Abstract
Background
Head and neck cancers are associated with high rates of depression, which may increase risk for poorer immediate and long-term outcomes. We hypothesized greater depressive symptoms would predict earlier mortality and examined behavioral (treatment interruption) and biological (treatment response) mediators.
Methods
Patients (N=134) reported depressive symptomatology at treatment planning. Clinical data were reviewed at two-year follow-up.
Results
Greater depressive symptoms were associated with significantly shorter survival (hazard ratio=0.868, 95% confidence interval [CI]=0.819–0.921, p<.001), higher rates of chemoradiation interruption (odds ratio [OR]=0.865; 95% CI=0.774–0.966, p=.010) and poorer treatment response (OR=0.879, 95% CI=0.803–0.963, p=.005). Poorer treatment response partially explained the depression-survival relationship. Other known prognostic indicators did not challenge these results.
Conclusions
Depressive symptoms at the time of treatment planning predict overall two-year mortality. Effects were partly influenced by treatment response. Depression screening and intervention may be beneficial. Future studies should examine parallel biological pathways linking depression to cancer survival, including endocrine disruption and inflammation.
Keywords: head and neck cancer, survival, depression, residual tumor
INTRODUCTION
Head and neck cancer patients endorse a wide range of depressive symptomatology. They experience rates of clinical depression higher than that of the general population, and some of the highest rates amongst cancer patients.1 Depression has been shown to predict early mortality across a number of different cancers.2,3 A meta-analysis examining multiple cancer types found that depressed patients may be at a 26–39% greater mortality risk than their non-depressed counterparts.4 The converse is also supported, as decreasing depression levels predict improved survival in metastatic breast cancer patients.5 Burgeoning evidence supports the relationship between depression and early mortality among head and neck cancer patients as well. Studies examining mixed head and neck tumor sites reveal depressive symptoms to be prognostic for two-year and three-year overall survival.6,7 Depression has also been shown to predict five-year overall survival in advanced stage oropharyngeal cancer patients.8 A recent epidemiological analysis using SEER data from 3466 head and neck cancer patients, demonstrated significantly elevated mortality rates for cancer patients diagnosed with depression.9 In contrast, two previous studies report non-significant associations between depressed mood and survival in head and neck samples.10,11 However, assessments employed may have been less sensitive12 or may not have provided valid or reliable assessment of depression in the samples.13
There are limited pathways by which psychosocial factors, including depression, may impact cancer survival. These include the receipt of treatment (adherence), biological pathways (e.g., interaction of tumor-host biology),5 and aspects of preventative self-care such as diet, exercise, smoking, etc.2 Cancer patients with depression may face additional obstacles related to depressive symptoms, such as low motivation, which may manifest in behavioral difficulties attending scheduled treatment appointments. Up to 70% of head and neck cancer patients experience an interruption in treatment,14 and head and neck cancer patients suffering from depression have triple the odds of noncompliance with medical treatment recommendations.15 Patients with laryngeal tumors who experienced a 5–30 day interruption in treatment had a 68% increased risk of mortality, compared with patients with no unscheduled interruptions.16 Further, head and neck cancer patients with moderate to severe pretreatment depression ratings exhibit lower rates of chemoradiation completion.17 The issue of radiotherapy and chemotherapy adherence is complicated by many factors including patient education, difficult side effects, extended treatment time, and patient-provider relationships.18 Depressed patients’ greater difficulty attending scheduled treatments is likely to result in worse treatment outcomes. Difficulty coping with negative affect, common in depression, may lead to a higher propensity to engage in high-risk behaviors, such as continued tobacco use after cancer diagnosis, poor diet, and poor sleep.19
Depression and survival may also be linked via biological pathways. Depression may accelerate cancer progression via endocrine and immune changes that result in tumor promotion.2,5,19 For example, autonomic sympathetic activation modulates gene expression in immune cells in the tumor microenvironment, resulting in promotion of metastasis, inflammation, angiogenesis, and suppression of cellular immune responses that combat tumor progression.20 Research has begun to examine the mechanisms by which depression or depressive symptoms may be linked with survival outcomes.21–23 These studies support the notion that endocrine and inflammatory pathways may underlie the depression-cancer survival associations.
