Abstract
Background
Poor enrollment of adolescents and young adults (AYAs, 15–39 years) onto cancer clinical trials (CCT) may contribute to inferior survival gains compared with children. This study assessed whether differences in CCT availability explain lower CCT enrollment for early AYAs (eAYAs, 15–21 years).
Methods
This prospective observational cohort study was conducted at a single academic children’s hospital. For consecutive patients newly-diagnosed with cancer over a 13 month period, it was determined whether an appropriate CCT existed nationally, was available locally, and whether enrollment on that CCT occurred. The proportions of eAYAs versus children in each category were compared using the χ2 test. The impact of age and other factors on enrollment status was assessed using logistic regression analysis.
Results
Among 216 patients, 58 were eAYAs and 158 were children. There was no difference in the proportion of eAYAs versus children who had an existing (28/58 [48.3%] versus 85/158 [53.8%]; p=0.47) or available (23/58 [39.7%] versus 75/158 [47.5%]; p=0.31) CCT. However, significantly fewer eAYAs were enrolled when a CCT was available (7/23 [30.4%] versus 50/75 [67.7%]; p=0.002). In multivariable analysis, eAYAs were significantly less likely than children to be enrolled in an available CCT (adjusted odds ratio 0.22, 95% confidence interval 0.08, 0.62).
Conclusions
Equal proportions of children and eAYAs had CCTs available but significantly fewer eAYAs were enrolled. This suggests that for eAYAs, factors other than CCT availability are important enrollment barriers and should be addressed.
Keywords: Adolescent, Clinical Oncology, Clinical Trial as Topic, Young Adult
INTRODUCTION
Cancer remains the leading cause of disease-related death in the United States (US) for adolescents and young adults (AYAs, 15–39 years old). Despite impressive survival gains achieved in both younger and older populations over the past thirty years, AYAs have not experienced the same improvements.1 In 2006, the US National Cancer Institute (NCI) identified AYAs as a health disparity population.2
The lagging survival improvement of AYAs is multifactorial.3–7 One fundamentally important contributor is the low participation of AYAs in NCI-sponsored cancer clinical trials (CCT). Numerous studies of CCT enrollment indicate that 40–60% of children < 15 years of age participate in CCTs compared with only 10–20% of early AYAs (eAYAs, 15–21 years old).1, 8–12 For AYAs older than 21 years, the proportion participating is even lower.10 This non-participation has serious consequences. Low CCT enrollment of AYAs has been directly correlated with significantly lower annual survival improvement compared with children.12 Non-enrollment onto CCT also prevents AYAs from gaining access to promising investigational therapies, providing biospecimens essential for basic and translational research, and benefiting from studies of supportive care, quality of life, cancer epidemiology, and other non-survival endpoints.13
Reasons for lower CCT enrollment of AYAs are not well understood. Studies suggest that AYA enrollment barriers include suboptimal insurance, low socioeconomic status, distance to the cancer center, older age, type of cancer specialist, and treatment by community-based providers who do not participate in NCI-sponsored CCT.9, 14, 15 Limited availability of CCTs for AYAs is another potential factor often asserted to be of major importance.9, 11, 16, 17 However, published data regarding CCT availability are scant and do not necessarily support this contention.18 Availability of CCTs is a crucial consideration because other factors that could influence enrollment, such as patient/family- and provider-level issues, presuppose availability of a CCT and are moot without one.13 Yet assessment of CCT availability is challenging because it is difficult for retrospective studies to ascertain eligibility of individual patients for CCTs that commonly open and close to enrollment over time. Also, it is difficult for population-based studies to determine whether CCTs that existed nationally were actually open at specific treatment sites. To overcome these challenges, we undertook this prospective study of consecutive eAYAs and children newly-diagnosed with cancer to evaluate potential age-related differences in CCT availability and enrollment. The primary study aim was to compare the proportions of eAYAs and children for whom an appropriate CCT existed, was available locally, and utilized for enrollment; secondary aims were to evaluate the effects of age and other factors upon CCT enrollment. Our overall hypothesis was that both availability of and enrollment onto CCTs would be significantly lower among eAYAs than children.
METHODS
Study Design, Setting and Case Ascertainment
This was a prospective observational cohort study conducted at Children’s Hospital Los Angeles (CHLA), an academic center located 6 miles from our affiliated adult-focused cancer hospital. CHLA provides specialized cancer care from birth through young adulthood, typically 21 years of age for newly-diagnosed patients.
