Abstract
Background
Chemotherapy for early breast cancer is associated with a small risk of developing myelodysplastic syndrome (MDS) and/or acute myeloid leukemia (AML). The aim of this study is to determine the risk of developing AML or MDS after modern adjuvant chemotherapy in older breast cancer patients and to further define the risk of individual chemotherapy regimens.
Methods
Patients diagnosed with stage I–III breast cancer from 2003 to 2009 were identified in the Surveillance Epidemiology and End Results Program (SEER)-Medicare and Texas Cancer Registry (TCR)-Medicare linked databases. Development of AML/MDS, chemotherapy use, and comorbidities were identified using International Classification of Diseases (ICD-9) and Healthcare Common Procedure Coding System (HCPCS) codes. Analyses included descriptive statistics, cumulative incidence, and Cox proportional hazards models to estimate the hazard of AML/MDS after adjusting for clinically relevant covariates.
Results
92,110 patients were included, after a median follow-up of 85 months, the overall rates per 1,000 person-years were 0.65 for AML and 1.56 for MDS. Patients who received an anthracycline (A) or an anthracycline/taxane (A+T) regimen were more likely to develop AML (HR1.70,95%CI 1.16–2.50 for A and HR1.68,95%CI 1.22–2.30 for A+T) or MDS (HR2.18,95%CI 1.70–2.80 for A and HR1.62,95%CI 1.29–2.03 for A+T) than patients who did not receive chemotherapy. Docetaxel/cyclophosphamide (TC) use was not at increased risk for AML and MDS.
Conclusions
Adjuvant chemotherapy is associated with a small but significant increase in the risk of AML and MDS, especially with regimens that include A. Longer follow-up is needed to confirm that risk is not increased with the recently adopted TC regimen.
Keywords: breast cancer, complications from chemotherapy, secondary malignancy, population-based, health-services
Introduction
Adjuvant chemotherapy for breast cancer has significantly improved the outcomes of patients of all ages [1]. Chemotherapy is not without risk, as both short and long-term complications can occur and likely contribute to decreased utilization in older patients. The risk of developing secondary malignancies has been well described with breast cancer regimens. Therapy-related acute myeloid leukemia (t-AML) that is associated with alkylating agents usually occurs 5 to 7 years after exposure to chemotherapy and is preceded by myelodysplastic syndrome (MDS). t-AML associated with topoisomerase II agents usually occurs with a shorter latency (less than 5 years) and is associated with 11q23 translocation. t-AML confers a poor prognosis and is particularly worse in patients with unfavorable cytogenetics [2].
The rates of t-AML and/or MDS after breast cancer have been described in multiple clinical trials and large database analyses ranging from 0.6% to 1.8% [3–5]. However, these evaluations do not take into account the current practice patterns of adjuvant chemotherapy use in the US. Since 2005, the use of docetaxel and cyclophosphamide (TC), a taxane-based regimen without an anthracycline has significantly increased in frequency [6]. Consequently, in this analysis, we investigate the rates of AML and MDS in an older breast cancer cohort who received modern adjuvant chemotherapy regimens including TC.
Methods
The Surveillance, Epidemiology and End Results (SEER)/Texas Cancer Registry (TCR)-Medicare linked databases were retrospectively reviewed and analyzed. The SEER program collects data from tumor registries covering 28% of the US population and is supported by the US National Cancer Institute (NCI). The Medicare program is administered by the Centers for Medicare & Medicaid Services (CMS) and covers 97% of the US population age 65 or older [7]. Under an agreement between the NCI and CMS, SEER participants are matched with their Medicare record. Of SEER participants who were diagnosed with cancer at age 65 years or older, 94% are matched with their Medicare enrollment records.
The TCR is a statewide population-based registry and is a component of the Texas Department of State Health Services. The TCR is not part of SEER, but collects data according to the standardized registry rules and is Gold Certified by the North American Association of Central Cancer Registries. The NCI linked the TCR databases with Texas Medicare data by means of a probabilistic linkage model, with the same methodology as the SEER–Medicare linkage.
We identified patients 65 years and older who were diagnosed with invasive breast cancer between the years of 2003 to 2009. SEER-Medicare patients were followed until December 31st 2014 and TCR-Medicare patients until December 31st, 2012. Only patients with localized or regional disease were included. Additionally, patients were required to have Medicare Part A and Part B coverage during the full year after diagnosis and not be enrolled in health maintenance organization (HMO) as Medicare claims are not complete for these members. Patients who received chemotherapy other than an anthracycline, a taxane, or cyclophosphamide, methotrexate, and 5-fluoruracil were excluded.
Patients who developed AML or MDS were identified by disease specific International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes extracted from inpatient, outpatient and provider claims. In an effort to ensure accuracy of the diagnosis for inpatient claims, we required ICD-9-CM code to be one of the first two diagnosis codes. For outpatient claims, patients were required to have at least two outpatient/provider claims with the ICD-9-CM code utilized at least 30 days apart.
Chemotherapy patients were defined by Medicare claims within one year of diagnosis and categorized as one of the following five cohorts: Anthracycline based (A-based) (claims with at least one of the Healthcare Common Procedure Coding System (HCPCS) codes for doxorubicin, liposomal-doxorubin, or epirubicin and no taxanes given), taxane based (patients with claims that had at least one of the HCPCS codes for docetaxel, nab-paclitaxel, or paclitaxel and no anthracycline given), anthracycline and taxane (A+T), or cyclophosphamide, methotrexate, or 5-fluoruracil (CMF) (patients with claims utilizing HCPCS codes utilizing all three drugs). The taxane group was further classified as docetaxel and cyclophosphamide (TC) and other taxanes of which most are trastuzumab-based FDA approved regimens or taxane without cyclophosphamide. ICD-9 and CPT/HCPCS codes used in the study are listed in the Supplementary Table 1. Frequencies of regimens in each chemotherapy group are listed in Supplementary Table 2.
