Abstract
Key to the pharmaceutical utility of certain macrocyclic drugs is a “chameleonic” ability to change their conformation to expose polar groups in aqueous solution, but bury them when traversing lipid membranes. Based on analysis of the structures of 20 macrocyclic compounds that are approved oral drugs, we propose that good solubility requires a topological polar surface area (TPSA, in Å2) of ≥0.2 × MW. Meanwhile, good passive membrane permeability requires a molecular (i.e. 3D) PSA in nonpolar environments of ≤140 Å2. We show that one or other of these limits is almost invariably violated for compounds with MW > 600 Da., suggesting that some degree of chameleonic behavior is required for most high MW oral drugs.
Keywords: druglikeness, polar surface area, permeability, solubility, conformational change, biopharmaceutical classification system, ABT737, ABT263
Introduction
It is well established that certain compounds with molecular weights (MW) in the range 500–1500 Da. can be useful as drugs, including as oral drugs [1,2], despite appearing to egregiously violate conventional definitions of druglikeness. Many of these high MW oral drugs are macrocycles [3–5]. The detailed molecular features that allow these high MW compounds to achieve good pharmaceutical properties remain incompletely understood, however, and this lack of knowledge presents a major obstacle to the broader exploitation of such non-canonical drug chemotypes [2,4,5]. A factor that contributes to the pharmacological utility of certain large macrocycles is an ability to adopt conformations in which multiple polar groups are internally complemented through intramolecular hydrogen bonding, or are otherwise shielded from solvent [6–10]. This property allows a compound to present a largely nonpolar surface when in nonpolar environments, such as when passing through a cell membrane, while changing conformation to present more polar functionality in aqueous conditions (Figure 1). However, our ability to exploit the phenomenon by designing such “chameleonic” behavior [11,12] into synthetic compounds remains rudimentary. In this article, we provide a quantitative description of the role that chameleonic behavior plays in the pharmaceutical properties of high MW drug chemotypes. We additionally propose an approach to estimating the extent of the need for chameleonic properties for a particular compound, and the degree to which an actual or proposed compound structure satisfies this need, with a view to advancing our ability to rationally exploit chameleonic properties when developing high MW compounds as drugs.
Figure 1. Cartoon illustrating how chameleonic compounds bury polar groups to improve passive membrane permeability.
The upper structures represent the molecular conformation favored in aqueous solution, with hydrogen bond donors (red dots) and acceptors (blue dots) fully exposed, and thus extensively solvated by water. This open conformation is in equilibrium with a “closed” conformation (lower structures), in which a subset of hydrogen bond acceptor and donor groups participate in intramolecular hydrogen bonds (red dashed lines), or are otherwise buried and sequestered from solvent. This conformational change reduces the number of water molecules engaged in strong solvating interactions with the compound, and lowers the energetic cost of desolvating the compound as it enters into and passes through the cell membrane.
The Theoretical Basis for Chameleonic Behavior
A useful way to think about chameleonic properties is in terms of the Biopharmaceutical Classification System (BCS) [13]. The BCS categorizes compounds in terms of their experimentally determined aqueous solubility and cell permeability (Figure 2A), two properties that are key for oral bioavailability and for activity against intracellular targets. Both solubility and passive membrane permeability are primarily functions of the balance between polar and nonpolar structure within the compound. A high content of polar groups favors aqueous solubility, but at the same time tends to confer a high desolvation energy barrier, retarding passage into and through the hydrophobic interior of cell membranes. Consequently, properties relating to compound polarity feature prominently in conventional metrics for druglikeness. Indeed, three of the four criteria that make up Lipinski’s Ro5 [14] involve limits on compound polarity. The Ro5 criterion that the calculated octanol-water partition coefficient (cLogP) should generally be ≤ 5 helps ensure that compounds are sufficiently polar to be soluble. Conversely, the Ro5 limits on the number of hydrogen bond acceptors (HBA ≤ 10) and particularly hydrogen bond donors (HBD ≤ 5) ensure that the energetic cost of desolvating the compound is not too high for good passive membrane permeation. As we will discuss, the remaining Ro5 criterion, MW ≤ 500 Da., also in part reflects the need for an appropriate polar-nonpolar balance, albeit indirectly. Similarly, one of the two druglikeness criteria proposed by Veber et al. [15] is that the polar surface area (PSA) of the compound should not exceed 140 Å2, representing an alternative expression of the need to limit the number of polar groups. Importantly, the measure of PSA used by Veber was the “topological polar surface area” (TPSA) [16], which depends only on atom content and does not take into account the degree to which given atoms are exposed to solvent in a given three dimensional conformation of the compound.
