Abstract
Objectives
Binaural pitch fusion is the fusion of stimuli that evoke different pitches between the ears into a single auditory image. Individuals who use hearing aids or bimodal cochlear implants (CIs) experience abnormally broad binaural pitch fusion, such that sounds differing in pitch by as much as 3–4 octaves are fused across ears, leading to spectral averaging and speech perception interference. The goal of this study was to determine if adult bilateral CI users also experience broad binaural pitch fusion.
Design
Stimuli were pulse trains delivered to individual electrodes. Fusion ranges were measured using simultaneous, dichotic presentation of reference and comparison stimuli in opposite ears, and varying the comparison stimulus to find the range that fused with the reference stimulus.
Results
Bilateral CI listeners had binaural pitch fusion ranges varying from 0–12 mm (average 6.1 ± 3.9 mm), where 12 mm indicates fusion over all electrodes in the array. No significant correlations of fusion range were observed with any subject factors related to age, hearing loss history, or hearing device history, or with any electrode factors including interaural electrode pitch mismatch, pitch-match bandwidth, or within-ear electrode discrimination abilities.
Conclusions
Bilateral CI listeners have abnormally broad fusion, similar to hearing aid and bimodal CI listeners. This broad fusion may explain the variability of binaural benefits for speech perception in quiet and in noise in bilateral CI users.
Keywords: pitch, fusion, binaural, bilateral cochlear implants
Introduction
Binaural fusion is the fusion of auditory stimuli across the ears into a single auditory object, analogous to the fusion of visual stimuli to the two eyes into a single image. Examples of binaural fusion include the fusion of stimuli with different arrival latencies (interaural time differences) or different spectral and temporal characteristics (speech fusion; Cutting, 1976). Here we describe another specific instance of fusion, binaural pitch fusion, which occurs when stimuli (such as tones) that evoke different pitches with each ear individually are fused into a single auditory object when played to both ears simultaneously. An associated phenomenon is the creation of a new pitch from the fusion of the monaurally heard pitches, which is often a weighted average of the two original pitches, i.e. not always in the middle between the two original pitches but anywhere in between (Reiss et al., 2014).
In acoustic hearing, binaural pitch fusion can be characterized by the frequency range of tones in one ear over which fusion occurs with a tone in the other ear, which we shall call the binaural fusion range for that tone and ear. Normal-hearing listeners exhibit “sharp fusion”, in which only tones in a narrow frequency range around the contralateral tone frequency are fused, i.e. the binaural fusion ranges are small, on the order of 0.1–0.2 octaves (Thurlow and Bernstein, 1957; Odenthal, 1963; van den Brink, 1976; Reiss et al., 2017).
Recent studies have shown that hearing-impaired individuals can exhibit much broader pitch fusion, fusing tones differing by as much as 3–4 octaves between ears. This “broad fusion”, characterized by binaural fusion ranges on the order of octaves, has been shown in both bilateral hearing aid (HA) users (Reiss et al., 2017) and bimodal cochlear implant (CI) users, who wear a CI in one ear and a HA in the other ear (Reiss et al., 2014). In addition, in both groups, broad fusion led to the averaging of different pitches between the ears for fused stimulus pairs (Reiss et al., 2014; Oh and Reiss, 2017). Such pitch averaging can lead to detrimental averaging of spectral information between the ears in hearing-impaired listeners, as has been shown for vowel perception in both bilateral HA and bimodal CI users (Reiss et al., 2016).
Broad binaural pitch fusion is associated with early onset and long durations of hearing loss in bilateral HA users (Reiss et al., 2017), suggesting a potential role of long-term experience with hearing loss and amplification in the development of broad fusion. This also suggests that broad fusion in CI users may be inherited from prior long-term experience with HAs. Fusion has also been suggested as a mechanism for minimizing the perception of diplacusis, in which different pitches are heard for the same tone under sequential presentation in the two ears, in normal-hearing listeners (van den Brink, 1976). Broad fusion is weakly correlated with pitch mismatch introduced by CI programming in bimodal CI users, suggesting that the brain may adapt to prevent the perception of pitch mismatches between electrode arrays by increasing fusion (Reiss et al., 2014). Bilateral CI users often have pitch mismatches between clinically paired electrodes due to large surgical variability in insertion depth of the electrode into the cochlea, with insertion depths varying between 8–21 mm (Lee et al., 2010) or cochlear place frequencies between 500–8000 Hz (Greenwood, 1990). Pitch mismatches in bilateral CI users may also arise due to asymmetries in nerve survival between the ears. These pitch mismatches can persist with experience (e.g. Aronoff et al., 2016; Kan et al., 2013), so it is possible that bilateral CI users also adapt to pitch mismatches via changes in fusion instead of pitch. In other words, it may be easier for the brain to adapt the range of binaural fusion than to re-map place pitch perception in tonotopic maps. Alternatively, pre-existing broad fusion inherited from prior experience with bilateral HAs and then bimodal CIs may prevent adaptation to pitch mismatch.
In electric hearing, the binaural fusion range would be the range of electrodes in one ear over which fusion occurs with a single electrode in the other ear. In the current study, binaural fusion ranges were measured in bilateral CI users, who use CIs in both ears, to determine whether this population exhibits broad binaural fusion similar to bilateral hearing aid users and bimodal CI users. Fusion range results were compared with various subject-specific and electrode-specific factors to examine potential relationships of these factors to broad fusion. First, the potential role of hearing history and hearing device experience was evaluated by correlation with the subject-specific factors of onset age of hearing loss, duration of moderate-to-severe hearing loss, and durations of bilateral HA, bimodal/unilateral, and bilateral CI use. A potential relationship of fusion ranges to diplacusis was evaluated by correlation with the electrode-specific factors of interaural electrode pitch mismatch and pitch match bandwidth. Relationships of fusion to overall age and peripheral resolution (as measured by electrode pitch ranking abilities) were also evaluated.