Descriptive statistics were used. Patient characteristic and treatments were compared according to chemotherapy categories utilizing the chi-squared test. Cumulative Incidence Function (CIF) of AML/MDS subject to competing risk of death were estimated with each chemotherapy group, and CIF between groups were compared with Gray’s test [8, 9]. Incidence rates were calculated by the number of AML/MDS events per 1,000 person-years at risk for each chemotherapy, and 95% confidence intervals (CIs) for incidence rates were computed by Poisson distribution. The 3, 5, and 8-year cumulative incidence with 95% CIs were estimated with univariable Kaplan-Meier method.
Multivariable cox regression models were applied to estimate hazard ratios (HR) and 95% confidence intervals (CI) for developing AML and MDS. The following variables were included in the final model: age, year of diagnosis, race, geographic region, marital status, urban/rural, socioeconomic variables (education and poverty levels), tumor grade, surgery type, radiation therapy exposure, other malignancies, and Charlson comorbidity index [10, 11]. Other malignancies were identified from breast cancer diagnosis to the end of follow-up or development of AML/MDS. This variable was treated as a time dependent covariable in the multivariable cox regression models. P-values less than 0.05 were considered statistically significant; all tests were two sided. All the statistical analyses were carried out using SAS 9.4 (SAS Institute Inc., Cary, NC). The research was reviewed by the institutional review board of The University of Texas MD Anderson Cancer Center and was exempt under the codes of regulations.
Results
At total of 92,110 (71,671 in SEER-Medicare and 20,439 in TCR-Medicare) patients were included in the analysis. The median age at breast cancer diagnosis was 74. A total of 20,224 (22%) patients received chemotherapy, among them with 3,797(18.8%) patients received anthracycline-based; 8,338 (41.2%) received an anthracycline and taxane-based regimen; 3,083 (15.2%) patients received TC; 2,851 (14.1%) other taxanes; and 2,155 (10.7%) received CMF. The proportion of patients who received A-based chemotherapy kept decreasing and the proportion who received TC regimen continued to increase after 2006 (Figure 1). Patient’s demographic and tumor characteristics are listed in Table 1.
Figure 1.
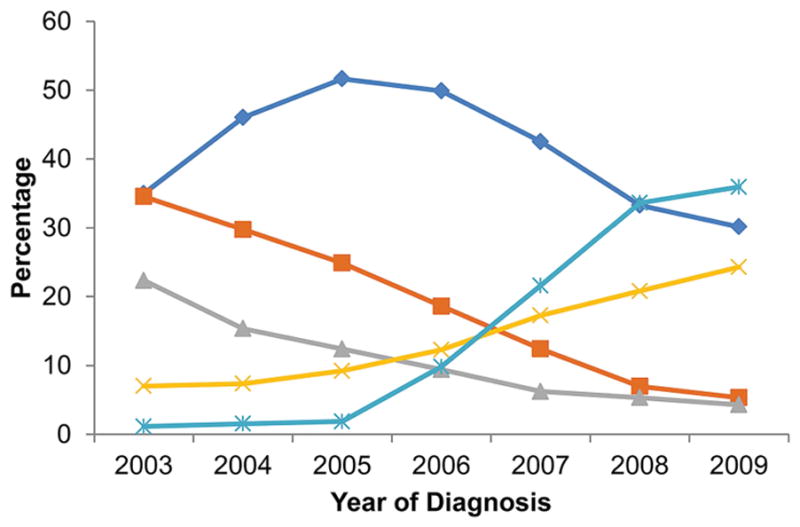
Trend of chemotherapy regimens change from 2003 to 2009 among patients received chemotherapy.
Table 1.
