Abstract
A novel method for desaturation of aliphatic amines into enamines as well as allylic and homoallylic amines has been developed. This general protocol operates via putative aryl hybrid Pd-radical intermediates, which combine the signature features of radical chemistry, a hydrogen atom transfer (HAT) process, and transition metal chemistry, a selective β-hydride elimination step, to achieve efficient and selective desaturation of amines. These hybrid Pd-radical intermediates are efficiently generated under mild photo-induced conditions and are capable of a 1,n-HAT (n = 5–7) event at C(sp3)–H sites. The selectivity of HAT is tunable by varying different auxiliaries, which highlight the generality of this method. Remarkably, this desaturation method, which operates under mild conditions and does not require employment of exogenous photosensitizers or oxidants, can be performed in a practical scalable fashion from simple amines.
Unsaturated amines are common functional groups found in natural products and bioactive molecules and are versatile synthetic building blocks.1 Thus, not surprisingly, an array of methods toward these important motifs have been developed. Conventional approaches to enamines2 involve condensations of the carbonyl group,2 cross-coupling reactions,3 or hydroaminations of alkynes.4 Main methods toward allylic- and homoallylic amines usually rely on alkylation of amines5 and imines6 with alkenyl halides. While most of the aforementioned methods are efficient, they all require prefunctionalized starting materials. It is apparent that straightforward conversion of cheap and abundant aliphatic amines into valuable alkenyl amines could serve as a more practical approach. However, methods for direct dehydrogenation of amines into unsaturated amines have not been broadly developed. One elegant strategy for converting amines into enamines employs a nondirected7a or directed7b transfer-hydrogenation process (Scheme 1a). Nonetheless, these methods have limited practicability, as they work on non-functionalizable tertiary alkyl amines7a or employ moisture-sensitive N-vinylsilane substrates.7b Another noteworthy, yet moderately efficient approach toward enamines by Doucet8a relies on conventional Pd-catalyzed C–H activation methodology. Both approaches suffer from harsh reaction conditions and are limited to secondary α-/β-desaturation of amines due to restricted size and steric hindrance of transition metal (TM)-cyclic intermediates.9 Conversely, radical strategies have shown exceptional capability for functionalization of unactivated tertiary and secondary C(sp3)–H sites due to a facile HAT event.10,11 The current state-of-the-art method for a remote desaturation of amines was developed by Baran,10a which follows a radical-polar crossover path (Scheme 1a). However, due to generation of cationic intermediates, the end game of this protocol, the proton elimination step, in some cases, results in low regioselectivity of desaturation. Based on these limitations, the development of a practical and universal method for site-selective desaturation of aliphatic amines is highly desirable. Herein, we report a mild, practical, and general protocol for selective desaturation of aliphatic amines (Scheme 1b). This method employs simple aliphatic amines, which, under practical conditions, are selectively and efficiently transformed into enamines, allylic amines, and homoallylic amines. The high selectivity of desaturation is achieved via an auxiliary-controlled 1,5-, 1,6-, and 1,7-HAT process of the photogenerated aryl hybrid Pd-radical intermediates12 (Scheme 1b, A → B), and a subsequent Pd-involved β-H-elimination step.13
Scheme 1.
