Abstract
The establishment of erythromycin production within the heterologous host E. coli marked an accomplishment in genetic transfer capacity. Namely, over 20 genes and 50 kb of DNA was introduced to E. coli for successful heterologous biosynthetic reconstitution. However, the prospect for production levels that approach those of the native host requires the application of engineering tools associated with E. coli. In this report, metabolic and genomic engineering were implemented to improve the E. coli cellular background and the plasmid platform supporting heterologous erythromycin formation. Results include improved plasmid stability and metabolic support for biosynthetic product formation. Specifically, the new plasmid design for erythromycin formation allowed for ≥89% stability relative to current standards (20% stability). In addition, the new strain (termed LF01) designed to improve carbon flow to the erythromycin biosynthetic pathway provided a 400% improvement in titer level.
Keywords: Erythromycin, antibiotic, E. coli, polyketide, heterologous
Introduction
Erythromycin is an antibiotic built from a complex biosynthetic pathway composed of two portions: polyketide formation and polyketide tailoring1–2. A polyketide intermediate, 6-doxyerythronolide B (6dEB), is produced by three large (~300 kDa each) polyketide synthases that resemble fatty acid synthases both in structure and catalytic mechanism3. 6dEB is then subjected to a series of tailoring reactions that include hydroxylations, methylation, and glycosylations4–5. Thus, the final erythromycin product results from a unique production pathway that offers numerous molecular engineering opportunities6 (Figure 1A).
Figure 1.
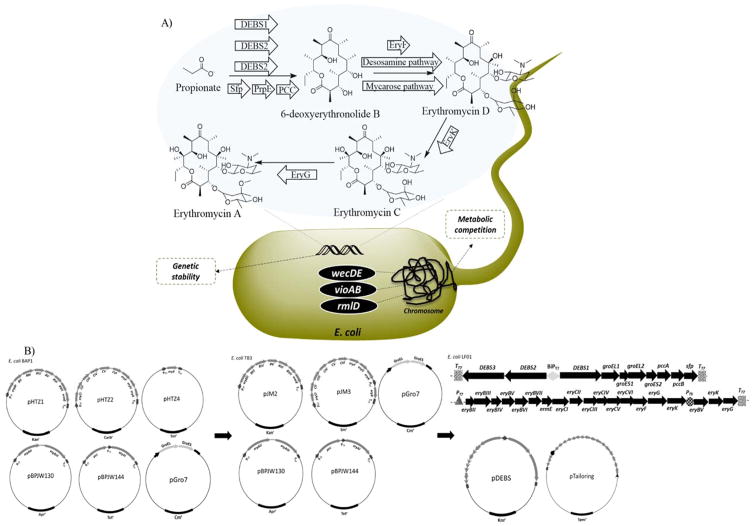
E. coli heterologous erythromycin biosynthesis and plasmid re-design. A) The heterologous erythromycin pathway established in E. coli featuring the DEBS1, 2, and 3 polyketide synthases, Sfp (required for polyketide synthase posttranslational modification), PrpE (a propionyl-CoA synthetase native to E. coli), PCC (a propionyl-CoA carboxylase), EryF (hydroxylase), the desosamine and mycarose deoxysugar pathways, EryK (hydoroxylase), and EryG (methylase). Engineering steps were taken to improve metabolic conversion and genetic stability. B) The left5, middle12, and right (this study) successive plasmid designs to address stability. PT7: T7 promoter; BiPT7: bi-directional T7 promoter; TT7: T7 terminator sequence; PT5: T5 promoter.
However, designed manipulations of erythromycin biosynthesis (and similarly complex natural products) are often complicated by the biological features of the native production hosts. In the case of erythromycin, the original production host is Saccharopolyspora erythraea7. In contrast to the innate features of most native natural product production hosts are the properties of Escherichia coli, with rapid growth kinetics and a wide range of molecular biology tools8–9. To access such properties, heterologous erythromycin biosynthesis was established in E. coli5, 10–11. Though this step confirmed that complex natural product systems could be reconstituted through a significantly different host system, accessing the engineering possibilities of the erythromycin pathway and the E. coli host is still developing.
