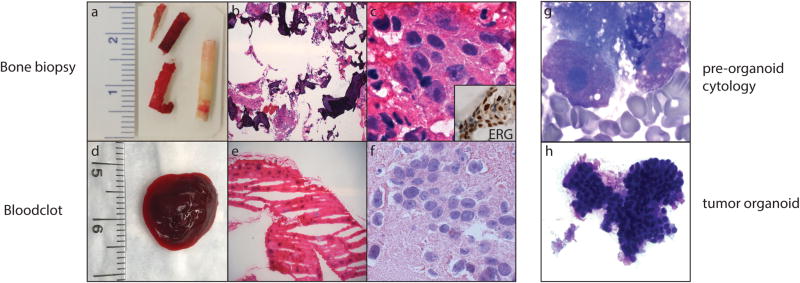
Figure 2.
Bone biopsy material used for diagnosis, next-generation sequencing (NGS), and preclinical models. (a) Macroscopic image of a bone biopsy. Compact bone (whitish core) and bone marrow (reddish core) are split upon receipt, and submitted for clinical diagnosis and NGS, respectively. (b and c) Histology of metastatic prostate adenocarcinoma using images from corresponding frozen bone marrow (H & E; low and high magnification). Inset: ETS-related gene (ERG)‐positive immunohistochemistry in tumor cells. (d) Macroscopic image of a fresh blood clot. (e and f) Histology of metastatic prostate adenocarcinoma using images from a corresponding frozen blood clot (H & E; low and high magnification). (g) Adequacy and tumor cell content are evaluated by cytologic smear examination of the biopsy and/or blood clot (Diff-Quik). (h) Morphologic evaluation of organoid in culture by cytologic smear examination (Diff-Quik).