Abstract
Background
There is a pressing need for robust longitudinal cohort studies in the modern treatment era of multiple sclerosis.
Objective
Build a MS cohort repository to capture the variability of disability accumulation, as well as provide the depth of characterization (clinical, radiologic, genetic, biospecimens) required to adequately model and ultimately predict a patient’s course.
Methods
SUMMIT (Serially Unified Multicenter Multiple Sclerosis Investigation) is an international multi-center, prospectively enrolled cohort with over a decade of comprehensive follow-up on more than 1,000 patients from 2 large North American academic MS Centers (Brigham and Women’s Hospital [CLIMB] and University of California, San Francisco [EPIC]). It is bringing online more than 2,500 patients from additional international MS Centers (Basel [UHB], VU University Medical Center MS Center Amsterdam [MSCA], Multiple Sclerosis Center of Catalonia-Vall d’Hebron Hospital ]Barcelona CIS cohort], and American University of Beirut Medical Center [AMIR]).
Results and Conclusion
We provide evidence for harmonization of two of the initial cohorts in terms of the characterization of demographic, disease and treatment-related variables; demonstrate several proof-of-principle analyses examining genetic and radiologic predictors of disease progression; and discuss the steps involved in expanding SUMMIT into a repository accessible to the broader scientific community.
Keywords: cohort, repository, multiple sclerosis, prediction, disability
INTRODUCTION
Although significant advances have been made in targeting the relapsing phase of multiple sclerosis (MS), fundamental questions remain about the pathophysiologic basis of its long-term course. For most individuals with early MS, the outcomes of interest – accumulation of disability and evolution from a relapsing to a progressive phase – require a decade or longer to become apparent. Many insights have been gained from previous natural history studies.1–9 A poorer long-term outcome was associated with male sex,1–3, 5, 8 older age at onset,1–3, 5, 6, 8, 9 symptom location at initial presentation,1, 2, 8, 9 number and pattern of attacks1–3, 5, 6, 8, 9 incomplete recovery from the first relapse,1–3 and progressive versus relapsing symptoms from onset.1–3, 5, 6, 8, 9
These natural history studies did not include advanced biomarkers such as magnetic resonance imaging (MRI) metrics (which have come to play a substantial role)10 or genetics, and the identified risk factors for long-term outcomes were neither sufficiently sensitive nor specific to be useful for individual decision-making regarding disease modifying therapies (DMTs). Most product registration trials are restricted to individual drugs (some use active comparators) and generally do not have systematic follow-up beyond 2-3 years.11 Moreover, these studies provide little guidance in determining which agent to use initially, how to monitor efficacy, or when and how to switch treatments. In the real world, patients are often treated with multiple therapies sequentially and occasionally in combination. Cause and effect relationships can best be determined through prospective studies, and many of the most pressing questions in MS today cannot be answered by industry-sponsored clinical trials. There are relatively few prospective, observational cohort studies in the current treatment era, and most studies are relatively small, single-center and retrospective.12–14 A notable exception is MSBase, a longitudinal multi-center international registry of over 15,000 individuals with MS15 that has provided a number of important insights relating to disease course; however, MRI and biomarkers are not collected.16
The key to better understanding and eventually slowing or preventing MS disability is to develop a much more sophisticated understanding of disease progression as it applies to individual patients, with the ultimate goal of being able to tailor treatment in a precise, evidence-based, manner. One strategy to achieve this goal is to investigate long-term, deeply phenotyped cohorts from multiple centers to achieve adequate statistical power to answer this pressing question. Harmonization of data capture is essential in order to compare and contrast disease behavior across cohorts and to move beyond local prescribing patterns, environmental exposures (UV, pathogens), or patient demographics (ancestry, smoking, diet) to understand other key contributors of disease progression.
Here, we introduce the Serially Unified Multicenter Multiple Sclerosis Investigation (SUMMIT), a prospectively ascertained multi-center international cohort with over a decade of systematic follow-up. We illustrate the main advantages of this prospective cohort, including deep phenotyping, long-term follow-up, and an efficient structure for discovery/validation analyses. We then provide a roadmap for establishing a suite of research and data management tools that will enable us to address many questions related to disability and progression. An important outcome of this program will be its ability to share these emerging resources so that others can contribute to SUMMIT and use its infrastructure to address their own questions regarding MS.
METHODS
Cohorts
Historical Developments
Spurred by an initial request for applications from the National Multiple Sclerosis Society (NMSS) to study the factors associated with MS progression, two MS cohorts (CLIMB at BWH and GeneMSA,17 a preexisting collaboration among UCSF’s EPIC cohort, Basel and Amsterdam), aligned to design and test a collaborative structure and methodology to study factors related to MS progression. Through this, they developed a shared framework for integrating and analyzing clinical, MRI, blood-based, and genomic data on existing MS patients and a plan to expand and improve upon this approach via collaborative development of new prospective patient cohorts. Given shared principles, scientific endeavors and cohort design, two additional academic MS Centers have joined this collaboration: the Barcelona CIS cohort (CEMCAT), and the more recent Beirut cohort (AMIR).
Boston
The Comprehensive Longitudinal Investigation of Multiple Sclerosis at the Brigham and Women’s Hospital (CLIMB; BWH) is a single-center prospective study that has enrolled more than 2,100 patients since the year 2000 (www.climbstudy.org).18 Patients are recruited directly from the clinical practice (median: 1 year since symptom onset), and followed longitudinally with standardized clinical exams every 6 months, and annual MRIs and stored blood samples. Within this umbrella, a subset of patients is enrolled in more detailed studies, including quality of life (QOL) and genetics studies. CLIMB patients’ clinical and neuroimaging measures, while standardized, are derived from routine clinical care. Overall, the dropout rate in CLIMB in 3% per year, and is largely due to patients moving, or the patient’s physician leaving the practice. From all CLIMB participants, we selected for inclusion into the CLIMB-SUMMIT cohort adult participants with a diagnosis of MS meeting 2010 International Panel criteria,19 who were recruited into the QOL arm of the CLIMB study (enrolled between 4/5/2000 and 9/3/2013), or those CLIMB subjects who had 10 years of follow-up since first symptom.
San Francisco
The Expression/genomics, Proteomics, Imaging, and Clinical (EPIC) study is a single-center prospective observational research cohort of MS patients evaluated annually since July 2004 (www.msepicstudy.com). Patients (age 18–65 years) receiving care at the University of California, San Francisco (UCSF) Multiple Sclerosis Center between July 2004 and September 2005 were invited to participate. Ambulatory subjects and those with a recent onset of clinically definite MS (2001 International Panel Diagnostic Criteria)20 or clinically isolated syndrome (CIS) were preferentially recruited, although individuals with all clinical subtypes of the disease participate. Patient retention at 10-year follow-up exceeds 91%.21
Basel
Recruitment of patients originally started in 2004 at the Universitätsspital Basel (UHB) as a part of the GeneMSA collaboration with UCSF and Amsterdam. Caucasian patients aged 18-70, with diagnosis of MS,20 or CIS if fulfilling 3 of the 4 Barkhof criteria for dissemination in space, and EDSS 0-7.5, were included. In 2011 new patients were added to compensate for drop outs of the initial study, comprising a total of 289. MS history, demographics, and exposures were collected at baseline. Patients receive annual clinical examinations including relapse history and EDSS, SDMT and MSFC assessments, as well as MRIs and blood sampling (serum and plasma). The follow-up time for the original cohort is more than 10 years, and for patients recruited after 2011 is up to 6 years. Drop-out rates for the since 2011 were 10% in the first year, 7% in year 1, and 2% after year 2.
Amsterdam
The cohort of the MS Center Amsterdam (MSCA) selected for SUMMIT is a single-center longitudinal prospective inception cohort of MS patients included within a year from diagnosis of MS according to the 2010 International Panel criteria,19 All patients were intensively followed up for 4 years using questionnaires, neurological evaluations, extensive testing and MRI scans. Subsequently patients were assessed at years 6 and 11. At the end of 2017, collection of year 11 data will be finished with a retention of 92%.
