Abstract
BACKGROUND
Children with acute lymphoblastic leukemia (ALL) can develop reduced bone mineral density (BMD). However, data from patients treated on a frontline regimen without cranial irradiation are limited, and no genome-wide analysis has been reported.
METHODS
Lumbar BMDs were evaluated by quantitative computed tomography at diagnosis, after 120 weeks of continuation therapy, and after 2 years off therapy in pediatric ALL patients (aged 2–18 years at diagnosis) treated on the St. Jude Total XV protocol. Clinical, pharmacokinetic, and genetic risk factors associated with decreased BMD Z-scores were evaluated.
RESULTS
The median BMD Z-score in 363 patients was 0.06 at diagnosis, declined to −1.08 at week 120, but partly recovered to −0.72 after 2 years off therapy; BMD in patients with low BMD Z-scores at diagnosis remained low after therapy. Older age (≥10 years vs. 2–9.9 years at diagnosis; P<0.001), higher BMD Z-score at diagnosis (P=0.001), and larger area under the plasma drug concentration–time curve (AUC) for dexamethasone in weeks 7 and 8 of continuation therapy (P=0.001) were associated with a greater decrease in BMD Z-score from diagnosis to week 120. Single-nucleotide polymorphisms in 2 genes important in osteogenesis and bone mineralization (COL11A1 [rs2622849, P=2.39×10−7] and NELL1 [rs11025915, P=4.07×10−6]) were associated with a decrease in BMD Z-score. NELL1 (P=0.003) was also associated with a larger dexamethasone AUC.
CONCLUSIONS
BMD Z-scores decreased during therapy, especially in patients with clinical, pharmacokinetic, and genetic risk factors. Early recognition of BMD changes and strategies to optimize bone health are essential.
Keywords: acute lymphoblastic leukemia, bone mineral density, children, chemotherapy, single-nucleotide polymorphism
INTRODUCTION
The survival rate for acute lymphoblastic leukemia (ALL) has increased to approximately 90% as a result of the introduction of risk-based treatment and improved supportive care.1 However, ALL treatment can itself have adverse effects. In healthy children, bone mineral density (BMD) continues to increase until early in the third decade of life,2 but children with ALL can develop reduced BMD due to the disease, its treatment, nutritional deficiencies, and/or physical inactivity.3–6 Glucocorticoids decrease bone formation by promoting apoptosis of osteoblasts and osteocytes, increasing bone resorption, and disturbing the calcium balance by reducing intestinal absorption of calcium and increasing its renal excretion.3 Endocrine abnormalities after cranial irradiation also contribute to bone morbidity.3 Although we have shown that prophylactic cranial irradiation can be safely omitted from the treatment of childhood ALL,7 there is only limited data on BMD in patients treated on a regimen without cranial irradiation.
Candidate gene studies have identified several single-nucleotide polymorphisms (SNPs) associated with low BMD in patients with ALL, including ones in the vitamin D receptor (VDR) 5′-end (Cdx-2/GATA polymorphism) haplotype 3 (P = 0.01),8 the genes encoding methylenetetrahydrofolate reductase (the MTHFR 677 T allele) and methionine synthase reductase (the MTRR 66 G allele) (P ≤ 0.01 with 2 or more risk alleles),9 CRHR1 (the G allele of rs1876828, P = 0.02 for boys),10 and RAPGEF5 (the T allele of rs6461639, P = 0.016).11 However, there has been no genome-wide analysis of low BMD in patients with ALL. Therefore, we evaluated clinical, pharmacokinetic, and genetic risk factors influencing BMD, especially those associated with a decline in BMD Z-score during therapy, in children with ALL treated on a contemporary protocol without cranial irradiation.
METHODS
Patients
Pediatric patients aged 1–18 years at diagnosis of ALL were enrolled on the Total XV protocol (ClinicalTrials.gov identifier: NCT00137111) at St. Jude Children’s Research Hospital (St. Jude) from 2000 to 2007.7 Patients aged 1–2 years at diagnosis and those with Down syndrome were excluded, and those who died, experienced relapse, or underwent hematopoietic stem cell transplant (HSCT) were removed from analyses involving subsequent BMD evaluations. The study was approved by the St. Jude Institutional Review Board.