In clinical settings, special attention to depressive symptoms may be warranted among patients with head and neck cancers given elevated depressive symptom reports1 and potential downstream effects of depression on tumor progression.24 This highlights the need for more prospective research that will allow examination of potential behavioral (treatment adherence and other health-related behaviors) versus biological pathways of the effects of depression in head and neck cancer. The current study explored two of these pathways using a longitudinal design that measured depressive symptoms at the outset of treatment, and subsequently recorded presence of treatment attendance interruptions, treatment response, and survival time. We employed a measure of depressive symptoms that has demonstrated high sensitivity among head and neck cancer patients12 and administered this measure early in the treatment planning phase. We hypothesized that patients who presented at treatment planning with greater depressive symptoms would experience significantly shorter overall cancer survival. In order to then elucidate two possible behavioral versus biological pathways by which depression may influence cancer survival, we examined data from a cohort of 134 head and neck cancer patients over an approximate two-year period. The behavioral pathway focused on the role of an interruption to scheduled treatment and the biological pathway tested the role of tumor response to treatment in the depression-survival relationship.
METHODS
Participants and Procedures
We reviewed all patients presenting to our institutional multidisciplinary head and neck cancer clinic from October 2012 to October 2013. This study received approval from our institutional review board with waiver of informed consent. Patients completed depression assessments upon presentation to the multidisciplinary clinic for radiation and/or chemotherapy treatment planning. Patients had typically received notification of their biopsy-proven diagnosis one to four weeks prior to presentation, with some of the sample (39%) already having undergone surgical procedure to confirm extent of malignancy or for extirpation. Of the 244 patients referred to our clinic during the one-year study period, 134 patients are included in the current analysis. The 110 remaining patients either did not return psychometric data (n=101) or were patients of the VA system or incarcerated (i.e., could not be included in research; n=9). There were no differences in age, site, or stage of disease between the patients who were or were not included in this dataset.
Measures
Clinical Variables
Patient demographics including age, sex, race, and alcohol and smoking history were collected from intake forms. American Joint Commission on Cancer staging at time of presentation was determined utilizing all available clinical, pathologic and radiographic data. Viral status of oropharyngeal cases was determined by either human papillomavirus (HPV; tested by in-situ hybridization) or p16 (immunohistochemistry) results. Tumor location was classified into five categories: oral, oropharyngeal, laryngeal, hypopharyngeal, and other (e.g., neck disease/tumors of unknown origin, thyroid, skin).
Medical records were reviewed after all participants had completed treatment, yielding data on radiotherapy or combined treatment (radiotherapy + chemotherapy) appointment attendance, the clinical response of the tumor to treatment, and two-year survival outcomes. Treatment interruption was defined as a dichotomous indicator of ≥ 3 consecutive days without treatment. This allowed for weekends to be considered as an intentional gap.14 Fourteen patients (10.5%) had at least one interruption of treatment according to this criterion. A dichotomous indicator of treatment response was calculated. Patients were coded as poorly responsive if there was clinical or radiological evidence of the persistence of disease, tumor presence, or early recurrence observed at three months post-treatment. Otherwise, patients were coded as responsive to treatment. Overall survival was calculated from the date of study entry, which was also the date at which patients visited with physicians to undertake planning for their (radiotherapy or combined chemoradiation) cancer treatment.
Depression
Depressive symptoms were measured at the time of study, before radiotherapy/chemotherapy treatment commenced, entry using the depression subscale of the Hospital Anxiety and Depression Scale (HADS).25 The HADS is a 14-item measure divided into two subscales (anxiety and depression). Total subscale scores range from 0–21. Scores ≥ 8 are indicative of clinically significant symptomatology. The HADS has previously been used with head and neck cancer patients26,27 and has demonstrated the highest screening accuracy compared to other depression measures for head and neck cancer patients.12
Statistical Analyses
Descriptive and summary statistics characterized clinical and demographic features. Prior to analyses, independent variables were centered,28 and all statistical assumptions were met.