Patient characteristics in final cohort of patients
| Variables | No Chemo n (%) |
A-based n (%) |
A+T n (%) |
TC n (%) |
Other Taxane n (%) |
CMF n (%) |
p value | Total n (%) |
|---|---|---|---|---|---|---|---|---|
| All subjects | 71886 (78.0) | 3797 (4.1) | 8338 (9.1) | 3083 (3.4) | 2851 (3.1) | 2155 (2.3) | 92,110 | |
| Age group (y) | ||||||||
| Range | 65–114 | 65–90 | 65–93 | 65–91 | 65–99 | 65–94 | <0.001 | 65–114 |
| Mean | 76±7 | 71±5 | 70±4 | 71±5 | 73±6 | 73±6 | 75±7 | |
| Median | 76 | 69 | 69 | 70 | 73 | 73 | 74 | |
| 65 – 70 | 18228 (62.6) | 2170 (7.5) | 5081 (17.4) | 1613 (5.5) | 1246 (4.3) | 792 (2.7) | 29,130 | |
| 71 – 75 | 15704 (74) | 1038 (4.9) | 2221 (10.5) | 869 (4.1) | 762 (3.6) | 628 (3) | 21,222 | |
| 76 – 80 | 16246 (85.6) | 450 (2.4) | 860 (4.5) | 452 (2.4) | 496 (2.6) | 485 (2.6) | 18,989 | |
| >80 | 21708 (95.3) | 139 (0.6) | 176 (0.8) | 149 (0.7) | 347 (1.5) | 250 (1.1) | 22,769 | |
| Race | <0.001 | |||||||
| NH_White | 59826 (79.4) | 2923 (3.9) | 6345 (8.4) | 2421 (3.2) | 2163 (2.9) | 1661 (2.2) | 75,339 | |
| Hispanic | 4618 (69.8) | 346 (5.2) | 884 (13.4) | 251 (3.8) | 297 (4.5) | 225 (3.4) | 6,621 | |
| NH_Black | 4930 (71.5) | 371 (5.4) | 804 (11.7) | 300 (4.4) | 279 (4.1) | 207 (3) | 6,891 | |
| NH_Other | 2512 (77.1) | 157 (4.8) | 305 (9.4) | 111 (3.4) | 112 (3.4) | 62 (1.9) | 3,259 | |
| Year of diagnosis | <0.001 | |||||||
| 2003 | 10974 (79.9) | 952 (6.9) | 964 (7) | 31 (0.2) | 193 (1.4) | 615 (4.5) | 13,729 | |
| 2004 | 10706 (78.5) | 874 (6.4) | 1352 (9.9) | 45 (0.3) | 216 (1.6) | 451 (3.3) | 13,644 | |
| 2005 | 10328 (78.5) | 705 (5.4) | 1464 (11.1) | 53 (0.4) | 261 (2) | 351 (2.7) | 13,162 | |
| 2006 | 10125 (77.8) | 539 (4.1) | 1444 (11.1) | 284 (2.2) | 355 (2.7) | 272 (2.1) | 13,019 | |
| 2007 | 10040 (77.2) | 368 (2.8) | 1262 (9.7) | 641 (4.9) | 512 (3.9) | 185 (1.4) | 13,008 | |
| 2008 | 9851 (77) | 205 (1.6) | 981 (7.7) | 991 (7.7) | 613 (4.8) | 157 (1.2) | 12,798 | |
| 2009 | 9862 (77.4) | 154 (1.2) | 871 (6.8) | 1038 (8.1) | 701 (5.5) | 124 (1) | 12,750 | |
| Urban/Rural | <0.001 | |||||||
| Big Metro | 37980 (78.7) | 1818 (3.8) | 4232 (8.8) | 1662 (3.4) | 1499 (3.1) | 1084 (2.3) | 48,275 | |
| Metro | 21036 (78) | 1163 (4.3) | 2465 (9.1) | 842 (3.1) | 786 (2.9) | 677 (2.5) | 26,969 | |
| Urban | 4309 (76.5) | 291 (5.2) | 523 (9.3) | 190 (3.4) | 191 (3.4) | 132 (2.3) | 5,636 | |
| Less Urban | 6693 (76.8) | 411 (4.7) | 868 (10) | 272 (3.1) | 276 (3.2) | 201 (2.3) | 8,721 | |
| Rural/Unknown | 1868 (148.6) | 114 (8) | 250 (19.6) | 117 (11.7) | 99 (8) | 61 (4.2) | 2,509 | |
| Marriage status | <0.001 | |||||||
| Married | 27941 (74.2) | 1915 (5.1) | 4079 (10.8) | 1558 (4.1) | 1246 (3.3) | 941 (2.5) | 37,680 | |
| Not married | 36029 (82) | 1425 (3.2) | 3151 (7.2) | 1146 (2.6) | 1234 (2.8) | 932 (2.1) | 43,917 | |
| Unknown | 7916 (75.3) | 457 (4.4) | 1108 (10.5) | 379 (3.6) | 371 (3.5) | 282 (2.7) | 10,513 | |
| Education | <0.001 | |||||||
| 1st Quartile (highest) | 18348 (80.6) | 756 (3.3) | 1833 (8.1) | 789 (3.5) | 619 (2.7) | 432 (1.9) | 22,777 | |
| 2nd Quartile | 17998 (79.1) | 887 (3.9) | 1951 (8.6) | 772 (3.4) | 648 (2.9) | 500 (2.2) | 22,756 | |
| 3rd Quartile | 17593 (77.3) | 1044 (4.6) | 2129 (9.4) | 697 (3.1) | 717 (3.2) | 570 (2.5) | 22,750 | |
| 4th Quartile (lowest) | 17947 (75.3) | 1110 (4.7) | 2425 (10.2) | 825 (3.5) | 867 (3.6) | 653 (2.7) | 23,827 | |
| Poverty | <0.001 | |||||||
| 1st Quartile (lowest) | 18166 (79.6) | 810 (3.6) | 1957 (8.6) | 777 (3.4) | 638 (2.8) | 489 (2.1) | 22,837 | |
| 2nd Quartile | 17955 (78.9) | 911 (4) | 1965 (8.6) | 765 (3.4) | 650 (2.9) | 498 (2.2) | 22,744 | |