Figure 2. Effect of chameleonic behavior on Biopharmaceutical Classification System (BCS) Class.
(A) Diagram representing the Biopharmaceutical Classification System for evaluating drug candidates [13]. For the purpose of the current discussion, the gridlines represent the degree of aqueous solubility (vertical gridline) and membrane permeability (horizontal gridline) required for the compound to be developable as an oral drug. A high MW compound that contains many polar atoms can have acceptable aqueous solubility. However, a high polar surface area will confer a high desolvation energy barrier to entering and passing through the low polarity interior of cell membranes, making the compound poorly soluble. A similarly large molecule that contains few polar atoms might readily permeate cell membranes, but due to its low polarity it will have poor aqueous solubility. A chameleonic compound contains enough polar atoms and groups for good aqueous solubility, but can undergo a conformational change that buries or internally complements multiple hydrogen bond donors and acceptors, thereby allowing good membrane permeability. This combination of aqueous solubility and membrane permeability places the compound in the most favorable BCS Class I. (B) TPSA values for all 20 approved oral drugs that are macrocycles (red circles) [4][5], plotted as a function of each compound’s molecular weight. The dashed line represents the minimum PSA per a.m.u. seen among this compound set, and has a slope of 0.20 Å2/Da. The green + shows the mean TPSA and MW values for 1193 oral drugs (taken from Veith et al. [17]). The blue dashed lines show the upper limits to MW and TPSA as defined by Lipinski’s Ro5 and Veber’s Rules, respectively. Inset plot: Relationship of compound polarity to compound size for the same set of macrocycle drugs, expressed in terms of polar atoms versus total heavy (i.e. nonhydrogen) atoms in each compound’s structure. The dashed and dotted lines correspond to the minimum and maximum polar atom content observed for this set of drugs, and have slopes corresponding to 20% and 30% polar atoms, respectively.
The aqueous solubility of a neutral compound depends, to a first approximation, on the balance of polar and nonpolar groups in the molecule. A molecule of almost any size can be water soluble, provided it contains a sufficient number of polar groups to counterbalance its nonpolar atom content. In contrast, the energetic penalty for desolvation and transfer into a nonpolar medium scales with the number (and type) of polar groups. Consequently, as molecular weight increases it becomes more difficult for a compound to possess high solubility in both aqueous and nonpolar solvents. For example, a compound with MW <500 that contains ~25% polar atoms, roughly average for oral small molecule drugs [17], will typically have an acceptable value for cLogP, and at the same time will contain <10 HBA/<5 HBD and have TPSA ≤ 140 Å2. However, for a much larger molecule, such as a large macrocycle with MW = 1000 Da., the same proportion of ~25% polar atoms will confer a similar cLogP, but the number of polar groups will have doubled, driving HBD, HBA and PSA above conventionally acceptable levels. If the number of polar atoms is reduced to avoid this problem, the ratio of nonpolar to polar atoms will increase, raising cLogP and reducing the aqueous solubility of the compound. Thus, high MW compounds tend to belong to BCS Categories II, III, or even IV, presenting significant challenges for their development as drugs.