Treatment
Total XV therapy and risk classification are described elsewhere.7 Patients received prednisone at 40 mg/m2/day for 28 days during induction and 5-day dexamethasone pulses every 4 weeks during continuation weeks 1 to 100 at 8 mg/m2/day for low-risk (LR) patients and 12 mg/m2/day for standard/high-risk (S/HR) patients. During re-induction I (weeks 7–9 of continuation) and II (weeks 17–19), all patients received dexamethasone at 8 mg/m2/day on days 1 to 8 and days 15 to 21, resulting in a cumulative prednisone dose of 1120 mg/m2 (for all patients) plus a dexamethasone dose of 1160 mg/m2 (for LR patients) or 1620 mg/m2 (for S/HR patients).7, 12 During remission induction, patients received 6 doses of L-asparaginase (10,000 units/m2/dose) during the first 19 days, and patients with minimal residual disease (MRD) of 1% or more on day 19 received 3 more doses. Low-risk patients received 9 doses of L-asparaginase (10,000 units/m2/dose) during each re-induction, whereas S/HR patients received 19 weekly doses (25,000 units/m2/dose) starting in week 1. Consolidation treatment consisted of high-dose methotrexate (a targeted steady-state concentration of 33 μM for LR patients and 65 μM for S/HR patients) given in 4 courses over 8 weeks with daily mercaptopurine. No cranial irradiation was used.
Bone mineral density
Quantitative computed tomography (QCT) data on vertebral trabecular BMD were obtained for the lumbar spine (L1/L2) at diagnosis, after 120 weeks of continuation therapy (when female patients completed treatment), after 146 weeks of continuation therapy (for male patients only, who then completed all treatment), and 2 years after completing therapy. Data were obtained with a Siemens SOMATOM-Plus spiral CT scanner (Siemens, Iselin, NY) and Mindways QCT calibration phantoms and software (Mindways Software, Austin, TX) as previously reported.13 References for vertebral BMD Z-scores based on age and sex were provided by the manufacturer of the QCT software (Mindways Software).14 The 2 vertebral BMD measurements were averaged, and the BMD Z-scores were used for analysis. A BMD Z-score of less than −1.5 was defined as indicating low BMD in this study.
Dexamethasone and methotrexate pharmacokinetics and cortisol and lipid levels
Blood samples for measuring dexamethasone levels were drawn before and after the morning dexamethasone doses (at 1, 2, 4, and 8 h) on days 1 and 8 of re-induction I (in weeks 7 and 8 of continuation treatment).12, 15 Dexamethasone pharmacokinetic parameters were estimated by fitting a 1-compartment model to the plasma concentration–time data by using maximum a posteriori probability estimation, as implemented in ADAPT II (Biomedical Simulations Resource, Los Angeles, CA). Plasma methotrexate concentrations were measured by a fluorescence polarization immunoassay (TDxFLx System, Abbott, Chicago, IL) at pre-dose and at between 0.5 and 6 h, 23 h, and 42 h after the start of methotrexate infusion.16, 17 Pharmacokinetic parameters were estimated with a 2-compartment first-order model in ADAPT II.
Heparinized blood samples were drawn on day 1 of week 7 of the continuation phase.12 Serum cortisol levels were determined by high-performance liquid chromatography. High- and low-density lipoproteins, cholesterol, and triglycerides were measured with a colorimetric enzymatic assay on a Cobas Integra system (Roche Diagnostics).