| 3rd Quartile | 17726 (77.9) | 990 (4.4) | 2029 (8.9) | 751 (3.3) | 749 (3.3) | 504 (2.2) | 22,749 | |
| 4th Quartile (highest) | 18039 (75.9) | 1086 (4.6) | 2387 (10) | 790 (3.3) | 814 (3.4) | 664 (2.8) | 23,780 | |
| Comorbidity | <0.001 | |||||||
| 0 | 43632 (78.2) | 2391 (4.3) | 5159 (9.2) | 1822 (3.3) | 1578 (2.8) | 1252 (2.2) | 55,834 | |
| 1 | 15311 (79.7) | 666 (3.5) | 1498 (7.8) | 609 (3.2) | 626 (3.3) | 494 (2.6) | 19,204 | |
| 2+ | 9165 (83.7) | 301 (2.8) | 579 (5.3) | 310 (2.8) | 359 (3.3) | 242 (2.2) | 10,956 | |
| Unknown | 3778 (61.8) | 439 (7.2) | 1102 (18) | 342 (5.6) | 288 (4.7) | 167 (2.7) | 6,116 | |
| Stage | <0.001 | |||||||
| Localized | 57156 (88) | 2075 (3.2) | 1782 (2.7) | 1551 (2.4) | 1269 (2) | 1110 (1.7) | 64,943 | |
| Regional | 14730 (54.2) | 1722 (6.3) | 6556 (24.1) | 1532 (5.6) | 1582 (5.8) | 1045 (3.9) | 27,167 | |
| Surgery | <0.001 | |||||||
| Breast conserving | 41922 (83.4) | 1821 (3.6) | 2921 (5.8) | 1526 (3) | 1079 (2.2) | 997 (2) | 50,266 | |
| Mastectomy | 25703 (70.7) | 1811 (5) | 4827 (13.3) | 1433 (3.9) | 1469 (4) | 1115 (3.1) | 36,358 | |
| No Surgery/Unknown | 4261 (77.7) | 165 (3) | 590 (10.8) | 124 (4.8) | 303 (10.9) | 43 (0.8) | 5,486 | |
| Radiation therapy | <0.001 | |||||||
| No | 35312 (83.5) | 1529 (3.6) | 2285 (5.4) | 1048 (2.5) | 1198 (2.8) | 945 (2.2) | 42,317 | |
| Yes | 36574 (73.5) | 2268 (4.6) | 6053 (12.2) | 2035 (4.1) | 1653 (3.3) | 1210 (2.4) | 49,793 | |
| Grade | <0.001 | |||||||
| Well Differentiated | 19516 (91.6) | 417 (2) | 658 (3.1) | 304 (1.4) | 155 (0.7) | 251 (1.2) | 21,301 | |
| Moderately Differentiated | 31232 (81.4) | 1447 (3.8) | 2838 (7.4) | 1197 (3.1) | 866 (2.3) | 800 (2.1) | 38,380 | |
| Poorly Differentiated | 15163 (60.5) | 1720 (6.9) | 4167 (16.6) | 1425 (5.7) | 1618 (6.5) | 955 (3.8) | 25,048 | |
| Unknown | 5975 (81) | 213 (2.9) | 675 (9.2) | 157 (2.1) | 212 (2.9) | 149 (2) | 7,381 | |
Among 92,110 patients, the median follow-up was 85 months (91 months among SEER-Medicare and 64 months for TCR-Medicare participants). Patients who were treated with A-based chemotherapy had longest median follow-up at 104 months, and all other chemotherapy groups had similar median follow-up months (84 for no chemotherapy group, 88 for A+T, 79 for TC, 73 for other Taxane, and 94 for CMF). The median number of cycles was 4 for A-based, 7 for A+T, 4 for TC, 5 for other Taxane, and 6 for CMF. A sensitivity analysis was performed to determine the dose-response effects of chemotherapy on AML/MDS, and no significant difference was detected (Supplementary Table 3). In addition, patients who received combination of chemotherapy and radiation therapy did not show significant increased risk of AML/MDS (Supplementary Table 4).
Of 92,110 breast cancer patients, the overall incidence rate for AML and MDS were 0.65 (95% CI 0.59–0.71) and 1.56 (95% CI 1.47–1.66) per 1,000 person-years. Among 71,886 patients with no chemotherapy the incidence rate of AML and MDS were 0.59 (95%CI 0.53–0.66) and 1.42 (95%CI 1.32–1.53) per 1,000 person-years. Of 20,224 patients who received chemotherapy, the incidence rate of AML and MDS were 0.85 (95% CI 0.71–1.01) and 2.05 (95% CI 1.83–2.30) per 1,000 person-years. The median time from breast cancer diagnosis to AML or MDS was 39 and 26 months respectively.
Patients treated with A-based (incidence rate for AML and MDS was 1.05 and 2.52 per 1,000 person-years ) and A+T (1.03 and 1.88) developed AML and MDS more than TC (0.32 and 1.22). CMF (0.74 and 2.39) and other taxanes (0.51 and 2.42) had lower AML incidence rates, but similar MDS incidence rates than A-based and A+T, as shown in Figure 2.
Figure 2.