Materials and Methods
Subjects
These studies were conducted according to the guidelines for the protection of human subjects as set forth by the Institutional Review Board (IRB) of Oregon Health & Sciences University (OHSU), and the methods employed were approved by that IRB. Eighteen adult subjects with bilateral cochlear implants participated in this study. All subjects were screened for normal cognitive function using the Mini Mental Status Examination with a minimum score of 25 out of 30 required to qualify (MMSE; Folstein et al., 1975; Souza et al., 2007). Subject factors were obtained through self-report in a questionnaire as well as from medical records. The subjects’ demographic information including age at time of testing, gender, duration of HA use prior to the first CI, duration of CI use for each ear, duration of bilateral CI use, and CI internal model(s) are shown in Table 1.
Table 1.
Demographic information for bilateral cochlear implant (CI) listeners: age, gender, duration of hearing aid (HA) use prior to the first CI, duration of CI use in each ear, duration of unilateral CI use, duration of bilateral CI use, CI internal model, pitch ranking score, minimum discriminable electrode spacing, and reference ear used in testing.
| Subj. ID | Age (yrs) | Gender | Dur. HA use before first CI (yrs) | Dur. CI use (yrs, L;R if diff.) | Dur. uni-lateral (yrs) | Dur. bi-modal (yrs) | Dur. bi-lateral (yrs) | CI internal device (L; R if diff.) | Pitch ranking score (%, L; R) | Min. discrimin. electrode spacing(mm, L; R) | Ref. Ear |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BI01 | 65 | M | 5 | 4.5; 3.0 | 1.5 | 1.5 | 3 | CI512; CI24RE | 91.8; 91.4 | 3.0; 3.0 | R |
| BI02 | 56 | M | 22 | 2.3; 2.1 | 0.2 | 0 | 2.1 | CI422 | 96.4; 91.8 | 1.2; 3.6 | R |
| BI03 | 46 | F | 9 | 5.5; 6.2 | 0.7 | 0.2 | 5.5 | CI512; CI24RE | 96.4; 98.6 | 1.6; 1.2 | R |
| BI04 | 56 | M | 0 | 5.3; 4.9 | 0.4 | 0 | 4.9 | CI512 | 79.9; 80.0 | 10.8; 8.3 | R |
| BI05 | 54 | F | 13 | 10.5; 3.8 | 6.6 | 0 | 3.8 | CI512; CI24RE | NR; 94.6 | NR; 1.2 | L |
| BI06 | 49 | F | 5 | 1.7; 1.3 | 0.4 | 0 | 1.3 | CI24RE | 89.1; 88.3 | 5.8; 4.8 | R |
| BI08 | 53 | M | 33 | 3.1; 0.5 | 2.6 | 2.3 | 0.5 | F28; Std Concerto | NR; 96.8 | NR; 3.8 | L |
| BI09 | 18 | M | 1.4 | 17; 11 | 6 | 1.6 | 11 | CI24R; CI24M | 68.0; 100 | 11.6; 1.2 | L |
| BI10 | 48 | M | 40 | 1.6; 2.9 | 1.4 | 0.3 | 1.6 | Std; F28 Concerto | 95.5; 96.2 | 5.1; 5.9 | R |
| BI11 | 23 | M | 13 | 13.5; 6.9 | 6.6 | 6 | 6.9 | CI24RE; CI24M | NR; 86.7 | NR; 4.6 | L |
| BI12 | 51 | M | 43 | 5.9; 15 | 9.1 | 7 | 5.8 | Std C40+; Std Sonata | 100; 98.6 | 1.9; 2.5 | L |
| BI13 | 19 | F | 0.1 | 5.4; 17 | 11.6 | 0 | 5.4 | Std C40+; Std Sonata | 85.5; NR | 6.1; NR | R |
| BI14 | 52 | F | 2 | 6.7; 13 | 6.3 | 0 | 6.7 | CI24R; CI24RE | 87.2; 92.3 | 4.2; 3.8 | L |
| BI15 | 54 | F | 0.5 | 1.7; 2.2 | 0.5 | 0 | 1.7 | CI24RE | 90.0; 94.6 | 4.7; 2.4 | L |
| BI17 | 24 | M | 0.25 | 20; 8 | 12 | 0 | 8 | CI24RE | 57.3; 63.6 | 13.2; 11.0 | R |
| BI19 | 64 | F | 0.5 | 2; 15 | 13 | 0 | 15 | CI422; CI24R | NR; NR | NR; NR | L |
| BI20 | 29 | M | 13 | 7; 7 | 7 | 1 | 7 | CI24R; CI24RE | 95.0; 92.3 | 2.5; 3.3 | L |
| BI21 | 64 | M | 2 | 11; 1.5 | 11 | 0 | 1.5 | CI24R; CI422 | 99.1; 96.5 | 1.2; 1.5 | R |
| Avg | 45.8 | 11.3 | 7.1; 6.4 | 5.4 | 1.1 | 4.0 | |||||
| Std | 15.9 | 14.1 | 6.0; 4.8 | 4.6 | 2.1 | 2.9 |
Std=Standard; F28=Flex28; NR = no data collected for that ear; R = right; and L=left. CI24R, CI24RE, and CI512 are all modiolar arrays. CI24M, CI422, Flex28, and Standard are all straight lateral wall arrays.