Statistical analysis
Associations of clinical characteristics with BMD Z-scores at each time point were evaluated with the rank-sum test or Spearman correlation test. Associations of BMD Z-score categories (≥0, ≥−1.5 to <0, and <−1.5) between two time points were tested with the chi-square test. To account for baseline data and the lack of a race-specific BMD Z-score reference, the analyses for the associations of clinical characteristics and BMD Z-score changes between 2 time points (from diagnosis to week 120 and from week 120 to 2 years off therapy) were adjusted for baseline BMD Z-score (at diagnosis or week 120) and race (white, black, or other) in the linear regression model. The BMD Z-score categories (≥ −1.5 vs. <−1.5) at week 120 and 2 years off therapy were modeled in logistic regression with clinical characteristics as predictors, adjusting for baseline BMD Z-score and race. The independent effect of each factor with a P-value of less than 0.10 was determined by multivariable analysis. The associations of dexamethasone and methotrexate pharmacokinetics and albumin, lipid, and cortisol levels with changes in BMD Z-scores from diagnosis to week 120 were evaluated after adjustments for BMD Z-score at diagnosis and race, and after additional adjustments for age and treatment risk in the multiple linear regression framework. In this analysis, the area under the plasma drug concentration–time curve (AUC) for dexamethasone was evaluated for each increment of 50 nM*h, and the triglyceride, total cholesterol, LDL, and HDL levels were considered for each increment of 20 mg/dL. Multivariable analysis was performed with factors identified by best-set model selection analysis using the Akaike information criterion (AIC). Statistical and computational analyses were performed using “R” Version 3.3.1 software (www.r-project.org) and SAS Version 9.4 software.
Genome-wide association study (GWAS)
Germline DNA samples were collected at remission and genotyped with the Affymetrix GeneChip Human Mapping 100K and 500K Array Sets. We studied 481,281 SNP genotypes after excluding 51,271 SNPs because of a low minor-allele frequency (<1%) or poor call rate (<95%).
For this genome-wide association study (GWAS), SNP genotypes were evaluated for associations with BMD Z-score changes from diagnosis to week 120 of continuation treatment with significance levels of P < 1 × 10−4, as selected by information-profiling criteria after adjustments for age, treatment risk, BMD Z-score at diagnosis, and genetic ancestry. The Spearman rank correlation test was used to evaluate the associations between the SNPs that are significantly associated with BMD Z-score changes from diagnosis to week 120 and the dexamethasone AUC.18
RESULTS
Patients and QCT data
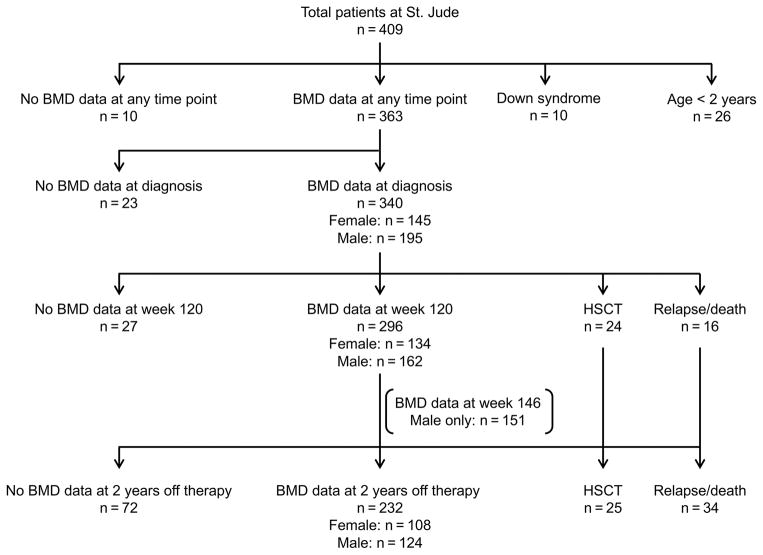
Of the 409 patients treated, 26 patients aged between 1 and 2 years at diagnosis and 10 patients with Down syndrome were excluded, and 10 had not undergone QCT evaluation of their BMD, leaving 363 patients who had undergone at least one QCT evaluation (Fig. 1 and Supplemental Table S1).
Figure 1. CONSORT diagram.