To confirm the feasibility of our methods, we first conducted an informal pilot study of ten children and eAYAs who were not included in this cohort. For this current study, all consecutive pathology reports from patients aged 0–21 years were screened in real-time by collaborating pediatric pathology fellows (HT, JS) and transmitted to the principal investigator (SMT) with name, medical record number, and final pathology diagnosis. Pathology reports were reviewed by SMT and disregarded if they represented pathology-only consultations for patients not receiving cancer care at CHLA, or pathology specimens from second surgeries of patients already included in this study. The remaining pathology reports were used to identify unique patients 0–21 years old with first diagnosis of cancer and cancer treatment initiated at CHLA. Exclusion criteria were relapsed cancer, subsequent malignant neoplasm (SMN), or transfers to CHLA having already starting cancer treatment elsewhere.
For each patient meeting eligibility criteria for this study, pertinent demographic (age, sex, and race/ethnicity) and disease-related (cancer diagnosis, stage, grade, risk group and relevant genomics) information was abstracted from the medical record. For analytic purposes, cancer type was grouped as leukemia/lymphoma and solid tumor. Patients were classified as children or eAYAs by age 0–14 or 15–21 years, respectively. This study was approved by the CHLA Institutional Review Board (IRB) with a waiver for informed consent, as only anonymous data were collected and there was no patient contact.
Determination of CCT Existence, Availability, and Enrollment Status
For purposes of this study, “clinical trial existence” was operationalized as a CCT appropriate for the patient’s age, diagnosis, and stage/risk-group registered nationally on ClinicalTrials.gov and listed as open and recruiting. “Clinical trial availability” was operationalized as an existing CCT that was IRB-approved, activated and open to enrollment at CHLA at the time of that patient’s diagnosis. “Clinical trial enrollment” was operationalized as the patient being successfully entered onto the CCT according to standard procedures of the Clinical Trials Office for the CHLA Children’s Center for Cancer and Blood Diseases. In regard to consenting procedures for CCTs, the CHLA IRB requires written informed assent and parental permission for cognitively intact patients aged 7–17 years, and written informed consent for patients 18 years and older. Regardless of patient age, permission for CCT participation is obtained in accordance with these requirements by the disease-specific clinical team.
For each patient, existence of a CCT was determined by SMT within two weeks of receiving the diagnosis by searching ClinicalTrials.gov for a relevant CCT. The two-week timeframe was established because trial sponsors are required to post updates on ClinicalTrials.gov within four weeks of changes in enrolling status. The diagnosis was entered into the search term area with studies limited to those “open and recruiting,” “interventional,” and available in the US. If a trial was so identified, patient-specific clinical information (e.g., histology, stage, grade, risk group, genomic status, and other characteristics) was employed to determine the patient’s specific eligibility for that trial. Those confirmed as eligible were thus classified as having an existing CCT. Trial-specific data available on ClinicalTrials.gov were recorded including the National Clinical Trial identification number, phase, categorical type (e.g., National Clinical Trials Network [NCTN], other national collaborative group, multi-center collaboration, industry, or institutional), the specific sponsor (e.g., Children’s Oncology Group [COG]), and the date the trial opened. Patients with an existing CCT were then assessed for availability of, and enrollment upon, the CCT as described above.
For patients who had more than one applicable trial listed on ClinicalTrials.gov but none available at CHLA, only one trial was recorded as existing using the following hierarchy: NCTN, other national collaborative group, multi-center collaboration, industry-sponsored, or institutional.
Statistical Analysis
Descriptive statistics were used to characterize the sample. Differences in demographics, CCT existence, CCT availability, and CCT enrollment among eAYAs versus children were evaluated using the χ2 test of proportions. The effects of demographics and disease characteristics on CCT enrollment were analyzed using logistic regression. Predictor variables consisting of age, sex, race/ethnicity, ethnicity, and cancer type were evaluated in univariable models. We tested for two-way interactions between all predictors with none discovered. Two multivariable models were assessed, one including ethnicity and the other including race/ethnicity, with both models including age, sex and cancer type because of their clinical relevance. The model selected for reporting adjusted for ethnicity because its effect was stronger than race/ethnicity in univariable analysis. All analyses were performed using 2-sided tests with a p-value of < 0.05 defined as significant. Stata statistical software was used for all analyses.19
RESULTS
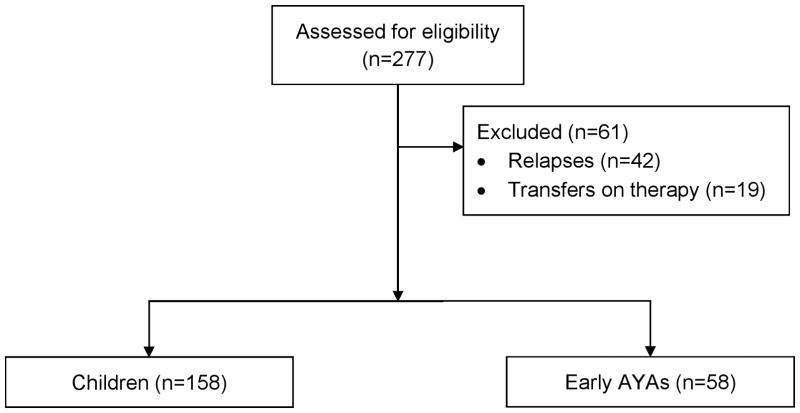
Between November 1, 2015 and December 1, 2016, a total of 277 unique patients were identified and screened for eligibility (Figure 1). Of these, 61 were excluded for having relapsed disease (n=42; 24 children, 18 eAYAs), or already starting cancer treatment prior to transfer to CHLA (n=19; 13 children, 6 eAYAs). There were no patients with SMN.
