google Scholar]
- Inoue Y, Howard AG, Thompson AL, Mendez MA, Herring AH, Gordon-Larsen P. The association between urbanization and reduced renal function: findings from the China Health and Nutrition Survey. BMC Nephrol. 2017;18:160. doi: 10.1186/s12882-017-0577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha V, Garcia–Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ Health. 2015a;14:89. doi: 10.1186/s12940-015-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria A, Trsande L, Trachtman H. The effects of environmental chemicals on renal function. Nat Rev Nephrol. 2015b;11:610–625. doi: 10.1038/nrneph.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan DA, Qayyum S, Saleem S, Ansari WM, Khan FA. Lead exposure and its adverse health effects among occupational worker’s children. Toxicol Ind Health. 2010;26:497–504. doi: 10.1177/0748233710373085. [DOI] [PubMed] [Google Scholar]
- Kooijman MN, Bakker H, Franco OH, Hofman A, Taal HR, Jaddoe VW. Fetal smoke exposure and kidney outcomes in school-aged children. Am J Kidney Dis. 2015;66:412–420. doi: 10.1053/j.ajkd.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Laamech J, Bernard A, Dumont X, Benazzouz B, Lyoussi B. Blood lead, cadmium and mercury among children from urban, industrial and rural areas of Fez Boulemane Region (Morocco): relevant factors and early renal effects. Int J Occup Med Environ Health. 2014;27:641–659. doi: 10.2478/s13382-014-0275-7. [DOI] [PubMed] [Google Scholar]
- Lagutina LE, Solov’eva V, Solomina II, Protopopov AA, Deriugina TI. Effects of some environmental factors on epidemiology of renal diseases in children in Saratov and Saratov Region. Pediatriia. 1979:41–43. [PubMed] [Google Scholar]
- Lam HS, Ng PC, Chu WC, Wong W, Chan DF, Ho SS, Wong KT, Ahuja AT, Li CK. Renal screening in children after exposure to low dose melamine in Hong Kong: cross sectional study. Bmj. 2008;337:a2991. doi: 10.1136/bmj.a2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landigan PJ, Sly JL, Ruchirawat M, Silva ER, Huo X, Diz-Barriga F, Zar HJ, King M, Ha EH, Asante KA, Ahanchian H, Sly PD. Health consequences of environmental exposures: changing global patterns of exposure and disease. Ann Glob Health. 2016;82:10–19. doi: 10.1016/j.aogh.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Liu JL, Xia T, Yu YY, Sun XZ, Zhu Q, He W, Zhang M, Wang A. The dose-effect relationship of water fluoride levels and renal damage in children. Wei Seng Yan Jiu. 2005;34:287–288. [PubMed] [Google Scholar]
- Loghan-Adham M. Aminoaciduria and glycosuria following severe childhood lead poisoning. Pediatr Nephrol. 1998;12:218–221. doi: 10.1007/s004670050441. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Brlin JA, Orza MJ, Chalmers TC. A meta-analysis of alcohol consumption in relation to risk of breast cancer. JAMA. 1988;260:652–656. [PubMed] [Google Scholar]
- Lutz E, Lind B, Herin P, Krakau I, Bui TH, Vahter M. Concentrations of mercury, cadmium and lead in brain and kidney of second trimester fetuses and infants. J Trace Elem Med Biol. 1996;10:61–67. doi: 10.1016/S0946-672X(96)80013-7. [DOI] [PubMed] [Google Scholar]
- Mcdonald SP, Craig JC Australian & New Zealand Paediatric Nephrology A. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, Chen J, He J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moel DI, Sachs HK. Renal function 17 to 23 years after chelation therapy for childhood plumbism. Kidney Int. 1992;42:1226–1231. doi: 10.1038/ki.1992.408. [DOI] [PubMed] [Google Scholar]