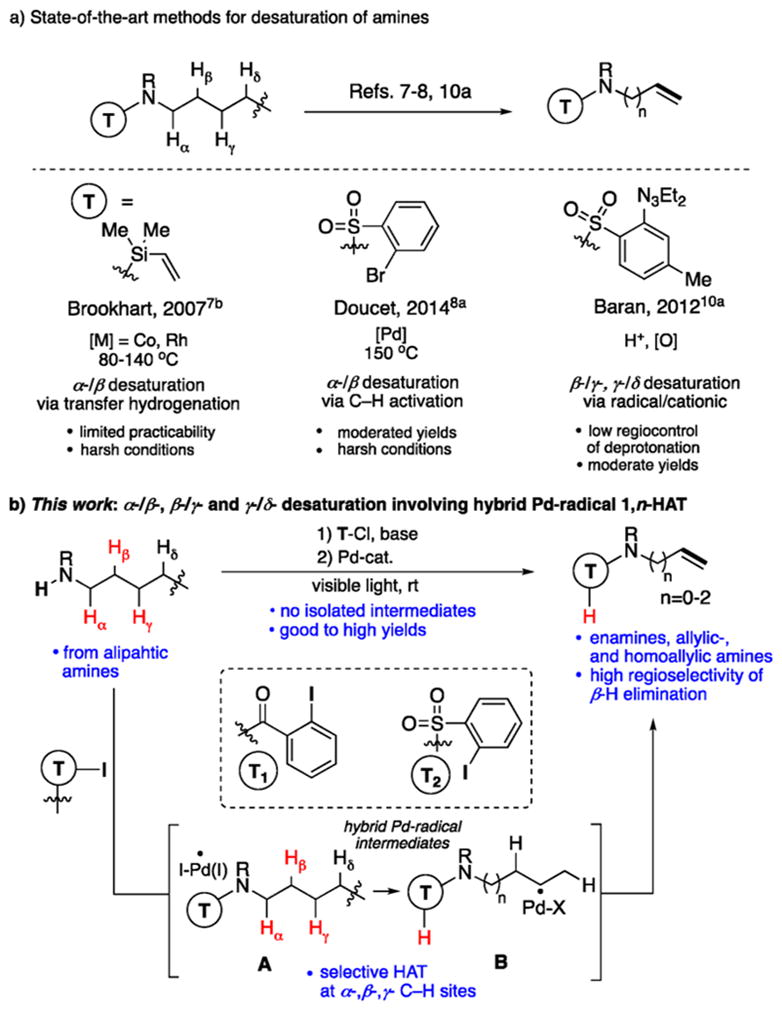
Methods for Directed Desaturation of Amines
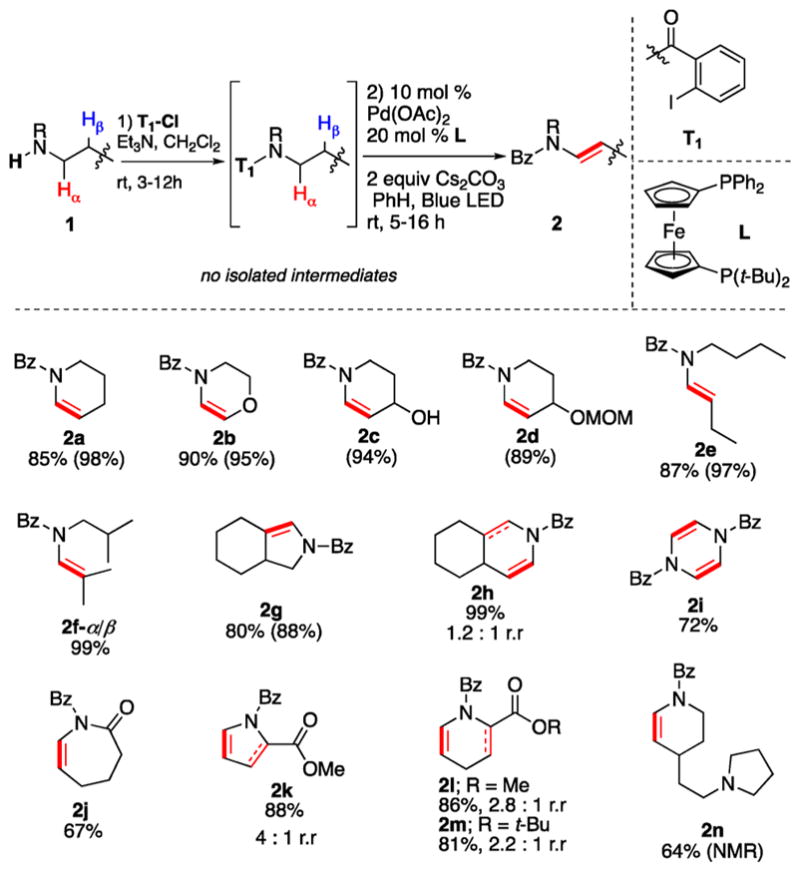
Recently, we developed Si-based auxiliaries for proximal and remote desaturation of aliphatic alcohols via photoinduced generation of hybrid aryl and alkyl Pd-radical species.14 Expectedly, due to the much lower hydrolytic stability of the N–Si bond compared to that of the O–Si bond,15 employment of these Si-based tethers for desaturation of aliphatic amines was not feasible. Thus, we examined more practical amide-based tethers. Delightfully, it was found that piperidine 1a, protected with commercially available o-iodobenzoyl chloride (tether T1), under our previously reported conditions14 underwent efficient α-/β- desaturation via a facile 1,5-HAT11 producing cyclic enamine 2a in nearly quantitative yield (Table 1). This represents the first room temperature dehydrogenation of an amine into an enamine, which operates under visible-light-induced16 Pd-catalyzed conditions without employment of exogenous photosensitizers17 or oxidants. Moreover, this method can efficiently be performed in a practical fashion without isolation of the tethered amine intermediate to produce enamine 2a directly from amine 1a in excellent yield over the two steps.