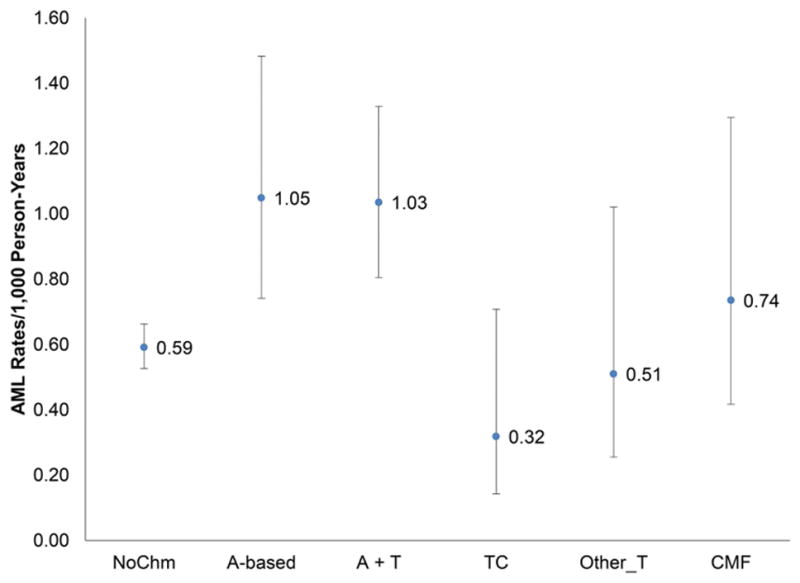
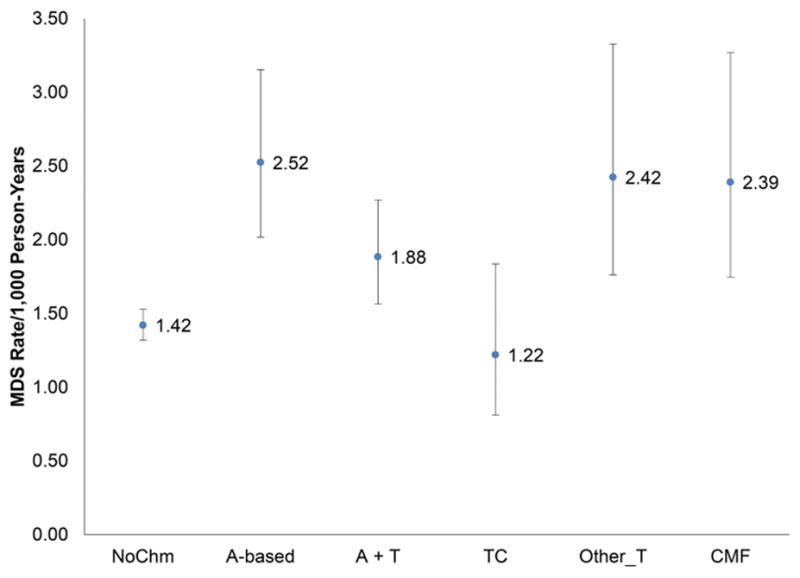
Incidence rates and 95% CI of secondary AML (A) and MDS (B) per 1,000 person-years by adjuvant chemotherapy use among older patients with localized and regional breast cancer.
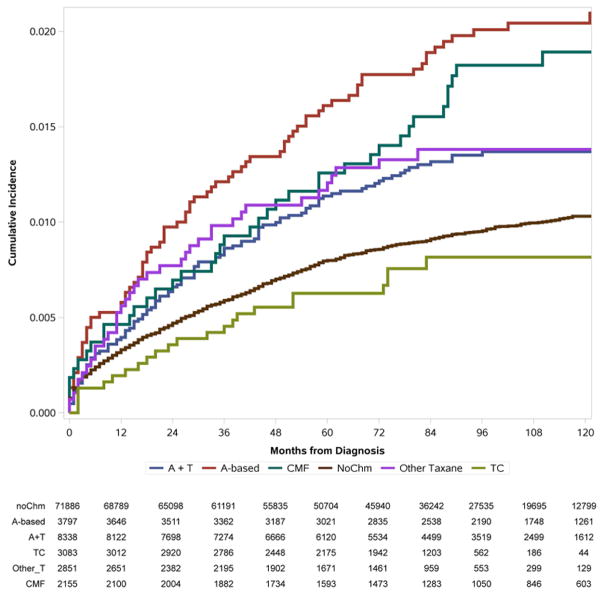
The cumulative incidence of developing AML and MDS is illustrated in Figure 3. Patients who received A-based and A+T containing regimens were more likely to develop AML than patients who did not received chemotherapy. No difference in AML incidence was found between patients who received taxane-based chemotherapy and CMF when compared with the non-chemotherapy cohort. Patients who received chemotherapy were more likely to develop MDS than patients who did not, except for the TC cohort. The specific 3, 5, and 8-year cumulative incidences of the outcomes under study are presented in Table 2. Similarly, patients received A-based and A+T regimens showed a higher cumulative incidence for AML and MDS at all years, comparing to patients without chemotherapy. Patients receiving the TC regimen were similarly likely to develop AML and MDS as patients without chemotherapy (0.13%, 0.17%, and 0.22% vs 0.21%, 0.32%, and 0.45% of patients at risk developed AML at 3,5, and 8-year for TC and no chemotherapy group; 0.47%, 0.67%, and 0.91% vs 0.62%, 0.88%, and 1.03% of patients at risk developed MDS at 3,5, and 8-year for TC and no chemotherapy group).
Figure 3.