To this end, one of the primary challenges in establishing heterologous E. coli erythromycin production was efficiently transferring the >20 genes and ~55 kbp of DNA required for biosynthesis. Original efforts relied on revisions to common expression plasmids to accommodate multiple genes per plasmid5. Later upgrades improved and expanded upon these efforts through improved operon design and plasmid consolidation12. However, each of these systems suffered from plasmid instability and the need to carry a minimum of five or six plasmids (Figure 1B).
In the current report, we present a new plasmid design based upon a bacterial artificial chromosome (BAC) means of stabilizing the polyketide and tailoring portions of erythromycin biosynthesis. Comparisons were made between previous plasmid designs regarding plasmid stability and biosynthetic production. In addition, the new BAC plasmids were compared across several E. coli strains metabolically engineered to improve production.
Materials and Methods
Chemicals and Reagents
The reagents and chemicals used in this study were purchased from Fisher Scientific (Pittsburgh, PA) or Sigma (St. Louis, MO). All restriction enzymes were purchased from New England Biolabs (Beverley, MA). The GeneArt™ pYES1L vector was purchased from ThermoFisher Scientific (Waltham, MA). Gotaq Green Master Mix from Promega (Madison, WI) was used for all PCR reactions in this study, and PCR primers were purchased from Eurofins MWF Operon (Huntsville, AL).
Strains and Plasmids
The 6dEB biosynthetic pathway (the genes encoding the deoxyerythronolide B synthase enzymes [DEBS1, 2, 3]), genes encoding the GroES/EL chaperonin, and the pccAB and sfp genes encoding for the enzymes to produce (2S)-methylmalonyl-CoA and to posttranslationally modify the DEBS enzymes, respectively, were codon-optimized via synthesis and placed under a bi-directional T7 promoter and assembled into vector pYES1L by Sapphire technology to generate pDEBS (Figure 1B). The resistance marker for pDEBS was converted from kanamycin to spectinomycin through lambda Red-mediated genetic exchange5, 13. Briefly, the kanamycin resistance gene was PCR-amplified from pKD4 and swapped with the spectinomycin resistance gene on pDEBS using the primers indicated in Table 1. Similar to pDEBS construction, the erythromycin tailoring pathways were consolidated and inserted to pYES1L under T7 and T5 promoter designs to yield pTailoring (Figure 1B).
Table 1.
Oligonucleotide primer and E. coli strain list.
| Name | Oligo Sequence |
|---|---|
| Sugar1 | CGCTCTAGAAGATTATCTCCAATAGCGCTTGGT |
| Sugar2 | GATCACAGATTATCTCCAATAGCGCTTGG |
| cat-MfeI1 | GCGCAATTGGTGTAGGCTGGAGCTGCTTC |
| cat-MfeI2 | GCGCAATTGCATATGAATATCCTCCTTAG |
| vioB Homo1 | ATGGCCTATTTAGATGAAATACAGTT |
| vioB Homo2 | TCACAGATTATCTCCAATAGCGCTTGG |
| wecD&E-NcoI | GCGCCATGGGTGCAGGCCAAAATTGCGGCATCA |
| wecD&E-XhoI | CGCCTCGAGTCAGGAAAAGTAGTTCAACAAAGTCGC |
| kan-BsaI | CGCCACAAGAGACCTGTGTAGGCTGGAGCTGCTTCG |
| kan-EcoRV | CGCGATATCCATTATGAATATCCTCCTTA |
| wecD&E Homo1 | GTGCAGGCCAAAATTGCGGCATCA |
| wecD&E Homo2 | TCAGGAAAAGTAGTTCAACAAAGTCGC |
| rmlA/D-BamHI | CGCGGATCCATGAATATCCTCCTATTCGGCAAAAC |
| rmlA/D-NotI | CGCGCGGCCGCATTAATAACCTTTAATCATTTTGAGCAGATAC |
| Kan-rmlD1 | GCGGTCGACTGTGTAGGCTGGAGCTGCTTCG |
| Kan-rmlD2 | GCGTACGTACATTATGAATATCCTCCTTAG |
| rmlA/D1 | ATGAATATCCTCCTATTCGGCAAAAC |
| rmlA/D2 | ATTAATAACCTTTAATCATTTTGAGCAG |