Stimuli and Procedures
All experiments were conducted in a double-walled, sound attenuated booth. Electric stimuli were delivered via computer directly to the CI using custom research software and hardware for each company. For Cochlear (Sydney, Australia), NIC2 software interface was used with two L34 processors, programming pods, and a synchronizing connection between the pods. For MED-EL (Innsbruck, Austria), RIB2 software interface and National Instruments (Austin, Texas) PCIe-6351 card were used to control stimulation through a research interface box with dual outputs. Stimuli consisted of biphasic pulse trains presented at 1200 pulses per second, using a monopolar ground. Loudness balancing was conducted prior to starting experiments. Electric pulse trains of 300 msec duration were initialized to “medium loud and comfortable” levels corresponding to 6 on a visual loudness scale from 0 (no sound) to 10 (too loud). Loudness was then adjusted for each electrode to be equally loud to a fixed electrode in the middle of the electrode array during sequential presentation both within and across the ears. For Cochlear, up to 11 even-numbered electrodes were tested, while for MED-EL, up to 12 electrodes were tested due to the wider electrode spacing (ranging from 1.9–2.4 mm for MED-EL compared to 0.4–0.9 mm for Cochlear). Only electrodes normally activated (i.e. no deactivated electrodes) in the subjects’ clinical programs were tested. Specifically, BI08, BI11, and BI12 had 3, 1, and 3 basal test electrodes deactivated, and BI13 had an apical electrode deactivated, in the clinical programs. Hence, these electrodes were also deactivated for testing in these experiments.
Within-ear electrode pitch discrimination was measured to assess peripheral resolution, which may be related to fusion. Electrode discrimination was measured using a pitch ranking test in which all possible pairs of electrodes were presented twice, in a two-interval, two-alternative forced choice procedure. In each trial, 500-msec pulse trains were delivered sequentially within one ear, with a 500-msec interstimulus interval. The interval order was randomized. The subject was asked to indicate which stimulus had the higher pitch. The total number of trials depended on the number of electrodes tested in that ear, and equaled two repeats times the sum of all possible combinations or 2 x (N-1)(N)/2, where N=the number of electrodes, for a total of up to 110 trials for Cochlear and up to 132 trials for MED-EL. Pitch ranking scores were computed as the percentage of responses where the more basal electrode was selected as higher in pitch, and are shown in Table 1. In order to equalize scores across devices with different electrode spacing, a percent correct versus electrode spacing curve was generated (for example, see Suppl. Fig. 1), and the minimum electrode spacing for 90% correct was calculated and converted to mm. Results were averaged over two repeat runs for each ear.
All listeners then performed interaural pitch matching and dichotic fusion range measurements as described below. First, a reference ear was selected for each subject. Thirteen subjects did the electrode discrimination task in both ears. If electrode discrimination performance was similar (within 5–7%) across ears, the reference ear was chosen randomly (twelve out of thirteen subjects); otherwise, the ear with worse electrode discrimination was assigned as the reference ear (the second implanted ear in one subject, BI09, with a score of 68% versus 100% in the first implanted ear). In five cases where electrode discrimination data was not present for both ears, the subject’s non-preferred ear (in all cases, the ear that was implanted second) was used as the reference ear. The worse ear was assigned to be the reference ear so that the resolution of comparison electrode testing would be maximized using the better ear, instead of limited by the worse ear. Reference ears are shown in Table 1. The contralateral ear was the comparison ear.
Up to three reference electrodes were tested; electrodes 18, 12, and 6 for Cochlear subjects and electrodes 5, 7, and 9 for MED-EL subjects. Subjects were given practice trials with feedback before each procedure. For both procedures, the method of constants was used, where the reference electrode was fixed and the comparison electrode varied in a pseudorandom (subset of Latin square) sequence across trials in order to average out sequence effects (for example, see Reiss et al., 2012). The comparison electrodes consisted of the electrodes that were loudness balanced in the loudness balancing procedure. The number of trials equaled the number of comparison electrodes times ten repeats for each electrode, for a total of up to 110 trials for Cochlear devices and 120 trials for MED-EL devices. At least two repeat runs were performed for each reference electrode and procedure, with order of runs randomized.
Dichotic fusion range measurements were performed to measure the fusion range, or the range of electrodes in one ear that were fused with a single electrode in the other ear during simultaneous presentation. In each trial, a 1500-msec pulse train was delivered to the reference electrode in the reference ear simultaneously with a comparison electrode in the contralateral ear. The subject was asked to indicate whether a single fused sound or two different sounds were heard, and given a matrix of five different buttons, so that there were five different options for this response (Reiss et al., 2014). If a single sound was heard, subjects were instructed to indicate whether they heard that sound in both ears (“Same”), in the left ear (“Left only”), or in the right ear (“Right only”). If two different sounds were heard, subjects were instructed to indicate which ear had the higher pitch (“Left higher” or “Right higher”). A “Repeat” button was also provided to allow subjects to listen to the stimuli again.
Fusion functions were generated as follows: Responses corresponding to fused sounds were assigned a value of 1 (“Same”, “Left only” and “Right only”). Responses corresponding to non-fused sounds were assigned a value of 0 (“Left higher” or “Right higher”). Values were averaged over all trials to generate a fusion function (solid lines with circles in Fig. 1D–F). The fusion range was defined as the range where averaged fusion values were above 0.5 (range between vertical lines in Fig. 1D–F), i.e. electrodes were fused more than 50% of the time.
