Abstract
Purpose
CEST-MRI is increasingly evolving from brain to body applications. One of the known problems in the body imaging is the presence of strong lipid signals. Although their influence on the CEST effect is acknowledged, there was no study focusing on the interplay among echo time (TE), fat fraction (FF) and Z-spectrum. This study strives to address these points, with the emphasis on the application in the breast.
Methods
Z-spectra were simulated at in-phase (IP) and out-of-phase (OP) of the main fat peak at −3.4 ppm with FF varying from 0 to 100%. MTRasym in two ranges centering at the exchanging pool and at 3.5 ppm approximately opposite the non-exchanging fat pool were calculated and were plotted against FF. The results were verified in phantoms and in vivo.
Results
The results demonstrate the combined influence of FF and TE on the Z-spectrum for gradient echo based CEST acquisitions. The influence is straightforward in the IP images, while it is more complicated in the OP images, potentially leading to erroneous CEST contrast.
Conclusion
The study provides a basis for understanding of the origin and appearance of lipid artifacts in CEST imaging and lays the foundations for their efficient removal.
Keywords: CEST, Lipid artifact, IP, OP, Breast imaging, Body imaging
Introduction
Chemical exchange saturation transfer (CEST) is a novel contrast mechanism (1), which is based on the chemical exchange processes between the protons in water and solutes (2–5). CEST can indirectly detect the low concentrated solutes which are not observable with conventional MRI (6). Moreover, due to the dependence on exchange rate, CEST is sensitive to the environmental parameters, such as pH and temperature (7–9). Therefore, CEST methodology is a focus of increased research interest and may have a significant clinical impact.
Often, CEST effect is measured using the Magnetization Transfer Ratio Asymmetry (MTRasym) analysis, MTRasym=[Mzw(−ωs)−Mzw(ωs)]/Mz0, where Mzw(−ωs) and Mzw(ωs) are the signal intensities acquired with the RF irradiation applied at a frequency offset ωs or −ωs, and Mz0 is the reference signal without the saturation.
Most human applications of CEST focused on the brain, utilizing solute amides resonating at 3.5 ppm, the so-called Amide Proton Transfer (APT) (8,10–12). Recently, promising applications were reported outside the brain, such as in prostate cancer (13), breast cancer (14–17) and renal ailments (18). One of the problems in body imaging is the presence of a large fat signal. Fat appears bright in both T1- and T2-weighted images, confounding image interpretation (19). The problem is very acute in breast, where fibroglandular tissue and fat are interleaved. The influence and the suppression of fat signals have been investigated long before CEST. However, the influence of fat in CEST imaging is relatively new and underexplored, although its confounding influence is acknowledged (20). 1H-NMR spectrum of fat displays multiple peaks with the main peak at −3.4 ppm from water (21). As a result, the MTRasym analysis becomes inaccurate in the presence of fat, affecting APT most. Moreover, fat overlaps with the Nuclear Overhauser Enhancement (NOE) (22–25).
Although methods to remove lipid artifacts in CEST imaging were reported (22,26,27), there was no study focusing on the interplay between image acquisition parameters, fat fraction (FF) and the Z-spectrum.
The goal of this paper is to discuss how the fat influences the Z-spectrum taking echo time (TE) into account in the gradient echo based sequences. The interplay is studied in simulations and verified in phantom and in vivo experiments. We predominantly focus on the CEST at 1 ppm, since previous studies indicated that it might be the most sensitive to the malignant alternations in breast (14,17,28–30). However, other offsets, exchange regimes and experimental parameters are briefly addressed. This study demonstrates and explains the abnormal appearance of Z-spectra when fat is present and emphasizes the importance of a correct choice of the imaging parameters. The goals are to help in recognizing lipid artifacts in CEST images, to provide suggestions on the selection of imaging parameters and to build a foundation for the most efficient removal of these artifacts in the future studies.
Methods
Simulation
Simulations (Matlab, The Mathworks, Natick, MA) used a three-pool Bloch-McConnell model including a bulk water pool (subscript w), a solute pool (s) and a fat pool (f). The water and solute pools are in chemical exchange and the solute/water ratio was kept constant. There is no exchange between fat and the other pools. To simulate different intravoxel FF, the pool sizes for water (M0w) and fat (M0f) varied between 0 and 1, with FF = M0f/(M0w+M0f) increasing from 0 to 100% while satisfying M0w+M0f=1. Other parameters included T1w = 1.5 s, T2w = 0.8 s, M0s/M0w = 0.005, T1s = 1 s, T2s = 1 s, Δws = 1 ppm, ksw = 100 Hz, T1f = 0.8 s, T2f = 0.5 s, Δwf = −3.4 ppm, where Δws/f is the chemical shift between water and the solute/fat pool, and ksw is the exchange rate from solute to the water pool. A CW pulse with length tsat= 5 s and B1~0.97 μT was used for all simulations. To study the influence of TE while avoiding the influence of other imaging parameters, the water and fat Z-magnetizations after the saturation pulse were tipped to the XY-plane assuming a perfect 90° pulse. Then, the signal was simulated assuming two conditions: in-phase (IP, 360° phase difference between water and fat) and out-of-phase (OP, 180° phase difference). The simulated MTRasym was averaged in two ranges: 0.8–1.2 ppm centering at the solute pool (hydroxyl-MTRasym) and 3.3–3.7 ppm centering at 3.5 ppm (amide-MTRasym, opposite to fat resonance, to monitor fat influence on the Z-spectra in the absence of an exchanging moiety). Additional simulations were performed to gauge the influence of different solute properties (Δws = 1, 2, 3.5 and 5.1 ppm in slow, intermediate and fast regime) as well as under different experimental conditions (B1=0.5–5 μT and tsat=0.5–7.5 s), and the results are presented in the Supporting Information.