| kan-spec1 | CCAGCCAGGACAGAAATGCCTCGACTTCGCTGCTACCCAAGGTTGCCGGGTGTGTAGGCTGGAGCTGCTTCG |
| kan-spec2 | TTATTTGCCGACTACCTTGGTGATCTCGCCTTTCACGTAGTGGACAAATTCCCATTATGAATATCCTCCTTAG |
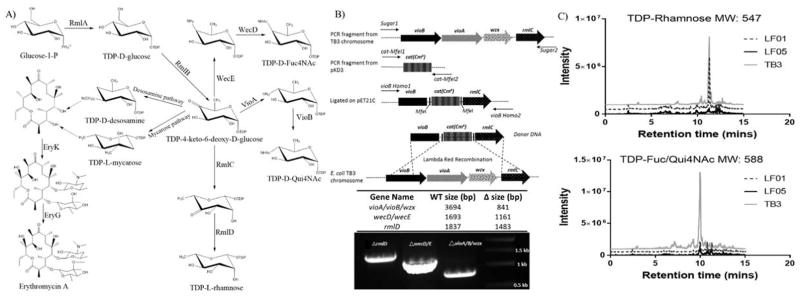
Lambda Red homologous recombination was also utilized to knock out vioA/vioB/wzx (wzx is next to the target vioA/vioB genes and was truncated in the following knockout procedure) and wecD/wecE from strain TB3 to create LF01 (Table 1). To produce donor linear DNA, vioA/vioB and wecD/wecE were first amplified by PCR and cloned into pET21c from the TB3 chromosome, followed by ligating corresponding FRT-flanked antibiotic resistance genes inside the pET21c inserts, yielding pET21c-vioAB-cat (cat resistance gene inserted using MfeI) and pET21c-wecDE-kan (kat resistance gene inserted using NcoI and XhoI). The plasmids were then used as templates to obtain donor DNA with homologous sequences to enable lambda Red-mediated chromosomal deletions as described previously (Figure 2B)13–15. The same method was applied to delete gene rmlD from LF01, which resulted in strain LF05.
Figure 2.
Metabolic pathway engineering to improve erythromycin formation. A) Competing pathways to desosamine and mycarose that were deleted in strains LF01 and LF05. B) Chromosomal gene deletion schematic (vioA/vioB/wzx deletion example) and verification by PCR. PCR bands represent the expected deletion fragments of indicated knockouts. C) Deoxysugar assessment by LC-MS supporting metabolic engineering design.
Cellular Cultures and Assessment
Strains were cultured in 25 mL production medium for six days at 22°C and 250 rpm. When the culture OD600nm reached 0.4~0.6, 250 μM of isopropyl β-D-1-thiogalactopyranoside (IPTG), 2 mg/mL arabinose, and 20 mM sodium propionate were added. Production medium was comprised of the following components (per 1 L): 5g yeast extract, 10 g tryptone, 15 g glycerol, 10 g sodium chloride, and 100 mM 4-(2-hyroxyethyl)-1-piperazineethanesuffonic acid (HEPES) buffer with the final medium adjusted to pH 7.5 using 1M NaOH. The starting OD600nm of each culture was adjusted to 0.1 by diluting from a seed culture (incubated in lysogeny broth [LB] at 37°C and 250 rpm) that had reached an OD600nm of 0.6. Culture samples were taken approximately every 24 hours for optical density measurements. Kanamycin (50 mg/L), chloramphenicol (12.5 mg/L), streptomycin (50 mg/L), apramycin (50 mg/L), spectinomycin (50 mg/L), and tetracycline (5 mg/L) were added, as needed, to maintain plasmids in corresponding cultures.
Plasmid Stability Assay
The erythromycin producing strains were cultured in 25 mL production medium containing appropriate antibiotics at 37°C and 250 rpm until the OD600nm reached 0.6. At this time, the culture temperature was reduced to 22°C and the cells were mixed with 250 μM IPTG, 2 mg/mL arabinose, and 20 mM sodium propionate. Plasmid stability analysis was completed on days 1 and 5 after culture temperatures were reduced to 22°C. At these time points, cells were streaked on LB agar plates and incubated at 37°C overnight to obtain single colonies. Twenty-eight colonies for each plate were selected and transferred to LB plates containing individual and combined antibiotics according to associated expression plasmids as indicated in Table 2. Resulting colony development was recorded, presented as a percentage of transferred colony growth on LB agar containing no antibiotics, and compared between strains.