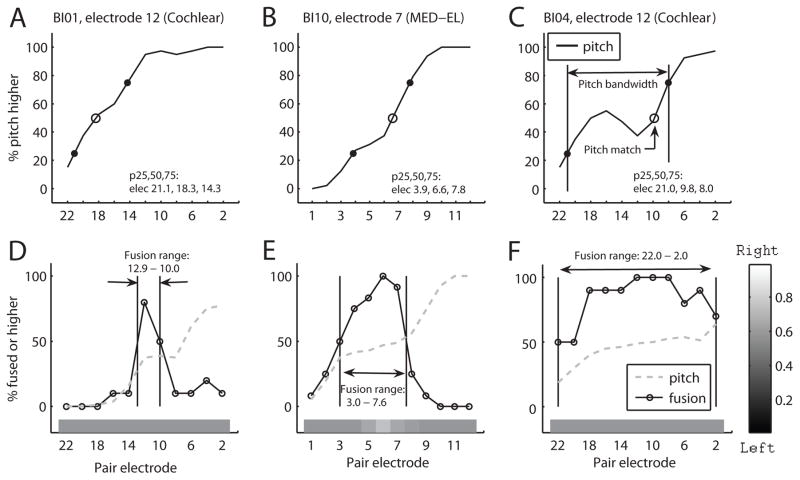
Figure 1.
Example pitch match and fusion range functions for three representative bilateral cochlear implant (CI) users. Functions were calculated using values ranging from 0 to 1, and multipled by 100 to show as percentages. Top row: Pitch match functions which indicate the proportion that the contralateral electrode was higher in pitch than the reference electrode. The pitch match is defined as the 50% point (open circle) and the pitch bandwidth is defined as the difference between the 25% and 75% points (small filled circles). Bottom row: Fusion functions. Solid curves indicate the proportion that each contralateral electrode was fused with the reference. Dashed gray lines indicate how often each contralateral electrode was heard as higher in pitch in the fusion task when two sounds were heard. Grayscale bars below the fusion functions in the bottom row indicate reported lateralization, with white indicating lateralization to the right and black indicating lateralization to the left. The fusion range is the range of contralateral electrodes that fused with the reference electrode more than 50% of the time.
Across-ear pitch comparisons recorded when two sounds were heard were also analyzed to generate “dichotic pitch” functions as follows. Responses were assigned a value of 0 when the comparison ear was lower in pitch than the reference ear (“Left higher” if the reference ear was the left ear, and vice versa), a value of 1 when the comparison ear was higher in pitch than the reference ear (“Right higher” if the reference ear was the left ear, and vice versa), and a value of 0.5 when only one sound was heard. Values were averaged across all trials to generate a dichotic pitch function (dashed gray lines in Fig. 1D–F).
Interaural pitch matches were performed to measure the degree of interaural mismatch in electrode pitch across the ears. In each trial, 500-msec pulse trains were delivered sequentially to the reference electrode in the reference ear and to the comparison electrode in the contralateral ear, with a 500-msec interstimulus interval and interval order randomized. The subject was asked to indicate which stimulus had the higher pitch, in a two-alternative forced choice procedure. Pitch matches were computed as the 50% point on the psychometric function generated from the average of the responses for each comparison electrode (open circles in Fig. 1A–C). The bandwidth of these pitch-matches was defined as the distance between the 25% and 75% points in the function (distance between the two filled circles in each plot in Fig. 1A–C).
In order to compare pitch match and fusion range results across devices with different inter-electrode distances, results in units of electrodes were converted into mm (measured between the centers of adjacent electrodes) using the following inter-electrode distances: 1.9 and 2.4 mm for MED-EL Flex 28 and Standard arrays, 0.75 mm for Cochlear CI24M, and variable distances between 0.4–0.81 for Cochlear CI24R/CI24RE/CI512 and between 0.85–0.95 for Cochlear CI422 arrays.
Results
Figure 1 shows example pitch match and fusion functions for three bilateral CI users. Pitch match functions are shown in the top row, with an open circle representing the pitch match (50% point) and small filled circles representing the 25% and 75% points, from which the pitch bandwidth is calculated. Fusion functions are shown in the bottom row, as solid lines with circle symbols. Vertical lines indicate 50% points on the fusion function used to calculate the fusion range. The monotonically increasing gray dotted lines also shown in the bottom row indicate the dichotic pitch functions (not to be confused with pitch match functions). The dichotic pitch functions indicate the percent of time that the comparison electrode was higher in pitch than the reference electrode when electrodes were not fused, and show that the subjects were generally able to indicate which ear had the higher pitch outside of the fusion range.
The three columns represent the three subjects as examples of narrow (<2 electrodes; Fig. 1D), medium (half of the array; Fig. 1E), and broad (over nearly the whole array; Fig. 1F) fusion ranges, respectively. Most subjects were able to pitch match the reference electrode to the contralateral ear; BI10 and BI04 had pitch matches within 3 electrodes which is equivalent to 1.5 mm (1.32 and 1.06 mm, respectively; Fig. 1B–C), while BI01 had a large interaural electrode mismatch of electrode 12 to electrode 18 (3.16 mm; Fig. 1A). All fusion functions showed a peak approximately centered on the reference electrode.