Phantom
Three phantoms containing water solutions of exchanging molecules and oil (Vegetable Oil, Crisco) were prepared. Phantom I contained 100 mM choline at pH=5.9 (24°C, no additional buffers). Phantoms II and III contained 194 mM iopamidol (Isovue-370, Bracco Diagnostics Inc.) with the pH adjusted by titration to 7.5 and 6.0 respectively. Since the water solutions and oil are immiscible, an interface between them builds up automatically (Fig. 1).
Figure 1.
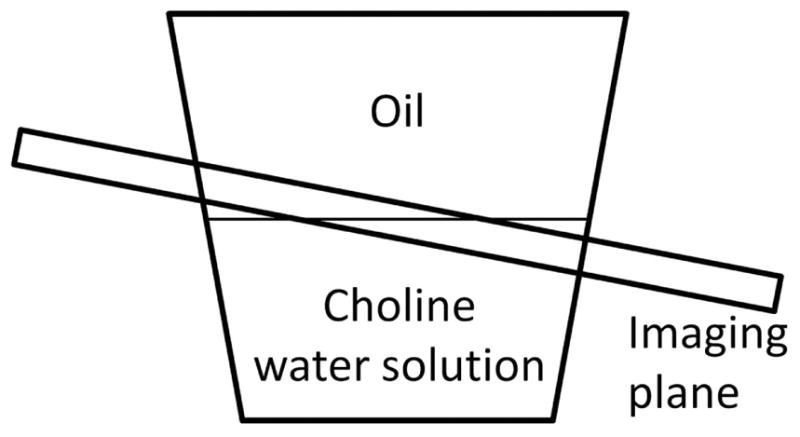
Schematic of the phantom and the imaging plane.
In vivo study
Three female volunteers without known breast ailments were recruited. The human studies were approved by the local Institutional Review Board (IRB) and performed in accordance with the guidelines.
MRI
All MRI scans were performed on a 3T whole-body scanner (Ingenia, Philips Healthcare). The phantom studies employed a 15-channel head-spine coil at room temperature (~22°C). CEST images were acquired using a 2D single-shot T1-weighted turbo field echo (TFE) sequence. Two scans were used to acquire CEST images with different TEs: i) IP at 2.30 ms and ii) OP at 3.45 ms, with the same TFE acquisition inter-pulse delay TR of 5 ms. The saturation pulse train was 4900 ms in length and consisted of 98 hyperbolic secant (HS) pulses, each 50 ms long, flip angle (FA) = 900° and no inter-pulse delay, equivalent to B1,CW = 1.17 μT (31). The total saturation and acquisition length was 5.1 s. Alternated parallel transmission is used to achieve an RF duty cycle of 100% (32). 41 points in the Z-spectrum in the range ±6 ppm for phantom I or ±10 ppm for phantoms II and III were acquired. Other imaging parameters were: centric k-space ordering, voxel size 2×2 mm2, slice thickness 8 mm and excitation FA = 45°. To obtain an image with varying FF, the image plane was placed oblique at the interface of oil and choline/iopamidol water solution (Fig. 1). Thus, the resulting image had a FF decreasing from 100% down to 0% from left to right (33).
The in vivo study employed a 16-channel bilateral breast coil. CEST images were acquired using the same TFE sequence with the same TEs (IP/OP) and inter-pulse TR as in the phantom study. The saturation pulse train (2 s) consisted of 40 HS pulses. Different saturation length was used in vivo to adhere to the stricter SAR limitations (<90% body) and to utilize shorter T1 in tissue (less time needed to reach the saturation steady-state). Total saturation and acquisition length was 2.6 s. 33 points in the Z-spectrum in the range ±6 ppm were acquired. Other imaging parameters were: centric k-space ordering, voxel size 2×2 mm2, slice thickness 5 mm and excitation FA = 10°.
In all studies, a separate B0 mapping sequence (dual echo FE with TE1/ΔTE=2.3/2.3 ms for two IP images) immediately followed the CEST imaging, without B0 re-adjustment between the scans.
Data processing
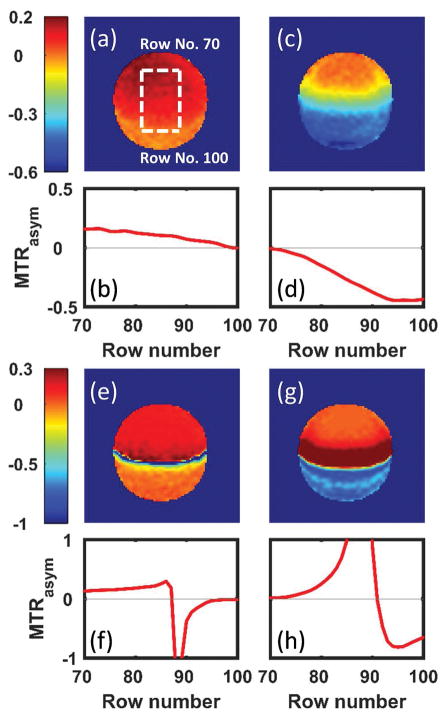
The data were processed using custom MATLAB codes on a pixel-by-pixel basis. The field inhomogeneity was corrected using the separate B0 map. The ROIs were placed manually on regions with different FFs to generate ROI-averaged Z-spectra and MTRasym. The exact FF in the experiments was not determined. The ROIs were selected qualitatively based on the position in the image. The IP and OP MTRasym were averaged in different ranges to generate CEST maps: i) 3.3–3.7 ppm for the influence of fat (no exchanging pool present) in all the phantoms; ii) 0.8–1.2 ppm or 4.0–4.4 ppm and 5.3–5.7 ppm for choline or iopamidol, respectively. For the image plane position (Fig. 1) used in the phantom study, increasing row number corresponds to the increasing FF. The MTRasym for each frequency range was averaged row-by-row and plotted against the row number (Fig. 4a).