Chameleonic compounds are able to square this circle by modulating how many polar atoms are exposed to solvent depending on the polarity of their environment. Thus, a large macrocycle containing ~25% polar atoms will likely be reasonably soluble in aqueous solution if it can adopt a conformation in which all or most of the polar atoms are exposed and therefore well solvated. This combination of properties would normally place the molecule in BCS Category II. However, upon encountering a nonpolar environment such as the interior of a cell membrane, a chameleonic compound changes conformation to shield a number of polar groups, as illustrated in Figure 1, resulting in a low effective PSA that in a rigid compound would likely confer membership in BCS Category III. The result is that the compound can, as needed, possess the high aqueous solubility and the high membrane permeability required to place it in the most favorable BCS Category I (Figure 2A). Most high MW oral drugs–and large macrocycles in particular–tend to conform to a polar:nonpolar atom ratio of ~25:75 (Figure 2B, inset), with clogP values in the same range as small oral drugs, but with much higher TPSA values typically in the range 180–320 Å2 [4,5]. Based on consideration of polar versus nonpolar content alone, therefore, such drugs should be Category II compounds, with very poor passive membrane permeability. The ability of such compounds to access intracellular targets, and particularly their oral bioavailability, likely depends in many cases on their possession of chameleonic properties.
Quantifying Chameleonic Properties
Predicting the aqueous solubility of organic compounds is notoriously difficult [18–21]. One reason is that even a relatively polar molecule can be poorly soluble if it possesses a particularly stable crystal form. It is, however, possible to identify features that are necessary for good solubility while not being sufficient to guarantee it. For example, Figure 2B shows that approved oral drugs that are large macrocycles tend to have a minimum TPSA of ~0.2 Å2 per unit of MW, corresponding to the dashed line in Figure 2B which defines the lower bound of the data set. This apparent requirement for a minimal level of polar content can alternatively be expressed in terms of fraction of polar atoms, as illustrated in Figure 2B, inset, which shows that oral drugs tend to contain ≥ 20% polar atoms (considering nonhydrogen atoms only). While exceeding the lower limits for polar content indicated in Figure 2B by no means guarantees that a compound will be soluble, it appears that if TPSA is significantly less than 0.2MW, then achieving the degree of aqueous solubility (and perhaps also other properties) required for oral activity is unlikely.
It is well established for conventional druglike compounds that PSA ≤ 140 Å2 is typically required to have sufficient membrane permeability for good oral bioavailability [15]. When applying this threshold to chameleonic compounds, it is clearly necessary to use a measure of PSA that takes into account the three-dimensional structure of the compound in a particular solvent environment, and thus whether polar atoms are buried or exposed to solvent [6,7,10]. The extent of PSA that is exposed to solvent in a particular three-dimensional conformation is called the “Molecular” PSA (MPSA) [22,23], or sometimes the “solvent-accessible” or “solvent-exposed” PSA. MPSA is significantly more complicated to calculate than TPSA, because for MPSA it is first necessary to compute what molecular conformation(s) will predominate in a given solvent environment. Nonetheless, methods exist for calculating MPSA with various degrees of theoretical rigor [22,23], and MPSA values have been determined for a number of macrocyclic drugs and other high MW compounds [23,24].
Based on the above reasoning, we propose that for good aqueous solubility PSAAq ≥ 0.2MW, and for good membrane permeability PSAnp ≤ 140 Å2, where PSAAq and PSAnp are the MPSA values for the molecular conformations that predominate, respectively, in aqueous solution and in the nonpolar membrane environment. For a reasonably flexible molecule, the major conformer in aqueous solution will have all or most polar groups exposed to solvent, such that MPSAAq can be approximated simply by using TPSA. However, a formally calculated MPSA can be used in the aqueous term also, if additional rigor is desired. These PSA thresholds are illustrated graphically in Figure 3A, which shows the range of MPSA values that correspond to a likelihood of good aqueous solubility (blue shading) and good membrane permeability (red shading) for compounds of a given MW. Figure 3A shows that a compound with MW < 600–700 Da. can have sufficient polar content to achieve PSAAq ≥ 0.2MW without exceeding PSA = 140 Å2. Thus, for low MW compounds, modulating the exposure of polar groups through conformational change is generally not required to balance aqueous solubility with membrane permeability, consistent with the observation that small drugs are often relatively rigid and generally are not thought to display chameleonic behavior. Indeed, this analysis likely accounts, at least in part, for the Ro5 criterion that MW ≤ 500 [14].