Occasionally, when sounds were fused, subjects were only able to hear the sound in one ear (even if the sounds were loudness balanced when presented sequentially). In those cases, subjects chose the ear that the sound was perceived in, providing additional data on lateralization, expressed as the fraction of trials in which a fused sound was lateralized to the right (shaded bars at bottom of Fig. 1D–F; white indicates lateralization to the right and black indicates lateralization to the left). Some lateralization to the right ear (the reference ear) was observed for subject BI10 (Fig. 1E) near the middle of the fusion range where fusion was strongest, for all electrodes tested. Overall, lateralization was observed for at least one electrode in 5 of the 18 subjects (BI10, BI14, BI15, BI17, and BI19). For some subjects with broad fusion, lateralization direction depended on the paired electrode. Subject BI14 lateralized electrodes 18 and 12 paired with more apical electrodes to the reference ear, and electrode 6 paired with basal electrodes to the contralateral ear. Subject BI19 showed the opposite trend, lateralizing electrode 6 paired with basal electrodes to the reference ear. For other subjects, lateralization was in the same direction for all paired electrodes; BI15 and BI17 both lateralized electrode 18 over a broad fusion range to the comparison ear.
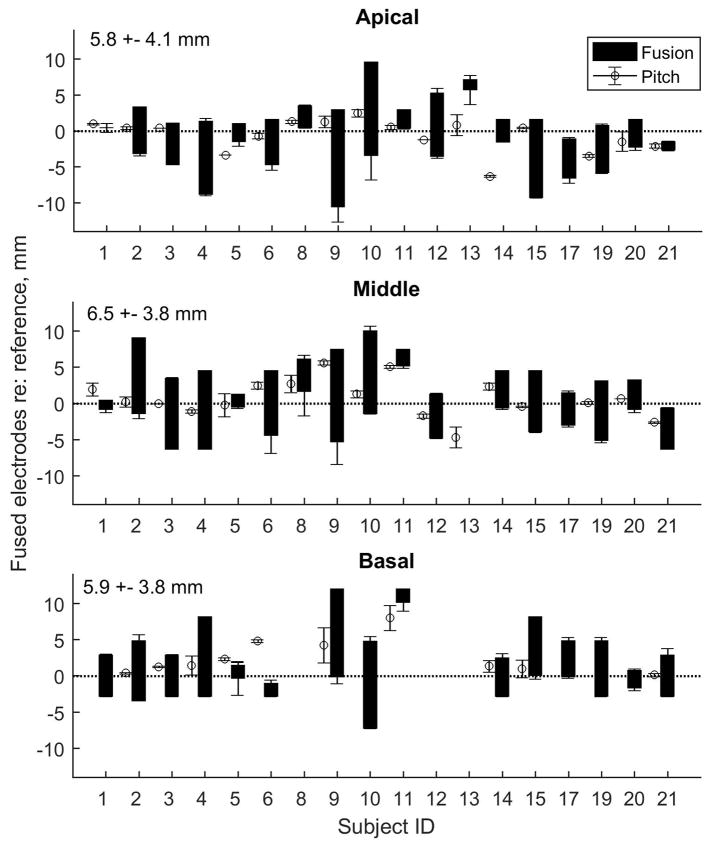
Figure 2 shows the summary results for all bilateral CI listeners. The top, middle, and bottom rows show individual results for apical, middle, and basal reference electrodes, respectively. Electrode pitch matches are shown as open circles and fusion ranges are shown as black bars; these correspond to the measurements from the top and bottom rows of Figure 1, respectively. Units are in mm relative to the reference electrode (indicated by dotted horizontal line), such that apical distances are positive (above the line) and basal distances are negative (below the line). A range of interaural electrode pitch mismatches (deviations of circles from horizontal dotted line) is evident across subjects as well as with electrode location. Similarly, fusion range varies across subjects and electrode locations (heights of black bars).
Figure 2.
Interaural pitch match and binaural fusion range results for all subjects. The top, middle, and bottom rows show individual results for all subjects for apical, middle, and basal reference electrodes (18, 12, and 6 for Cochlear and 5, 7, and 9 for MED-EL), respectively. Dotted horizontal lines indicate the reference electrode. Fusion ranges are shown as black bars, with across-trial standard deviations of endpoints shown. Interaural pitch match 50% points for each reference electrode are shown as open circles, with across-trial standard deviations also shown. Some subjects were not able to pitch match, indicated by the absence of a circle symbol. Mean values across subjects are shown in text at the top left of the subplot for each electrode region.
Most subjects had similar fusion range widths across the array, though one subject, BI06, had fusion differing across electrodes with broad fusion for the apical and middle electrodes and narrow fusion for the basal electrode. BI13 did not fuse any electrodes at all for the middle and basal electrodes. Bilateral CI electrode fusion ranges ranged from 0 to 12 mm, with average fusion ranges of 5.8, 6.5, and 5.9 mm for the apical, middle, and basal electrodes, and standard deviations of 4.1, 3.8, and 3.8 mm, respectively. These average fusion ranges correspond to fusion of one electrode with approximately 2 MED-EL or 8–10 Cochlear electrodes in the other ear. Because of the non-normality of the fusion range data (assessed using a Kolmogorov-Smirnov test, p>0.05), the 2-way Friedman test was used instead of a repeated measures ANOVA to evaluate effects of electrode location on fusion range. No significant effects of electrode region were observed (χ2=1.677, p=0.432).
Figure 2 shows that in most cases, the fusion ranges overlapped with interaural electrode pitch match, such that pitch-matched electrodes were fused together. The exceptions were for BI01 (middle electrode), BI05 (apical), BI06 (basal), BI13 (all electrodes), and BI14 (apical). Note that in some cases, such as BI08 (apical and middle), BI11 (middle and basal), and BI21 (apical and middle), fusion ranges overlapped with pitch-matched electrodes, but did not overlap with the reference electrode (0 or dotted line in panels in Fig. 2). The reference electrode fused binaurally with the pitch-matched electrode, but not the electrode matched in number. There were also some cases in which no pitch matches could be obtained, and so are not shown (indicated by the absence of a symbol for that subject in Fig. 2): BI01 (basal), BI04 (apical), BI10 (basal), BI13 (basal), BI17 (all electrodes), BI19 (basal), and BI20 (basal). These were mostly basal electrodes for which the reference electrode pitch was out of the pitch range of the contralateral electrodes and could not be pitch matched. No pitch match or fusion range data was obtained for BI08 or BI12 for basal electrodes.