Figure 4.
The MTRasym maps of choline-oil phantom (Phantom I) were generated by averaging the MTRasym in two frequency ranges: 0.8–1.2 ppm (a,e) and 3.3–3.7 ppm (c,g) for both IP (a,c) and OP (e,g). The MTRasym in each case are then averaged in the middle part of the CEST maps (vertical dashed line in a, omitted in other images) row-by-row (horizontal dashed line in a, omitted in other images) and plotted against the image row number (b,d,f,h). Part of the MTRasym (very large negative and positive values due to normalization to a reference signal close to 0) are not shown in (f) and (h) for display convenience.
Results
Simulation
Figure 2 shows the representative simulated IP and OP Z-spectra and MTRasym with different intravoxel FFs. Here both the Z-spectra and MTRasym are normalized to the sum of the water and fat pool sizes. Importantly, in the experiment, the results are normalized by the reference signal, which is also affected by the presence of fat and choice of TE. The influence of fat is further studied in two particular ranges, hydroxyl and amide, using continuously increasing FF (Fig. 3). Here, the MTRasym was normalized to the reference signal (for comparison, normalization by the sum of the pool sizes is shown in Supporting Fig. S1).
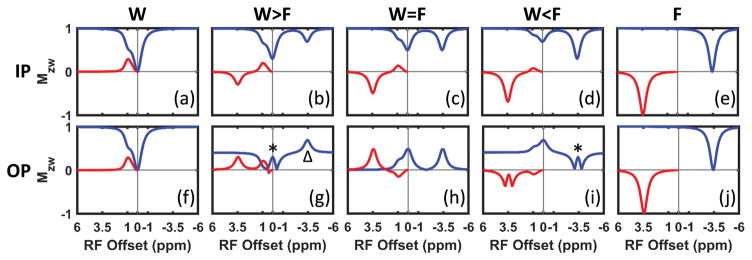
Figure 2.
Simulated (a–e) IP and (f–j) OP Z-spectra (blue line) and MTRasym (red line) with fat fraction of 0% (W), 30% (W>F), 50% (W=F), 70% (W<F) and 100% (F). The Z-spectra were normalized to the sum of the water and fat pool size for display convenience. (* in g and i) mark the “fold-back” of the negative values in the Z-spectra when magnitude is taken. (Δ in g) labels a fat related peak (see text for further discussion).
Figure 3.
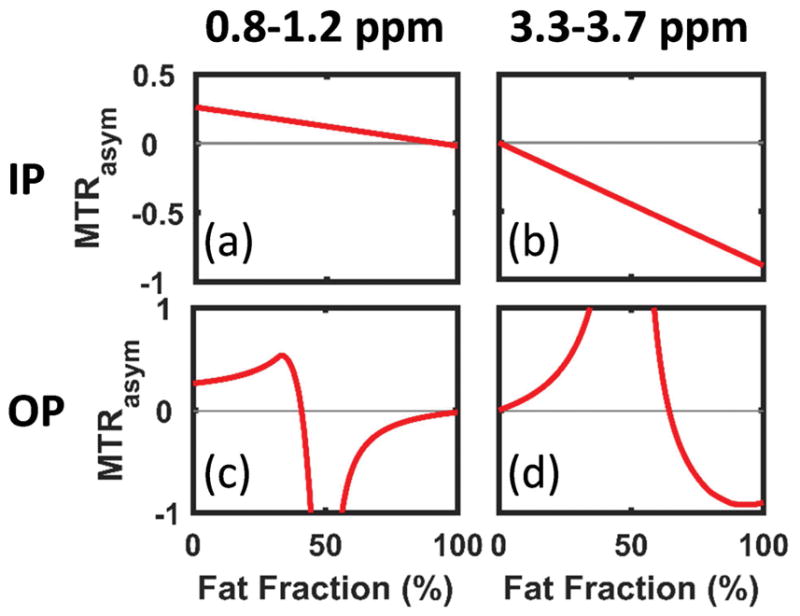
The simulated MTRasym averaged in two ranges: 0.8–1.2 ppm (a,c) and 3.3–3.7 ppm (b,d) for IP (a,b) and OP (c,d) respectively. The MTRasym were normalized to the reference signal, which is the sum of the water and fat signals with their relative phase taken into account. Part of the MTRasym (very large negative and positive values due to normalized to a reference signal close to 0) are not shown in (c) and (d) for display convenience.