Table 2.
Plasmid stability assays across strains. Strain 1: TB3 (pBPJW130/pBPJW144/pJM03/pJM02/pGro7); Strain 2: LF01(pDEBS/pTailoring); Strain 3: TB3(pDEBS/pTailoring); Strain 4: LF05(pDEBS/pTailoring). Cm: chloramphenicol; Tet: tetracycline; Apr: apramycin; Sm: streptomycin; Km: kanamycin; Spec: spectinomycin.
| 1ST DAY | Cm | Tet | Apr | Sm | Km | Spec | Combined |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Plasmid | pGro7 | pBPJW144 | pBPJW130 | pJM03 | pJM02/pDEBS | pTailoring | |
| Strain 1 | 100% | 46% | 40% | 73% | 83% | N/A | 27% |
| Strain 2 | N/A | N/A | N/A | N/A | 100% | 100% | 100% |
| Strain 3 | N/A | N/A | N/A | N/A | 100% | 100% | 100% |
| Strain 4 | N/A | N/A | N/A | N/A | 100% | 100% | 100% |
| 5TH Day | Cm | Tet | Apr | Sm | Km | Spec | Combined |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Plasmid | pGro7 | pBPJW144 | pBPJW130 | pJM03 | pJM02/pDEBS | pTailoring | |
| Strain 1 | 100% | 23% | 23% | 67% | 70% | N/A | 20% |
| Strain 2 | N/A | N/A | N/A | N/A | 90% | 90% | 90% |
| Strain 3 | N/A | N/A | N/A | N/A | 89% | 89% | 89% |
| Strain 4 | N/A | N/A | N/A | N/A | 93% | 93% | 93% |
Erythromycin A and TDP-deoxysugar Analysis
For erythromycin A assessment, culture samples (0.75 mL) were extracted twice with an equal volume of ethyl acetate each time. The extract was allowed to dry before being dissolved in 75 μL methanol containing 0.25 μg/mL roxithromycin to serve as an internal standard during subsequent analysis. The erythromycin relative titer represents the ratio of the peak area of erythromycin over the peak area of the internal standard. Experimental samples were analyzed using an AP I3000 Triple Quad LC-MS with a Turbo Ion Spray source (PE Sciex) coupled with a Shimadzu Prominence LC system. Chromatography was performed on a Waters X Terra C18 column (2.1 mm × 250 mm). All MS analyses were conducted in positive ion mode. A linear gradient of 70% buffer A (95% water/5% acetonitrile/0.1% formic acid) to 100% buffer B (5% water/95% acetonitrile/0.1% formic acid) was used at a flow rate of 0.2 mL/min for the LC.
For TDP-deoxysugar analysis, overnight cultures of LF01, LF05, TB3 were inoculated (1% v/v) into 25 mL production medium for culturing at 37°C and 250 rpm for 96 hours. Cells were collected by centrifugation, stored at −20°C overnight, resuspended in 2 mL phosphate-buffered saline (PBS), and sonicated for 2 minutes (Fisher Scientific Model FB50 at an amplitude of 35). Cell lysates were centrifuge at 4000 rpm (ThermoFisher Scientific Sorvall Primo Benchtop centrifuge) for 10 minutes, and the supernatant was analyzed by LC-MS (AP I3000 Triple Quad LC-M S with a Turbo Ion Spray source [PE Sciex] coupled with a Shimadzu Prominence LC system). Chromatography was performed on a Waters Xbridge amide 3.5 μm (2.1 mm × 150 mm) column. All MS analyses were conducted in negative ion mode. A linear gradient of 100% buffer B (10% water/90% acetonitrile/5mM ammonium acetate) to 100% buffer A (100% water and 5 mM ammonium acetate [pH 9]) was used at a flow rate of 0.2 mL/min for the LC.