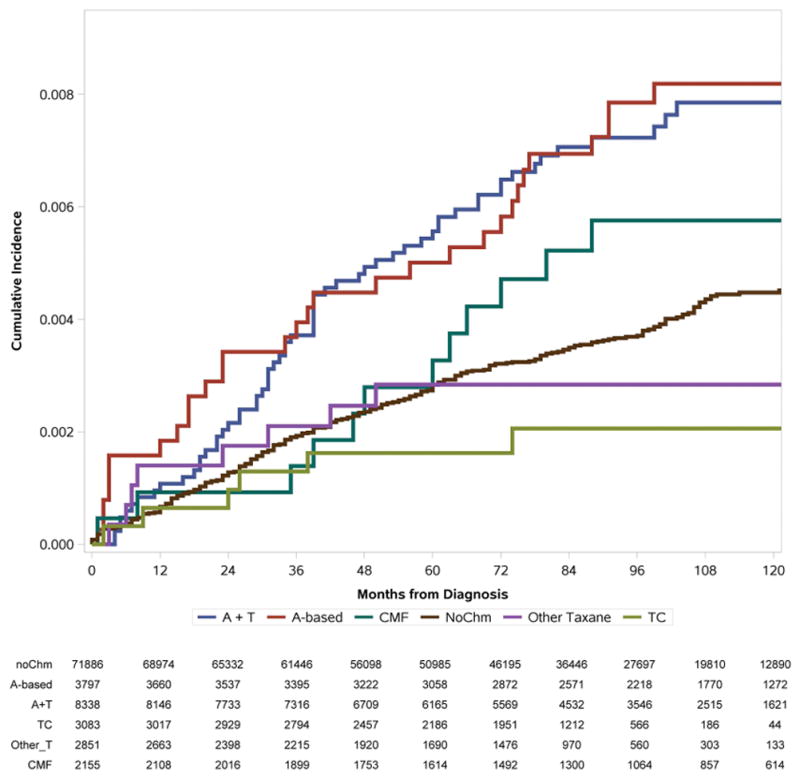
AML (A) and MDS (B) Cumulative Incidence (%) by adjuvant chemotherapy use among older patients with localized and regional breast cancer, subjects to competing risk of death. Numbers of patients at risk at each time point are listed.
Table 2.
3-year, 5-year and 8-year Cumulative Incidence (%) of AML/MDS by Chemotherapy Regimens*
| AML | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N patients | 3-year Cumulative Incidence | 5-year Cumulative Incidence | 8-year Cumulative Incidence | ||||
|
| |||||||
| N at Risk | % (95% CI) | N at Risk | % (95% CI) | N at Risk | % (95% CI) | ||
| All Subjects | 92,110 | ||||||
| Chemo Regimen | |||||||
| No Chemo | 71,886 | 61,446 | 0.21 (0.18 – 0.25) | 50,985 | 0.32 (0.13– 0.37) | 27,697 | 0.45 (0.40 – 0.51) |
| A-based | 3,797 | 3,395 | 0.41 (0.25 – 0.68) | 3,058 | 0.53 (0.34 – 0.83) | 2,218 | 0.90 (0.62 – 1.30) |
| A+T | 8,338 | 7,316 | 0.40 (0.28 – 0.56) | 6,165 | 0.61 (0.46 – 0.82) | 3,546 | 0.84 (0.64 – 1.08) |
| TC | 3,083 | 2,794 | 0.13 (0.05 – 0.36) | 2,186 | 0.17 (0.07 – 0.41) | 566 | 0.22 (0.10 – 0.51) |
| Other Taxane | 2,851 | 2,215 | 0.23 (0.10 – 0.51) | 1,690 | 0.33 (0.16 – 0.66) | 560 | 0.33 (0.17 – 0.66) |
| CMF | 2,155 | 1,899 | 0.15 (0.05 – 0.45) | 1,614 | 0.37 (0.18 – 0.79) | 1,064 | 0.72 (0.41 – 1.28) |
|
| |||||||
| MDS | |||||||
|
| |||||||
| N patients | 3-year Cumulative Incidence | 5-year Cumulative Incidence | 8-year Cumulative Incidence | ||||
|
| |||||||
| N at Risk | % (95% CI) | N at Risk | % (95% CI) | N at Risk | % (95% CI) | ||
|
| |||||||
| All Subjects | 92,110 | ||||||
| Chemo Regimen | |||||||
| No Chemo | 71,886 | 61,191 | 0.62 (0.56 – 0.68) | 50,704 | 0.88 (0.81 – 0.96) | 27,535 | 1.11 (1.03 – 1.20) |
| A-based | 3,797 | 3,362 | 1.26 (0.94 – 1.67) | 3,021 | 1.72 (1.34 – 2.21) | 2,190 | 2.24 (1.78 – 2.80) |
| A+T | 8,338 | 7,274 | 0.90 (0.71 – 1.13) | 6,120 | 1.23 (1.00 – 1.50) | 3,519 | 1.55 (1.28 – 1.87) |
| TC | 3,083 | 2,786 | 0.47 (0.28 – 0.79) | 2,175 | 0.67 (0.42 – 1.04) | 562 | 0.91 (0.59 – 1.38) |
| Other Taxane | 2,851 | 2,195 | 1.07 (0.74 – 1.55) | 1,671 | 1.38 (0.99 – 1.94) | 553 | 1.66 (1.20 – 2.30) |
| CMF | 2,155 | 1,882 | 0.97 (0.62 – 1.49) | 1,593 | 1.37 (0.94 – 1.99) | 1,050 | 2.19 (1.78 – 2.80) |
Estimated from Kaplan-Meier Method; CI - Confidence Interval
In a Cox regression model, patients who received A-based regimen (HR= 1.70; 95%CI 1.16–2.50) and A+T (HR= 1.68; 95%CI 1.22–2.30) were more likely to develop AML when compared with patients not treated with chemotherapy. There was no significant increase risk when comparing patients who received TC (HR=0.62; 95CI 0.27–1.41), other taxanes (HR=0.88; 95%CI 0.43–1.79) or CMF (HR=1.11, 95%CI 0.62–1.99), compared to patients not treated with chemotherapy. In the model evaluating MDS as an outcome, we observed that treatment with A-based chemotherapy (HR=2.18; 95%CI 1.70–2.80), A+T-based (HR=1.62; 95%CI 1.29–2.03), other Taxane (HR=1.99; 95%CI 1.42–2.80), and CMF (HR=1.71, 95%CI 1.23–2.37) were all associated with an increased risk of developing secondary MDS when comparing to patients who did not receive chemotherapy. TC was the only cohort that received chemotherapy that was not associated with a statistically significant increased risk in developing MDS (HR=1.18; 95CI 0.77–1.81), with the similar follow-up months after diagnosis comparing to no chemotherapy group (79 months vs 84 months). Comorbid conditions also increased risk of both MDS and AML, while advancing age and receiving radiation increased risk for only MDS. The complete model is shown in Table 3.