Primary Hypothesis
The relationship between depressive symptoms and overall survival was examined using a Cox proportional hazards model.
Secondary Hypothesis – Possible Mediators
Separate logistic regressions tested relationships between depressive symptoms and treatment interruption and treatment response. Tests of mediation on the relationship between depression and survival were then performed using the MacArthur approach,29 in which predictors were centered and interaction terms were included. To show that B mediates A on C, it must be shown that A, B, C, happen in that order, that A and B are correlated, and that B explains all (complete) or part (partial) of the association between A and C. In a linear model, that means that either the main effect of B and/or the interaction between A and B is statistically significant. Cox models were constructed to include entry depressive symptoms, post-entry treatment interruption or treatment response respectively, and the interaction between the two.
Secondary Hypothesis – Possible Proxies
Spearman rank correlations assessed the contribution of traditional prognostic indicators, including cancer stage, site of disease, age at diagnosis, sex, race, marital status, and tobacco history in pack-years. Those that correlated with both depression and survival were considered possible proxies according to the MacArthur definition.30 When this occurred, Cox models were constructed to include the entry depressive symptoms, the possible entry proxy, and the interaction between the two.
All statistical tests were two-sided, with alpha set at .05, using SPSS version 21 (IBM; Armonk, NY). Power analysis was based on a comparable study reporting depressive symptoms as a significant predictor of head and neck cancer survival in 130 patients experiencing 18 deaths.8 Results suggested that our sample of 134 patients with 39 deaths would attain 100% power to detect significant effects.
RESULTS
Patient and Clinical Characteristics
Sample characteristics are presented in Table 1. Treatment interruptions typically occurred early (i.e., within the first three weeks) in treatment, for reasons of acute toxicity (e.g. nausea/vomiting; n=2), decline in functional status (e.g. falls, stroke; n=7), or social factors (e.g. legal, transportation issues; n=5). We followed every patient until death or until two years post study entry, whichever came first. No patients were lost to follow-up; when date-of-death was unavailable in multidisciplinary clinic records, data were gathered from outside medical records, obituaries, and state death records.
Table 1.
Clinical and demographic characteristics (N=134).
| N | (%) | Median | M | (SD) | ||
|---|---|---|---|---|---|---|
| Age at Diagnosis (range 22 – 87) | 60.6 | 61.2 | (13.0) | |||
| Male Sex | 99 | (73.9%) | ||||
| Race | ||||||
| Non-Hispanic white | 113 | (84.3%) | ||||
| African-American | 20 | (14.9%) | ||||
| Asian | 1 | (0.7%) | ||||
| Pack-Years (range 1.5 – 120) | 30.0 | 36.9 | (24.9) | |||
| Site of Disease | Oral | 19 | (14.2%) | |||
| Oropharyngeal | ||||||
| HPV positive | 20 | (14.9%) | ||||
| HPV negative | 8 | (6.0%) | ||||
| HPV status unknown | 7 | (5.2%) | ||||
| Laryngeal | 22 | (16.4%) | ||||
| Hypopharyngeal | 4 | (3.0%) | ||||
| Other | 54 | (40.3%) | ||||
| Summary Stage | I | 21 | (21.4%) | |||
| II | 10 | (9.7%) | ||||
| III | 22 | (21.4%) | ||||
| IV | 50 | (48.5%) | ||||
| Recurrent | 31 | (23.1%) | ||||
| Treatment Received | No Data or Observation Only | 6 | (4.5%) | |||
| Surgery only | 5 | (3.7%) | ||||
| Surgery + Radiation | 18 | (13.4%) | ||||
| Radiation Alone | 44 | (32.8%) | ||||
| Radiation + Chemotherapy | 61 | (45.5%) | ||||
| HADS Depression Score (range 0 – 20) | 5.0 | 5.9 | (4.9) | |||
| Above Clinical Cutoff (HADS-Depression ≥ 8) | 45 | (33.6%) | ||||
| Treatment Interruption | 14 | (10.4%) | ||||
| Treatment Interruption Total Days (range 1 – 42) | 3.5 | 7.6 | (12.4) | |||
| Treatment Response (Evidence of Disease at Completion) | 25 | (18.7%) | ||||
| Deaths at Two-Year Follow-Up | 39 | (29.1%) | ||||
| Two-Year Overall Survival, Days (range 11 – 730) | 589.5 | 496.4 | (239.3) | |||
Note. HPV = human papillomavirus; HADS = Hospital Anxiety and Depression Scale
Tests of Hypotheses
Primary Hypothesis
Depressive symptoms measured at the time of treatment planning predicted subsequent two-year overall survival (p(Wald) < .001, hazard ratio [HR] = 0.868, 95% confidence interval [CI] = 0.819–0.921; Table 2).