As expected, if only water or fat is present, the IP and OP Z-spectra are identical (Fig. 2a vs. f and e vs. j). In-phase the water and fat signals overlap and the IP Z-spectra (Fig. 2a–e, blue line) are straightforward to understand: the water dip shrinks while the fat dip increases as the FF increases. Linear monotonic decrease of both hydroxyl- and amide-MTRasym are observed in Figure 2a–e (red line) and Figure 3a,b.
The OP Z-spectra and MTRasym are more complicated than the IP, since water and fat have a phase difference of (2n+1)π (n∈ℤ). As shown in Figure 2f–j, both water and fat could form ‘dips’ and ‘ascends’ in the Z-spectra depending on the FF. The reference signal approaches zero as FF approaching 50% (Fig. 2g–i). As a result, close to W~F the MTRasym normalized to the reference signal may become a very large positive or negative number, depending on the sign of the MTRasym before normalization. As shown in Figure 3c, the hydroxyl-MTRasym as a function of FF increases at first, indicating that the decrease of the reference signal is faster than the decrease of the hydroxyl-MTRasym before normalization. As FF approaches 50%, the hydroxyl-MTRasym rapidly drops to a large negative value and gradually increases back to approximately zero for FF>50%. Conversely, the amide-MTRasym increases from 0 to a very large value as FF increases from 0 to 50% and then decreases as FF further increases to 100% (Fig. 3d). The MTRasym normalized to the sum of the water and fat pool sizes (Fig. 2, red line) shows similar trend to the MTRasym normalized to the reference (Fig. 3) except for the decreasing OP hydroxyl-MTRasym at low to mid FF (Supporting Fig. S1).
Additional simulations (Supporting Figs. S2–S9) demonstrate that while the details change, the overall behavior remains consistent with the Figure 3 for a variety of experimental and solute parameters.
Phantom Experiments
Figure 4 and Supporting Figure S8 show the results of the phantom I. The normalized IP and OP Z-spectra and MTRasym were averaged in four ROIs: W only, W>F, W<F and F only. The choline peak is relatively far from the direct fat influence, especially at the low power levels used here. Comparing Figure 2 and Supporting Figure S8, the experimental Z-spectra and MTRasym maps display the same trends as the simulation. For the IP case, both the hydroxyl- (Fig. 4b) and amide- (Fig. 4d) MTRasym decrease as FF increases, similar to the simulation (Fig. 3a,b). The hydroxyl-MTRasym decreases from ~15% to ~0% (Fig. 4b) while the amide-MTRasym decreases approximately linearly from 0% to about −45% (Fiure. 4d). As shown in Figure 4e–h, the OP MTRasym maps display very large negative or positive values in the middle where W~F due to normalization by the reference values close to zero. In the regions W>F and W<F, the behavior of both the hydroxyl- and amide- MTRasym (Fig. 4f,h) are also in exact agreement with the trend observed in the simulation (Fig. 3c,d).
Supporting Figures S11–S14 show the results of the phantoms II and III. The 4.2 ppm peak of iopamidol is close to direct influence of fat, while the 5.5 ppm peak is further away and thus is less influenced by fat. Moreover, there are two exchanging pools and exchange-mediated T2 shortening (34). Despite these differences from the simple model systems above, the IP and OP CEST maps and MTRasym (Supporting Figs. S11–S14) show similar behavior as phantom I and simulations (Figs. 3 and 4).
In vivo study
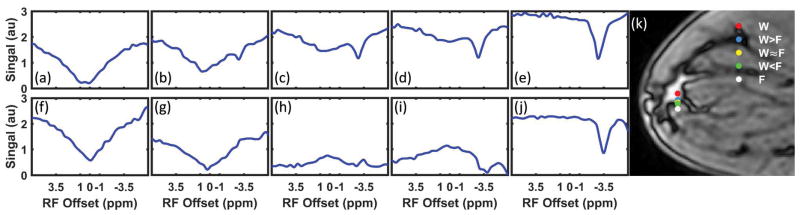
The representative in vivo IP and OP Z-spectra are shown in Figure 5. The Z-spectra were not normalized. The pixels were chosen on a straight line going from pure fat into pure fibroglandular tissue (Fig. 5k). Comparing Figures 2, 4 and 5, it is evident that the in vivo Z-spectra display exactly the same behavior as was predicted in simulations and phantoms.
Figure 5.
Representative IP (a–e) and OP (f–j) Z-spectra without normalization and B0 correction for different fat fractions from a healthy volunteer. The Z-spectra were from separate pixels shown as dots in the OP image acquired at TE = 3.45 ms (k).
Discussion
In the simulations, the MTRasym of the solute is only weakly influenced by fat, because the two pools are relatively far from each other (|2.4| ppm). The linear decrease of IP MTRasym at both frequency ranges (Fig. 3a,b) is due to the monotonic decrease of the relative size of the water pool.
The intensity of the OP Z-spectrum at any frequency can be quantitatively explained by the equation: intensity = |Z-Intensity(water)-Z-Intensity(fat)|. Consider the reference signal: it is equal to the absolute value of fraction difference between water and fat; e.g. resulting in 0.4 in the cases of W>F (|0.7–0.3|=0.4) and W<F (|0.3–0.7|=0.4) and 0 in the case of W=F (|0.5–0.5|=0). The reference signal may become lower than the rest of the Z-spectrum. For example, consider W>F and the saturation is at −3.4 ppm (labeled by Δ in Fig. 2g). Assuming that fat is fully saturated at this frequency while water is unperturbed, the OP Z-spectrum value becomes |0.7–0|=0.7 which is larger than the reference signal value of 0.4.