Figure 1.
CONSORT Diagram. Children = < 15 years old; early AYA = 15–21 years old.
Patient Characteristics
A total of 216 consecutive patients, 158 children and 58 eAYAs, constituted the analytic sample (Table 1). The median ages of the childhood and eAYA groups were 6 years (range, 0–14) and 17 years (range, 15–20), respectively. Other demographic characteristics were similar except there was a higher proportion of males in the eAYA group. In both groups, more than half the patients were Hispanic, consistent with the population served by CHLA. A significantly higher proportion of diagnoses were leukemia and brain tumors among children, whereas lymphoma and non-brain solid tumors were more common among eAYAs.
Table 1.
Patient Characteristics1
| Total (%) | Children (%) | Early AYA (%) | p | |
|---|---|---|---|---|
| Total | 216 | 158 (73.1) | 58 (26.9) | -- |
| Age (years) | ||||
| Median | 10 | 6 | 17 | -- |
| Range | 0–20 | 0–14 | 15–20 | |
| Sex | ||||
| Male | 111 (51.4) | 73 (46.2) | 38 (65.5) | 0.012 |
| Female | 105 (48.6) | 85 (53.8) | 20 (34.5) | |
| Race/Ethnicity | ||||
| Hispanic | 120 (55.6) | 84 (53.2) | 36 (62.1) | 0.53 |
| White/Non-Hispanic | 50 (23.1) | 41 (25.9) | 9 (15.5) | |
| Asian | 13 (7.4) | 10 (6.3) | 3 (5.2) | |
| Black | 12 (5.6) | 9 (5.7) | 3 (5.2) | |
| Other | 21 (9/7) | 14 (8.9) | 7 (12.1) | |
| Diagnosis | ||||
| Leukemia/lymphoma | 114 (52.8) | 81 (51.3) | 33 (56.9) | 0.0015 |
| Leukemia | 85 (39.4) | 68 (43.0) | 17 (29.3) | |
| Lymphoma | 29 (13.4) | 13 (8.2) | 16 (27.6) | |
| Solid Tumor | 102 (47.2) | 77 (48.7) | 25 (43.1) | |
| Brain Tumor | 25 (11.6) | 21 (13.3) | 4 (6.9) | |
| Non-brain Solid Tumor | 77 (35.6) | 56 (35.4) | 21 (36.2) |
Children = < 15 years old; early AYA = 15–21 years old.