Table 2.
Associations between depressive symptoms, two-year overall survival and potential behavioral and biological mediators.
| Survival Analysis: Depressive Symptoms | |||||
| Predictor | B | df | P | Hazard Ratio | 95% CI |
| Depression | −0.141 | 1 | <.001 | 0.868 | 0.819 – 0.921 |
| Survival Mediation Analysis: Depressive Symptoms and Treatment Interruption | |||||
| Predictors | B | df | P | Hazard Ratio | 95% CI |
| Depression | −0.106 | 1 | .026 | 0.900 | 0.819 – 0.987 |
| Treatment Interruption | 0.359 | 1 | .527 | 1.432 | 0.471 – 4.351 |
| Depression * Interruption | 0.036 | 1 | .703 | 1.036 | 0.863 – 1.245 |
| Survival Mediation Analysis: Depressive Symptoms and Treatment Response | |||||
| Predictors | B | df | P | Hazard Ratio | 95% CI |
| Depression | −0.096 | 1 | .005 | 0.908 | 0.849 – 0.971 |
| Treatment Response | 1.729 | 1 | <.001 | 5.637 | 2.683 – 11.845 |
| Depression * Response | 0.098 | 1 | .153 | 1.103 | 0.964 – 1.261 |
Note. All predictors were centered prior to analyses.
Secondary Hypothesis – Possible Mediators
Entry depressive symptoms were significantly associated with subsequent treatment interruption (odds ratio [OR] = 0.865, 95% CI = 0.774–0.966, p = .010) as well as poorer treatment response (OR = 0.879, 95% CI = 0.803–0.963, p = .005). The relationship between depressive symptoms and survival could not be shown to be mediated by treatment interruption (Table 2). The relationship between depressive symptoms and two-year overall survival was shown to be significantly mediated by treatment response (Table 2; Figure 1). Analytic assumptions were carefully checked to confirm there was no violation due to potential multicollinearity between depressive symptoms and treatment response given the observed significant relationship between the two noted above. All MacArthur criteria for mediation29 were met; depressive symptoms (A) preceded treatment response (B), A was significantly associated with B, the interaction term was not significant, and there was a significant main effect of B.
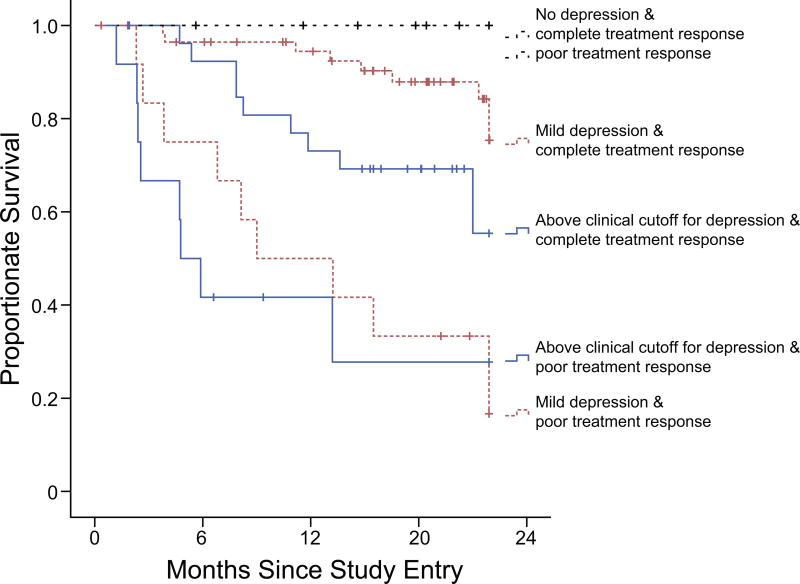
Figure 1.