In practice, the reference signal is always nonnegative since magnitude images are used most of the time. Thus, the sign of MTRasym before normalization determines the sign of the MTRasym after normalization by the reference signal. The hydroxyl-MTRasym becomes negative when the water “dip” at 0 ppm in the Z-spectrum changes to an “ascent” due to the increasing FF (Fig. 2f–i). At low FF, the amide-MTRasym is positive because there is an “ascent” at the lipid frequency in the Z-spectrum (Fig. 2g–h); at larger FF, amide-MTRasym becomes negative as Z-spectrum intensities form a “dip” around −3.4 ppm.
The oil used in the phantom study has multiple peaks (35). The main peak is at about −3.4 ppm, followed by the peaks of decreasing sizes at −3.8 ppm and 0.6 ppm. The experimental IP and OP images refer to the main peak of the oil. The other peaks may introduce additional asymmetry of the Z-spectrum as reflected in Supporting Figures S10–S11.
The field inhomogeneity was corrected using the B0 maps generated by a sequence with two IP echoes. This method may not be as accurate as WASSR (36) in pixels without fat and underperforms in pixels with large FF. However, WASSR is suboptimal for B0 correction in the images containing pixels with FF in the broad range, from 0 to 100%, since WASSR fails in voxels with little or no water (36).
Saturation parameters and the solute properties influence the Z-spectrum and the MTRasym. However, as the simulations included in the Supporting Information demonstrate, these parameters did not largely alter the overall behavior of the IP and OP MTRasym against FF (Supporting Figs. S2–S7). While these parameters influenced the specific values of the MTRasym, the overall trends (i.e. decreases with FF and singularity areas) remained the same. Thus, the explanation suggested above is largely valid.
Other experimental parameters including T1, T2, T2*, excitation FA, and TR together determine the acquired signal (including the reference) and thus will further influence the Z-spectrum appearance. Moreover, presence of multiple fat peaks or multiple exchanging sites at high concentration may potentially lead to a non-zero reference signal at FF=50% and smoothing of the singularities. However, the detailed exploration of the influence of these factors is beyond the scope of this note.
Two well-defined acquisition conditions, IP and OP, were investigated here. Other TEs will lead to complicated behavior consisting of a mix of the effects. Moreover, while quantification methods other than MTRasym may be used successfully in IP Z-spectra to eliminated lipid/NOE peak (15), these methods would fail in the OP Z-spectra.
Conclusions
We demonstrate that the TE and FF together determine the appearance of the Z-spectrum for gradient echo based sequences. Phantom and in vivo studies agree with the simulation. Although several lipid artifact removal methods were reported in CEST (3,15–17,37), additional studies are needed to examine the efficiency of various fat removal methods and their influence on the CEST contrast.
Supplementary Material
Supporting Figure S1. The simulated MTRasym averaged in two ranges: 0.8–1.2 ppm (a,c) and 3.3–3.7 ppm (b,d) for IP (a,b) and OP (c,d) respectively. The MTRasym is normalized to the sum of the water and fat pool size.
Supporting Figure S2. The simulated MTRasym for varying B1 and FF. The exchanging pool located 1 ppm downfield respect of water. The MTRasym were averaged in two ranges: 0.8 – 1.2 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c, i–k) and OP (e–g, m–o). The exchange rates were 20 Hz (a,e,i,m), 100 Hz (b,f,j,n), and 1000 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S3. The simulated MTRasym for varying saturation time and FF. The exchanging pool located 1 ppm downfield respect of water. The MTRasym were averaged in two ranges: 0.8 – 1.2 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 100 Hz (b,f,j,n), and 1000 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S4. The simulated MTRasym for varying B1 and FF. The exchanging pool located 2 ppm downfield respect of water. The MTRasym were averaged in two ranges: 1.8 – 2.2 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 250 Hz (b,f,j,n), and 2500 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S5. The simulated MTRasym for varying saturation time and FF. The exchanging pool located 2 ppm downfield respect of water. The MTRasym were averaged in two ranges: 1.8 – 2.2 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 250 Hz (b,f,j,n), and 2500 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S6. The simulated MTRasym for varying B1 and FF. The exchanging pool located 3.5 ppm downfield respect of water. The MTRasym were averaged in one range: 3.3 – 3.7 ppm (a–c,e–g) for IP (a–c) and OP (e–g). The exchange rates were 20 Hz (a,e), 450 Hz (b,f), and 4500 Hz (c,g) respectively. The MTRasym against FF plots at the dashed lines in (b,f) are shown in (d,h) accordingly as an example.
Supporting Figure S7. The simulated MTRasym for varying saturation time and FF. The exchanging pool located 3.5 ppm downfield respect of water. The MTRasym were averaged in one range: 3.3 – 3.7 ppm (a–c,e–g) for IP (a–c) and OP (e–g). The exchange rates were 20 Hz (a,e), 450 Hz (b,f), and 4500 Hz (c,g) respectively. The MTRasym against FF plots at the dashed lines in (b,f) are shown in (d,h) accordingly as an example.