Table 1.
α-β Desaturation of Amines with T1a
|
Isolated yields for two steps (1 → 2) are shown. Yields for one-step desaturation (from amine protected with reactive tether) are shown in parentheses. r.r, regiomeric ratio. Bz, benzoyl.
Next, the generality of the α-/β-desaturation of aliphatic amines was examined. Morpholine derivative 1b was found to be a competent substrate, producing dihydrooxazine 2b in 95% yield. Piperidines, possessing an unprotected secondary alcohol moiety (1c) or a labile acetal group (1d), also reacted well, producing enamines 2c and 2d in high yields. These results represent the efficient synthesis of endocyclic enamines, which are difficult to access via traditional methods mentioned above.2–4 Linear amines were also amenable, yielding almost quantitative yields of enamine products (2e, 2f-α/β). It is worth mentioning that amine 1f failed to react under reported C–H activation conditions.8a Gratifyingly, desaturation of a pyrrolidine derivative and a bicyclic substrate produced the corresponding enamines in good yields (2g, 2h). Interestingly, double-fold desaturation of piperazine, decorated with two reacting tethers, produced dehydropyrazine 2i in good yield. Notably, a caprolactam derivative was selectively desaturated at an unusual reaction site (2j).18 Dehydrogenation of proline-and pipecolic esters (1k–m) furnished separable mixtures (except for 2k) of regioisomers, with the less substituted alkenes being the major products. Finally, desaturation of substrate 1n, containing two amine moieties, underwent selective directed reaction at the proximal site producing enamine 2n in 64% NMR yield.
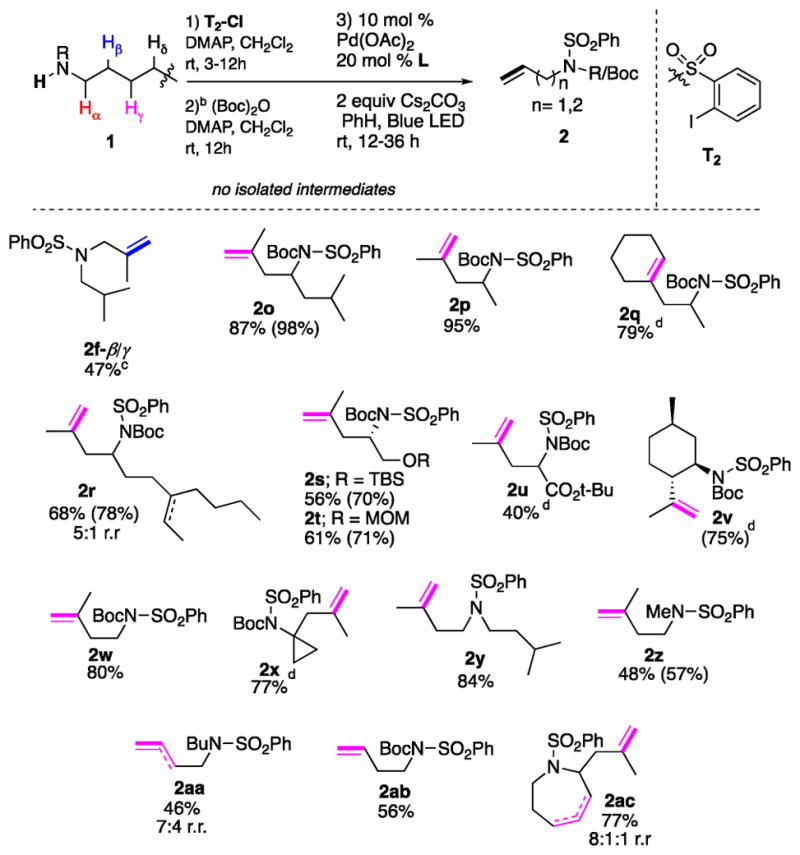
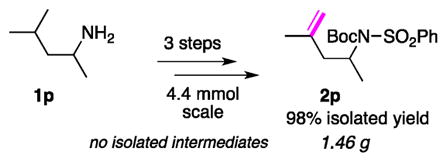
After establishing the scope of α-/β-desaturation, we turned our attention to remote desaturation of amines (Table 2). Apparently, for achieving this goal, the development of the tether capable of HAT at a more distant C–H site was necessary. Based on the efficient and selective remote HAT of Baran’s tosyl triazene auxiliary (Scheme 1a),10a we assumed that the aryl iodide derivative of it could be an appropriate choice of tether (T2, Scheme 2). Indeed, this tether can easily be installed at the amine group using a commercially available o-iodobenzenesulfonyl chloride. Upon generation of the hybrid Pd-radical species, it was expected to undergo 1,n-HAT (n = 6, 7) at an unactivated C(sp3)–H site,10a followed by Pd-involved elimination to selectively furnish a remote alkene moiety. Remarkably, it was found that amine 1f, now protected by the T2 tether, underwent efficient 1,6-HAT to produce a β-/γ-desaturation product in 47% yield together with 31% of the cyclic sulfonamide, a