Abbreviations: BMD, bone mineral density; HSCT, hematopoietic stem cell transplant
Table 1 show the cross-sectional BMD Z-scores and their categories at each time point. The median BMD Z-score was 0.06 at diagnosis, decreased to −1.08 at week 120, but improved to −0.72 at 2 years after therapy. There were no differences in BMD Z-score between male and female patients. Of note, although other clinical characteristics did not differ, the available BMD Z-scores at diagnosis and week 120 in 232 patients who had a QCT examination at 2 years off therapy were lower than those in 72 patients who did not have a QCT examination (Supplemental Table S2). The median Z-scores at diagnosis were −0.05 (range, −3.27 to 3.56) for 216 patients who had a QCT examination and 0.30 (range, −2.12 to 2.68) for 66 patients who did not have an examination (P = 0.009). The median Z-scores at week 120 were −1.13 (range, −5.93 to 2.05) for 219 patients who had a QCT examination and −0.92 (range, −4.73 to 1.31) for 58 patients who did not have an examination (P = 0.043). When the BMD Z-scores were categorized as ≥0, ≥ −1.5 to <0, or <−1.5, the percentage of patients with a BMD Z-score of <−1.5 was 6.5%, 37.8%, and 25.4% at diagnosis, week 120, and 2 years off therapy, respectively. There were significant associations in the BMD Z-score categories between any two time points (P < 0.001 for all 3 association tests); most of the patients with BMD Z-scores of less than −1.5 at diagnosis remained in the same category at the subsequent time points (Supplemental Table S3). However, 14 of 108 patients (13.0%) with BMD Z-scores of 0 or higher and 31 of 95 patients (32.6%) with BMD Z-scores of at least −1.5 but less than 0 at diagnosis had BMD Z-scores of less than −1.5 at 2 years off therapy.
Table 1.
Cross-sectional data on bone mineral density Z-score
| Z-score | All patients N |
Median (range) | Female N |
Female, median (range) | Male N |
Male, median (range) | P-value |
|---|---|---|---|---|---|---|---|
| Diagnosis | |||||||
| All patients | 340 | 0.06 (−3.27 to 3.56) | 145 | 0.09 (−3.27 to 3.56) | 195 | 0.06 (−2.52 to 2.68) | 0.995 |
| ≥0 | 182 (53.5%) | 76 (52.4%) | 106 (54.4%) | ||||
| ≥ −1.5 to 0 | 136 (40.0%) | 58 (40.0%) | 78 (40.0%) | ||||
| < −1.5 | 22 (6.5%) | 11 (7.6%) | 11 (5.6%) | ||||
|
| |||||||
| Week 120 | |||||||
| All patients | 296 | −1.08 (−5.93 to 2.05) | 134 | −1.10 (−5.93 to 2.05) | 162 | −1.05 (−5.62 to 1.43) | 0.714 |
| ≥0 | 51 (17.2%) | 25 (18.7%) | 26 (16.1%) | ||||
| ≥ −1.5 to 0 | 133 (44.9%) | 57 (42.5%) | 76 (46.9%) | ||||
| < −1.5 | 112 (37.8%) | 52 (38.8%) | 60 (37.0%) | ||||
|
| |||||||
| (Week 146) | |||||||
| All patients | 151 | 151 | −0.92 (−4.16 to 2.13) | N/A | |||
| ≥0 | 34 (22.5%) | ||||||
| ≥ −1.5 to 0 | 68 (45.1%) | ||||||
| < −1.5 | 49 (32.5%) | ||||||
|
| |||||||
| 2 years off therapy | |||||||
| All patients | 232 | −0.72 (−3.56 to 2.46) | 108 | −0.66 (−3.1 to 2.46) | 124 | −0.75 (−3.56 to 2.45) | 0.446 |
| ≥0 | 71 (30.6%) | 37 (34.3%) | 34 (27.4%) | ||||
| ≥ −1.5 to 0 | 102 (44.0%) | 44 (40.7%) | 58 (46.8%) | ||||
| < −1.5 | 59 (25.4%) | 27 (25.0%) | 32 (25.8%) | ||||
Abbreviation: N/A, not applicable
Clinical characteristics associated with QCT BMD Z-score values
Supplemental Table S4 and Supplemental Figure S1 show the associations between BMD Z-scores and presenting clinical factors. At diagnosis, patients aged 10 years or older had a median BMD-Z-score of 0.50, which was higher than that of the normal controls and those aged 2–9.9 years had a median Z-score of (−0.09), which was comparable to that of the controls. In multivariable analysis, age 10 years or older (vs. 2–9.9 years) was significantly associated with a higher BMD Z-score (P = 0.030). Age 10 years or older at diagnosis and a lower BMD Z-score at diagnosis were significantly associated with a lower BMD Z-score at week 120 (P < 0.001 for both). Lower BMD Z-scores at week 120 correlated with lower BMD Z-scores at 2 years after therapy (P < 0.001).