Barcelona
The Barcelona CIS cohort is a single-center prospective open cohort initiated in 1995. It includes patients aged under 50 years of age with a CIS suggestive of central nervous system demyelination not attributable to other diseases and with onset of symptoms within 3 months of the first clinical evaluation at the Cemcat MS center. Patients are regularly followed on a biannual basis following a pre-defined clinical and MRI protocol. CIS topography, use of steroids, and EDSS assessment are evaluated. IgG oligoclonal bands (OB) are examined within the first 3 months of disease onset. The remaining serum and cerebrospinal fluid (CSF) samples are stored at −80°C. Brain MRI is performed 3-5 months after the CIS and repeated after twelve months and every five years. MR is performed on a 1.5 or 3.0T magnet with a standard head coil. MR analysis is routinely performed by one of two neuroradiologists with expertise in inflammatory-demyelinating diseases. Overall, the dropout rate in the Barcelona CIS cohort is 3.2% per year.22
Beirut
The AUBMC-Multiple Sclerosis Interdisciplinary Research (AMIR) is a single-center longitudinal prospective study that has enrolled 891 patients since 2012. Patients are recruited directly from the clinical practice, and followed longitudinally with standardized clinical exams every 6 months, annual or bi-annual MRIs, and QOL questionnaires. Most patients opt to participate in the blood, DNA, and urine biobank (annual collection). Patients may also participate in longitudinal optical coherence tomography (OCT) yearly measurements. Patients with all forms of MS (including CIS, RIS) and with NMO are included in this cohort.
As outlined in Table 1, each cohort has distinct features in terms of subject selection and depth of phenotyping; nonetheless, there is consistency in primary MS-related data elements. Data harmonization between the UCSF and BWH cohorts has been completed, and is ongoing with the other cohorts. For this reason, we will focus below how on two of these cohorts, BWH and UCSF, offer complementary and synergistic approaches to understanding the course of MS in the modern era. The Committee on Human Research at each institution approved the SUMMIT protocol, and informed consent was obtained from all participants. Detailed information about the methods deployed for each illustrative project are provided in Appendix 1, and about study retention and data completeness is provided in Appendix 2.
Table 1.
Prospective data collection across the SUMMIT cohorts
| Characteristics | Boston CLIMB (N=511) | UCSF EPIC (N=517) | Basel (N=289) | Amsterdam MSCA (N=250) | Barcelona CIS Cohort (N=1172) | Beirut AMIR (N=891) |
|---|---|---|---|---|---|---|
| Patient Visits | Clinic-based, locally followed | Yearly research-based, referred from UCSF MS Clinic | Clinic-based, locally followed | Clinic-based, locally followed | Clinic-based, locally followed | Clinic-based, locally followed |
| Year Started | 2000 | 2004-2005 enrollment for original 517 | 2004, ongoing recruitment started 2011 | 2004 | 1995 | 2012 |
| Study Retention | 3% attrition rate per year | 91% retention rate (incl. follow-up on deceased); 450 currently being followed for YR12 | Initial cohort: drop outs 10% year 1, 7% Y2, 2% after year 2 and 0% thereafter | 92% retention at Y11 | 3.2% attrition rate per year | |
| Data Elements | ||||||
| Demographics | Age, race/ethnicity, age at symptom onset, age at diagnosis Locations lived, smoking, alcohol, education, reproductive history | Age, race/ethnicity, age at symptom onset, age at diagnosis Locations lived, smoking, alcohol, education, reproductive history | Age, race/ethnicity, age at symptom onset, age at diagnosis Education, Allergies, Alcohol, Smoking, Vit. D, family history | Age, race/ethnicity, age at symptom onset, age at diagnosis | Age, age at symptom onset, age at diagnosis | Age, age at symptom onset, age at diagnosis Smoking, alcohol, caffeine, education, physical activity, family history, relationship status, reproductive history |
| Clinical Measures | Biannually: Disease type, EDSS, DMT, steroids, symptomatic therapies, OTC medications, BMI, BP, SDMT, MSQOL54, CESD, MFIS | Annually: Disease type, EDSS, DMT, steroids, symptomatic therapies, OTC medications, BMI, BP, PASAT/SDMT, MSFC, FAMS, WHODAS | Annually: Disease type, EDSS, DMT, relapses, relapse treatment, medication, SDMT, MSFC | Annually until Y4, then Y6 and Y11: EDSS, questionnaires | Biannually: Disease type, EDSS, DMT, steroids | Annually: Disease type, EDSS, DMT, steroids, symptomatic therapies, OTC medications, vitamin D supplementation, Eps, BMI, BP, SDMT, 9HPT, 25FW, OCT, MusiQoL |
| Relapses | Date, steroids used, medical record review for any prior to baseline visit, symptom location, objective findings (exam, MRI), recovery | Date, steroids used, medical record review for any prior to baseline visit | Date, treatment, symptom location, recovery | Date, treatment, symptom location, recovery | Date, steroids used, symptom location, objective findings (exam), recovery | Date, steroids used, medical record review for any prior to baseline visit, symptom location, objective findings (exam, MRI), duration history and resolution |
| MRI Measures | Annually: 3T MRI scanner (Siemens Skyra) | Annually: 3T MRI scanner (Siemens Skyra) | Annually: MRI scan (2011-2016: 1.5 T Avanto 2016- : 3 T Skyra | Annually: 3T MRI scanner | Baseline, 12M, 5Y and every 5Y thereafter: 1.5T and 3T MRI scanner | Annually/Biannually: 1.5T and 3T MRI scanner |
| Blood | Annually: DNA, RNA, serum, PBMCs | Annually: DNA, RNA, serum, PBMCs | Annually: serum, plasma, BL-Y2 in addition whole blood (EDTA) | Annually: DNA, serum, plasma, PBMCs | Baseline: serum, plasma, PBMCs | Annually: DNA, serum, plasma, PBMCs |
| Other | Stool | Stool | CSF | Urine | ||
Abbreviations: EDSS = expanded disability status scale; DMT = disease modifying therapy; OTC = over the counter; BMI = body mass index; BP = blood pressure; SDMT = symbol digit modalities test; MSQOL54 = multiple sclerosis quality of life-54; CESD = Center for Epidemiologic Studies Depression scale; MFIS = modified fatigue impact scale; PASAT = paced auditory serial addition test; MSFC = multiple sclerosis functional composite; FAMS = functional assessment of multiple sclerosis; WHODAS = World Health Organization disability assessment schedule; PBMC = peripheral blood mononuclear cell; CSF= cerebrospinal fluid
RESULTS
Illustrative Projects
(1) Is the course of MS becoming milder? Clinical features in two modern cohorts
Whether due to earlier diagnosis, evolving diagnostic criteria that now include MRI, rapid initiation of DMTs, inclusion of milder forms of MS, or other environmental/epigenetic phenomena, it has been hypothesized that MS now appears to have an overall milder course than in the pre-treatment era.7, 23
To address this question, we included all SUMMIT participants. Using simple descriptive statistics, we compared demographic and clinical features at the baseline enrollment visits between the two sites (Table 2). At enrollment, CLIMB participants were younger than EPIC participants; however, because enrollment began in 2000 for CLIMB and in 2004 for EPIC, at the most recent visit the two cohorts were similar in age (50.2 years for CLIMB vs. 51.2 for EPIC) and disease duration (14 vs. 15 years respectively). In both groups there was a preponderance (>90%) of individuals with relapsing onset MS.
Table 2.