Table 3.
Multivariable Cox Regression Model for Secondary AML and MDS among older breast cancer patients*
| Variables | Adjusted HR for AML | 95% CI | P value | Adjusted HR for MDS | 95% CI | P value |
|---|---|---|---|---|---|---|
| Chemotherapy Types | ||||||
| No Chemotherapy | [reference] | [reference] | ||||
| Anthracycline Based | 1.70 | 1.16 – 2.50 | 0.007 | 2.18 | 1.70 – 2.80 | <.001 |
| Anthracycline + Taxane | 1.68 | 1.22 – 2.30 | 0.001 | 1.62 | 1.29 – 2.03 | <.001 |
| TC | 0.62 | 0.27 – 1.41 | 0.254 | 1.18 | 0.77 – 1.81 | 0.461 |
| Other Taxane Based | 0.88 | 0.43 – 1.79 | 0.714 | 1.99 | 1.42 – 2.80 | <.001 |
| CMF | 1.11 | 0.62 – 1.99 | 0.733 | 1.71 | 1.23 – 2.37 | 0.002 |
| Other Malignancies | ||||||
| No | [reference] | [reference] | ||||
| Yes | 1.15 | 0.81 – 1.62 | 0.436 | 0.58 | 0.43 – 0.77 | <.001 |
| Age | ||||||
| 65–70 | [reference] | [reference] | ||||
| 71–75 | 1.22 | 0.94 – 1.58 | 0.145 | 1.36 | 1.13 – 1.64 | 0.001 |
| 76–80 | 1.26 | 0.95 – 1.68 | 0.114 | 1.81 | 1.50 – 2.18 | <.001 |
| >80 | 1.27 | 0.92 – 1.73 | 0.143 | 1.97 | 1.62 – 2.41 | <.001 |
| Comorbidity | ||||||
| 0 | [reference] | [reference] | ||||
| 1 | 1.30 | 1.02 – 1.65 | 0.034 | 1.31 | 1.12 – 1.53 | <.001 |
| 2+ | 1.43 | 1.04 – 1.97 | 0.028 | 2.06 | 1.72 – 2.46 | <.001 |
| Unknown | 1.11 | 0.74 – 1.66 | 0.616 | 0.81 | 0.59 – 1.12 | 0.208 |
| Radiation Therapy | ||||||
| No | [reference] | [reference] | ||||
| Yes | 1.20 | 0.93 – 1.55 | 0.165 | 1.42 | 1.21 – 1.67 | <.001 |
Other variables included in the models are year of diagnosis, race, marital status, region, year of diagnosis, education, poverty, urban/rural, tumor grade and surgery
Discussion
In our analysis, we identified the risk of AML and MDS for older patients with local or regional stage breast cancer. The 8-year cumulative incidence for patients who did not receive chemotherapy for AML and MDS was 0.45% and 1.11% respectively. For patients who received A-based and A+T regimens the cumulative incidence was significantly higher for both AML (0.90% and 0.84%) and MDS (2.24% and 1.55%). These results add to previous estimates of secondary myeloid malignancies after breast cancer.
In an analysis of 3 large Cancer and Leukemia Group B (CALGB) adjuvant trials in which 6,174 women with node-positive breast cancer received doxorubicin and cyclophosphamide-based therapy, the 5-year cumulative incidence for AML and MDS combined was 0.6%. Among patients 65 years old and greater, 1.8% developed AML/MDS. While this estimate suggests that older patients are at much higher risk, this cumulative incidence should be viewed with caution as only 7% of the participants were 65 years old and greater[4]. Despite a concerted effort from cooperative groups to increase recruitment of older patients[12], including designing trials specifically for this patient population [13], older patient enrollment in clinical trials remains low. In a recent review of all breast cancer Alliance clinical trials from 1985–2012, 17% of study participants were 65 years or older[14]. With limited numbers of patients in clinical trials, retrospective analyses can help to define benefit and risks involved in treatments.
In an study utilizing the SEER-Medicare linked database of breast cancer patients diagnosed from 1992 to 2002, a 10-year cumulative incidence for AML in breast cancer patients of 1.2% was observed among patients who did not receive chemotherapy and 1.8% for patients treated with adjuvant chemotherapy [5]. Differing from our analysis, this study did not evaluate MDS as Medicare did not have a unique ICD-9-CM code for MDS at that time. Additionally, the chemotherapy regimens included are likely no longer representative of current practices as the group of patients receiving non-anthracycline, non-taxane chemotherapy was the largest cohort of patient (45.8%).