Supporting Figure S8. The simulated MTRasym for varying B1 and FF. The exchanging pool located 5.1 ppm downfield respect of water. The MTRasym were averaged in two ranges: 4.9 – 5.3 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 650 Hz (b,f,j,n), and 6500 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S9. The simulated MTRasym for varying saturation time and FF. The exchanging pool located 5.1 ppm downfield respect of water. The MTRasym were averaged in two ranges: 4.9 – 5.3 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 650 Hz (b,f,j,n), and 6500 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S10. ROI averaged IP (a) and OP (b) Z-spectra and MTRasym of the choline-oil phantom (Phantom I).
Supporting Figure S11. ROI averaged IP (a) and OP (b) Z-spectra and MTRasym of the iopamidol pH 7.5-oil phantom (Phantom II).
Supporting Figure S12. The MTRasym maps of the iopamidol pH 7.5-oil phantom (Phantom II) were generated by averaging the MTRasym in three frequency ranges: 4.0 – 4.4 ppm (a,g), 5.3 – 5.7 (c,i) and 3.3 – 3.7 ppm (e,k) for both IP (a,c,e) and OP (g,i,k). The MTRasym in each case are then averaged in the middle part of the CEST maps (vertical dashed line in a, omitted in other images) row-by-row (horizontal dashed line in b, omitted in other images) and plotted against the image row number (b,d,f,h,j,l). Part of the MTRasym (very large negative and positive values due to normalization to a reference signal close to 0) are not shown in (h,j,l) for display convenience.
Supporting Figure S13. ROI averaged IP (a) and OP (b) Z-spectra and MTRasym of the iopamidol pH 6.0-oil phantom (Phantom III).
Supporting Figure S14. The MTRasym maps of the iopamidol pH 6.0-oil phantom (Phantom III) were generated by averaging the MTRasym in three frequency ranges: 4.0 – 4.4 ppm (a,g), 5.3 – 5.7 (c,i) and 3.3 – 3.7 ppm (e,k) for both IP (a,c,e) and OP (g,i,k). The MTRasym in each case are then averaged in the middle part of the CEST maps (vertical dashed line in a, omitted in other images) row-by-row (horizontal dashed line in b, omitted in other images) and plotted against the image row number (b,d,f,h,j,l). Part of the MTRasym (very large negative and positive values due to normalization to a reference signal close to 0) are not shown in (h,j,l) for display convenience.
Acknowledgments
The research was supported by the NIH grant R21EB020245 and the UTSW Radiology Research fund.
References
- 1.Ward K, Aletras A, Balaban R. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Lal B, Wilson DA, Laterra J, van Zijl P. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50(6):1120–1126. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]
- 3.Kogan F, Haris M, Singh A, Cai K, Debrosse C, Nanga RP, Hariharan H, Reddy R. Method for high-resolution imaging of creatine in vivo using chemical exchange saturation transfer. Magn Reson Med. 2014;71(1):164–172. doi: 10.1002/mrm.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, Richardson S, Goncalves M, Parkes HG, Arstad E, Thomas DL, Pedley RB, Lythgoe MF, Golay X. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med. 2013;19(8):1067–1072. doi: 10.1038/nm.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagher AP, Aletras A, Choyke P, Balaban RS. Imaging of urea using chemical exchange-dependent saturation transfer at 1. 5 T. J Magn Reson Imaging. 2000;12(5):745–748. doi: 10.1002/1522-2586(200011)12:5<745::aid-jmri12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Zijl PCMv. Chemical exchange saturation transfer imaging and spectroscopy. Progress in Nuclear Magnetic Resonance Spectroscopy. 2006;48(2–3):109–136. doi: 10.1016/j.pnmrs.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Ward K, Balaban R. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST) Magn Reson Med. 2000;44(5):799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Payen J-F, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 9.Li AX, Wojciechowski F, Suchy M, Jones CK, Hudson RHE, Menon RS, Bartha R. A sensitive PARACEST contrast agent for temperature MRI: Eu 3+-DOTAM-glycine (Gly)-phenylalanine (Phe) Magn Reson Med. 2008;59(2):374–381. doi: 10.1002/mrm.21482. [DOI] [PubMed] [Google Scholar]
- 10.Jones CK, Schlosser MJ, van Zijl P, Pomper MG, Golay X, Zhou J. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006;56(3):585–592. doi: 10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu D-X, Ford E. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med. 2011;17(1):130–134. doi: 10.1038/nm.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagiyama K, Mashimo T, Togao O, Vemireddy V, Hatanpaa KJ, Maher EA, Mickey BE, Pan E, Sherry AD, Bachoo RM, Takahashi M. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci USA. 2014;111(12):4542–4547. doi: 10.1073/pnas.1323855111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia G, Abaza R, Williams JD, Zynger DL, Zhou J, Shah ZK, Patel M, Sammet S, Wei L, Bahnson RR. Amide proton transfer MR imaging of prostate cancer: a preliminary study. J Magn Reson Imaging. 2011;33(3):647–654. doi: 10.1002/jmri.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt B, Zamecnik P, Zaiss M, Rerich E, Schuster L, Bachert P, Schlemmer HP. A New Contrast in MR Mammography by Means of Chemical Exchange Saturation Transfer (CEST) Imaging at 3 Tesla: Preliminary Results. Fortschr Röntgenstr. 2011;183(11):1030–1036. doi: 10.1055/s-0031-1281764. [DOI] [PubMed] [Google Scholar]