Comparison of demographic characteristics across the Boston and San Francisco cohorts
| Characteristic | All (N=1028) | EPIC (N=517) | CLIMB (N=511) | p-Value |
|---|---|---|---|---|
| Baseline Demographic | ||||
| Age at exam, mean ± sd | 40.8 ± 10.3 | 42.5 ± 9.8 | 39 ± 10.5 | 3.54e-08 |
| Sex | ||||
| Women, n (%) | 744 (72.4%) | 355 (68.7%) | 389 (76.1%) | 0.008 |
| Men, n (%) | 284 (27.6%) | 162 (31.3%) | 122 (23.9%) | 0.008 |
| Number of visits, mean (IQR) (range) | 9 (7, 22) (1-44) | 7 (6, 8) (1-11) | 22 (17, 25) (5-44) | 4.41e-165 |
| Years of follow-up, mean (IQR) (range) | 10.2 (8.7, 11.5) (0-18) | 9.1 (8.3, 10.2) (0-11.4) | 11.5 (10, 12.9) (3-18) | 2.53e-70 |
| Mean visits per year, mean (IQR) (range) | 1 (0.7, 1.8) (0.2-2.9) | 0.7 (0.6, 0.8) (0.2-1) | 1.8 (1.5, 1.9) (0.4-2.9) | 7.78e-164 |
|
Baseline Clinical | ||||
| Age of onset, mean ± sd | 33.9 ± 9.7 | 33.4 ± 9.3 | 34.4 ± 10.1 | 0.09 |
| Disease Duration, median (IQR) (range) | 3 (1, 10) (0-45) | 6 (2, 13) (0-45) | 2 (1, 6) (0-44) | 1.27e-21 |
| Disease Course | ||||
| CIS, n (%) | 165 (16.1%) | 82 (15.9%) | 83 (16.4%) | 0.865 |
| RR, n (%) | 698 (68.2%) | 366 (70.8%) | 332 (65.6%) | 0.07 |
| SP, n (%) | 61 (6%) | 48 (9.3%) | 13 (2.6%) | 4.36e-06 |
| PP, n (%) | 42 (4.1%) | 20 (3.9%) | 22 (4.3%) | 0.755 |
| EDSS score, median (IQR) (range) | 1.5 (1, 2.5) (0-7.5) | 1.5 (1, 3) (0-7) | 1.5 (1, 2) (0-7.5) | 0.005 |
| T25W score trial 1, median (IQR) (range) | 5 (4.2, 5.8) (0-53.8) | 4.8 (4.2, 5.8) (2.9-53.8) | 5 (4.1, 6) (0-29.7) | 0.268 |
| FSS | ||||
| Visual, median (IQR) (range) | 0 (0, 0) (0-6) | 0 (0, 0) (0-6) | 0 (0, 0) (0-5) | 0.026 |
| Brainstem, median (IQR) (range) | 0 (0, 0) (0-4) | 0 (0, 0) (0-4) | 0 (0, 0) (0-3) | 0.764 |
| Pyramidal, median (IQR) (range) | 1 (0, 1) (0-5) | 1 (0, 1) (0-5) | 0 (0, 1) (0-5) | 0.438 |
| Cerebellar, median (IQR) (range) | 0 (0, 1) (0-4) | 0 (0, 1) (0-4) | 0 (0, 0) (0-3) | 1.67e-09 |
| Sensory, median (IQR) (range) | 0 (0, 1) (0-4) | 0 (0, 1) (0-4) | 0 (0, 1) (0-4) | 9.46e-04 |
| Mental, median (IQR) (range) | 0 (0, 0) (0-5) | 0 (0, 0) (0-4) | 0 (0, 0) (0-5) | 0.577 |
| Bowel bladder, median (IQR) (range) | 0 (0, 1) (0-5) | 0 (0, 1) (0-4) | 0 (0, 0) (0-5) | 1.57e-05 |
| Relapse history | ||||
| Number of prior relapses, median (IQR) (range) | 2 (1, 3) (0-20) | 3 (2, 4) (1-20) | 1 (1, 2) (0-9) | 1.06e-61 |
|
Baseline Treatment * | ||||
| Treatment type | ||||
| DMT first line, n (%) | 862 (93%) | 410 (93.4%) | 452 (92.6%) | 0.7 |
| DMT oral, n (%) | 7 (0.8%) | 3 (0.7%) | 4 (0.8%) | 1 |
| DMT high, n (%) | 26 (2.8%) | 7 (1.6%) | 19 (3.9%) | 0.045 |
| Experimental, n (%) | 5 (0.5%) | 3 (0.7%) | 2 (0.4%) | 0.672 |
| Immune, n (%) | 2 (0.2%) | 2 (0.5%) | ||
| Steroid, n (%) | 7 (0.8%) | 7 (1.6%) | ||
| MS other, n (%) | 18 (1.9%) | 7 (1.6%) | 11 (2.3%) | 0.488 |
|
Last Visit | ||||
| Age at exam, median (IQR) (range) | 51 (44, 58) (23-81) | 51 (44,59) (27-76) | 50 (43, 57) (23-81) | 0.218 |
| Disease duration, median (IQR) (range) | 14 (11, 20) (0-54) | 15 (11, 22) (0-54) | 14 (11, 17) (3-52) | 0.017 |
| EDSS score, median (IQR) (range) | 2 (1.5, 3.5) (0-9.5) | 2.5 (1.5, 4) (0-9.5) | 2 (1, 3) (0-9.5) | 1.16e-15 |
| Treatment type | ||||
| DMT first line, n (%) | 421 (45.4%) | 252 (57.4%) | 169 (34.6%) | 3.47e-12 |
| DMT oral, n (%) | 278 (30%) | 87 (19.8%) | 191 (39.1%) | 1.3e-10 |
| DMT high, n (%) | 89 (9.6%) | 42 (9.6%) | 47 (9.6%) | 1 |
| Experimental, n (%) | 28 (3%) | 13 (3%) | 15 (3.1%) | 1 |
| Immune, n (%) | 3 (0.3%) | 2 (0.5%) | 1 (0.2%) | 0.606 |
| Steroid, n (%) | 22 (2.4%) | 22 (5%) | ||
| MS other, n (%) | 86 (9.3%) | 21 (4.8%) | 65 (13.3%)) | 7.12e-06 |
| Never on treatment, n (%) | 101 (9.8%) | 78 (15.1%) | 23 (4.5%) | 7.45e-09 |
| FSSC Scores | ||||
| Visual, median (IQR) (range) | 0 (0, 1) (0-6) | 1 (0, 1) (0-5) | 0 (0, 0) (0-6) | 3.72e-18 |
| Brainstem, median (IQR) (range) | 0 (0, 0) (0-5) | 0 (0, 1) (0-4) | 0 (0, 0) (0-5) | 1.38e-13 |
| Pyramidal, median (IQR) (range) | 1 (0, 2) (0-6) | 1 (0, 2.2) (0-5) | 0 (0, 2) (0-6) | 9.72e-11 |
| Cerebellar, median (IQR) (range) | 1 (0, 1) (0-5) | 1 (0, 2) (0-5) | 0 (0, 1) (0-4) | 1.22e-12 |
| Sensory, median (IQR) (range) | 1 (0, 2) (0-6) | 1 (1, 2) (0-4) | 1 (0, 2) (0-6) | 1.69e-13 |
| Mental, median (IQR) (range) | 0 (0, 1) (0-4) | 1 (0, 1) (0-4) | 0 (0, 1) (0-4) | 1.58e-04 |
| Bowel bladder, median (IQR) (range) | 0 (0, 1) (0-5) | 1 (0, 1) (0-5) | 0 (0, 1) (0-5) | 5.69e-05 |
P-values compare EPIC and Harvard subjects. For normally distributed data, mean and standard deviation are shown and Student’s t-test was used. For data that are no normally distributed, median, interquartile, and range are shown and a Wilcoxon test was used. For qualitative data, counts and percentages are shown and Fisher’s exact test was used. Abbreviations: CIS = clinically isolated syndrome; RR = relapsing-remitting multiple sclerosis; SP = secondary progressive multiple sclerosis; PP = primary progressive multiple sclerosis; EDSS = expanded disability status scale; T25W = timed 25 foot walk; FSS = functional systems score; DMT = disease modifying therapy
DMT first line treatments include interferons (Avonex, Betaseron, Extavia, Rebif, and Plegridy) and glatiramer acetate (Copaxone, Glatopa). DMT high treatments include Campath, natalizumab, ocrelizumab, alemtuzumab, and Novantrone. DMT oral treatments include fingolimod, dimethyl fumarate, and teriflunomide. Experimental treatments include Cladribine, doxycycline, MBP8298, Minocycline, T Cell Vaccination study, TCR Peptide trial, Low Dose Naltrexone, Biotin, CTLA4-Ig, Orencia, Rapamune, Rilutek, Simulect, Zenapax, and Ozanimod. Immune treatments include IVIG and plasmapheresis. MS other treatments include rituximab, chemotherapy, cyclophosphamide, Cellcept, Methotrexate, Imuran, and Ciclosporin. Steroid treatments include oral steroids, intravenous steroids, intravenous methylprednisolone, dexamethasone, prednisolone, prednisone, ACTH, Hydrocortisone, and Test Steroid.