CCT Existence, Availability, and Enrollment Proportions
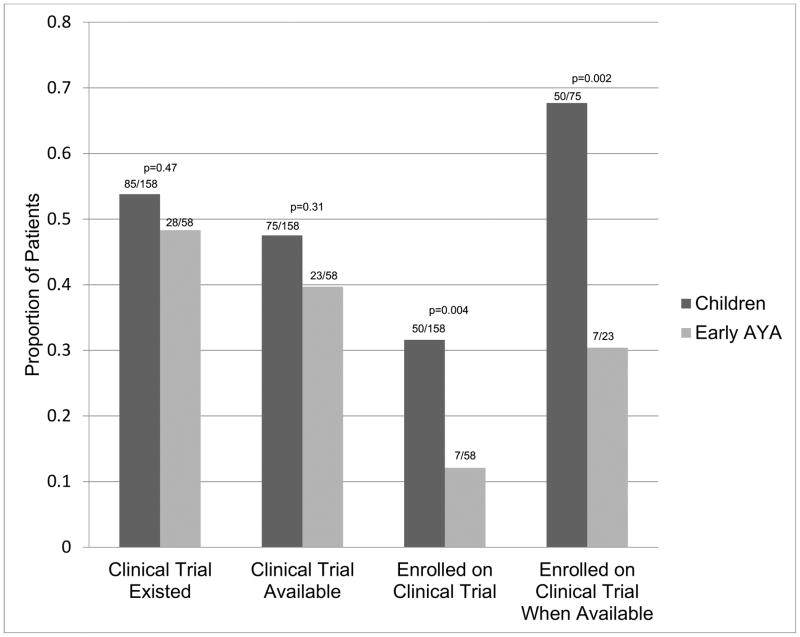
The proportions of CCT existence, availability, and enrollment were compared for eAYAs and children (Figure 2). The proportions of eAYAs and children were similar for both having an existing (28/58 [48.3%] versus 85/158 [53.8%], p=0.47) and having an available (23/58 [39.7%] versus 75/158 [47.5%], p=0.31) CCT. However, a significantly lower proportion of eAYAs than children was enrolled onto CCT. Among those with an existing CCT, only 7/58 (12.1%) eAYAs enrolled versus 50/158 (31.6%) children (p=0.004). Among patients who had an available CCT, 7/23 (30.4%) eAYAs versus 50/75 (67.7%) children were enrolled (p=0.002). In seeking to localize the age where enrollment declines, we found the enrollment proportions among both children < 10 years old and children 10–14 years old with available CCT were significantly higher than eAYAs (34/49 [69.4%], p=0.002; and 16/26 [61.5%], p=0.030, respectively). Additionally, we compared CCT enrollment between younger eAYAs who gave informed assent (15–17 years old; n=45) and older eAYAs who gave informed consent (18 years and older; n=13); among those with an available CCT, the proportions enrolled were 4/16 (25.0%) and 3/7 (42.8%), respectively (p=0.39).
Figure 2.
Cancer Clinical Trial Existence, Availability, and Enrollment by Age Group. Children = < 15 years (n=158); early AYA = 15–21 years old (n=58). See Methods for operational definitions of cancer clinical trial existence, availability and enrollment.
As shown in Supplemental Table 1, for both eAYAs and children a diagnosis of leukemia had the highest proportion of existing and available CCTs, whereas a diagnosis of non-brain solid tumors had the lowest. However, within diagnoses there were no compelling age-related differences in CCT existence or availability. For our sample, most diagnoses without an available CCT occurred in both children and eAYAs. These included astrocytoma, Ewing sarcoma (localized), germ cell tumors, Hodgkin lymphoma (low stage), melanoma, mixed phenotypic acute leukemia, neuroblastoma, non-Hodgkin lymphoma, osteosarcoma, rhabdomyosarcoma, and thyroid carcinoma. Among children, diagnoses where CCTs were not available included chondrosarcoma, chordoma, hepatoblastoma, glioblastoma multiforme, infantile fibrosarcoma, inflammatory myofibroblastic tumor, Langerhans cell histiocytosis, rhabdoid tumor, renal cell carcinoma, retinoblastoma, solid pseudopapillary tumor of the pancreas, synovial sarcoma, transitional liver cell tumor, and undifferentiated sarcoma. There were no diagnoses encountered only among eAYAs where no CCT was available.
Predictors of CCT Enrollment
Logistic regression analysis was used to evaluate predictors for both overall CCT enrollment (i.e., among all patients included in the study [n=216]), as well as for CCT enrollment when the existing trial was also available at CHLA (n=98). For overall CCT enrollment (Table 2), univariable analysis found there was a significantly lower likelihood (OR [95%CI]) for eAYAs (0.30 [0.13, 0.70], p= 0.002) and patients diagnosed with solid tumors (0.09 [0.04, 0.22], p= <0.001) to be enrolled, whereas there was a significantly greater likelihood for Hispanic patients to be enrolled compared with non-Hispanic patients (2.09 [1.10, 3.95], p= 0.021). In multivariable analysis adjusted for sex, ethnicity, cancer type and age, overall CCT enrollment was significantly less likely to occur for both eAYAs (0.22 [0.09, 0.58], p= 0.001) and those with solid tumors (0.07 [0.03, 0.18], p= <0.001). In contrast, among those for whom existing CCTs were available at CHLA (Table 3), the only factor associated with CCT enrollment was age, where eAYAs were significantly less likely to be enrolled in both univariable (0.22 [0.08, 0.60], p= 0.002) and multivariable (0.21 [0.07, 0.61], p= 0.003) analysis. Predictors of enrollment within the eAYA group were unable to be assessed due to small numbers.
Table 2.