- 15.Dula AN, Arlinghaus LR, Dortch RD, Dewey BE, Whisenant JG, Ayers GD, Yankeelov TE, Smith SA. Amide proton transfer imaging of the breast at 3 T: establishing reproducibility and possible feasibility assessing chemotherapy response. Magn Reson Med. 2013;70(1):216–224. doi: 10.1002/mrm.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klomp DW, Dula AN, Arlinghaus LR, Italiaander M, Dortch RD, Zu Z, Williams JM, Gochberg DF, Luijten PR, Gore JC. Amide proton transfer imaging of the human breast at 7T: development and reproducibility. NMR Biomed. 2013;26(10):1271–1277. doi: 10.1002/nbm.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dula AN, Dewey BE, Arlinghaus LR, Williams JM, Klomp D, Yankeelov TE, Smith S. Optimization of 7-T chemical exchange saturation transfer parameters for validation of glycosaminoglycan and amide proton transfer of fibroglandular breast tissue. Radiology. 2014;275(1):255–261. doi: 10.1148/radiol.14140762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longo DL, Dastrù W, Digilio G, Keupp J, Langereis S, Lanzardo S, Prestigio S, Steinbach O, Terreno E, Uggeri F. Iopamidol as a responsive MRI-chemical exchange saturation transfer contrast agent for pH mapping of kidneys: In vivo studies in mice at 7 T. Magn Reson Med. 2011;65(1):202–211. doi: 10.1002/mrm.22608. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein MA, King KF, Zhou XJ. Handbook of MRI pulse sequences. Elsevier; 2004. [Google Scholar]
- 20.van Zijl P, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011;65(4):927–948. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60(5):1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Zhou J, Cai C, Cai S, Chen Z. Observation of true and pseudo NOE signals using CEST-MRI and CEST-MRS sequences with and without lipid suppression. Magn Reson Med. 2015;73(4):1615–1622. doi: 10.1002/mrm.25277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proceedings of the National Academy of Sciences. 2008;105(7):2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones CK, Polders D, Hua J, Zhu H, Hoogduin HJ, Zhou J, Luijten P, van Zijl P. In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magn Reson Med. 2012;67(6):1579–1589. doi: 10.1002/mrm.23141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones CK, Huang A, Xu J, Edden RA, Schär M, Hua J, Oskolkov N, Zacà D, Zhou J, McMahon MT. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. Neuroimage. 2013;77:114–124. doi: 10.1016/j.neuroimage.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun PZ, Zhou J, Sun W, Huang J, van Zijl P. Suppression of lipid artifacts in amide proton transfer imaging. Magn Reson Med. 2005;54(1):222–225. doi: 10.1002/mrm.20530. [DOI] [PubMed] [Google Scholar]
- 27.Zhu H, Jones CK, van Zijl PCM, Barker PB, Zhou JY. Fast 3D Chemical Exchange Saturation Transfer (CEST) Imaging of the Human Brain. Magn Reson Med. 2010;64(3):638–644. doi: 10.1002/mrm.22546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song X, Airan RD, Arifin DR, Bar-Shir A, Kadayakkara DK, Liu G, Gilad AA, van Zijl PCM, McMahon MT, Bulte JWM. Label-free in vivo molecular imaging of underglycosylated mucin-1 expression in tumour cells. Nature Communications. 2015;6:6719. doi: 10.1038/ncomms7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan KW, Jiang L, Cheng M, Wijnen JP, Liu G, Huang P, Zijl P, McMahon MT, Glunde K. CEST-MRI detects metabolite levels altered by breast cancer cell aggressiveness and chemotherapy response. NMR Biomed. 2016;29(6):806–816. doi: 10.1002/nbm.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S, Seiler S, Madhuranthakam AJ, Keupp J, Dimitrov IE, Lenkinski RE, Vinogradov E. CEST-mDixon for Breast Lesion Characterization at 3T. Proceedings of the ISMRM 24th Annual Meeting; 2016; p. 2894. [Google Scholar]
- 31.Zu Z, Li K, Janve VA, Does MD, Gochberg DF. Optimizing pulsed-chemical exchange saturation transfer imaging sequences. Magn Reson Med. 2011;66(4):1100–1108. doi: 10.1002/mrm.22884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keupp J, Baltes C, Harvey P, Van den Brink J. Parallel RF transmission based MRI technique for highly sensitive detection of amide proton transfer in the human brain. Proceedings of the ISMRM 19th Annual Meeting; 2011; p. 710. [Google Scholar]
- 33.Hussain HK, Chenevert TL, Londy FJ, Gulani V, Swanson SD, McKenna BJ, Appelman HD, Adusumilli S, Greenson JK, Conjeevaram HS. Hepatic Fat Fraction: MR Imaging for Quantitative Measurement and Display—Early Experience 1. Radiology. 2005;237(3):1048–1055. doi: 10.1148/radiol.2373041639. [DOI] [PubMed] [Google Scholar]
- 34.Soesbe TC, Merritt ME, Green KN, Rojas-Quijano FA, Sherry AD. T2 exchange agents: a new class of paramagnetic MRI contrast agent that shortens water T2 by chemical exchange rather than relaxation. Magn Reson Med. 2011;66(6):1697–1703. doi: 10.1002/mrm.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soriano NU, Jr, Venditti R, Argyropoulos DS. Biodiesel synthesis via homogeneous Lewis acid-catalyzed transesterification. Fuel. 2009;88(3):560–565. [Google Scholar]
- 36.Kim M, Gillen J, Landman BA, Zhou J, van Zijl P. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61(6):1441–1450. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia G, Wei W, Yang X, Flanigan DC, Keupp J, Zhou J, Knopp MV. Improving mobile protein level detection using mDIXON-based APT-MRI in bone marrow edema. Proceedings of the ISMRM 20th Annual Meeting; 2012; p. 3373. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1. The simulated MTRasym averaged in two ranges: 0.8–1.2 ppm (a,c) and 3.3–3.7 ppm (b,d) for IP (a,b) and OP (c,d) respectively. The MTRasym is normalized to the sum of the water and fat pool size.