While at baseline most participants in both cohorts were treated with first-line injectable therapies, at the most recent clinical visit there was a broader distribution of therapies, reflecting a general transition to oral therapies in CLIMB contrasted with a “treat to target” approach (i.e. advancing therapy based on evidence of disease activity) in EPIC. These differences in practice patterns highlight the benefits of being able to seize on patterns from one cohort to prospectively examine differences in another.
Our next goal was to examine aspects of disability progression in a contemporary clinical setting. We used an interval-censored approach for estimating time to sustaining expanded disability status scale (EDSS) score of 624 (Figure 1), and secondarily to sustained EDSS of 3, in all SUMMIT participants. The proportion of EPIC participants sustaining EDSS 3 by 15 years was 42% (56% by 20 years; 67% by 25 years). The proportion of subjects in CLIMB sustaining EDSS 3 by 15 years was 39% (50% by 20 years; 59% by 25 years). The proportion of subjects in EPIC sustaining EDSS 6 by 15 years was 10% (13% by 20 years; 17% by 25 years). In CLIMB, the proportion of subjects sustaining EDSS 6 by 15 years was 15% (22% by 20 years; 27% by 25 years). In both cohorts, disability accumulation was less pronounced than in earlier natural history studies; where up to 50% of the cohort had reached an EDSS 6 by 15-16 years.3, 25
Figure 1.
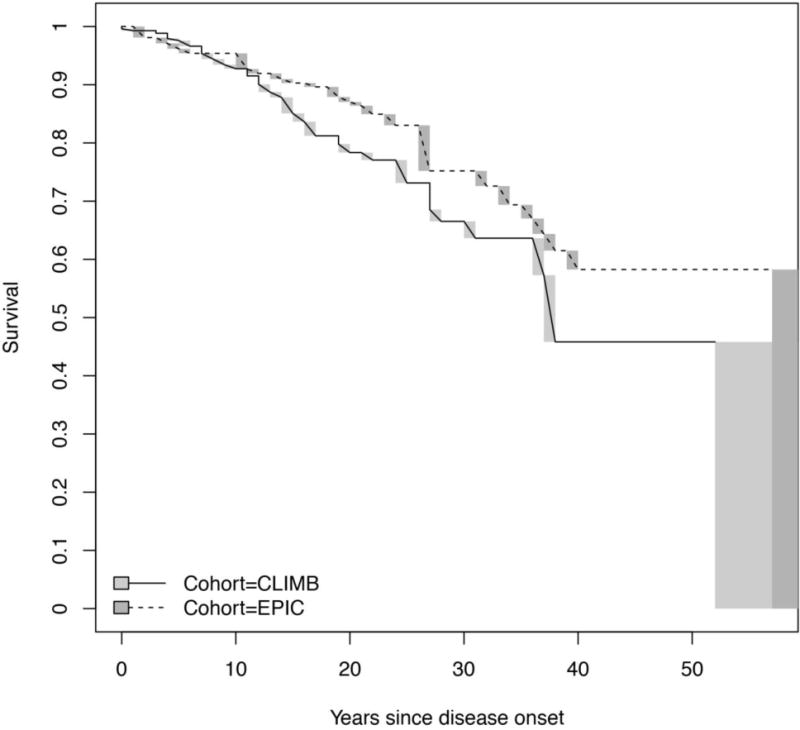
Comparison of time to sustained EDSS 6 between CLIMB and EPIC.
Time to sustained EDSS 6 in the CLIMB and EPIC cohorts (p=0.011 for the difference between the two cohorts); solid line is the CLIMB cohort and dotted line is the EPIC cohort.
Abbreviations: EDSS: expanded disability status scale
Overall, the EPIC and CLIMB cohorts are similar at present in demographic and disease characteristics. Further, while both cohorts showed a slower time to EDSS 6 than previously reported in natural history studies, the small differences between the two cohorts provide fertile ground for future detailed work on environmental, genomic and pharmacological contributors to disability progression.
(2) Harmonizing MRI data to evaluate the utility of NEDA in predicting long-term disease progression
The concept of “no evidence of disease activity” (NEDA) has been gaining traction as a goal of DMT management in relapsing forms of MS, and yet its utility in predicting long-term outcomes is uncertain.26 Both sites previously reported NEDA analyses,21, 27 with divergent findings: in the BWH cohort, NEDA (clinical + MRI) at 2 years had a positive predictive value of 78.3% for no progression (EDSS score change ≤0.5) at 7 years;27 while in EPIC, participants meeting NEDA (clinical + MRI) at 2 years had long-term outcomes at 10 years that were no different from those of the cohort as a whole.21
We piloted our ability to evaluate the long-term predictive utility of NEDA using our cohorts. First, we compared the rate of clinical NEDA (no relapses and no clinically significant increase in EDSS from baseline through the second year of the study) over 2 years in both cohorts. In CLIMB, 186 (56%) of 335 subjects fulfilled clinical NEDA criteria, as did 214 (55%) of 390 EPIC participants (p=0.88).
Then, we piloted our ability to apply a common clinical and MRI data processing methodology in a subset of 43 bout-onset individuals in each cohort, matched for age (39.6 years), sex (72.1% female), disease duration (3 years) and disease course (9.3% CIS; 90.7%RRMS) (Table 3). In the pooled cohort, 37% subjects satisfied clinical NEDA, and 11% satisfied both clinical + MRI NEDA. Clinical + MRI NEDA at 2 years was not predictive of changes in EDSS between years 2 and 10 (p=1.0). This pilot finding with low numbers will require validation in the entire cohort. Using a newly-developed MRI database and common image processing pipeline, the project will include all 511 CLIMB and 517 EPIC subjects, providing an opportunity to examine the impact of the newly-described NEDA-4 metric,28 which adds a brain atrophy change metric to the traditional NEDA assessments.
Table 3.