Predictors of Enrollment onto Cancer Clinical Trials (n = 216)
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Predictor | Levels | OR (95% CI) | p | OR (95% CI) | p‡ |
| Age group1 | Children (ref) | 1 | 0.002 | 1 | 0.001 |
| Early AYA | 0.30 (0.13, 0.70) | 0.23 (0.09, 0.58) | |||
| Sex | Female (ref) | 1 | 0.18 | 1 | 0.090 |
| Male | 0.66 (0.36, 1.22) | 0.54 (0.26, 1.11) | |||
| Race/Ethnicity | White/Non-Hispanic (ref) | 1 | 0.067 | Not included | |
| Hispanic | 1.93 (0.87, 4.25) | ||||
| Others | 0.84 (0.30, 2.36) | ||||
| Ethnicity | Non-Hispanic (ref) | 1 | 0.021 | 1 | 0.085 |
| Hispanic | 2.09 (1.10, 3.95) | 1.89 (0.91 3.92) | |||
| Disease category | Leukemia/lymphoma (ref) | 1 | <0.001 | 1 | <0.001 |
| Solid tumors | 0.09 (0.04, 0.22) | 0.08 (0.03, 0.19) | |||
Children = < 15 years; early AYA = 15–21 years old.
Table 3.
Predictors of Enrollment onto Cancer Clinical Trials when Available (n = 98)
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Predictor | Levels | OR (95% CI) | p | OR (95% CI) | p |
| Age group1 | Children (ref) | 1 | 0.002 | 1 | 0.003 |
| Early AYA | 0.22 (0.08, 0.60) | 0.22 (0.08, 0.62) | |||
| Sex | Female (ref) | 1 | 0.15 | 1 | 0.22 |
| Male | 0.55 (0.25, 1.25) | 0.58 (0.24, 1.40) | |||
| Race/Ethnicity | White/Non-Hispanic (ref) | 1 | 0.10 | Not included | |
| Hispanic | 1.30 (0.43, 3.91) | ||||
| Others | 0.43 (0.12, 1.59) | ||||
| Ethnicity | Non-Hispanic (ref) | 1 | 0.085 | 1 | 0.057 |
| Hispanic | 2.06 (0.90, 4.73) | 2.37 (0.96, 5.81) | |||
| Disease Category | Leukemia/lymphoma (ref) | 1 | 0.69 | 1 | 0.64 |
| Solid tumors | 1.30 (0.35, 4.75) | 1.39 (0.35, 5.52) | |||
Children = < 15 years; early AYA = 15–21 years old.
CCT Characteristics
The characteristics of the CCTs that existed, were available, and were utilized for enrollment are summarized in Table 4 and Supplemental Table 1. Twenty-six unique trials existed for the patients in this study, 20 for children and 12 for eAYAs; six spanned both age groups (leukemia=4, lymphoma=1, and solid tumor=1). Of these, 13 were available locally compared with 7 for eAYAs. Target cancers addressed by these trials were similar across both age groups except that children had more brain tumor studies available. Phase 3 trials accounted for the vast majority of available trials in both age groups. For both age groups, most trials were sponsored by the NCTN; all of the available Phase 3 trials were sponsored by COG. Among children, all five of the existing single-institution studies were sponsored by a single pediatric cancer research hospital, whereas among AYAs both existing single institution studies were from adult-focused cancer research centers. Within phases and types of CCT, the distribution of patients by age was not appreciably different (Supplemental Table 1).
Table 4.
Number and Types of Cancer Clinical Trials (n=26)
| Cancer Clinical Trials Among Children1 | Cancer Clinical Trials Among Early AYAs | |||||
|---|---|---|---|---|---|---|
| Existed2 | Available2 | Utilized for Enrollment2 | Existed | Available | Utilized for Enrollment | |
| Total | 20 | 13 | 9 | 12 | 7 | 4 |
| Target Cancer | ||||||
| Leukemia | 6 | 5 | 3 | 4 | 4 | 2 |
| Lymphoma | 4 | 2 | 2 | 5 | 1 | 1 |
| Non-brain Solid Tumor | 4 | 2 | 1 | 2 | 1 | -- |
| Brain Tumor | 6 | 4 | 3 | 1 | 1 | 1 |
| Phase | ||||||
| Phase 1/2 | 1 | -- | -- | -- | -- | -- |
| Phase 2 | 5 | 2 | 2 | 5 | 0 | -- |
| Phase 2/3 | 1 | -- | -- | -- | -- | -- |
| Phase 3 | 12 | 10 | 6 | 7 | 7 | 4 |
| Phase 4 | 1 | 1 | 1 | -- | -- | -- |
| Sponsor | ||||||
| NCTN | 12 | 12 | 8 | 9 | 7 | 4 |
| Multi-site | 3 | 1 | 1 | 1 | -- | -- |
| Single Institution | 5 | -- | -- | 2 | -- | -- |
Children = age < 15 years; early AYA = 15–21 years old.