Supporting Figure S2. The simulated MTRasym for varying B1 and FF. The exchanging pool located 1 ppm downfield respect of water. The MTRasym were averaged in two ranges: 0.8 – 1.2 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c, i–k) and OP (e–g, m–o). The exchange rates were 20 Hz (a,e,i,m), 100 Hz (b,f,j,n), and 1000 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S3. The simulated MTRasym for varying saturation time and FF. The exchanging pool located 1 ppm downfield respect of water. The MTRasym were averaged in two ranges: 0.8 – 1.2 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 100 Hz (b,f,j,n), and 1000 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S4. The simulated MTRasym for varying B1 and FF. The exchanging pool located 2 ppm downfield respect of water. The MTRasym were averaged in two ranges: 1.8 – 2.2 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 250 Hz (b,f,j,n), and 2500 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S5. The simulated MTRasym for varying saturation time and FF. The exchanging pool located 2 ppm downfield respect of water. The MTRasym were averaged in two ranges: 1.8 – 2.2 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 250 Hz (b,f,j,n), and 2500 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S6. The simulated MTRasym for varying B1 and FF. The exchanging pool located 3.5 ppm downfield respect of water. The MTRasym were averaged in one range: 3.3 – 3.7 ppm (a–c,e–g) for IP (a–c) and OP (e–g). The exchange rates were 20 Hz (a,e), 450 Hz (b,f), and 4500 Hz (c,g) respectively. The MTRasym against FF plots at the dashed lines in (b,f) are shown in (d,h) accordingly as an example.
Supporting Figure S7. The simulated MTRasym for varying saturation time and FF. The exchanging pool located 3.5 ppm downfield respect of water. The MTRasym were averaged in one range: 3.3 – 3.7 ppm (a–c,e–g) for IP (a–c) and OP (e–g). The exchange rates were 20 Hz (a,e), 450 Hz (b,f), and 4500 Hz (c,g) respectively. The MTRasym against FF plots at the dashed lines in (b,f) are shown in (d,h) accordingly as an example.
Supporting Figure S8. The simulated MTRasym for varying B1 and FF. The exchanging pool located 5.1 ppm downfield respect of water. The MTRasym were averaged in two ranges: 4.9 – 5.3 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 650 Hz (b,f,j,n), and 6500 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S9. The simulated MTRasym for varying saturation time and FF. The exchanging pool located 5.1 ppm downfield respect of water. The MTRasym were averaged in two ranges: 4.9 – 5.3 ppm (a–c,e–g) and 3.3 – 3.7 ppm (i–k,m–o) for IP (a–c,i–k) and OP (e–g,m–o). The exchange rates were 20 Hz (a,e,i,m), 650 Hz (b,f,j,n), and 6500 Hz (c,g,k,o) respectively. The MTRasym against FF plots at the dashed lines in (b,f,j,n) are shown in (d,h,l,p) accordingly as an example.
Supporting Figure S10. ROI averaged IP (a) and OP (b) Z-spectra and MTRasym of the choline-oil phantom (Phantom I).
Supporting Figure S11. ROI averaged IP (a) and OP (b) Z-spectra and MTRasym of the iopamidol pH 7.5-oil phantom (Phantom II).
Supporting Figure S12. The MTRasym maps of the iopamidol pH 7.5-oil phantom (Phantom II) were generated by averaging the MTRasym in three frequency ranges: 4.0 – 4.4 ppm (a,g), 5.3 – 5.7 (c,i) and 3.3 – 3.7 ppm (e,k) for both IP (a,c,e) and OP (g,i,k). The MTRasym in each case are then averaged in the middle part of the CEST maps (vertical dashed line in a, omitted in other images) row-by-row (horizontal dashed line in b, omitted in other images) and plotted against the image row number (b,d,f,h,j,l). Part of the MTRasym (very large negative and positive values due to normalization to a reference signal close to 0) are not shown in (h,j,l) for display convenience.
Supporting Figure S13. ROI averaged IP (a) and OP (b) Z-spectra and MTRasym of the iopamidol pH 6.0-oil phantom (Phantom III).
Supporting Figure S14. The MTRasym maps of the iopamidol pH 6.0-oil phantom (Phantom III) were generated by averaging the MTRasym in three frequency ranges: 4.0 – 4.4 ppm (a,g), 5.3 – 5.7 (c,i) and 3.3 – 3.7 ppm (e,k) for both IP (a,c,e) and OP (g,i,k). The MTRasym in each case are then averaged in the middle part of the CEST maps (vertical dashed line in a, omitted in other images) row-by-row (horizontal dashed line in b, omitted in other images) and plotted against the image row number (b,d,f,h,j,l). Part of the MTRasym (very large negative and positive values due to normalization to a reference signal close to 0) are not shown in (h,j,l) for display convenience.