Comparison of demographic and disease characteristics in EPIC and CLIMB subjects used for NEDA analyses.
| Characteristic | All (N=86) | EPIC (N=43) | CLIMB (N=43) | p-Value |
|---|---|---|---|---|
| Demographic | ||||
| Age at exam, mean ± sd | 39.6 ± 9 | 39.6 ± 8.9 | 39.6 ± 9.1 | 0.981 |
| Sex | ||||
| Women, n (%) | 62 (72.1%) | 31 (72.1%) | 31 (72.1%) | 1 |
| Men, n (%) | 24 (27.9%) | 12 (27.9%) | 12 (27.9%) | 1 |
| Number of visits, mean (IQR) (range) | 10 (8, 20) (4-34) | 8 (7, 8) (4-10) | 20 (17.5, 22.5) (9-34) | 1.36e-15 |
| Years of follow-up, mean (IQR) (range) | 10.2 (9, 11.2) (6.5-14.7) | 10.1 (8.7, 11) (7.1-11.4) | 10.4 (9.5, 11.4) (6.5-14.7) | 0.051 |
| Mean visits per year, mean (IQR) (range) | 0.9 (0.7, 1.8) (0.4-2.9) | 0.7 (0.7, 0.7) (0.4-0.9) | 1.8 (1.6, 1.9) (1-2.2) | 1.46e-15 |
|
Clinical | ||||
| Age of onset, mean ± sd | 36.5 ± 8.9 | 36.3 ± 8.8 | 36.8 ± 9 | 0.79 |
| Disease Duration, median (IQR) (range) | 3 (1.2, 4) (0-5) | 3 (1.5, 4) (0-5) | 3 (1.5, 4) (0-5) | 1 |
| Disease Course | ||||
| CIS, n (%) | 8 (9.3%) | 4 (9.3%) | 4 (9.3%) | 1 |
| RR, n (%) | 78 (90.7%) | 39 (90.7%) | 39 (90.7%) | 1 |
| EDSS score, median (IQR) (range) | 1 (0, 1.5) (0-3.5) | 1.5 (1, 2) (0-3.5) | 1 (0, 1.5) (0-3) | 0.001 |
| T25W score trial 1, median (IQR) (range) | 4.2 (3.8, 5) (2.7-6.7) | 4.2 (3.8, 4.8) (2.6-6.7) | 4.3 (3.7, 5) (3.3-6) | 0.94 |
| FSS | ||||
| Visual, median (IQR) (range) | 0 (0, 0) (0-4) | 0 (0, 0) (0-4) | 0 (0, 0) (0-1) | 0.024 |
| Brainstem, median (IQR) (range) | 0 (0, 0) (0-2) | 0 (0, 0) (0-2) | 0 (0, 0) (0-2) | 0.048 |
| Pyramidal, median (IQR) (range) | 0 (0, 1) (0-2) | 0 (0, 1) (0-2) | 0 (0, 1) (0-2) | 0.767 |
| Cerebellar, median (IQR) (range) | 0 (0, 0) (0-1) | 0 (0, 0) (0-1) | 0 (0, 0) (0-1) | 0.142 |
| Sensory, median (IQR) (range) | 0 (0, 1) (0-3) | 0 (0, 1) (0-2) | 0 (0, 0) (0-3) | 0.037 |
| Mental, median (IQR) (range) | 0 (0, 0) (0-2) | 0 (0, 0) (0-2) | 0 (0, 0) (0-1) | 0.022 |
| Bowel bladder, median (IQR) (range) | 0 (0, 0) (0-2) | 0 (0, 1) (0-2) | 0 (0, 0) (0-2) | 0.043 |
|
Treatment * | ||||
| Treatment history | ||||
| DMT first line, n (%) | 55 (68.8%) | 33 (86.8%) | 22 (52.4%) | 0.001 |
| DMT oral, n (%) | ||||
| DMT high, n (%) | 2 (2.5%) | 2 (4.8%) | ||
| Experimental, n (%) | 1 (1.2%) | 1 (2.6%) | ||
| Immune, n (%) | ||||
| Steroid, n (%) | 18 (22.5%) | 18 (42.9%) | ||
| MS other, n (%) | 4 (5%) | 4 (10.5%) | ||
| Never on treatment, n (%) | 6 (7%) | 5 (11.6%) | 1 (2.3%) | 0.202 |
Baseline data for 43 CLIMB subjects and 43 EPIC subjects matched by disease duration, age at exam, and gender. P-Values compare CLIMB and EPIC subjects. For normally distributed data, mean and standard deviation are shown and Student’s t-test was used. For data that are not normally distributed, median, interquartile, and range are shown and a Wilcoxon test was used. For qualitative data, counts and percentages are show and Fisher’s exact test was used. Abbreviations: CIS = clinically isolated syndrome; RR = relapsing-remitting multiple sclerosis; EDSS = expanded disability status scale; T25W = timed 25 foot walk; FSS = functional systems score; DMT = disease modifying therapy
DMT first line treatments include interferons (Avonex, Betaseron, Extavia, Rebif, and Plegridy) and glatiramer acetate (Copaxone, Glatopa). DMT high treatments include Campath, natalizumab, ocrelizumab, alemtuzumab, and Novantrone. DMT oral treatments include fingolimod, dimethyl fumarate, and teriflunomide. Experimental treatments include Cladribine, doxycycline, MBP8298, Minocycline, T Cell Vaccination study, TCR Peptide trial, Low Dose Naltrexone, Biotin, CTLA4-Ig, Orencia, Rapamune, Rilutek, Simulect, Zenapax, and Ozanimod. Immune treatments include IVIG and plasmapheresis. MS other treatments include rituximab, chemotherapy, cyclophosphamide, Cellcept, Methotrexate, Imuran, and Ciclosporin. Steroid treatments include oral steroids, intravenous steroids, intravenous methylprednisolone, dexamethasone, prednisolone, prednisone, ACTH, Hydrocortisone, and Test Steroid.
Further, for prospective collection of MRI data, the scanners (3T Skyra, Siemens Healthcare, Erlangen, Germany) and acquisition protocols at the two sites have been harmonized. The two sites share three identical core sequences (3D T2, fluid-attenuated inversion-recovery, and T1-weighted gradient-echo acquisitions), and have furthermore completed human phantom calibration studies that enable calibration of regional volumes in MS patients across the sites.29
(3) Harmonizing genetic data to better understand MS risk
Both sites have a long history of productive collaborations on major MS genetics efforts including participation in the International MS Genetics Consortium (IMSGC)30–33 that discovered over 140 single nucleotide polymorphisms (SNPs) associated with MS risk and accounted for approximately 28 % of the sibling recurrence risk.33
To illustrate the wealth of data and the value of efficient discovery/replication analyses, we assessed genetic risk markers in a subset of 1014 BWH subjects (including 248 subjects from the CLIMB-SUMMIT group who had genotyping available) and 467 EPIC subjects. We focused on two genetic measures: (1) the rs3129889 SNP, which captures the HLA DRB1*1501 MS susceptibility haplotype, is associated with an earlier age at MS onset,32, 34, 35 and (2) a Genetic Risk Score integrating the effect of 95 susceptibility alleles (GRS95) outside of the major histocompatibility complex.36 We asked whether (1) the two cohorts differed in terms of genetic risk burden; (2) given established sex-based differences in MS risk, there might be similar differences in the distribution of genetic measures by gender; and (3) the GRS95, in addition to HLA-DRB1*15:01, was associated with age at initial MS symptom. The details of the genotyping are summarized in the supplementary materials.
As shown in Table 4, the cohorts were not significantly different in terms of the frequency of individuals carrying the HLA DRB1*1501 risk haplotype or in the mean GRS95. Women had a higher mean HLA DRB1*1501 score than men (in each cohort, as well as the pooled analysis where p=0.0012), but a lower non-HLA GRS95 (true in EPIC [p=0.029] and in the pooled analysis [p=0.023]). Finally, with respect to age at MS onset, HLA DRB1*1501 was associated with earlier MS onset in the pooled analysis (p=0.0011). Age of onset was not significant for GRS95 (p=0.10).
Table 4.