- Moel DI, Sachs HK, Cohn RA, Drayton MA. Renal function 9 to 17 years after childhood lead poisoning. J Pediatr. 1985;106:729–733. doi: 10.1016/s0022-3476(85)80344-9. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- GBD. Mortality and Causes of Death Collaborators, 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2013;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. http://dx.doi.org/10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento S, Baierle M, Goethel G, Barth A, Brucker N, Charao M, Sauer E, Gauer B, Arbo MD, Altknecht L, Jager M, Dias AC, DE Salle JF, Pierre TS, Gioda A, Moresco R, Garcia SC. Associations among environmental exposure to manganese, neuropsychological performance, oxidative damage and kidney biomarker in children. Environ Res. 2016;147:32–43. doi: 10.1016/j.envres.2016.01.035. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009;170:1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan CW, Sarasua SM, Campagna D, Kathman SJ, Lybarger JA, Mueller PW. Effects of exposure to low levels of environmental cadmium on renal biomarkers. Environ Health Perspect. 2002;110:151–155. doi: 10.1289/ehp.02110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittler MH. Systematic Reviews in Health Care: Meta-analysis in Context. Focus on Alternative and Complementary Therapies. 2002;7:206–207. [Google Scholar]
- Rakhmanin IUA, Mukhambetova L, Pinigin MA. Study of the influence of chemical pollution of the environment on the health status of children by noninvasive biochemical diagnosis. Gig Sanit. 2004:6–9. [PubMed] [Google Scholar]
- Ramirez-Rubio O, Amador JJ, Kaufman JS, Weiner DE, Parikh CR, Kahn U, Mcclean MD, Laws RL, Lopez-Pilarte D, Friedman DJ, Kupferman J, Brooks DR. Urine biomarkers of kidney injury among adolescents in Nicaragua, a region affected by an epidemic of chronic kidney disease of unknown aetiology. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee CM, Kovesdy CP. Epidemiology: Spotlight on CKD deaths-increasing mortality worldwide. Nat Rev Nephrol. 2015;11:199–200. doi: 10.1038/nrneph.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharer K, Vets G, Brockhaus A, Ewers U. High lead content of deciduous teeth in chronic renal failure. Pediatr Nephrol. 1991;5:704–707. doi: 10.1007/BF00857878. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(Suppl 3):S451–S455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skroder H, Hawkesworth S, Kippler M, EL Arifeen S, Wagatsuma Y, Moore SE, Vahter M. Kidney function and blood pressure in preschool-aged children exposed to cadmium and arsenic - potential alleviation by selenium. Environ Res. 2015;140:205–213. doi: 10.1016/j.envres.2015.03.038. [DOI] [PubMed] [Google Scholar]
- Sly PD, Flack F. Susceptibility of children to environmental pollutants. Ann N Y Acad Sci. 2008;1140:163–183. doi: 10.1196/annals.1454.017. [DOI] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ Health Perspect. 2012;120:1527–1531. doi: 10.1289/ehp.1104867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Corvalan CF, Kjellstrom T. How much global ill health is attributable to environmental factors? Epidemiology. 1999;10:573–584. [PubMed] [Google Scholar]
- Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS. Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Ds. 2010;17:254–264. doi: 10.1053/j.ackd.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Nawrot T, Hond ED, Thijs L, Fagard R, Hoppenbrouwers K, Koppen G, Nelen V, Schoeters G, Vanderschueren D, VAN Hecke E, Verschaeve L, Vlietinck R, Roels HA. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: a feasibility study of biomarkers. Lancet. 2001;357:160–1669. doi: 10.1016/s0140-6736(00)04822-4. [DOI] [PubMed] [Google Scholar]
- Swaddiwudhipong W, Mahasakpan P, Jeekeeree W, Funkhiew T, Sanjum R, Apiwatpaiboon T, Phopueng I. Renal and blood pressure effects from environmental cadmium exposure in Thai children. Environ Res. 2015;136:82–87. doi: 10.1016/j.envres.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Taal HR, Geelhoed JJ, Seegers EA, Hofman A, Moll HA, Lequin M, Van Der Heijden AJ, Jaddoe VW. Maternal smoking during pregnancy and kidney volume in the offspring: the Generation R Study. Pediatr Nephrol. 