Table 2 shows correlations of fusion range results with subject-specific and electrode-specific factors. Because of the non-normality of the fusion range data, two-tailed Spearman correlation tests were used for all correlation analyses with Bonferroni corrections for a total of 13 tests of fusion range (p<0.004 required for significance).
Table 2.
Table of correlations of fusion results with various subject- and electrode-specific variables for bilateral CI listeners.
| Apical | Middle | Basal | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Subject-specific factors | ||||||
| Age | −0.14 | 0.563 | 0.02 | 0.945 | 0.16 | 0.662 |
| Duration of M/S hearing loss up to first CI | −0.01 | 0.984 | 0.05 | 0.842 | −0.09 | 0.803 |
| Age of HL onset | −0.08 | 0.760 | 0.11 | 0.662 | −0.02 | 0.960 |
| Duration of bilateral HA use | −0.09 | 0.714 | −0.20 | 0.421 | −0.22 | 0.411 |
| Duration of bimodal CI use | 0.03 | 0.883 | −0.08 | 0.741 | 0.05 | 0.886 |
| Duration of unilateral CI use | −0.36 | 0.142 | −0.53 | 0.023 | −0.32 | 0.365 |
| Duration of bilateral CI use | 0.01 | 0.968 | −0.04 | 0.874 | 0.20 | 0.580 |
| Electrode pitch ranking score: Reference ear | −0.13 | 0.655 | −0.06 | 0.845 | −0.38 | 0.360 |
| Electrode pitch ranking score: Comparison ear | 0.23 | 0.370 | 0.39 | 0.118 | 0.42 | 0.226 |
| Minimum discriminable electrode spacing: Reference ear | 0.57 | 0.044 | 0.47 | 0.103 | 0.67 | 0.083 |
| Minimum discriminable electrode spacing: Comparison ear | 0.09 | 0.722 | −0.09 | 0.729 | −0.13 | 0.712 |
| Electrode-specific factors | ||||||
| ||Electrode Pitch Mismatch|| | 0.08 | 0.771 | −0.20 | 0.432 | −0.39 | 0.263 |
| Electrode Pitch Match Bandwidth | 0.02 | 0.927 | −0.07 | 0.801 | 0.79 | 0.006 |
| Fusion Center vs Electrode Pitch Mismatch | 0.41 | 0.116 | 0.70 | 0.0036* | 0.25 | 0.489 |
indicates significant p-values after Bonferroni correction (p<0.004). Italics indicate marginal correlations (p<0.05). Correlations are with fusion ranges unless indicated otherwise.
M/S = moderate/severe; HL=hearing loss; HA=hearing aid.
No significant correlations were seen with any subject-specific factors, including age, onset age of moderate-to-severe hearing loss, duration of bilateral HA use prior to the first CI, duration of bimodal CI experience prior to the second CI, and duration of bilateral CI use. In addition, no significant differences were seen when subjects were subdivided based on modiolar or lateral wall electrode array type in the reference or comparison ear (p>0.5, two-tailed Wilcoxon rank-sum test; array type indicated in Table 1).
No significant correlations were seen between fusion range and within-ear electrode pitch ranking abilities or minimum discriminable electrode spacing. This is consistent with the broad fusion observed even for subjects with high pitch ranking scores for both ears (e.g. BI03 and BI12; compare Table 1 and Fig. 2). However, narrow fusion was only observed for subjects with good pitch ranking ability.
Relationships of fusion ranges and fusion center offsets with electrode-specific factors of pitch match and bandwidth were also analyzed. No significant correlations of fusion range were seen with electrode mismatch between the ears, although a nearly significant positive correlation was seen between fusion range and pitch match bandwidth for the basal electrode region only (r=0.79, p=0.006; p<0.004 required for significance with Bonferroni correction; Suppl. Fig. 2).
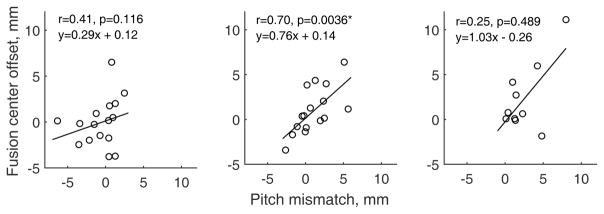
Consistent with the visual correspondence of fusion range and pitch matched electrodes seen in Fig. 2, a highly significant positive correlation was seen between the fusion center offset and the interaural electrode pitch mismatch for the middle electrode region only (r=0.70, p=0.0036; Fig. 3).
Figure 3.
Scatter plots of fusion center offset versus interaural electrode pitch mismatch for all subjects. Left, middle, and right panels show correlations for the apical, middle, and basal electrode regions, respectively. * indicates significant p-values (p<0.004).
Discussion
This study showed that bilateral CI users can experience broad binaural pitch fusion, similar to bilateral HA users and bimodal CI users (Reiss et al., 2014; 2017). On average across subjects and reference electrode locations, subjects fused a reference electrode in one ear with electrodes over a 5–6 mm range in the other ear, corresponding to approximately 8–10 Cochlear electrodes or 3 MED-EL electrodes. However, as in the other subject populations with hearing aids or bimodal CIs, there was a range of fusion range widths, with some subjects experiencing zero fusion and others fusing over the entire electrode array in the other ear. This average fusion range in mm corresponds to slightly over an acoustic octave in the human ear (in the 1–8 kHz range likely to be stimulated by the electrodes; Greenwood, 1990), and is of a similar scale to the average fusion range of ~1.7 octaves in bilateral HA users, but much wider than the fusion ranges of 0.1–0.2 octaves seen in NH listeners (Reiss et al., 2017).