See Methods for operational definitions of cancer clinical trial existence, availability and enrollment.
DISCUSSION
The overall objective of this study was to evaluate the relative importance of trial availability as a cause of under-enrollment of eAYAs onto CCTs. Consistent with our hypothesis, we found that a significantly lower proportion of eAYAs than children was enrolled onto CCTs that existed nationally. However, contrary to our hypothesis, we found that there was no significant difference in CCT availability among children and eAYAs. Importantly, we found that among patients who had a CCT available, a significantly lower proportion of eAYAs was enrolled. To our knowledge, this is the first study to evaluate potential differences in CCT availability and their impact on CCT enrollment for eAYAs versus children. Our principal finding of lower eAYA enrollment in, but equivalent availability of, CCTs is consequential because it indicates that factors other than CCT availability serve as barriers to eAYA enrollment. This study addresses a clinically important issue because low CCT enrollment of AYAs has been correlated with poorer cancer survival improvement.12
To date, explaining low AYA participation in CCTs has been the subject of considerable speculation but limited research. In this study, we focused on potential differences in CCT availability. Several authors have argued that there are an insufficient number and variety of CCTs available for the cancers that are most common among AYAs, and that opening more trials should be a high priority.9, 16, 17 This is a crucial question because many factors that influence enrollment become relevant only when a CCT is available. However, in this study we found there was no difference in the proportion of eAYAs and children for whom a CCT either existed at the national level or was available within our institution. On the other hand, the proportion of those eAYAs actually enrolled onto available CCTs was less than half that of children. In adjusted multivariable analysis, eAYAs were nearly 80% less likely to be enrolled. Few literature reports exist to provide a context for these findings. A recent children’s hospital-based retrospective study reported that 62% of eAYAs versus 47% of children lacked an available CCT, which had not improved from a previous study.11,17 In a large, retrospective, case-linked study of CCT enrollment performed in our cancer center that documented markedly lower CCT enrollment of AYAs than children, we found that, for the top 10 diagnoses in each age group, AYAs had 8 available CCTs compared with 10 for children.10 In contrast to these two studies, a SEER-based study of AYA CCT enrollment by the NCI estimated that actual enrollment exceeded expected enrollment for several cancer types.18 All three of these studies reflect the difficulty of collecting robust CCT enrollment data retrospectively. To our knowledge, no published study prior to this has used prospective, case-linked methodology to assess the impact of CCT availability on AYA enrollment. This approach offers substantial benefits. For example, we discerned that although fewer CCTs did, in fact, exist for eAYAs than children (12 versus 20), the proportions of patients who had CCTs available were equivalent. For discussions of CCT availability, this illustrates the importance of accounting for site and patient characteristics in addition to the absolute number of existing trials.
The setting and design of our study afforded important strengths with some limitations. A significant strength was prospective case ascertainment combined with real-time, patient-specific evaluation of CCT existence and availability. This provided a level of accuracy that is difficult to achieve retrospectively. The volume and heterogeneity of patients at CHLA made it feasible to conduct this study over a relatively short period at a single institution, yet the sample was not large enough to assess definitively all covariates of interest. For example, Hispanic ethnicity was suggestive of facilitating CCT enrollment in our adjusted multivariable analysis, a finding consistent with our previous retrospective study,10 but did not reach statistical significance. Also, results obtained at a large, urban, academic children’s hospital may be different from those obtained in either a community-based setting where many AYAs are treated, or at an academic hospital serving the full AYA age spectrum.20–22 Similarly, our sample with a maximum age of 21 years may not be reflective of CCT activity among older AYAs. Our focus on patients with first cancers limits our ability to comment on the impact of CCT availability in relapsed cancer, which could be important for AYAs who often have high-risk disease. Finally, we relied on pathology specimens to trigger case ascertainment, which could have resulted in missing a small number of patients where diagnosis is based on imaging, such as diffuse intrinsic pontine glioma.