product of cyclization of the formed alkyl radical at the aromatic ring of the arylsulfonyl group19 (Table 2). On the other hand, the same amine (1f), while decorated with the T1 tether (vide supra), underwent efficient α-/β-desaturation to produce enamine 2f-α/β (Table 1). These results, where two auxiliaries completely switched the regioselectivity of desaturation of the same substrate,20 clearly indicate the potential of this strategy toward development of auxiliary-controlled site-selective C–H functionalizations. Due to the observed competing cyclization of the formed β-alkyl radical at the arylsulfonyl group, at the moment, the attempts on further exploration of the β-/γ-desaturation of amines were halted, and we turned our attention to the γ-/δ-desaturation reaction. Delightfully, it was found that γ-/δ-desaturation of the amine 1o via a three-step procedure (without isolation of the intermediates) produced 2o as a sole product in excellent yield, which is superior, in both yield and regioselectivity, to those reported via the radical/cationic approach.10a Evidently, the observed highly regioselective outcome reflects the nature of the “controlled” β-Pd–H elimination step of the operative hybrid Pd-radical mechanism (vide infra). Desaturation of various aliphatic amines (1p–1r) proceeded uneventfully, resulting in the formation of homoallylic amines (2p–2r) in good yields. Particularly, acid-sensitive groups, such as primary TBS-ether (1s) or acetal groups (1t) which do not withstand the reported acidic conditions,10a,19 were well-preserved in this reaction. Leucine ester (1u) and menthyl-amine (1v) were found to be competent substrates yielding the corresponding desaturation products 2u–2v in reasonable yields. Amines bearing primary (1w) and tertiary (1x) alkyl carbon chains also provided high yields of the corresponding desaturation products. Notably, unprecedented remote desaturation of secondary amines 1y, 1z proceeded smoothly, furnishing the corresponding products (2y, 2z) in good to excellent yields. Markedly, functionalization of inert secondary C–H bonds occurred efficiently (1aa, 1ab), resulting in homoallylic amines 2aa, 2ab in respectable yields, where a substrate possessing a bulkier substituent at the N-atom (1ab) resulted in better selectivity of the HAT event. A heterocyclic substrate, azepane derivative 1ac, reacted efficiently to produce 2ac in 77% yield. Importantly, under the reported conditions,15 the benzenesulfonyl group in homoallylic amine 2y can easily be removed.19 Finally, the practicality of this method was illustrated by a gram-scale desaturation of amine 1p into homoallylic amine 2p in nearly quantitative yield over the three steps (eq 1).