Another relevant study evaluating data form the National Comprehensive Cancer Network (NCCN) revealed that 0.24% at 5 years and 0.48% at 10 years of patients with early stage breast cancer developed a marrow neoplasm including malignancies of myeloid and lymphoid origin[3]. The authors estimated the incidence rate of marrow neoplasm among patients receiving chemotherapy of 0.46 per 1,000 person-years. Unique to our analysis is that we evaluated the risk associated with all modern regimens including TC. This is relevant since this adjuvant chemotherapy regimen has become widely used. In a separate population-based study, TC was identified as the most frequently used regimen among breast cancer patients younger than 65 [15]. Our results indicate that TC was not associated with an increased risk of AML or MDS when compared to patients that did not receive chemotherapy. However, it is known that the exposure to the alkylating agent cyclophosphamide is associated with risk of developing secondary myeloid malignancies that occur 7 years after exposure. Given the relatively recent adaptation of TC, longer follow-up and additional patients are needed to truly define risk for AML and/or MDS associated with this regimen. This limitation can possibly be seen in our evaluation of MDS as the hazard ratio for TC is in the direction of risk but does not reach statistical significance. We cannot exclude that with larger numbers of patients and longer follow-up, an increase risk be identified in future analysis. Despite this limitation, in our cohort, patients treated with TC had a median follow-up of 79 months and provide an informative estimate considering that this regimen has become the most common utilized regimen in 2008 and 2009. Our study is limited by its retrospective nature and characteristics inherent in claims-based research. While unlikely, it is possible that patients may have been lost or misclassified, explaining the lower rates of cases when compared to other studies focusing on elderly patients.
The question of efficacy of TC in comparison to taxane and anthracycline based therapy had not been fully addressed until recently. The results of the joint analysis of the ABC (anthracycline in early breast cancer) trials revealed a significant different 4 year invasive disease free survival with TC at 88.2% while for anthracycline and taxane based regimen was 90.7% (p=0.04) [16]. In this joint analysis, at a median follow-up of 3.3 years the cumulative incidence of acute leukemia was 0.24% (5/2062) in the anthracycline and taxane cohort. No cases of leukemia were identified in the TC cohort. This finding was consistent with our results that TC regimen was associated with lower risk of AML than other regimens, although the follow-up time in this study was not long enough to draw an affirmative conclusion.
Overall, our data provides valid estimations of the risk of a devastating complication secondary to chemotherapy usage. The greatest strength of our analysis is that it is the largest study to date evaluating breast cancer patients’ incidence rate of AML and MDS among patients received no chemotherapy and patients treated with contemporary adjuvant chemotherapy regimens. The risk of AML/MDS remains small but significant. It is important to carefully select patients that truly benefit from chemotherapy and select the appropriate regimen depending on the patient’s risk. Gene signature assays can be used in appropriate scenarios to identify appropriate candidates for chemotherapy. Utilizing the results from our study, practicing oncologists can more accurately estimate the risk involved with developing secondary myeloid diseases.
Supplementary Material
Acknowledgments
RESEARCH SUPPORT
This research was supported in part by the National Cancer Institute’s Cancer Center support grant awarded to MD Anderson Cancer Center (2P30 CA016672), by the Cancer Prevention and Research Institute of Texas (CPRIT) grant (RP160674) and by the Duncan Family Institute. Mariana Chavez-MacGregor, Sharon H. Giordano, and Antonio C. Wolff are supported by the Susan G. Komen Breast Cancer Foundation (SAC150061 and SAC110053).
The ideas and opinions expressed herein are those of the authors. The SEER program, the TCR or the funding agencies had no role in the design, conduction or interpretation of the study.
Footnotes
Disclosures: No conflicts of interests from any authors
Author Contributions:
Conceptualization: AR, SG, MC
Methodology: AR, JN, HZ, AW, MC
Software: JN, HZ
Validation: AR, SG, AW, MC
Formal analysis: JN, HZ
Investigation: JN
Resources: JN, SG, HZ, MC
Data curation: AR, JN, HZ, MC
Writing-original: AR
Writing-review and editing: AR, JN, SG, HZ, AW, MC
Visualization: AR, JN
Supervision: AG, MC
Projection administration: AR, MC
Funding acquisition: SG, AW, MC
References
- 1.Muss HB, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293(9):1073–81. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 2.Schoch C, et al. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18(1):120–5. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 3.Wolff AC, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. 2015;33(4):340–8. doi: 10.1200/JCO.2013.54.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muss HB, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25(24):3699–704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 5.Patt DA, et al. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J Clin Oncol. 2007;25(25):3871–6. doi: 10.1200/JCO.2007.12.0832. [DOI] [PubMed] [Google Scholar]
- 6.Giordano SH, et al. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30(18):2232–9. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potosky AL, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–48. [PubMed] [Google Scholar]
- 8.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 9.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 10.Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–9. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–90. [DOI] [PubMed] [Google Scholar]
- 12.Kimmick GG, et al. Improving accrual of older persons to cancer treatment trials: a randomized trial comparing an educational intervention with standard information: CALGB 360001. J Clin Oncol. 2005;23(10):2201–7. doi: 10.1200/JCO.2005.01.222. [DOI] [PubMed] [Google Scholar]
- 13.Hurria A, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol. 2014;32(24):2587–94. doi: 10.1200/JCO.2013.55.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman RA, et al. Accrual of Older Patients With Breast Cancer to Alliance Systemic Therapy Trials Over Time: Protocol A151527. J Clin Oncol. 2017;35(4):421–431. doi: 10.1200/JCO.2016.69.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barcenas CH, et al. Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer. J Clin Oncol. 2014;32(19):2010–7. doi: 10.1200/JCO.2013.49.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blum JL, et al. Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology) J Clin Oncol. 2017 doi: 10.1200/JCO.2016.71.4147. p. JCO2016714147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.