MS genetic risk alleles: Sex differences, and association with age at MS onset.
| A. Summary measures* | |||
|---|---|---|---|
| BWH (N=1023) | EPIC (N=467) | Comparison | |
| DRB1*1501 | 0.272 | 0.269 | 0.69 |
| GRS95, mean | 5.1 | 5.1 | 0.86 |
| B. Comparison of women and men** | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BWH | EPIC | Combined | |||||||
| Women (N=776) | Men (N=247) | p-value | Women (N=318) | Men (N=149) | p-value | Women (N=1094) | Men (N=396) | p-value | |
| DRB1*1501 | 0.283 | 0.239 | 0.0278 | 0.294 | 0.215 | 0.0149 | 0.286 | 0.23 | 0.0012 |
| GRS95, mean | 5.09 | 5.13 | 0.193 | 5.08 | 5.14 | 0.0287 | 5.09 | 5.14 | 0.0229 |
| C. Association with age at MS onset *** | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women (N=1087) | Men (N=394) | Combined (N=1481) | ||||||||||
| beta | SE | t-stat | p-value | beta | SE | t-stat | p-value | beta | SE | t-stat | p-value | |
| DRB1*1501 | −1.64 | 0.464 | −3.54 | 0.000422 | −0.465 | 0.846 | −0.55 | 0.583 | −1.33 | 0.407 | −3.27 | 0.00108 |
| GRS95, mean | −0.433 | 0.454 | −0.954 | 0.34 | −1.15 | 0.763 | −1.5 | 0.134 | −0.64 | 0.39 | −1.64 | 0.101 |
Wilcoxon Rank Test
Wilcoxon-rank test. For the pooled analysis (CLIMB+EPIC), we controlled for cohort.
Linear regressions additive model (treating SNPs as a continuous variable (0,1,2), controlling for cohort. For the pooled analysis (men + women), we controlled for sex and cohort. Abbreviations: SE = standard estimate; t-stat = t statistic
Altogether, we were able to support a previously reported association between the HLA DRB1*1501 allele with an earlier age of MS onset37 in these two cohorts. More interestingly, we were able to apply a discovery/replication model to validate sex differences in genetic measures. Our findings highlight the value of sex-stratified genetic analyses and genetic risk scores in future genotype-phenotype studies in MS.38
(4) Towards a universal data repository
Our vision for the SUMMIT project is to create an open network, through which scientists would contribute to, and have access to, de-identified high-quality, deeply profiled, prospectively collected data. By analogy, the SUMMIT concept shares attributes with the open-access Alzheimer’s Disease Neuroimaging Initiative (ADNI) model (adni-info.org)39 or to the seminal, but population-based, Framingham Heart Study40, 41 which enabled its founding investigators, and subsequently many others, to understand chronic heart disease in defined cohorts of patients over long periods of time. One important difference of the SUMMIT model is the requirement that external investigators obtain datasets from the SUMMIT Data Coordinating Center, after review of their scientific proposal. In MS, the MSBase registry has provided a number of important insights relating to disease course, but MRIs and biomarker data are not collected in the registry.16 What distinguishes SUMMIT from major clinical trial cohorts in MS, of similar cohort size, is its (1) longer duration of follow-up; (2) inclusion of all MS phenotypes (CIS, RRMS, PPMS, SPMS); (3) ascertainment of clinical metrics that extend beyond the EDSS, plus collection of genomics and other biomarkers; (4) scope of the questions that will be addressed; and (5) the opportunity for discovery and validation of biomarkers in two distinct cohorts.
Common Platform
Our first goal was to fully integrate the clinical, imaging, and biomarker data from the two centers into a common informatics platform capable of tracking, synthesizing and analyzing data. Several principles are paramount, including the development of rules for sharing finite resources (e.g. cerebrospinal fluid or cells); insuring privacy protection for participants, as well as the capability to remove data from those who wish to withdraw from participation; and creating a long-term governance structure for the maintenance, expansion, and beneficent use of SUMMIT resources that can function beyond the tenure of its founders (included in Supplementary Materials). To ensure the judicious use of finite biosamples, our Biorepository Governance Committee has the primary responsibility to design and execute appropriate studies using the material available. Their decisions will be based on a sound biological rationale, proper statistical handling and expert execution. The Committee also has the responsibility of evaluating external requests for biosamples. Some collected materials in the repository, such as DNA, are abundant while others such as serum, plasma, and RNA, are in limited supply.
Sequential Expansion into an Open Access Platform
This network is sequentially expanding along a multistage process to include other academic centers with the ability to add prospectively followed patients into the system. We will share all of the relevant protocols and invite other MS investigators to upload comparable data from their own patients. Ultimately, this would involve direct on-line data entry from both large and small MS centers that wish to contribute to the dataset, creating a community-wide resource. We have generated the following criteria for inclusion of a new Center into SUMMIT: (1) Prospective data collection with visits at least annually; (2) contribution of at least 50 new participants a year, or a total of 300 participants from onset; (3) contribution of at least clinical and MRI data meeting basic feature list as detailed in Table 1; (4) institutionally-approved informed consent forms indicating participant approval that anonymized data be shared outside of the home institution; (5) clearly identified site PI, as well as clinical, MRI and data lead investigators; and (6) approval by the SUMMIT Governance Group.
CONCLUSIONS
In summary, the SUMMIT cohort combines the ongoing efforts of several large referral centers to create a uniform deeply characterized cohort of thousands of patients followed for an extended period of time. Built into the program are independent validation cohorts to ensure the accuracy and generalizability of the findings; to capture any heterogeneity between sites in terms of individual practice patterns, environmental exposures, and ancestry; and to rapidly apply novel diagnostic markers, including newly-developed imaging sequences, as these become available.
In addition to ongoing augmentation of the SUMMIT cohorts at the two North American sites (BWH and UCSF) the addition of new centers to the SUMMIT consortium is in progress. This growth will align a global group of MS centers with proven records of effective collaboration to create the most rigorous long-term cohort possible for open use by the global research community. These centers will benefit from the existing prototype platform for managing, accessing and displaying multi-dimensional patient information. Importantly, additional cohorts would also capture broader racial/ethnic diversity, as well as differences in management (including prescribing patterns).
In the near future, our independent cohorts should provide the much-needed statistical power to evaluate new biomarkers and therapies, particularly with respect to their long-term implications. In the domain of disease prediction, this will include validating existing biomarkers, such as transcription factor Tob142 (previously reported as marking the transition from CIS to RRMS),43 a transcriptional (MSA/MSB) signature from peripheral blood mononuclear cells,44 or lipid antibodies from antigen arrays,45 among others. It will also promote adoption of new serum markers (such as circulating miRNA,46 with mRNA targets integrated into biologically meaningful pathways) and emerging MRI sequences (such as quantitation of regional brain,47, 48 gray matter45, 49–51 and spinal cord atrophy52, 53) that show stronger associations with subsequent disability than traditional measurement of white matter burden. For example, an efficient phase sensitive inversion recovery method termed SF-SIGMA has now been implemented at both sites, permitting automated segmentation of spinal cord grey matter54, 55 shown to be the strongest MRI predictor yet developed for MS disability.56, 57
In the domain of disease treatment, combining fully harmonized, deeply-phenotyped, long-term cohorts will provide power to analyze the impact of a variety of therapies (including therapies which are not FDA-approved for MS) on clinical progression outside of the structure of trial-based cohorts. It should also be possible to monitor adherence to DMTs (likely lower in real-world settings than in clinical trials) and assess its effect on disease progression. Also, the current practice, supported by many payers, to promote step-up therapies rather than aggressive therapies in the initial management of early MS, makes the current SUMMIT cohort an ideal platform to study this “real-world” question. Assessing the value of diagnostic and monitoring tools, including costly imaging studies, on the long-term management of established MS could be another objective. Finally, these robust primary data will also advance development of evidence-based precision medicine and individualized care for the MS patient, through new areas of inquiry such as bioinformatics, machine-learning tools, user-friendly dashboards,58 and remote medicine.
Novel analyses that combine longitudinal clinical and MRI information along with biomarker and genomic (including pharmacogenomic) data enabled by the SUMMIT database and repositories will enable investigators worldwide to access robust primary data to address critical questions about long-term progression of disease and ask new sets of questions as the dynamic landscape of MS prevention and management continues to evolve.
Supplementary Material
Acknowledgments
The authors are grateful to the participants from each cohort who have contributed to our decades-long research efforts, and to the numerous administrative staff, coordinators, and other research personnel. The SUMMIT study was funded by the National Multiple Sclerosis Society, NIH Grant R01NS026799, and collection of the cohorts was also made possible through grants received from the Valhalla Foundation, GlaxoSmithKline, and Biogen.