Results and Discussion
Heterologous Erythromycin Plasmid Design and Stability
A significant challenge in establishing heterologous production of erythromycin in E. coli was the transfer of the prerequisite genetic pathway. Comprised of >20 genes and spanning ~50 kbp of DNA, the pathway posed several issues to conventional E. coli expression plasmids which were generally designed for single genes16–21. Further complicating the reconstitution process was the significant size (~10 kb each) of the polyketide synthase genes17–18.
The initial approach to deal with the transfer of a complex modular polyketide system such as erythromycin was to re-build existing expression plasmids (from the pET family) with constructed operons containing the erythromycin pathway genes22. As opposed to localizing all of the pathway to one expression plasmid, several pET vectors were utilized in part because of the difficulties in cloning >20 kbp into individual plasmids. There were also concerns about the stability of such plasmids (both in terms of culture maintenance and unwanted recombination events) with large inserts. As a result, the original system to enable complete erythromycin production from E. coli required six different expression plasmids which was later refined to five plasmids12. As expected, due to a combination of incompatible origins of replication and insert size, plasmid stability was an issue5, 12.
In the current study, the biosynthetic process was divided between polyketide formation and downstream tailoring (Figure 1). In addition, a systematic plasmid construction was undertaken to address issues of instability and enhance eventual gene expression. Namely, genetic content was synthesized to optimize codon usage and incorporate all needed genes into two bacterial artificial chromosome (BAC) plasmids. The BAC plasmids utilize the F plasmid sop system to maintain plasmid number to one copy per cell, which aids the prospect for proper plasmid segregation during replication and subsequent plasmid stability over time (in contrast, the original pET plasmids utilized for initial production rely upon the pBR322 origin of replication and are expected to reside at ~40 copies per cell)23. Furthermore, the separation of polyketide formation and downstream tailoring to two separate plasmids enables future efforts to systematically manipulate either aspect of biosynthesis for the purpose of generating erythromycin analogs4, 24–25.
Utilizing this new plasmid design resulted in substantially improved plasmid stability (Table 2). The stability afforded is attributed to reduced copy number since the BAC plasmids possess the same origin of replication, which could complicate plasmid stability over extended timeframes. As such, future directions include the localization of the entire erythromycin pathway to one BAC plasmid.
Metabolic Engineering to Improve Downstream Tailoring
Metabolic engineering was applied to strain TB3 (Table 1). Specifically, genes identified from pathways capable of siphoning carbon from desoxysugar biosynthesis were sequentially deleted. In so doing, the objective was to improve glycosylation of the erythromycin product and subsequent final titers. Figure 2 shows the pathways where deletions were made including the knockouts of vioA/vioB/wzx and wecD/wecE to generate LF01 and the additional rmlD deletion to generate LF05. Supporting the engineering steps taken is a profile of specific sugar content within the TB3, LF01, and LF05 strains (Figure 2C).
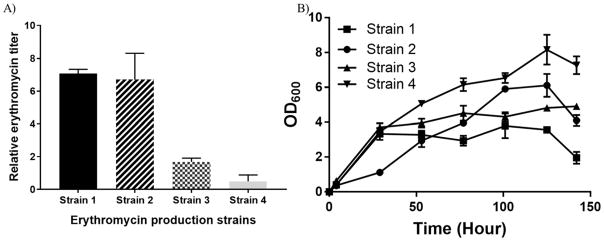
Erythromycin production comparison at 22°C is presented in Figure 3. Improved production of LF01 (strain #2 in Figure 3A) compared to TB3 (strain #3 in Figure 3A) when using the new BAC plasmids indicates a successful engineering alteration, especially when biomass levels are comparable (Figure 3B). Interestingly, when the additional rmlD deletion is included in LF05 (strain #4 in Figure 3A), production levels drop below those for strain LF01.
Figure 3.
Erythromycin production across different strains. Relative erythromycin titer (A; measure at final time point) and optical density at 600 nm (OD600; B) comparison. Strain 1: TB3 (pBPJW130/pBPJW144/pJM03/pJM02/pGro7); Strain 2: LF01(pDEBS/pTailoring); Strain 3: TB3(pDEBS/pTailoring); Strain 4: LF05(pDEBS/pTailoring). Error bars represent standard deviation values from three independent experiments.