Table 2 and supplemental Figures S1 and S2 show clinical factors associated with the changes in BMD Z-score from diagnosis to week 120 and from week 120 to 2 years off therapy, with adjustment for baseline BMD Z-score and race. In multivariable analysis, age 10 years or older at diagnosis, compared with 2–9.9 years, and a greater decrease in height Z-score from diagnosis to week 120 were significantly associated with a greater decline in BMD Z-score from diagnosis to week 120 (P < 0.001 and P = 0.009, respectively). Patients aged 10 years or older at diagnosis had a significantly greater increase in BMD Z-score from week 120 to 2 years off therapy than did those aged 2–9.9 years (P < 0.001).
TABLE 2.
Factors associated with bone mineral density Z-score changes
| Clinical characteristics | 2 years off therapy from week 120 | Week 120 from diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variables | N | Coefficient | 95% CI | Individual factora P |
Multivariableb P |
N | Coefficient | 95% CI | Individual factora P |
| Age | |||||||||
| 2–9.9 years | 202 | 0.976 | 0.685 to 1.268 | <0.001 | <0.001 | 164 | −0.524 | −0.768 to −0.280 | <0.001 |
| ≥10 years | 74 | 55 | |||||||
| Sex | |||||||||
| Female | 124 | 0.031 | −0.242 to 0.303 | 0.826 | |||||
| Male | 152 | ||||||||
| WBC at diagnosis | |||||||||
| <50 × 109/L | 209 | −0.054 | −0.373 to 0.266 | 0.743 | 167 | −0.028 | −0.286 to 0.231 | 0.834 | |
| ≥50 × 109/L | 67 | 52 | |||||||
| Lineage | |||||||||
| B lineage | 233 | 0.474 | 0.102 to 0.847 | 0.013 | 0.440 | 186 | −0.133 | −0.447 to 0.181 | 0.407 |
| T lineage | 43 | 33 | |||||||
| CNS involvement | |||||||||
| CNS negative | 216 | 0.146 | −0.181 to 0.473 | 0.382 | 175 | −0.168 | −0.439 to 0.103 | 0.224 | |
| CNS positive | 60 | 44 | |||||||
| Risk | |||||||||
| Low | 149 | 0.646 | 0.375 to 0.917 | <0.001 | 0.218 | 124 | −0.159 | −0.383 to 0.066 | 0.167 |
| Standard/high | 127 | 95 | |||||||
| Height Z-score change | |||||||||
| Week 120 from diagnosis | 276 | 0.335 | 0.104 to 0.566 | 0.005 | 0.009 | ||||
| 2 years off therapy from week 120 | 218 | −0.022 | −0.287 to 0.242 | 0.868 | |||||
| BMI Z-score change | |||||||||
| Week 120 from diagnosis | 276 | 0.201 | 0.063 to 0.339 | 0.005 | 0.146 | ||||
| 2 years off therapy from week 120 | 218 | −0.097 | −0.233 to 0.039 | 0.163 | |||||
Data are adjusted for baseline BMD Z-scores and race. For 2 years off therapy from week 120, data are additionally adjusted for sex.
Multivariable analysis was performed to determine the independent effect of each individual factor that had a P–value of less than 0.10.
Abbreviations: CI, confidence interval; WBC, white blood cell count; CNS, central nervous system; BMI, body mass index
When clinical characteristics associated with a low BMD Z-score (<−1.5) are evaluated after adjustment for baseline BMD Z-score and race, patients aged 10 years or older at diagnosis were at significantly higher risk for a low BMD Z-score at week 120 when compared with patients aged 2–9.9 years (P = 0.003) (Supplemental Table S5).