To aid understanding of the statistically significant interaction between depressive symptoms and treatment response (i.e., for descriptive and not statistical purposes), patients were split into six groups according to their depressive symptom scores (none, mild/moderate, or above clinical cutoff) and the response of their tumor to radiation/combined treatment (complete response or poor response). Kaplan-Meier curves depict all-cause mortality for each of these groups. Depressive symptoms were measured at the treatment planning visit using the Hospital Anxiety and Depression Scale. Scores were: No symptoms (0), Mild to Moderate (1–7), and Clinically Significant (≥ 8). Response to treatment was assessed after completion of radiotherapy or combined treatment, and categorized as complete (no evidence of disease), or poor (some evidence of disease present). No deaths occurred among patients who reported no depressive symptoms. Thus, the two survival curves for these patients (no depression + complete response, no depression + poor treatment response) overlap.
Secondary Hypothesis – Possible Proxies
Of the possible proxies measured, the only variable associated both with depressive symptoms and overall survival was clinical stage at time of diagnosis (depressive symptoms, rho = .204, p = .019; survival, rho = .220, p = .011). Associations with disease site, age, sex, race, marital status, and pack-years were all nonsignificant (all rho’s > |.164|, p’s > .060. Stage was classified into early (summary stages I, II), advanced (summary stages III, IV), and recurrent in order to meet analytic assumptions. The Cox proportional hazards model was rerun entering depressive symptoms, summary stage, and their interaction, to test whether the finding of poorer survival among those with higher depressive symptoms would remain. Depressive symptoms remained a significant predictor of overall survival.
DISCUSSION
Depressive symptoms prior to chemoradiation treatment predict shortened survival among patients with head and neck cancer. Our findings are consistent with recent studies showing depression predicts early mortality in head and neck cancer patients.6–9 Although we found that depressive symptoms were associated with increased treatment disruption, we did not find evidence that this mediated survival, likely due to the low percentage of patients with treatment disruption. However, we document one plausible mediating pathway of the depression-survival relationship, via response of the tumor to treatment.
Our data demonstrate that patients with greater depressive symptoms were significantly more likely to experience interruptions in treatment attendance, despite the low number of patients who experienced such interruption (i.e. 14 out of 134). However, in our study, treatment interruptions could not be shown to mediate the relationship between depression and overall survival. Responses to individual items on the HADS suggested that although patients scored the highest on the item “I feel as if I am slowed down,” it may be that levels of this symptom were not high enough to significantly interfere with attendance at scheduled treatment appointments. Alternatively, due to the low number of patients with treatment interruption and their various antecedents, the ability to detect effects on survival was likely underpowered. Thus, future research with larger sample numbers of events should continue to explore the hypothesis that treatment interruption may relate to both depression and medical outcomes.
Importantly, several findings were observed when testing the biological pathway. First, depressive symptoms were significantly related to treatment response. To our knowledge, this is the first study to test the impact of depression on head and neck tumor response to treatment. Second, when depression and treatment response were entered simultaneously in the survival model, depressive symptoms remained a significant prognostic factor that was partially mediated by treatment response. Thus, it is possible that biological processes can partially explain the effect of depression on treatment response.
In our sample, only four of the patients who experienced a treatment interruption demonstrated poor treatment response (i.e., clinical or radiographic evidence of disease after treatment completion). The correlation between treatment interruption and treatment response was also small (Spearman r = .281). This suggests that these two variables may be independent, and supports the notion that depressive symptoms may adversely impact host defenses even for the compliant patient. In light of the impact to overall cancer mortality demonstrated by this and previous studies, the mechanisms of depression deserve further elucidation and are especially relevant to cancer control.