The original production strain (TB3/pBPJW130/pBPJW144/pJM03/pJM02/pGro7; strain #1 in Figure 3A) is included as a reference and shows nearly identical production levels to LF01(pDEBS/pTailoring). However, it is difficult to compare these systems for several reasons. First, plasmid stability, as indicated in Table 2, and copy level are substantially different. The comparable erythromycin production from LF01(pDEBS/pTailoring) is impressive when considering that plasmid levels of pDEBS/pTailoring will be substantially reduced; however, this is countered by the fact that the reference TB3/pBPJW130/pBPJW144/pJM03/pJM02/pGro7 strain demonstrates reduced plasmid stability. Hence, only a fraction of cells within the reference culture will be producing erythromycin; whereas, nearly all the cells of LF01(pDEBS/pTailoring) will be generating erythromycin. Using this same rationale in comparing production from strains 1 and 3 in Figure 3A, even with the improved plasmid stability of TB3(pDEBS/pTailoring), production is well below titers from the plasmid-unstable TB3/pBPJW130/pBPJW144/pJM03/pJM02/pGro7 system. However, likely offsetting plasmid instability of reference stain 1 is the heightened level of plasmid copy and associated enzyme number in the cells generating erythromycin, which offers an explanation for the production differences observed.
By observing static metabolic maps (Figure 2A), it is unclear why the additional deletion of rmlD within LF05 did not further improve or at least maintain production levels relative to strain LF01. However, the LF05 strain consistently produced the lowest levels of erythromycin. A possible explanation relates to the Figure 2A map and the genomic localization of rmlD in close proximity to rmlA. Hence, the rmlD deletion may have had a polarity effect on rmlA, which as indicated in Figure 2A, encodes for the required deoxysugar initiation step in erythromycin glycosylation.
Conclusions
In summary, we report upon engineering steps to address deficiencies of the current E. coli erythromycin heterologous production system. Through BAC plasmid pathway construction, plasmid stability has been improved to ~90% retention. Similarly, metabolic engineering improved deoxysugar conversion of the final erythromycin product. These and related engineering steps readily available to E. coli will continue to be applied with the ultimate goal of matching erythromycin productivity to that of current industrial systems (reliant on advanced S. erythraea strains) with the additional goals of directing the engineering platform at erythromycin analog production and altogether different complex natural product discovery and production projects.
Acknowledgments
Financial support is recognized from awards AT008756 (NIH), AI126367 (NIH), and CHE-1410075 (NSF).
References
- 1.Haight TH, Finland M. The antibacterial action of erythromycin. Proc Soc Exp Biol Med. 1952;81(1):175–83. doi: 10.3181/00379727-81-19815. [DOI] [PubMed] [Google Scholar]
- 2.Kibwage IO, Hoogmartens J, Roets E, Vanderhaeghe H, Verbist L, Dubost M, Pascal C, Petitjean P, Levol G. Antibacterial activities of erythromycins A, B, C, and D and some of their derivatives. Antimicrob Agents Chemother. 1985;28(5):630–3. doi: 10.1128/aac.28.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carreras C, Pieper R, Khosla C. The Chemistry and Biology of Fatty Acid, Polyketide, and Nonribosomal Peptide Biosynthesis. Vol. 188. Springer-Verlag; Heidelberg: 1997. pp. 86–126. [Google Scholar]
- 4.Zhang G, Li Y, Fang L, Pfeifer BA. Tailoring pathway modularity in the biosynthesis of erythromycin analogs heterologously engineered in E. coli. Sci Adv. 2015;1(4):e1500077. doi: 10.1126/sciadv.1500077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Wang Y, Wu J, Skalina K, Pfeifer BA. Complete biosynthesis of erythromycin A and designed analogs using E. coli as a heterologous host. Chem Biol. 2010;17(11):1232–40. doi: 10.1016/j.chembiol.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Cane DE. Programming of erythromycin biosynthesis by a modular polyketide synthase. J Biol Chem. 2010;285(36):27517–23. doi: 10.1074/jbc.R110.144618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minas W. Production of erythromycin with Saccharopolyspora erythraea. Humana Press; Totowa, NJ: 2004. pp. 65–89. [Google Scholar]