These findings of broad binaural pitch fusion in bilateral CI users are consistent with others that have examined binaural fusion in the context of sound localization. In these studies, fusion of sounds across ears was considered to be a prerequisite for being able to lateralize sounds, so the initial step was to find the electrode pairs that fused into a single auditory image. For instance, early case studies report a single electrode in one ear fusing with up to 6–15 electrodes in the other ear (van Hoesel et al., 1993; van Hoesel and Clark, 1997; Long et al., 2003). A later study by Steel et al. (2015) showed some cases of broad fusion over a range of electrode place mismatches. Recently, a study by Kan et al. (2013) examined the effects of interaural electrode place-mismatches up to ±8 electrodes (±5 mm) on fusion and localization. They found that the range of electrodes fused varied from 0 up to 16 electrodes across the array, with 6 of 10 subjects experiencing fusion over the whole range. Lateralization also occurred with electrode place-mismatches in the absence of interaural time or level differences, and tended to vary with the electrode pair tested, occurring more often with more extreme electrode place-mismatches, as also seen in some subjects in this study. A parallel study by Goupell et al. (2013) showed that a simulated spread of excitation in normal-hearing listeners could replicate the patterns of fusion observed in bilateral CI listeners, suggesting that current spread may contribute to broad fusion.
Interestingly, some subjects fused electrodes that were mismatched rather than matched in pitch. This suggests that some bilateral CI users are fusing mismatched pitches across the electrode array and thus averaging spectrally complex stimuli across mismatched ears, which may cause binaural speech perception interference as has been shown in bimodal CI users (Reiss et al., 2016).
Various subject-specific and electrode-specific factors were examined to explore potential relationships to broad binaural pitch fusion in bilateral CI users. Electrode-specific analyses indicate that, unlike in bimodal CI users, fusion range widths were not significantly correlated with absolute interaural electrode pitch mismatch. However, it should be noted that the majority of subjects had broader fusion that encompassed any mismatch, thus preventing the perception of mismatch. Bilateral CI users did show a significant correlation of fusion center offset with interaural electrode pitch mismatch, in the middle electrode region, which is consistent with one previous study in normal-hearing listeners (van den Brink, 1976) and with the general concept that fusion prevents the perception of any pitch mismatch. It is not clear why the correlation was seen in the middle region only. This correlation may have been underpowered in the basal region due to difficulties in obtaining pitch matches for basal electrodes, while the apical region may have been subject to greater influence from bimodal experience history due to a greater likelihood of overlap with low-frequency acoustic hearing, as discussed below. More data is needed to determine whether this trend is consistent and confined to the middle electrode region.
No significant correlations were seen between fusion range and overall electrode discrimination abilities as measured by the pitch ranking task, though a marginal positive correlation was seen with pitch match bandwidths in the basal region. These findings suggest that broad fusion ranges may be only weakly related to peripheral factors such as broad electrical tuning due to current spread, which would limit electrode discrimination abilities and broad pitch match bandwidths relative to the other ear. More direct measures of current spread, such as electrically evoked compound action potentials, are needed to fully assess the importance of peripheral factors in fusion, especially for basal electrodes.
Subject-specific analyses showed no significant correlations between fusion range widths and age, age of onset of hearing loss, duration of hearing loss, duration of HA use, duration of bimodal CI use, or duration of bilateral CI use. The lack of correlations differ from previous findings in HA users, in which broad fusion was positively associated with the covarying factors of younger age, younger age of onset of hearing loss, long duration of hearing loss, and long duration of HA use (Reiss et al., 2017). The lack of correlations with subject factors related to hearing loss and HA use here is likely due to the greater complexity of hearing device experience history in bilateral CI users compared to HA users. Specifically, while broad fusion may be inherited from initial experience with hearing loss and low-frequency amplification, fusion may be modified by later experience with bimodal CI use and interaural pitch mismatch, as suggested by recent findings in bimodal CI users (Reiss et al., 2014). More complexity may be added with subsequent experience with bilateral CI use and interaural electrode pitch mismatch due to mismatches in electrode array insertion depth between the ears.
Hence, there are multiple potential factors underlying broad fusion in bilateral CI users, which include and may be a combination of early/long-term experience with hearing loss and amplification, plasticity due to interaural pitch mismatch with bimodal CI experience, and peripheral limitations such as current spread at the electrode-neural interface. Larger subject numbers and more detailed subject histories, or even longitudinal studies over time at each stage, are needed along with direct measures of peripheral function to separate these potential factors.
Why is broad binaural pitch fusion a concern, especially when fusion facilitates the ability to process interaural time and level difference cues for sound localization? While slightly broadened binaural fusion may be beneficial for sound localization in the presence of small place-mismatches (Blanks et al., 2008), extremely broad fusion on the order of octaves has been shown to lead to interference in speech perception, especially when large interaural pitch mismatches are present (Reiss et al., 2016). This interference occurs due to spectral averaging between ears when dichotic stimuli are fused, such that pitches or even speech spectra are averaged between the ears (Reiss et al., 2014, 2016; Oh and Reiss, 2017). Another confound of broad fusion is that, as shown in this study as well as previous studies in HA, bimodal CI, and bilateral CI users, fusion of extremely mismatched frequencies or electrodes can lead to illusions of lateralization in the absence of interaural time or intensity differences (Goupell et al., 2013; Reiss et al., 2014, 2017; Kan et al., 2013). Finally, in order to benefit from localization abilities for speech perception in noise, multiple sounds need to be segregated and localized. This will not be possible if all sounds are fused into a single sound. Therefore, the ideal scenario for bilateral CI listeners is likely to be narrowly tuned, pitch-matched fusion across ears similar to that experienced by NH listeners.