Table 2.
β-γ and γ-δ Desaturation of Amines with T2a
|
Isolated yields for two steps (1 → 2) are shown. Yields for one-step desaturation (from amine protected with reactive tether) are shown in parentheses. r.r, regiomeric ratio.
The Boc installation was not applicable for secondary amines (1f, 1y–1aa, 1ac).
31% of HAT/cyclization at aromatic ring product was also isolated.
Contains minor amount of hydrodehalogenation byproduct.
Scheme 2.
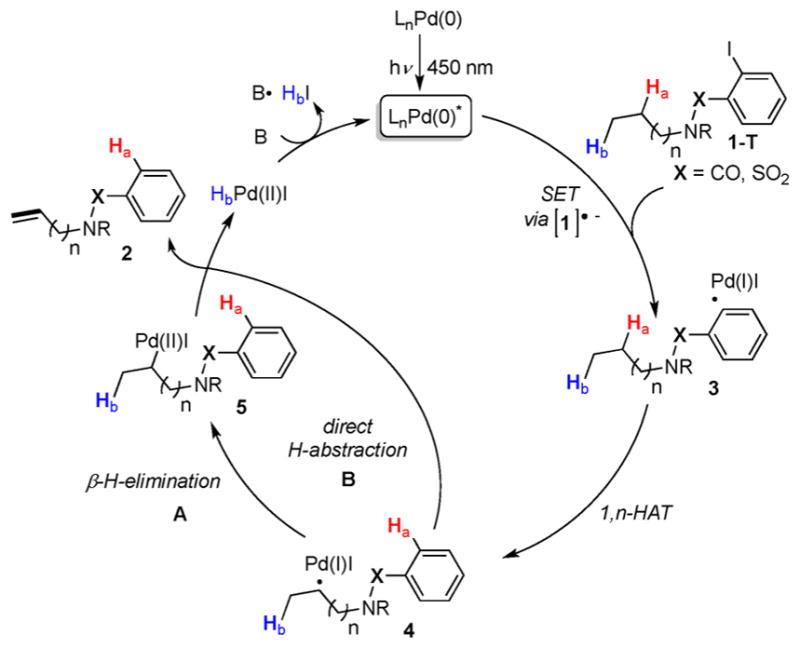
Proposed Mechanism
 |
(1) |
Based on the literature reports13,14 and our initial mechanistic studies,19 including the radical scavenger experiments, deuterium labeling studies, and Stern–Volmer quenching studies, the following mechanism for this remote desaturation reaction of amines is proposed (Scheme 2). The formed in situ Pd(0) complex undergoes excitation by visible light to form the active Pd(0)* catalyst. The latter engages in an SET event with aryl iodide 1-T to generate aryl hybrid Pd-radical species 3, which via 1,n-HAT (n = 5–7) produces alkyl hybrid Pd-radical species 4. A subsequent β-hydrogen elimination (path A)13 or a direct hydrogen abstraction (path B)21 generates the desaturated product 2 and regenerates the catalyst.
In summary, a general, mild, efficient, and selective method for desaturation of aliphatic amines has been developed. This method employs easily installable/removable15,19 aryl iodide containing tethers, which upon visible light irradiation/Pd-catalysis generate an aryl radical at the tether, which triggers an auxiliary-controlled 1,n-HAT event at the aliphatic amine moiety, followed by the Pd-assisted β-H elimination step. It is expected that this operationally simple and easily scalable method, which does not require employment of exogenous photosensitizers or external oxidants, will find broad applications in synthesis.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM120281) for financial support of this work. We also thank Dr. Yang Wang (Northwestern University) for helpful discussions.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b00488.