Disclosures
T.C. has received consulting fees from Biogen-Idec, Novartis, and Roche-Genentech; she serves on advisory boards for pediatric clinical trials for Novartis and Genzyme–Sanofi; and she has received research support from Merck Serono, Biogen-Idec, Verily Life Sciences, and Novartis. B.A.C.C. has received consulting fees from Abbvie, Biogen, EMD Serono, Novartis and Shire. S.L.H. serves on the Scientific Advisory Boards of Annexon, Bionure, Symbiotix, and Molecular Stethoscope, and on the Board of Trustees of Neurona; also reports receiving travel reimbursement and writing assistance from F. Hoffmann-La Roche for CD20-related meetings and presentations. H.L.W. has served as consultant to Genentech and Tiziana Life Sciences, and received research support from EMD Serono, Miragen, Sanofi, Teva, and Verily Life Sciences. L.K. has reveived personal compensation for activities with University Hospital Basel as an advisory board member, has received research support from Swiss Multiple Sclerosis Society, Swiss National Research Foundation, European Union, Gianni Rubatto Foundation, Novartis Research Foundation, and Roche Research Foundation. B.M.J.U. has received personal compensation for consulting from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and Teva. X.M. has received honoraria and travel expenses for speaking at scientific meetings, has been a steering committee member of clinical trials, or participated in advisory boards of clinical trials in the past years with Almirall, Bayer Schering Pharma, Biogen Idec, EMD Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, and Teva Pharmaceutical Industries. M.T. has received compensation for consulting services and speaking honoraria from Bayer, Biogen, Merk-Serono, Teva, Novartis, Sanofi-Aventis, Genzyme and Roche. S.J.K. has received compensation in an editorial capacity for Clinical Immunology and has received research support from Novartis.
Footnotes
SUMMIT Study Group
Principal Investigators: Rohit Bakshi, Sergio Baranzini, Riley Bove, Tanuja Chitnis, Bruce Cree, Philip De Jager, Stephen Hauser, Roland Henry, Jorge Oksenberg, Nikolaos Patsopoulos, Francisco Quintana, Howard Weiner, Alex Rovira, Mar Tintore, Xavier Montalban, Bernard Uitdehaag, Yvonne Nagelin, Ludwig Kappos and Samia Khoury.
CLIMB Investigators: Rohit Bakshi, Riley Bove, Guy Buckle, Tanuja Chitnis, Philip De Jager, Dorlan Kimbrough, Maria Houtchens, Christopher Severson, James Stankiewicz, Bonnie Glanz, Brian Healy, Adrian Ivinson, Mariann Polgar.
EPIC Investigators: Carolyn Bevan, Riley Bove, Elizabeth Crabtree-Hartman, Bruce Cree, Jeffrey Gelfand, Jennifer Graves, Ari Green, Samuel Pleasure, Emmanuelle Waubant, Michael Wilson, Scott Zamvil, Refujia Gomez, Adam Santaniello, Jill Hollenbach, Chris Lin.
UHB Investigators: Yvonne Naegelin, Jens Kuhle, Jens Würfel, Ludwig Kappos.
MSCA Investigators: Frederik Barkhof, Danko Coric, Iris Dekker, Marloes Hagens, Cyra Leurs, Jessica Nielssen, Judith Sonder, Mike Wattjes, Bernard Uitdehaag.
Cemcat Investigators: Mar Tintore, Susana Otero, Georgina Arrambide, Jaume Sastre-Garriga, Manuel Comabella, Jordi Rio, Cristina Auger, Joaquín Castilló, Angela Vidal, Carlos Nos, Patricia Mulero, Luciana Midaglia, Santiago Perez-Hoyos, Alex Rovira, Xavier Montalban.
AMIR Investigators: Samia Khoury, Nabil El Ayoubi, Basem Yamout
References
- 1.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain: a journal of neurology. 2003;126:770–82. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- 2.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. The New England journal of medicine. 2000;343:1430–8. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 3.Debouverie M, Pittion-Vouyovitch S, Louis S, Guillemin F. Natural history of multiple sclerosis in a population-based cohort. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2008;15:916–21. doi: 10.1111/j.1468-1331.2008.02241.x. [DOI] [PubMed] [Google Scholar]
- 4.Pittock SJ, Mayr WT, McClelland RL, et al. Disability profile of MS did not change over 10 years in a population-based prevalence cohort. Neurology. 2004;62:601–6. doi: 10.1212/wnl.62.4.601. [DOI] [PubMed] [Google Scholar]
- 5.Tremlett H, Paty D, Devonshire V. The natural history of primary progressive MS in British Columbia, Canada. Neurology. 2005;65:1919–23. doi: 10.1212/01.wnl.0000188880.17038.1d. [DOI] [PubMed] [Google Scholar]
- 6.Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology. 2006;66:172–7. doi: 10.1212/01.wnl.0000194259.90286.fe. [DOI] [PubMed] [Google Scholar]
- 7.Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology. 2010;74:2004–15. doi: 10.1212/WNL.0b013e3181e3973f. [DOI] [PubMed] [Google Scholar]
- 8.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain: a journal of neurology. 1989;112(Pt 1):133–46. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- 9.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain: a journal of neurology. 1989;112(Pt 6):1419–28. doi: 10.1093/brain/112.6.1419. [DOI] [PubMed] [Google Scholar]
- 10.Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. The New England journal of medicine. 2002;346:158–64. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 11.Cree BA, Hartung HP. Steering through complexity: management approaches in multiple sclerosis. Curr Opin Neurol. 2016;29:263–71. doi: 10.1097/WCO.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 12.Prosperini L, Gallo V, Petsas N, Borriello G, Pozzilli C. One-year MRI scan predicts clinical response to interferon beta in multiple sclerosis. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2009;16:1202–9. doi: 10.1111/j.1468-1331.2009.02708.x. [DOI] [PubMed] [Google Scholar]
- 13.Romeo M, Martinelli-Boneschi F, Rodegher M, Esposito F, Martinelli V, Comi G. Clinical and MRI predictors of response to interferon-beta and glatiramer acetate in relapsing-remitting multiple sclerosis patients. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2013;20:1060–7. doi: 10.1111/ene.12119. [DOI] [PubMed] [Google Scholar]
- 14.Trojano M, Pellegrini F, Paolicelli D, et al. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Annals of neurology. 2009;66:513–20. doi: 10.1002/ana.21757. [DOI] [PubMed] [Google Scholar]
- 15.Butzkueven H, Chapman J, Cristiano E, et al. MSBase: an international, online registry and platform for collaborative outcomes research in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2006;12:769–74. doi: 10.1177/1352458506070775. [DOI] [PubMed] [Google Scholar]
- 16.Jokubaitis VG, Spelman T, Kalincik T, et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Annals of neurology. 2016 doi: 10.1002/ana.24682. [DOI] [PubMed] [Google Scholar]
- 17.Baranzini SE, Galwey NW, Wang J, et al. Pathway and network-based analysis of genome-wide association studies in multiple sclerosis. Human molecular genetics. 2009;18:2078–90. doi: 10.1093/hmg/ddp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauthier SA, Glanz BI, Mandel M, Weiner HL. A model for the comprehensive investigation of a chronic autoimmune disease: the multiple sclerosis CLIMB study. Autoimmun Rev. 2006;5:532–6. doi: 10.1016/j.autrev.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Polman CH, Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology. 2010;74(Suppl 3):S8–15. doi: 10.1212/WNL.0b013e3181dbb571. [DOI] [PubMed] [Google Scholar]
- 20.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of neurology. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 21.Cree BA, Gourraud PA, Oksenberg JR, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Annals of neurology. 2016;80:499–510. doi: 10.1002/ana.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tintore M, Rovira A, Rio J, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain: a journal of neurology. 2015;138:1863–74. doi: 10.1093/brain/awv105. [DOI] [PubMed] [Google Scholar]
- 23.Goodin DS, Traboulsee A, Knappertz V, et al. Relationship between early clinical characteristics and long term disability outcomes: 16 year cohort study (follow-up) of the pivotal interferon beta-1b trial in multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry. 2012;83:282–7. doi: 10.1136/jnnp-2011-301178. [DOI] [PubMed] [Google Scholar]