Figure 3. Quantifying Chameleonic Behavior.
(A) Molecular (3D) polar surface area (MPSA) values that we propose to be compatible with good aqueous solubility (blue area) and good membrane permeability (red area), as a function of compound molecular weight. The green + shows mean values reported for 1193 oral drugs, from Veith et al. [17]. Red symbols show MPSA values for approved macrocyclic drugs, for the compound conformation that predominates in aqueous solution (O) and in nonpolar solvent (x). Blue symbols describe the properties of the Bcl-xL inhibitors ABT737 (open triangle) and ABT263 (filled triangle). (B) Properties of the six macrocyclic drugs plotted in (A). All PSA and oral bioavailability data are from references [23] and [24]. ΔPSA = TPSA − MPSAnp. The target value for the extent of ΔPSA required for effective chameleonic behavior is the larger of 0.2MW – 140 Å2 or TPSA - 140 Å2, according to Equations 1 and 2.
For compounds with MW > 700 Da., however, there is a gap between the blue and red shaded areas in Figure 3A, indicating that for these high MW compounds there is no single PSA value that can simultaneously satisfy the conditions 0.2MW ≤ PSA ≤ 140 Å2. The higher the MW, the larger the gap between the minimum PSA required for good aqueous solubility and the maximum that is compatible with good membrane permeability. For example, for a compound with MW = 800 Da., 0.2MW = 160 Å2, suggesting that, absent a chameleonic conformational change, a compound of this size that has enough polar content for good aqueous solubility will have a PSA that is at least ~20 Å2 higher than is compatible with good membrane permeation. Similarly, for a compound with MW = 1200, approximately the size of the macrocyclic drug cyclopsporin A, 0.2MW = 240 Å2, implying that an aqueous soluble compound of this size must find a way to bury ~40% of its polar groups to achieve good membrane permeability.
The above analysis suggests that the minimum PSA that a chameleonic compound must bury to possess both good aqueous solubility and good membrane permeability can be estimated from Equation 1.
| Eq. 1 |
For a compound with TPSA that exceeds 0.2MW, as will often be the case, the minimum amount of PSA that must be buried is given by Equation 2.
| Eq. 2 |
We emphasize that TPSA ≥ 0.2MW does not guarantee good aqueous solubility, and MPSAnp ≤ 140 Å2 does not guarantee good membrane permeability. Many compound structures might exist that violate these expectations. However, compounds (especially high MW compounds) with TPSA significantly below 0.2MW are unlikely to have good solubility, and those with MPSAnp much above 140 Å2 are unlikely to have good permeability. Therefore, these proposed thresholds represent reasonable approximations of the minimal requirements for possession of these key pharmaceutical properties, and provide a useful framework for evaluating the need for chameleonic properties in the most favorable cases.