Experimental procedures and compound characterization data (PDF)
References
- 1.(a) Besada P, Mamedova L, Thomas CJ, Costanzi S, Jacobson KA. Org Biomol Chem. 2005;3:2016. doi: 10.1039/b416349d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rai US, Isloor AM, Shetty P, et al. Med Chem Res. 2012;21:1090. [Google Scholar]; (c) Steele AD, Keohane CE, Knouse KW, Rossiter SE, Williams SJ, Wuest WM. J Am Chem Soc. 2016;138:5833. doi: 10.1021/jacs.6b03373. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tabor MG, Shenvi RA. Org Lett. 2015;17:5776. doi: 10.1021/acs.orglett.5b02992. [DOI] [PubMed] [Google Scholar]
- 2.(a) Cook G, editor. Enamines: Synthesis: Structure, and Reactions. 2. Marcel Dekker; NY: 1988. [Google Scholar]; (b) Rappoport Z, editor. The Chemistry of Enamines, Parts 1 & 2. Wiley; New York: 1994. [Google Scholar]; (c) Hickmott PW. Tetrahedron. 1982;38:3363. [Google Scholar]; (d) Tada N, Jansen DJ, Mower MP, Blewett MM, Umotoy JC, Cravatt BF, Wolan DW, Shenvi RA. ACS Cent Sci. 2016;2:401. doi: 10.1021/acscentsci.6b00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For a review, see: Dehli JR, Legros J, Bolm C. Chem Commun. 2005:973. doi: 10.1039/b415954c.
- 4.(a) Pohlki F, Doye S. Chem Soc Rev. 2003;32:104. doi: 10.1039/b200386b. [DOI] [PubMed] [Google Scholar]; (b) Severin R, Doye S. Chem Soc Rev. 2007;36:1407. doi: 10.1039/b600981f. [DOI] [PubMed] [Google Scholar]
- 5.For reviews, see: Johannsen M, Jørgensen KA. Chem Rev. 1998;98:1689. doi: 10.1021/cr970343o.Ramirez TA, Zhao B, Shi Y. Chem Soc Rev. 2012;41:931. doi: 10.1039/c1cs15104e.
- 6.For a review, see: Campos KR. Chem Soc Rev. 2007;36:1069. doi: 10.1039/b607547a.
- 7.Zhang X, Fried A, Knapp S, Goldman AS. Chem Commun. 2003:2060.Bolig AD, Brookhart M. J Am Chem Soc. 2007;129:14544. doi: 10.1021/ja075694r.. For reviews on iridium pincer complex catalyzed transfer–hydrogenation, see: Choi J, MacArthur AHR, Brookhart M, Goldman AS. Chem Rev. 2011;111:1761. doi: 10.1021/cr1003503.Daugulis O, MacArthur AHR, Rix FC, Templeton JL. ACS Catal. 2016;6:1518.
- 8.Bheeter CB, Jin R, Bera JK, Dixneuf PH, Doucet H. Adv Synth Catal. 2014;356:119.Rousseaux S, Gorelsky SI, Chung BKW, Fagnou K. J Am Chem Soc. 2010;132:10692. doi: 10.1021/ja103081n.. For desaturation of nonamine tethered substrates, see Giri R, Maugel N, Foxman BM, Yu JQ. Organometallics. 2008;27:1667.Baudoin O, Herrbach A, Gueritte F. Angew Chem, Int Ed. 2003;42:5736. doi: 10.1002/anie.200352461.Motti E, Catellani M. Adv Synth Catal. 2008;350:565.
- 9.He J, Wasa M, Chan KSL, Shao Q, Yu JQ. Chem Rev. 2017;117:8754. doi: 10.1021/acs.chemrev.6b00622.Daugulis O, Roane J, Tran LD. Acc Chem Res. 2015;48:1053. doi: 10.1021/ar5004626.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem - Eur J. 2010;16:2654. doi: 10.1002/chem.200902374.Topczewski JJ, Cabrera PJ, Saper NI, Sanford MS. Nature. 2016;531:220. doi: 10.1038/nature16957.