One plausible mediator of depression, immune disruption, has garnered substantial research attention in the healthcare context. Depression is associated with systemic inflammation31,32 and higher levels of proinflammatory cytokines interleukin (IL)-6, IL-1, and C-reactive protein.33,34 The relationship between depression and hypothalamic-pituitary-adrenal (HPA) axis dysregulation has also been well established;35 depression has been associated with hyper-secretion of the hormone cortisol,36 a blunted cortisol awakening response,37,38 and flattened diurnal cortisol profiles.32 In the cancer context, chronic inflammation has been established as a contributor to cancer progression,39,40 and flattened diurnal cortisol profiles have predicted shortened survival in examinations across a number of cancer types.23,41–43 Taken together, these biological processes likely decrease host ability to fight tumors effectively, potentially impeding response to medical treatment. These biological pathways may run parallel to the effects of depression and warrant further attention, as depression remained a significant and independent predictor of survival in our study.
Our study underlines several important clinical implications. First, while cancer stage at time of diagnosis was associated with depressive symptoms and overall survival, depression appeared to be as important a predictor of survival as traditional clinical prognostic indicators. Among early, late stage, and recurrent disease cases, higher levels of depressive symptoms consistently predicted earlier mortality. A similar finding was observed by Lazure and colleagues6 and in a study by Kim and colleagues,7 which adjusted for clinical factors (including stage), and found depression to be related to disease-free and overall oropharyngeal cancer survival. Similar research evaluating effects of treatment interruption also has not demonstrated effects of patient age, sex, race, primary tumor site, or summary stage on treatment outcomes.44 Thus, an important point remains: depressive symptoms may be as powerful as the clinical features that healthcare professionals typically use as prognostic indicators for head and neck cancer patients. This finding strengthens the importance of screening for depressive symptoms in all head and neck cancer patients, particularly during the cancer treatment planning phase.
Second, 67.2% of the participants in this study endorsed depressive symptoms at or below a score of 8 on the HADS depression subscale, the identified cutoff for clinically significant symptomatology. This emphasizes the importance of capturing or attending to subclinical symptoms of depression in head and neck cancer patients, as they may significantly influence overall survival. Our data are similar to those of other head and neck cancer samples where the majority of patients have also endorsed subclinical depressive symptomatology.6,8,10 Thus, our data highlight the importance of screening for depression among all head and neck cancer patients. This information may then be used to facilitate discussion with patients and expedite development of targeted behavioral interventions by psycho-oncologists or behavioral oncology specialists.
Last, our study may serve to inform future research regarding depression treatment and medical outcomes. Although some evidence indicates psychological intervention may be associated with improved cancer treatment outcomes,5,45 no studies to our knowledge have investigated the potential for a depression intervention to specifically impact medical treatment outcomes among head and neck cancer patients. The need for advancements in psychosocial care with the potential to impact cancer survivorship has recently been highlighted46, as evidence-based psychosocial treatments have high potential to complement biomedical treatment efficacy.47
Several limitations exist in the current study. First, not all patients in this sample can be considered completely treatment naïve, as some had already undergone a surgical procedure prior to presentation for definitive radiation or combined therapy treatment planning. Second, only a few patients experienced treatment interruptions directly related to psychosocial factors. Some suggest that treatment interruptions less than or equal to five days do not detectably influence outcomes.14,48 Direct toxic effects of radiation therapy were also not specifically examined, thus their role in unscheduled treatment interruptions is unknown. Third, pathological or radiological confirmation of disease status after completion of treatment was also not readily available for some of the cases. We also do not have complete information on cause of death available. Head and neck cancer morbidity is often associated with disease burden (e.g., refractory or advancing tumors, aspiration, carotid infiltration). However, competing causes cannot be ruled out. These are important to consider as they may account for up to one third of deaths in some head and neck cancer samples.49 Though this study was more than sufficiently powered to detect effects of depressive symptoms on overall survival, replication in a sample with a more substantial number of plausible mediating events (e.g., treatment interruptions) would be useful in clarifying nuanced relationships between depressive symptoms, medical and biological factors.