- 24.Bove RM, Healy B, Augustine A, Musallam A, Gholipour T, Chitnis T. Effect of gender on late-onset multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2012;18:1472–9. doi: 10.1177/1352458512438236. [DOI] [PubMed] [Google Scholar]
- 25.Trojano M, Pellegrini F, Fuiani A, et al. New natural history of interferon-beta-treated relapsing multiple sclerosis. Annals of neurology. 2007;61:300–6. doi: 10.1002/ana.21102. [DOI] [PubMed] [Google Scholar]
- 26.Bevan CJ, Cree BA. Disease activity free status: a new end point for a new era in multiple sclerosis clinical research? JAMA neurology. 2014;71:269–70. doi: 10.1001/jamaneurol.2013.5486. [DOI] [PubMed] [Google Scholar]
- 27.Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA neurology. 2015;72:152–8. doi: 10.1001/jamaneurol.2014.3537. [DOI] [PubMed] [Google Scholar]
- 28.Kappos L, De Stefano N, Freedman MS, et al. Inclusion of brain volume loss in a revised measure of ‘no evidence of disease activity’ (NEDA-4) in relapsing-remitting multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2016;22:1297–305. doi: 10.1177/1352458515616701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keshavan A, Paul F, Beyer MK, et al. Power estimation for non-standardized multisite studies. NeuroImage. 2016;134:281–94. doi: 10.1016/j.neuroimage.2016.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patsopoulos NA, Barcellos LF, Hintzen RQ, et al. Fine-mapping the genetic association of the major histocompatibility complex in multiple sclerosis: HLA and non-HLA effects. PLoS Genet. 2013;9:e1003926. doi: 10.1371/journal.pgen.1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patsopoulos NA, Esposito F, Reischl J, et al. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Annals of neurology. 2011;70:897–912. doi: 10.1002/ana.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–9. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beecham AH, Patsopoulos NA, Xifara DK, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45:1353–60. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masterman T, Ligers A, Olsson T, Andersson M, Olerup O, Hillert J. HLA-DR15 is associated with lower age at onset in multiple sclerosis. Ann Neurol. 2000;48:211–9. [PubMed] [Google Scholar]
- 35.Cree BA, Reich DE, Khan O, et al. Modification of Multiple Sclerosis Phenotypes by African Ancestry at HLA. Arch Neurol. 2009;66:226–33. doi: 10.1001/archneurol.2008.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Jager PL, Chibnik LB, Cui J, et al. Integration of genetic risk factors into a clinical algorithm for multiple sclerosis susceptibility: a weighted genetic risk score. Lancet Neurol. 2009;8:1111–9. doi: 10.1016/S1474-4422(09)70275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensiek AE, Sawcer SJ, Feakes R, et al. HLA-DR 15 is associated with female sex and younger age at diagnosis in multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry. 2002;72:184–7. doi: 10.1136/jnnp.72.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Z, White CC, Owen EK, et al. GEMS Project: A Platform to Investigate Multiple Sclerosis Risk. Annals of neurology. 2015 doi: 10.1002/ana.24560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller SG, Weiner MW, Thal LJ, et al. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin N Am. 2005;15:869–77. xi–xii. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–81. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long MT, Fox CS. The Framingham Heart Study–67 years of discovery in metabolic disease. Nat Rev Endocrinol. 2016;12:177–83. doi: 10.1038/nrendo.2015.226. [DOI] [PubMed] [Google Scholar]
- 42.Schulze-Topphoff U, Casazza S, Varrin-Doyer M, et al. Tob1 plays a critical role in the activation of encephalitogenic T cells in CNS autoimmunity. The Journal of experimental medicine. 2013;210:1301–9. doi: 10.1084/jem.20121611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corvol JC, Pelletier D, Henry RG, et al. Abrogation of T cell quiescence characterizes patients at high risk for multiple sclerosis after the initial neurological event. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11839–44. doi: 10.1073/pnas.0805065105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ottoboni L, Keenan BT, Tamayo P, et al. An RNA profile identifies two subsets of multiple sclerosis patients differing in disease activity. Science translational medicine. 2012;4:153ra31. doi: 10.1126/scitranslmed.3004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakshi R, Yeste A, Patel B, et al. Serum lipid antibodies are associated with cerebral tissue damage in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e200. doi: 10.1212/NXI.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gandhi R, Healy B, Gholipour T, et al. Circulating MicroRNAs as biomarkers for disease staging in multiple sclerosis. Annals of neurology. 2013;73:729–40. doi: 10.1002/ana.23880. [DOI] [PubMed] [Google Scholar]
- 47.Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69:1213–23. doi: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]
- 48.Rocca MA, Mesaros S, Pagani E, Sormani MP, Comi G, Filippi M. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology. 2010;257:463–9. doi: 10.1148/radiol.10100326. [DOI] [PubMed] [Google Scholar]
- 49.Chu R, Tauhid S, Glanz BI, et al. Whole Brain Volume Measured from 1.5T versus 3T MRI in Healthy Subjects and Patients with Multiple Sclerosis. J Neuroimaging. 2016;26:62–7. doi: 10.1111/jon.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudick RA, Lee JC, Nakamura K, Fisher E. Gray matter atrophy correlates with MS disability progression measured with MSFC but not EDSS. Journal of the neurological sciences. 2009;282:106–11. doi: 10.1016/j.jns.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Annals of neurology. 2008;64:255–65. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- 52.Horsfield MA, Sala S, Neema M, et al. Rapid semi-automatic segmentation of the spinal cord from magnetic resonance images: application in multiple sclerosis. NeuroImage. 2010;50:446–55. doi: 10.1016/j.neuroimage.2009.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen AB, Neema M, Arora A, et al. The relationships among MRI-defined spinal cord involvement, brain involvement, and disability in multiple sclerosis. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2012;22:122–8. doi: 10.1111/j.1552-6569.2011.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papinutto N, Schlaeger R, Panara V, et al. Age, gender and normalization covariates for spinal cord gray matter and total cross-sectional areas at cervical and thoracic levels: A 2D phase sensitive inversion recovery imaging study. PloS one. 2015;10:e0118576. doi: 10.1371/journal.pone.0118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papinutto N, Schlaeger R, Panara V, et al. 2D phase-sensitive inversion recovery imaging to measure in vivo spinal cord gray and white matter areas in clinically feasible acquisition times. Journal of magnetic resonance imaging: JMRI. 2015;42:698–708. doi: 10.1002/jmri.24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schlaeger R, Papinutto N, Zhu AH, et al. Association Between Thoracic Spinal Cord Gray Matter Atrophy and Disability in Multiple Sclerosis. JAMA neurology. 2015;72:897–904. doi: 10.1001/jamaneurol.2015.0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlaeger R, Papinutto N, Panara V, et al. Spinal cord gray matter atrophy correlates with multiple sclerosis disability. Annals of neurology. 2014;76:568–80. doi: 10.1002/ana.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: The multiple sclerosis BioScreen. Annals of neurology. 2014;76:633–42. doi: 10.1002/ana.24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finkelstein DM. A proportional hazards model for interval-censored failure time data. Biometrics. 1986;42:845–54. [PubMed] [Google Scholar]
- 60.Healy BC, Liguori M, Tran D, et al. HLA B*44: protective effects in MS susceptibility and MRI outcome measures. Neurology. 2010;75:634–40. doi: 10.1212/WNL.0b013e3181ed9c9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