Association of dexamethasone and methotrexate pharmacokinetics and albumin and lipid levels with QCT BMD Z-scores at week 120
We evaluated the associations of changes in BMD Z-score from diagnosis to week 120 with dexamethasone and methotrexate pharmacokinetics and with albumin, lipid, and cortisol levels adjusted for BMD Z-score at diagnosis and race, and also with additional adjustments for age at diagnosis and treatment risk, by using a multiple linear regression model (Table 3). In the latter model, the average AUCs for dexamethasone in continuation weeks 7 and 8 (P < 0.001) and triglyceride (P = 0.021) and cholesterol (P = 0.018) levels in week 7 were negatively associated with changes in BMD Z-score, and the albumin level in week 7 (P = 0.017) was positively associated with such changes. These suggest that larger dexamethasone AUCs, higher triglyceride and cholesterol levels, and lower albumin levels result in a greater decrease in BMD Z-score from diagnosis to week 120.
TABLE 3.
Association of bone mineral density Z-score changes from diagnosis to week 120 with dexamethasone and methotrexate pharmacokinetics and albumin, lipid, and cortisol levels
| Variables | N | Individual factora | Multiple linear regressionb | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | ||
| Average of dexamethasone AUCs in weeks 7 and 8 of continuation | 241 | −0.070 | −0.094 to −0.046 | <0.001 | −0.047 | −0.073 to −0.021 | <0.001 |
| Average methotrexate AUCs during consolidation | 276 | −0.025 | −0.036 to −0.013 | <0.001 | −0.025 | −0.052 to 0.001 | 0.062 |
| Albumin level (g/dL) in week 7 of continuation | 263 | 0.512 | 0.313 to 0.711 | <0.001 | 0.342 | 0.063 to 0.622 | 0.017 |
| Triglyceride level (mg/dL) in week 7 of continuation | 250 | −0.038 | −0.054 to −0.021 | <0.001 | −0.021 | −0.038 to −0.003 | 0.021 |
| Total cholesterol level (mg/dL) in week 7 of continuation | 250 | −0.122 | −0.183 to −0.061 | <0.001 | −0.073 | −0.134 to −0.013 | 0.018 |
| LDL level (mg/dL) in week 7 of continuation | 250 | −0.037 | −0.120 to 0.047 | 0.394 | −0.035 | −0.115 to 0.045 | 0.396 |
| HDL level (mg/dL) in week 7 of continuation | 250 | 0.135 | −0.034 to 0.305 | 0.119 | −0.004 | −0.169 to 0.161 | 0.961 |
| Cortisol level (μg/dL) in week 7 of continuation | 263 | 0.017 | −0.003 to 0.037 | 0.101 | 0.007 | −0.012 to 0.026 | 0.468 |
P-value after adjustment for bone mineral density Z-score at diagnosis and race
P-value after adjustment for bone mineral density Z-score at diagnosis, race, age, and treatment risk
Average of dexamethasone AUCs in weeks 7 and 8 was evaluated for the change of 50 nM*h, and triglyceride, total cholesterol, LDL, and HDL levels were examined for each increment of 20 mg/dL.
Abbreviations: CI, confidence interval; AUC, area under the plasma drug concentration–time curve; LDL, low-density lipoprotein; HDL, high-density lipoprotein
Multivariable analysis for factors associated with BMD Z-scores at week 120
To evaluate factors significant for the changes in BMD Z-score from diagnosis to week 120 with multivariable analysis, we performed best-set model selection analysis with the AIC by using the age group, leukemia risk, BMD Z-score at diagnosis, race, lineage, Z-score changes for height and body mass index from diagnosis to week 120, average of the week 7 and week 8 dexamethasone AUCs, average methotrexate AUCs during consolidation, and albumin, triglyceride, and total cholesterol levels in week 7 of continuation therapy (Supplemental Table S6). The best model fit (AIC 649.924) included the age group, leukemia risk, BMD Z-score at diagnosis, race, average of the week 7 and week 8 dexamethasone AUCs, average methotrexate AUCs during consolidation, and total cholesterol level in week 7. In multivariable analysis with these factors, age 10 years or older at diagnosis (P < 0.001), higher BMD Z-score at diagnosis (P = 0.001), and greater average of the week 7 and week 8 dexamethasone AUCs (P = 0.001) were associated with a greater decrease in BMD Z-score from diagnosis to week 120 (Table 4).