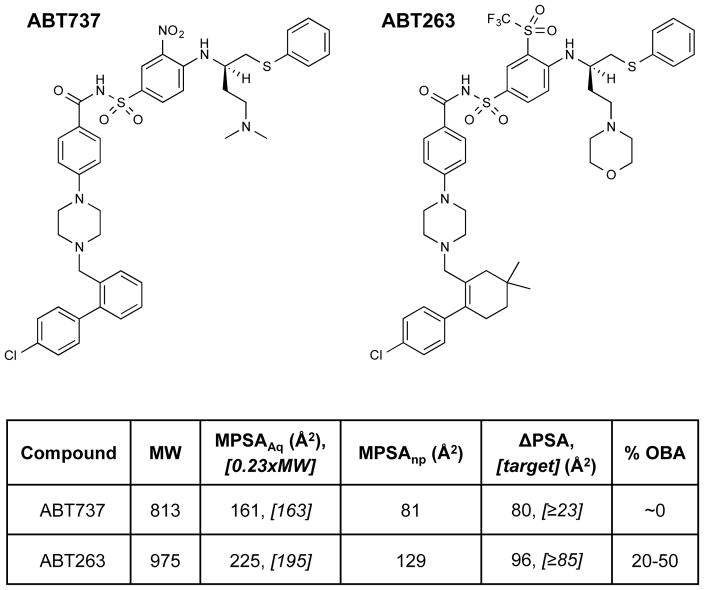
We tested the validity of this hypothesis with respect to the properties of known chameleonic compounds from the literature. Figure 3B shows reported TPSA and MPSAnp values for six macrocyclic drugs. Five of these compounds, clarithromycin, rifampicin, roxithromycin, rapamycin, and cyclosporine A, have oral bioavailabilities in man of ≥14%, and are all oral drugs [23,24]. Figure 3A shows that all five of these compounds meet, or come very close to meeting, our proposed guidelines that TPSA ≥ 0.2MW and MPSAnp ≤ 140 Å2, showing chameleonic changes in exposed PSA that roughly conform to our proposed threshold, from Eq. 2, of ΔPSA ≥ TPSA – 140 Å2. Cyclosporin A, in particular, shows the very large ΔPSA of 174 Å2, burying ~62% of its PSA when in nonpolar environments [25], in close agreement with the expectation from Equation 2. Another macrocyclic drug, paclitaxel, has some degree of chameleonic behavior (ΔPSA = 38 Å2), but even in nonpolar environments has a high MPSA of 183 Å2. Paclitaxel thus violates one of our two guidelines (Figure 3A), resulting in a low oral bioavailability of 6.5% and enabling only parenteral administration [23]. Additional validation involving a non-macrocyclic chemotype was obtained by considering the Bcl-xL inhibitors ABT737 (MW = 813) and ABT263 (MW = 975) (Figure 4), well-known examples of high MW drug candidates from the recent literature. ABT737 provided initial proof of concept for the pharmacological utility of the chemotype [26], but showed extremely poor aqueous solublility and was not orally bioavailable [27]. ABT263 was developed to address this limitation and, despite its even higher MW, has good oral availability [27], and is currently in clinical development [28,29]. We calculated that ABT737, while possessing some degree of chameleonic behavior, has MPSAAq that is well below the level of 0.2MW required for any prospect of good aqueous solubility, and thus does not meet our proposed criteria. In contrast, the orally available ABT263 matches our guidelines almost exactly, having a substantially higher MPSAAq that now equals 0.2MW, but at the same time a large degree of chameleonic behavior that brings MPSAnp down to <140 Å2 in nonpolar environments (Figures 3A and 4). ABT263 is itself poorly soluble [27], but the improvements in its properties compared to ABT737 are evidently sufficient to confer oral bioavailability and to enable its further development as an oral drug. The higher polarity of ABT263 is largely due to the replacement of a nitro group with the much more polar trifluoroemthylsulfone. Its increased ΔPSA is due to the fact that, in nonpolar environments, this sulfone group can be essentially entirely shielded from solvent by the neighboring thiophenyl moiety (see Supplementary Information for additional details). Taken together, the above results provide strong support that the rule of thumb we propose provides a reasonable approximation for the degree of chameleonic change in PSA required for high MW compounds to achieve both good aqueous solubility and good membrane permeability.
Figure 4. Chemical structures of ABT737 and ABT263, with calculated values for MPSAAq, MPSAnp, and target and actual ΔPSA.
Data are plotted in Figure 3B. The target value for the extent of ΔPSA required for effective chameleonic behavior is the larger of 0.2MW – 140 Å2 or TPSA - 140 Å2, according to Equations 1 and 2. See supplementary Information for details of the MPSA calculations.