Nonetheless, our study offers important insights concerning barriers and facilitators of CCT enrollment among AYAs. What implications should be drawn from these data? First, it is clear that factors other than CCT availability influence AYA enrollment and need further study. These “downstream” factors may include provider-level choices not to present the CCT option due to medical and perceived social circumstances, limited professional time, poor reimbursement, and lack of research infrastructure.23, 24 At the patient level, several psychosocial factors may influence AYA enrollment, including coping status, perceptions of clinical research, informed consent issues, and social relationships.25–27 Because our study was not designed to capture such information, further research is needed to understand and address these factors. Second, with our study showing that only about half of both children and eAYAs had existing CCTs, there may indeed be opportunities to increase the number of CCTs for both age groups. Our data do not provide a clear indication of where such efforts should be focused, as the diagnoses where CCTs were not available spanned the spectrum of age, incidence and outcomes. Thus, for improving survival it seems reasonable to maximize CCT availability for the more common, highest-risk cancers. However, this seems unlikely to be an effective stand-alone strategy for improving AYA-specific CCT enrollment. Similarly, with a high proportion of existing trials already being available locally, our results suggest that broad efforts to increase CCT activation at the site level may not necessarily yield higher enrollment for either age group. On the other hand, it must be acknowledged that some institutions may face substantial resource limitations preventing activation of all CCTs. Also, the number of existing CCTs nationally may fluctuate over time as they are individually launched and completed. Therefore, it is safe to assume that maximizing both development and availability of impactful CCTs for AYAs will always be necessary and appropriate. Finally, because this study reflects the CCT enrollment fate only of patients who “made it through the door” at CHLA, it does not negate the importance of pre-hospital barriers that impede AYA CCT participation.9, 20, 21 Thus, the most important implication of this study is that for AYAs, future research should be focused on provider- and patient/family-level factors affecting CCT enrollment. These factors are the subject of a funded study we are now conducting.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by the David Stroud Adolescent and Young Adult Oncology Fellowship Fund (SMT) and by grant UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health (SMT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts: The authors have no conflicts of interest to disclose.
Author Contributions:
Dr. Thomas conceptualized and designed the study, conducted data collection and initial statistical analyses, drafted the initial manuscript, and reviewed and revised the manuscript.
Ms. Malvar carried out the final statistical analyses, and reviewed and revised the manuscript.
Dr. Tran and Dr. Shows coordinated case ascertainment, contributed data, and critically reviewed the manuscript.
Dr. Freyer conceptualized and designed the study, participated in the initial and final statistical analyses, drafted the initial manuscript, and critically reviewed and revised the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.Bleyer WA, Tejeda H, Murphy SB, Robison LL, Ross JA, Pollock BH, et al. National cancer clinical trials: children have equal access; adolescents do not. J Adolesc Health. 1997;21(6):366–373. doi: 10.1016/S1054-139X(97)00110-9. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute LAF. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer: Report of the Adolescent and Young Adult Oncology Progress Review Group. 2006 [Google Scholar]
- 3.Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8(4):288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 4.Canner J, Alonzo TA, Franklin J, Freyer DR, Gamis A, Gerbing RB, et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: a report from the Children’s Oncology Group. Cancer. 2013;119(23):4162–4169. doi: 10.1002/cncr.28342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butow P, Palmer S, Pai A, Goodenough B, Luckett T, King M. Review of adherence-related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28(32):4800–4809. doi: 10.1200/JCO.2009.22.2802. [DOI] [PubMed] [Google Scholar]
- 6.Zebrack BJ. Psychological, social, and behavioral issues for young adults with cancer. Cancer. 2011;117(10 Suppl):2289–2294. doi: 10.1002/cncr.26056. [DOI] [PubMed] [Google Scholar]
- 7.Martin S, Ulrich C, Munsell M, Taylor S, Lange G, Bleyer A. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist. 2007;12(7):816–824. doi: 10.1634/theoncologist.12-7-816. [DOI] [PubMed] [Google Scholar]