2011;26:1275–1283. doi: 10.1007/s00467-011-1848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenbeg FL, Shrader P, Barregard L, Maserejian NN. Dental composite materials and renal function in children. Br Dent J. 2014;216:E4. doi: 10.1038/sj.bdj.2014.36. [DOI] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Trachtman H. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 2013;83:741–748. doi: 10.1038/ki.2012.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Sathyanarayan S, Trachtman H. Dietary phthalates and low-grade albuminuria in US children and adolescents. Clin J Am Soc Nephrol. 2014;9:100–109. doi: 10.2215/CJN.04570413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HJ, Chen BH, Wu CF, Wang SL, Huang PC, Tsai YC, Chen ML, Ho CK, Hsiung CA, Wu MT. Intake of phthalate-tainted foods and micro-albuminuria in children: The 2011 Taiwan food scandal. Environ Int. 2016;89–90:129–137. doi: 10.1016/j.envint.2016.01.015. [DOI] [PubMed] [Google Scholar]
- U.S. Renal Data System (USRDS) Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. [Google Scholar]
- Verberk MM, Willems TE, Verplanke AJ, Wolff FA, DE Environmental lead and renal effects in children. Arch Environ Health. 1996;51:83–87. doi: 10.1080/00039896.1996.9935998. [DOI] [PubMed] [Google Scholar]
- Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22:1999–2009. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, Savitz DA, Fletcher T, Wellenius GA. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect. 2013;121:625–630. doi: 10.1289/ehp.1205838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Kotchmar DJ, Fadrowski JJ, Silbergeld EK. Challenges for environmental epidemiology research: are biomarker concentrations altered by kidney function or urine concentration adjustment? J Expo Sci Environ Epidemiol. 2016;26:1–8. doi: 10.1038/jes.2015.8. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Vargas GG, Silbergeld EK, Rothenberg SJ, Fadrowski JJ, Rubio-Andrade M, Parsons PJ, Steuerwald AJ, Navas-Acien A, Guallar E. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ Res. 2014;132:226–232. doi: 10.1016/j.envres.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann D, Kuo CC, Nvas-Acien A, Abraham AG, Weaver V, Fadrowski J. Association of arsenic with kidney function in adolescents and young adults: Results from the National Health and Nutrition Examination Survey 2009–2012. Environ Res. 2015a;140:317–324. doi: 10.1016/j.envres.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann DK, Weaver VM, Fadrowski JJ. Toxic environmental exposures and kiney health in children. Pediatr Nephrol. 2015b doi: 10.1007/s00467-015-3222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL. CKiD (CKD in children) prospective cohort study: a review of current findings. Am J Kidney Dis. 2012;60:1002–1011. doi: 10.1053/j.ajkd.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Martin MD, Leroux BC, Derouen TA, Benardo MF, Luis HS, Leitao JG, Kushleika JV, Rue TC, Korpak AM. Biomarkers of kidney integrity in children and adolescents with dental amalgam mercury exposure: findings from the Casa Pia children’s amalgam trial. Environ Res. 2008;108:393–399. doi: 10.1016/j.envres.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Burden of Disease from Ambient and Household Air Pollution. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- Xiong X, Liu J, He W, Xia T, He P, Chen X, Yang K, Wang A. Dose-effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children. Environ Res. 2007;103:112–116. doi: 10.1016/j.envres.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Yeh HC, Lin YS, Kuo CC, Weidemann D, Weaver V, Fadrowski J, Neu A, Navas-Acien A. Urine osmolality in the US population: implications for environmental biomonitoring. Environ Res. 2015;136:482–490. doi: 10.1016/j.envres.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Kuo CC, Fadrowski J, Agnew J, Weaver V, Navas-Acien A. Arsenic and chronic kidney disease: a systematic review. Curr Environ Health Rep. 2014a;1:192–207. doi: 10.1007/s40572-014-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]