Overall, head and neck cancer patients experiencing greater depressive symptoms demonstrate poorer overall survival, as well as greater risk for treatment interruption and poorer therapeutic response. Treatment response may partly mediate the relationship between depression and survival. These findings highlight the need for careful assessment of depressive symptoms among head and neck cancer patients preparing to undergo treatment, which may help identify patients who may be at risk for incomplete therapeutic response and poorer long-term outcomes.
Acknowledgments
Funding: A portion of this work was supported by a grant from the National Cancer Institute at the National Institutes of Health, grant number R25 CA134283-06
Footnotes
Disclosures: All authors do not have any conflicts of interest to disclose.
Author Contributions: Lauren A. Zimmaro: Data collection, analysis, and interpretation, manuscript preparation. Sandra E. Sephton: Study design, data interpretation, manuscript preparation. Chelsea Siwik: Data interpretation, manuscript preparation. Kala Phillips: Data interpretation, manuscript preparation. Whitney N. Rebholz: Data collection, data interpretation, manuscript preparation. Helena C. Kraemer: Data interpretation, manuscript preparation. Janine Giese-Davis: Data interpretation, manuscript preparation. Liz Wilson: Study design, data collection. Jeffrey M. Bumpous: Study concept and design, manuscript preparation. Elizabeth Cash: Study concept and design, data collection, analysis, and interpretation, manuscript preparation.
References
- 1.Massie MJ. Prevalence of Depression in Patients With Cancer. J Natl Cancer Inst Monogr. 2004;2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiat. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 3.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 5.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazure KE, Lydiatt WM, Denman D, Burke WJ. Association between Depression and Survival or Disease Recurrence in Patients with Head and Neck Cancer Enrolled in a Depression Prevention Trial. Head Neck-J Sci Spec. 2009;31:888–892. doi: 10.1002/hed.21046. [DOI] [PubMed] [Google Scholar]
- 7.Kim SA, Roh JL, Lee SA, et al. Pretreatment depression as a prognostic indicator of survival and nutritional status in patients with head and neck cancer. Cancer. 2016;122:131–140. doi: 10.1002/cncr.29693. [DOI] [PubMed] [Google Scholar]
- 8.Shinn EH, Valentine A, Jethanandani A, et al. Depression and Oropharynx Cancer Outcome. Psychosom Med. 2016;78:38–48. doi: 10.1097/PSY.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rieke K, Schmid KK, Lydiatt W, Houfek J, Boilesen E, Watanabe-Galloway S. Depression and survival in head and neck cancer patients. Oral Oncol. 2017;65:76–82. doi: 10.1016/j.oraloncology.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Graeff A, de Leeuw JR, Ros WJG, Hordijk GJ, Blijham DH, Winnubst JAM. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37:332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 11.Coyne JC, Pajak TF, Harris J, et al. Emotional well-being does not predict survival in head and neck cancer patients. Cancer. 2007;110:2568–2575. doi: 10.1002/cncr.23080. [DOI] [PubMed] [Google Scholar]
- 12.Katz MR, Kopek N, Waldron J, Devins GM, Tomlinson G. Screening for depression in head and neck cancer. Psycho-Oncol. 2004;13:269–280. doi: 10.1002/pon.734. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel D, Kraemer HC. Emotional well-being does not predict survival in head and neck cancer patients. Cancer. 2008;112:2326–2327. doi: 10.1002/cncr.23435. [DOI] [PubMed] [Google Scholar]
- 14.Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Rasiat Oncol. 2003;56:399–412. doi: 10.1016/s0360-3016(02)04469-3. [DOI] [PubMed] [Google Scholar]
- 15.DiMatteo MR, Lepper HS, Croghan TW. Depression Is a Risk Factor for Noncompliance With Medical Treatment. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 16.Fesinmeyer MD, Mehta V, Blough D, Tock L, Ramsey SD. Effect of radiotherapy interruptions on survival in medicare enrollees with local and regional head-and-neck cancer. Int J Radiat Oncol. 2010;78:675–681. doi: 10.1016/j.ijrobp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Barber B, Dergousoff J, Nesbitt M, et al. Depression as a predictor of postoperative functional performance status (PFPS) and treatment adherence in head and neck cancer patients: a prospective study. J Otoaryngol Head Neck Surg. 2015;44:38. doi: 10.1186/s40463-015-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edmonds MF, McGuire DB. Treatment adherence in head and neck cancer patients undergoing radiation therapy: challenges for nursing. Journal of Radiology Nursing. 2007;26:87–92. [Google Scholar]