- 8.Krailo MD, Bernstein L, Sullivan-Halley J, Hammond GD. Patterns of enrollment on cooperative group studies. An analysis of trends from the Los Angeles County Cancer Surveillance Program. Cancer. 1993;71(10 Suppl):3325–3330. doi: 10.1002/1097-0142(19930515)71:10+<3325::aid-cncr2820711731>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045–4053. doi: 10.1200/JCO.2011.36.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins CL, Malvar J, Hamilton AS, Deapen DM, Freyer DR. Case-linked analysis of clinical trial enrollment among adolescents and young adults at a National Cancer Institute-Designated comprehensive cancer center. Cancer. 2015;121(24):4398–4406. doi: 10.1002/cncr.29669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw PH, Ritchey AK. Different rates of clinical trial enrollment between adolescents and young adults aged 15 to 22 years old and children under 15 years old with cancer at a children’s hospital. J Pediatr Hematol Oncol. 2007;29(12):811–814. doi: 10.1097/MPH.0b013e31815814f3. [DOI] [PubMed] [Google Scholar]
- 12.Bleyer A, Montello M, Budd T, Saxman S. National survival trends of young adults with sarcoma: lack of progress is associated with lack of clinical trial participation. Cancer. 2005;103(9):1891–1897. doi: 10.1002/cncr.20995. [DOI] [PubMed] [Google Scholar]
- 13.Freyer DR, Seibel NL. The Clinical Trials Gap for Adolescents and Young Adults with Cancer: Recent Progress and Conceptual Framework for Continued Research. Current Pediatrics Reports. 2015;3(2):137–145. doi: 10.1007/s40124-015-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felgenhauer J, Hooke MC. Regulatory barriers to clinical trial enrollment of adolescent and young adult oncology patients. Pediatrics. 2014;133(Suppl 3):S119–122. doi: 10.1542/peds.2014-0122H. [DOI] [PubMed] [Google Scholar]
- 15.Parsons HM, Harlan LC, Schmidt S, Keegan TH, Lynch CF, Kent EE, et al. Who Treats Adolescents and Young Adults with Cancer? A Report from the AYA HOPE Study. J Adolesc Young Adult Oncol. 2015;4(3):141–150. doi: 10.1089/jayao.2014.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuk MK, Mulugeta Y, Roth-Cline M, Mehrotra N, Reaman GH. Enrolling Adolescents in Disease/Target-Appropriate Adult Oncology Clinical Trials of Investigational Agents. Clin Cancer Res. 2017;23(1):9–12. doi: 10.1158/1078-0432.CCR-16-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob SA, Shaw PH. No improvement in clinical trial enrollment for adolescents and young adults with cancer at a children’s hospital. Pediatr Blood Cancer. 2017 doi: 10.1002/pbc.26638. [DOI] [PubMed] [Google Scholar]
- 18.Seibel N, Hunsberger S, O’Mara AM, Budd T, Friedman SH, Finnigan S, et al. Adolescent and young adult oncology (AYAO) patient enrollments onto National Cancer Institute (NCI)-supported trials from 2000 to 2010. Journal of Clinical Oncology. 2014;32(15_suppl):10058–10058. [Google Scholar]
- 19.StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 20.Yeager ND, Hoshaw-Woodard S, Ruymann FB, Termuhlen A. Patterns of care among adolescents with malignancy in Ohio. J Pediatr Hematol Oncol. 2006;28(1):17–22. [PubMed] [Google Scholar]
- 21.Albritton KH, Wiggins CH, Nelson HE, Weeks JC. Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol. 2007;25(29):4616–4621. doi: 10.1200/JCO.2006.08.4103. [DOI] [PubMed] [Google Scholar]
- 22.Tai E, Buchanan N, Westervelt L, Elimam D, Lawvere S. Treatment setting, clinical trial enrollment, and subsequent outcomes among adolescents with cancer: a literature review. Pediatrics. 2014;133(Suppl 3):S91–97. doi: 10.1542/peds.2014-0122C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunfeld E, Zitzelsberger L, Coristine M, Aspelund F. Barriers and facilitators to enrollment in cancer clinical trials: qualitative study of the perspectives of clinical research associates. Cancer. 2002;95(7):1577–1583. doi: 10.1002/cncr.10862. [DOI] [PubMed] [Google Scholar]
- 24.Meropol NJB, JS, Millard J, Damjanov N, Miller SM, Ridgway C, Ross EA, Sprandio JD, Watts P. Barriers to Clinical Trial Participation as Perceived by Oncologists and Patients. J Natl Compr Canc Netw. 2007;5(8):655–664. doi: 10.6004/jnccn.2007.0067. [DOI] [PubMed] [Google Scholar]
- 25.Buchanan ND, Block R, Smith AW, Tai E. Psychosocial barriers and facilitators to clinical trial enrollment and adherence for adolescents with cancer. Pediatrics. 2014;133(Suppl 3):S123–130. doi: 10.1542/peds.2014-0122I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Read K, Fernandez CV, Gao J, Strahlendorf C, Moghrabi A, Pentz RD, et al. Decision-making by adolescents and parents of children with cancer regarding health research participation. Pediatrics. 2009;124(3):959–965. doi: 10.1542/peds.2008-2878. [DOI] [PubMed] [Google Scholar]
- 27.Barakat LP, Schwartz LA, Reilly A, Deatrick JA, Balis F. A Qualitative Study of Phase III Cancer Clinical Trial Enrollment Decision-Making: Perspectives from Adolescents, Young Adults, Caregivers, and Providers. J Adolesc Young Adult Oncol. 2014;3(1):3–11. doi: 10.1089/jayao.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.