- 10.(a) Voica AF, Mendoza A, Gutekunst WR, Fraga JO, Baran PS. Nat Chem. 2012;4:629. doi: 10.1038/nchem.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu T, Mei TS, Yu JQ. J Am Chem Soc. 2015;137:5871. doi: 10.1021/jacs.5b02065. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu T, Myers MC, Yu JQ. Angew Chem, Int Ed. 2017;56:306. doi: 10.1002/anie.201608210. [DOI] [PubMed] [Google Scholar]; (d) Wappes EA, Fosu SC, Chopko TC, Nagib DA. Angew Chem, Int Ed. 2016;55:9974. doi: 10.1002/anie.201604704. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wappes EA, Nakafuku KM, Nagib DA. J Am Chem Soc. 2017;139:10204. doi: 10.1021/jacs.7b05214. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen K, Richter JM, Baran PS. J Am Chem Soc. 2008;130:7247. doi: 10.1021/ja802491q. [DOI] [PubMed] [Google Scholar]; (g) Wang YF, Chen H, Zhu X, Chiba S. J Am Chem Soc. 2012;134:11980. doi: 10.1021/ja305833a. [DOI] [PubMed] [Google Scholar]; (h) Lu H, Zhang X, et al. Angew Chem, Int Ed. 2010;49:10192. doi: 10.1002/anie.201005552. [DOI] [PubMed] [Google Scholar]; (i) Chu JCK, Rovis T. Nature. 2016;539:272. doi: 10.1038/nature19810. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Choi GJ, Zhu Q, Miller DC, Gu CJ, Knowles RR. Nature. 2016;539:268. doi: 10.1038/nature19811. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Chen DF, Chu JCK, Rovis T. J Am Chem Soc. 2017;139:14897. doi: 10.1021/jacs.7b09306. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Shu W, Nevado C. Angew Chem, Int Ed. 2017;56:1881. doi: 10.1002/anie.201609885. [DOI] [PubMed] [Google Scholar]; (m) Shu W, Genoux A, Li Z, Nevado C. Angew Chem, Int Ed. 2017;56:10521. doi: 10.1002/anie.201704068. [DOI] [PubMed] [Google Scholar]; (n) Du S, Kimball EA, Ragains JR. Org Lett. 2017;19:5553. doi: 10.1021/acs.orglett.7b02650. [DOI] [PubMed] [Google Scholar]; (o) Hollister KA, Conner ES, Spell ML, Deveaux K, Maneval L, Beal MW, Ragains JR. Angew Chem, Int Ed. 2015;54:7837. doi: 10.1002/anie.201500880. [DOI] [PubMed] [Google Scholar]; (p) Breslow R, Baldwin S, Flechtner T, Kalicky P, Liu S, Washburn W. J Am Chem Soc. 1973;95:3251. doi: 10.1021/ja00791a031. [DOI] [PubMed] [Google Scholar]
- 11.For a review, see: Nechab M, Mondal S, Bertrand MP. Chem - Eur J. 2014;20:16034. doi: 10.1002/chem.201403951.
- 12.For a review, see: Liu Q, Dong X, Li J, Xiao J, Dong Y, Liu H. ACS Catal. 2015;5:6111.. See also: Bonney KJ, Proutiere F, Schoenebeck F. Chem Sci. 2013;4:4434.Venning ARO, Kwiatkowski MR, Peña JER, Lainhart BC, Guruparan AA, Alexanian EJ. J Am Chem Soc. 2017;139:11595. doi: 10.1021/jacs.7b06794.. For a review on UV-induced Pd-catalyzed transformations involving alkyl iodides, see: Sumino S, Fusano A, Fukuyama T, Ryu I. Acc Chem Res. 2014;47:1563. doi: 10.1021/ar500035q.
- 13.(a) Bloome KS, McMahen RL, Alexanian EJ. J Am Chem Soc. 2011;133:20146. doi: 10.1021/ja2091883. [DOI] [PubMed] [Google Scholar]; (b) Parasram M, Iaroshenko VO, Gevorgyan V. J Am Chem Soc. 2014;136:17926. doi: 10.1021/ja5104525. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kurandina D, Parasram M, Gevorgyan V. Angew Chem, Int Ed. 2017;56:14212. doi: 10.1002/anie.201706554. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kurandina D, Rivas M, Radzhabov M, Gevorgyan V. Org Lett. 2018;20:357. doi: 10.1021/acs.orglett.7b03591. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wang GZ, Shang R, Cheng WM, Fu Y. J Am Chem Soc. 2017;139:18307. doi: 10.1021/jacs.7b10009. [DOI] [PubMed] [Google Scholar]; (f) Zhou WJ, Cao GM, Shen G, Zhu XY, Gui YY, Ye JH, Sun L, Liao LL, Li J, Yu DG. Angew Chem, Int Ed. 2017;56:15683. doi: 10.1002/anie.201704513. [DOI] [PubMed] [Google Scholar]
- 14.Parasram M, Chuentragool P, Sarkar D, Gevorgyan V. J Am Chem Soc. 2016;138:6340. doi: 10.1021/jacs.6b01628.Parasram M, Chuentragool P, Wang Y, Shi Y, Gevorgyan V. J Am Chem Soc. 2017;139:14857. doi: 10.1021/jacs.7b08459.. For a review on employment of Si-tethers in C–H functionalizations, see: Parasram M, Gevorgyan V. Acc Chem Res. 2017;50:2038. doi: 10.1021/acs.accounts.7b00306.