Value of Analysis for Compound Design and Optimization
The ability to quantify chameleonic properties does not directly address the difficult problem of how to design chameleonic properties into specific molecules, something that has rarely been attempted outside the arena of cyclic peptides [6–10]. However, we believe the analysis can help inform such efforts. Firstly, in considering what compounds to make, for large macrocycles and other high MW chemotypes it is important to realize that Veber’s limit of TPSA ≤ 140 Å2 does not apply in the conventional sense, and that exceeding this limit is not only permitted but is actually required for good aqueous solubility. Indeed, of the 22 approved oral macrocycle drugs [4,5], none has TPSA ≤ 140 Å2, and approved macrocyclic drugs that are administered parenterally have even higher TPSA values [2,4,5]. The analysis presented here suggests that, for any prospect of good aqueous solubility, compound designs should aim for TPSA ≥ 0.2MW, roughly corresponding to a minimum of ~20% of heavy atoms being N or O. A second insight is that some degree of chameleonic behavior will likely be required for virtually any oral drug with MW greater than ~700 Da., as well as for some high MW compounds intended for parenteral administration if any degree of membrane permeability is required, for example to access an intracellular target. The analysis also provides a rough guide as to what proportion of a compound’s polar content must be shielded for it to pass readily through a cell membrane. For example, one might consider a hypothetical large macrocycle containing a total of 60 nonhydrogen atoms, with 16 N or O atoms leading to a TPSA ~ 220 Å2. The above analysis indicates that, for effective chameleonic behavior, such a compound must be able to adopt a conformation that buries approximately 220 - 140 = 80 Å2 of PSA, corresponding to roughly one third of its polar groups, for example through formation of intramolecular hydrogen bonds. While designing a compound that possesses this property is clearly nontrivial, having even an approximate target for what must be achieved should aid decisions about which compounds to make or test, and what to look for as outcomes from computational studies that model the effect of solvent polarity on compound conformation.
Supplementary Material
Acknowledgments
The authors thank Alessio Ciulli for helpful discussions.
Funding: This work was supported, in part, by NIH grant 1R01GM094551.
Footnotes
Supplementary Information: Description of the calculation of MPSAAq and MPSAnp for compounds ABT737 and ABT263 (PDF file).
References
- 1.Ganesan A. The impact of natural products upon modern drug discovery. Current Opinion in Chemical Biology. 2008;12(3):306–317. doi: 10.1016/j.cbpa.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Doak BC, et al. Oral Druggable Space beyond the Rule of 5: Insights from Drugs and Clinical Candidates. Chemistry & Biology. 2014;21(9):1115–1142. doi: 10.1016/j.chembiol.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Driggers EM, et al. The exploration of macrocycles for drug discovery - an underexploited structural class. Nature Reviews Drug Discovery. 2008;7(7):608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- 4.Villar EA, et al. How proteins bind macrocycles. Nature Chemical Biology. 2014;10(9):723–731. doi: 10.1038/nchembio.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordanetto F, Kihlberg J. Macrocyclic Drugs and Clinical Candidates: What Can Medicinal Chemists Learn from Their Properties? Journal of Medicinal Chemistry. 2014;57(2):278–295. doi: 10.1021/jm400887j. [DOI] [PubMed] [Google Scholar]
- 6.Rezai T, et al. Conformational flexibility, internal hydrogen bonding, and passive membrane permeability: Successful in silico prediction of the relative permeabilities of cyclic peptides. Journal of the American Chemical Society. 2006;128(43):14073–14080. doi: 10.1021/ja063076p. [DOI] [PubMed] [Google Scholar]
- 7.Bockus AT, et al. Probing the Physicochemical Boundaries of Cell Permeability and Oral Bioavailability in Lipophilic Macrocycles Inspired by Natural Products. Journal of Medicinal Chemistry. 2015;58(11):4581–4589. doi: 10.1021/acs.jmedchem.5b00128. [DOI] [PubMed] [Google Scholar]