- 19.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 20.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen BL, Thornton LM, Shapiro CL, et al. Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants. Clin Cancer Res. 2010;16:3270–3278. doi: 10.1158/1078-0432.CCR-10-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton LM, Andersen BL, Schuler TA, Carson WE., III A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosom Med. 2009;71:715–724. doi: 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen L, Cole SW, Sood AK, et al. Depressive Symptoms and Cortisol Rhythmicity Predict Survival in Patients with Renal Cell Carcinoma: Role of Inflammatory Signaling. PLoS ONE. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eismann EA, Lush E, Sephton SE. Circadian effects in cancer-relevant psychoneuroendocrine and immune pathways. Psychoneuroendocrino. 2010;35:963–976. doi: 10.1016/j.psyneuen.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiat Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Pandey M, Devi N, Ramdas K, Krishnan R, Kumar V. Higher distress relates to poor quality of life in patients with head and neck cancer. Int JOral Max Surg. 2009;38:955–959. doi: 10.1016/j.ijom.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Neilson KA, Pollard AC, Boonzaier AM, et al. Psychological distress (depression and anxiety) in people with head and neck cancers. Med J Australia. 2010;193:S48. doi: 10.5694/j.1326-5377.2010.tb03928.x. [DOI] [PubMed] [Google Scholar]
- 28.Kraemer HC, Blasey CM. Centring in regression analyses: a strategy to prevent errors in statistical inference. IntJ Methods Psych. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27:S101. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiat. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 31.Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord. 2012;2:4. doi: 10.1186/2045-5380-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maes M, Bosmans E, De Jongh R, Kenis F, Vandoolarghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 34.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 35.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 36.Linkowski P, Mendlewicz J, Leclercq R, et al. The 24-Hour Profile of Adrenocorticotropin and Cortisol in Major Depressive Illness. J Clin Endo Metab. 1985;61:429–438. doi: 10.1210/jcem-61-3-429. [DOI] [PubMed] [Google Scholar]
- 37.Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182:54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- 38.Huber TJ, Issa K, Schik G, Wolf OT. The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrin. 2006;31:900–904. doi: 10.1016/j.psyneuen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Keibel A, Singh V, Sharma MC. Inflammation, microenvironment, and the immune system in cancer progression. Current Pharm Design. 2009;15:1949–1955. doi: 10.2174/138161209788453167. [DOI] [PubMed] [Google Scholar]
- 41.Schrepf A, Thaker PH, Goodheart MJ, et al. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinol. 2015;53:256–267. doi: 10.1016/j.psyneuen.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 43.Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30(Suppl):S163–70. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Patel UA, Patadia MO, Holloway N, Rosen F. Poor radiotherapy compliance predicts persistent regional disease in advanced head/neck cancer. Laryngoscope. 2009;119:528–533. doi: 10.1002/lary.20072. [DOI] [PubMed] [Google Scholar]
- 45.Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: overview and recommendations for future research. J Natl Cancer I. 2002;94:558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 46.Klein WMP, Bloch M, Hesse BW, et al. Behavioral Research in Cancer Prevention and Control. Am J Prev Med. 2014;46:303–311. doi: 10.1016/j.amepre.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoni MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain Behav Immun. 2013;30(Suppl):S88–98. doi: 10.1016/j.bbi.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bese NS, Hendry J, Jeremic B. Effects of Prolongation of Overall Treatment Time Due To Unplanned Interruptions During Radiotherapy of Different Tumor Sites and Practical Methods for Compensation. Int J Radiat Oncol. 2007;68:654–661. doi: 10.1016/j.ijrobp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Massa ST, Osazuwa-Peters N, Christophe KM, et al. Competing causes of death in the head and neck cancer population. Oral Oncol. 2007;65:8–15. doi: 10.1016/j.oraloncology.2016.12.006. [DOI] [PubMed] [Google Scholar]