- 15.Wuts PGM, Greene TW, editors. Greene’s protective groups in organic synthesis. 4. Wiley; New York: 2007. Sabitha G, Reddy BVS, Abraham S, Yadav JS. Tetrahedron Lett. 1999;40:1569.
- 16.For selected reviews on photocatalytic transformations, see: Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322. doi: 10.1021/cr300503r.Skubi KL, Blum TR, Yoon TP. Chem Rev. 2016;116:10035. doi: 10.1021/acs.chemrev.6b00018.Romero NA, Nicewicz DA. Chem Rev. 2016;116:10075. doi: 10.1021/acs.chemrev.6b00057.Staveness D, Bosque I, Stephenson CRJ. Acc Chem Res. 2016;49:2295. doi: 10.1021/acs.accounts.6b00270.Tellis JC, Kelly CB, Primer DN, Jouffroy M, Patel NR, Molander GA. Acc Chem Res. 2016;49:1429. doi: 10.1021/acs.accounts.6b00214.Meggers E. Chem Commun. 2015;51:3290. doi: 10.1039/c4cc09268f.Xie J, Jin H, Hashmi ASK. Chem Soc Rev. 2017;46:5193. doi: 10.1039/c7cs00339k.
- 17.For a review on exogenous photosensitizer-free, visible light-induced TM-catalyzed transformations, see: Parasram M, Gevorgyan V. Chem Soc Rev. 2017;46:6227. doi: 10.1039/c7cs00226b.
- 18.For examples of desaturation of lactam systems, generally producing alkene proximal to the carbonyl group, see: Chen Y, Turlik A, Newhouse TR. J Am Chem Soc. 2016;138:1166. doi: 10.1021/jacs.5b12924.Pineschi M, Del Moro F, Di Bussolo V, Macchia F. Adv Synth Catal. 2006;348:301.Chen M, Dong G. J Am Chem Soc. 2017;139:7757. doi: 10.1021/jacs.7b04722.Garnier EC, Liebeskind LS. J Am Chem Soc. 2008;130:7449. doi: 10.1021/ja800664v.Wang Z, He Z, Zhang L, Huang Y. J Am Chem Soc. 2018;140:735. doi: 10.1021/jacs.7b11351.. For α/β-desaturation of esters, see: Chen Y, Romaire JP, Newhouse TR. J Am Chem Soc. 2015;137:5875. doi: 10.1021/jacs.5b02243.
- 19.See Supporting Information for details.
- 20.Generally, the 1,5-HAT process is the most favorable for carbon-tethered radicals due to the conformationally favored six-membered transition state. See ref 11; see also: Yoshikai N, Mieczkowski A, Matsumoto A, Ilies L, Nakamura E. J Am Chem Soc. 2010;132:5568. doi: 10.1021/ja100651t.However, for the sulfonyl tether, owing to longer C–S bonds, larger rings to adopt a quasi-linear transition state for an HAT process are required, which results in preferred 1,6-or 1,7-HAT events. See refs 10a and 11.
- 21.(a) Kuo JL, Hartung J, Han A, Norton JR. J Am Chem Soc. 2015;137:1036. doi: 10.1021/ja511883b. [DOI] [PubMed] [Google Scholar]; (b) Hu Y, Shaw AP, Estes DP, Norton JR. Chem Rev. 2016;116:8427. doi: 10.1021/acs.chemrev.5b00532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