- 8.Hewitt WM, et al. Cell-Permeable Cyclic Peptides from Synthetic Libraries Inspired by Natural Products. Journal of the American Chemical Society. 2015;137(2):715–721. doi: 10.1021/ja508766b. [DOI] [PubMed] [Google Scholar]
- 9.Bockus AT, et al. Form and Function in Cyclic Peptide Natural Products: A Pharmacokinetic Perspective. Current Topics in Medicinal Chemistry. 2013;13(7):821–836. doi: 10.2174/1568026611313070005. [DOI] [PubMed] [Google Scholar]
- 10.Rand AC, et al. Optimizing PK properties of cyclic peptides: the effect of side chain substitutions on permeability and clearance. Medchemcomm. 2012;3(10):1282–1289. doi: 10.1039/C2MD20203D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escalera JB, et al. Predicting the solubility of drugs in solvent mixtures: multiple solubility maxima and the chameleonic effect. J Pharm Pharmacol. 1994;46(3):172–176. doi: 10.1111/j.2042-7158.1994.tb03772.x. [DOI] [PubMed] [Google Scholar]
- 12.Vistoli G, et al. Assessing drug-likeness--what are we missing? Drug Discov Today. 2008;13(7–8):285–294. doi: 10.1016/j.drudis.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Amidon GL, et al. A Theoretical Basis for a Biopharmaceutic Drug Classification - the Correlation of in-Vitro Drug Product Dissolution and in-Vivo Bioavailability. Pharmaceutical Research. 1995;12(3):413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 14.Lipinski CA, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 1997;23(1–3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 15.Veber DF, et al. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. Journal of Medicinal Chemistry. 2002;45(12):2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 16.Ertl P, et al. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem. 2000;43(20):3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 17.Vieth M, et al. Characteristic physical properties and structural fragments of marketed oral drugs. Journal of Medicinal Chemistry. 2004;47(1):224–232. doi: 10.1021/jm030267j. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Hou T. Recent advances on aqueous solubility prediction. Comb Chem High Throughput Screen. 2011;14(5):328–338. doi: 10.2174/138620711795508331. [DOI] [PubMed] [Google Scholar]
- 19.Abramov YA. Major Source of Error in QSPR Prediction of Intrinsic Thermodynamic Solubility of Drugs: Solid vs Nonsolid State Contributions? Mol Pharm. 2015;12(6):2126–2141. doi: 10.1021/acs.molpharmaceut.5b00119. [DOI] [PubMed] [Google Scholar]
- 20.Salahinejad M, et al. Aqueous solubility prediction: do crystal lattice interactions help? Mol Pharm. 2013;10(7):2757–2766. doi: 10.1021/mp4001958. [DOI] [PubMed] [Google Scholar]
- 21.Chevillard F, et al. In silico prediction of aqueous solubility: a multimodel protocol based on chemical similarity. Mol Pharm. 2012;9(11):3127–3135. doi: 10.1021/mp300234q. [DOI] [PubMed] [Google Scholar]
- 22.Ertl P, et al. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. Journal of Medicinal Chemistry. 2000;43(20):3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 23.Guimaraes CRW, et al. Use of 3D Properties to Characterize Beyond Rule-of-5 Property Space for Passive Permeation. Journal of Chemical Information and Modeling. 2012;52(4):882–890. doi: 10.1021/ci300010y. [DOI] [PubMed] [Google Scholar]
- 24.Levin JI. Macrocycles in Drug Discovery. Royal Society of Chemistry; 2014. [Google Scholar]
- 25.el Tayar N, et al. Solvent-dependent conformation and hydrogen-bonding capacity of cyclosporin A: evidence from partition coefficients and molecular dynamics simulations. J Med Chem. 1993;36(24):3757–3764. doi: 10.1021/jm00076a002. [DOI] [PubMed] [Google Scholar]
- 26.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 27.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 28.Roberts AW, et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20(+) lymphoid malignancies. Br J Haematol. 2015;170(5):669–678. doi: 10.1111/bjh.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kipps TJ, et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015:1–8. doi: 10.3109/10428194.2015.1030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.