Supplementary Material
Acknowledgments
Source of Funding: This research was supported by grants R01 DC013307 and P30 DC010755 from the National Institutes of Deafness and Communication Disorders, National Institutes of Health.
We would like to thank Cochlear and MED-EL for providing equipment and software support for interfacing with the cochlear implant, and Razi Muhammed for assistance with programming the software interface. L.A.J.R. designed experiments, analyzed data, and wrote the paper; J.R.F., C.L.H., and Y.O. performed experiments. All authors discussed the results, and commented on the manuscript at all stages.
Footnotes
Conflicts of Interest: There are no conflicts of interest to report.
References
- Aronoff JM, Padilla M, Stelmach J, Landsberger DM. Clinically paired electrodes are often not perceived as pitch matched. Trends in Hearing. 2016;20:1–9. doi: 10.1177/2331216516668302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks DA, Buss E, Grose JH, Fitzpatrick DC, Hall JW. Interaural time discrimination of envelopes carried on high-frequency tones as a function of level and interaural carrier mismatch. Ear Hear. 2008;29(5):674–683. doi: 10.1097/AUD.0b013e3181775e03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting JE. Auditory and linguistic process in speech perception: Inferences from six fusions in dichotic listening. Psychol Rev. 1976;83(2):114–140. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goupell MJ, Stoelb C, Kan A, Litovsky RY. Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening. J Acoust Soc Am. 2013;133(4):2272–2287. doi: 10.1121/1.4792936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D. A cochlear frequency-position function for several species--29 years later. J Acoust Soc Am. 1990;87(6):2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Kan A, Stoelb C, Litovsky RY, Goupell MJ. Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear implant listeners. J Acoust Soc Am. 2013;134(4):2923–2936. doi: 10.1121/1.4820889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Nadol JB, Eddington DK. Depth of electrode insertion and postoperative performance in humans with cochlear implants: A histopathologic study. Audiol Neurotol. 2010;15(5):323–331. doi: 10.1159/000289571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CJ, Eddington DK, Colburn HS, Rabinowitz WM. Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user. J Acoust Soc Am. 2003;114(3):1565–1574. doi: 10.1121/1.1603765. [DOI] [PubMed] [Google Scholar]
- Odenthal DW. Perception and neural representation of simultaneous dichotic pure tone stimuli. Acta Physiol Pharmacol: Neerl. 1963;b:453–496. [PubMed] [Google Scholar]
- Oh Y, Reiss LA. Toward a systematic analysis of binaural pitch averaging trends in hearing impaired listeners. J Acoust Soc Am. 2017;142(2):780–791. doi: 10.1121/1.4997190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LA, Eggleston JL, Walker EP, Oh Y. Two ears are not always better than one: Mandatory vowel fusion across spectrally mismatched ears in hearing-impaired listeners. J Assoc Res Otolaryngol. 2016;17(4):341–356. doi: 10.1007/s10162-016-0570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LA, Perreau AE, Turner CW. Effects of lower frequency-to-electrode allocations on speech and pitch perception with the Hybrid short-electrode cochlear implant. Audiol Neurotol. 2012;17(6):357–372. doi: 10.1159/000341165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LAJ, Shayman CS, Walker EP, Bennett KO, Fowler JR, Hartling CL, Glickman B, Oh Y. Binaural pitch fusion: Comparison of normal-hearing and hearing-impaired listeners. J Acoust Soc Am. 2017;141(3):1909–1920. doi: 10.1121/1.4978009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LAJ, Ito RA, Eggleston JL, Wozny DR. Abnormal binaural spectral integration in cochlear implant users. J Assoc Res Otolaryngol. 2014;15(2):235–248. doi: 10.1007/s10162-013-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza PE, Boike KT, Witherall K, Tremblay K. Prediction of speech recognition from audibility in older listeners with hearing loss: Effects of age, amplification, and background noise. J Am Acad Audiol. 2007;18(1):54–65. doi: 10.3766/jaaa.18.1.5. [DOI] [PubMed] [Google Scholar]
- Steel MM, Papsin BC, Gordon KA. Binaural fusion and listening effort in children who use bilateral cochlear implants: A psychoacoustic and pupillometry study. PLOS One. 2015;10(2):e0117611. doi: 10.1371/journal.pone.0117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow WR, Bernstein S. Simultaneous two-tone pitch discrimination. J Acoust Soc Am. 1957;29(4):515–519. [Google Scholar]
- Van den Brink G, Sintnicolaas K, van Stam WS. Dichotic pitch fusion. J Acoust Soc Am. 1976;59(6):1471–1476. doi: 10.1121/1.380989. [DOI] [PubMed] [Google Scholar]
- Van Hoesel RJM, Clark GM. Psychophysical studies with two binaural cochlear implant subjects. J Acoust Soc Am. 1997;102(1):495–507. doi: 10.1121/1.419611. [DOI] [PubMed] [Google Scholar]
- Van Hoesel RJM, Tong YC, Hollow RD, Clark GM. Psychophysical and speech perception studies: A case report on a binaural cochlear implant subject. J Acoust Soc Am. 1993;94(6):3178–3189. doi: 10.1121/1.407223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.