- 8.Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 1999;10(5):411–21. doi: 10.1016/s0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Boghigian BA, Armando J, Pfeifer BA. Methods and options for the heterologous production of complex natural products. Nat Prod Rep. 2011;28(1):125–51. doi: 10.1039/c0np00037j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiru S, Menzella HG, Rodriguez E, Carney J, Gramajo H. Production of the potent antibacterial polyketide erythromycin C in Escherichia coli. Appl Environ Microbiol. 2005;71(5):2539–47. doi: 10.1128/AEM.71.5.2539-2547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HY, Khosla C. Bioassay-guided evolution of glycosylated macrolide antibiotics in Escherichia coli. PLoS Biol. 2007;5(2):e45. doi: 10.1371/journal.pbio.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang M, Fang L, Pfeifer BA. Improved heterologous erythromycin a production through expression plasmid re-design. Biotechnol Prog. 2013;29(4):862–9. doi: 10.1002/btpr.1759. [DOI] [PubMed] [Google Scholar]
- 13.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97(12):6640–5. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Boghigian BA, Pfeifer BA. Investigating the role of native propionyl-CoA and methylmalonyl-CoA metabolism on heterologous polyketide production in Escherichia coli. Biotechnol Bioeng. 2010;105(3):567–73. doi: 10.1002/bit.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Fang L, Osburne MS, Pfeifer BA. The continuing development of E. coli as a heterologous host for complex natural product biosynthesis. Methods Mol Biol. 2016;1401:121–34. doi: 10.1007/978-1-4939-3375-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mierendorf RC, Morris BB, Hammer B, Novy RE. Expression and purification of recombinant proteins using the pET system. Methods Mol Med. 1998;13:257–92. doi: 10.1385/0-89603-485-2:257. [DOI] [PubMed] [Google Scholar]
- 17.Cortes J, Haydock SF, Roberts GA, Bevitt DJ, Leadlay PF. An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. Nature. 1990;348(6297):176–8. doi: 10.1038/348176a0. [DOI] [PubMed] [Google Scholar]
- 18.Donadio S, Staver MJ, McAlpine JB, Swanson SJ, Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991;252(5006):675–9. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- 19.Reeves AR, English RS, Lampel JS, Post DA, Vanden Boom TJ. Transcriptional organization of the erythromycin biosynthetic gene cluster of Saccharopolyspora erythraea. J Bacteriol. 1999;181(22):7098–106. doi: 10.1128/jb.181.22.7098-7106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summers RG, Donadio S, Staver MJ, Wendt-Pienkowski E, Hutchinson CR, Katz L. Sequencing and mutagenesis of genes from the erythromycin biosynthetic gene cluster of Saccharopolyspora erythraea that are involved in L-mycarose and D-desosamine production. Microbiology. 1997;143(Pt 10):3251–62. doi: 10.1099/00221287-143-10-3251. [DOI] [PubMed] [Google Scholar]
- 21.Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189(1):113–30. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 22.Pfeifer BA, Admiraal SJ, Gramajo H, Cane DE, Khosla C. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science. 2001;291(5509):1790–2. doi: 10.1126/science.1058092. [DOI] [PubMed] [Google Scholar]
- 23.Hiraga S. Chromosome and plasmid partition in Escherichia coli. Annu Rev Biochem. 1992;61:283–306. doi: 10.1146/annurev.bi.61.070192.001435. [DOI] [PubMed] [Google Scholar]
- 24.Jiang M, Pfeifer BA. Metabolic and pathway engineering to influence native and altered erythromycin production through E. coli. Metab Eng. 2013;19C:42–49. doi: 10.1016/j.ymben.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Jiang M, Zhang H, Park SH, Li Y, Pfeifer BA. Deoxysugar pathway interchange for erythromycin analogues heterologously produced through Escherichia coli. Metab Eng. 2013;20:92–100. doi: 10.1016/j.ymben.2013.09.005. [DOI] [PubMed] [Google Scholar]