TABLE 4.
Multivariable analysis for factors associated with bone mineral density Z-score changes from diagnosis to week 120
| Characteristics | Estimate | Standard error | P |
|---|---|---|---|
| Low vs. standard/high risk | −0.461 | −1.189 to 0.267 | 0.216 |
| Race, black vs. white | 0.290 | −0.072 to 0.651 | 0.117 |
| Race, other vs. white | −0.575 | −1.267 to 0.117 | 0.105 |
| Age, ≥10 vs. 2–9.9 years | −0.732 | −1.119 to −0.344 | <0.001 |
| BMD Z-score at diagnosis | −0.224 | −0.356 to −0.092 | 0.001 |
| Average of dexamethasone AUCs for weeks 7 and 8 of continuation | −0.047 | −0.074 to −0.021 | 0.001 |
| Total cholesterol level (mg/dL) in week 7 of continuation | −0.057 | −0.118 to 0.005 | 0.074 |
| Average methotrexate AUCs during consolidation | −0.023 | −0.051 to 0.005 | 0.102 |
Abbreviations: BMD, bone mineral density; AUC, area under the plasma drug concentration–time curve
Average of dexamethasone AUCs in weeks 7 and 8 was evaluated for the change of 50 nM*h, and total cholesterol was examined for each increment of 20 mg/dL.
Genomic study
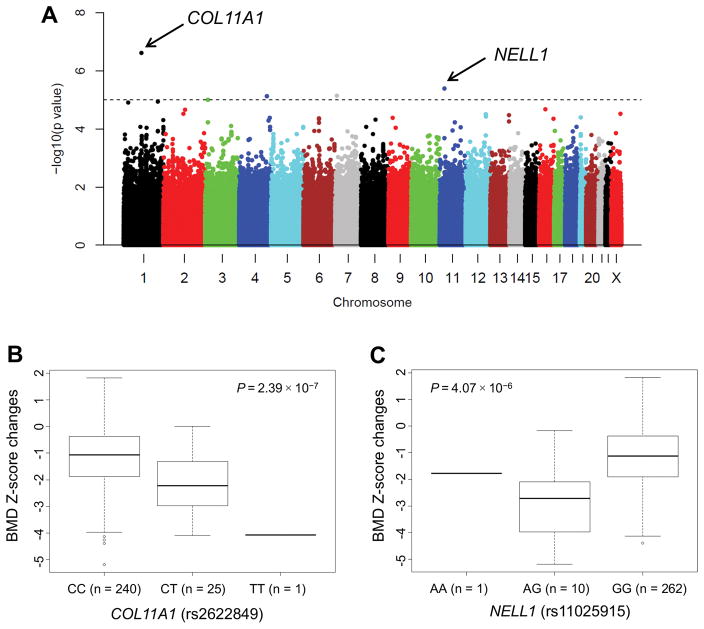
We investigated which of 481,281 germline SNP genotypes were associated with BMD Z-score changes from diagnosis to continuation week 120 (Fig. 2A). Table 5 shows the 38 SNPs with P-values of less than 1.0 × 10−4, of which 19 were annotated to 16 genes.
Figure 2. Single-nucleotide polymorphisms that are associated with bone mineral density (BMD) Z-score changes from diagnosis to week 120 of continuation therapy.
Data are adjusted for age at diagnosis, treatment risk, BMD Z-scores at diagnosis, and ancestry group. Manhattan plots (A) and box plots (B and C) for COL11A1 and NELL1 are shown.
Table 5.
Single-nucleotide polymorphisms that are associated with bone mineral density Z-score changes from diagnosis to week 120 and dexamethasone AUC
| SNP information
|
Association with bone mineral density Z-score change from diagnosis to week 120a
|
Association with dexamethasone AUC
|
|||||
|---|---|---|---|---|---|---|---|
| SNPID | Gene | Risk allele frequency | Risk allele | P | Coefficient | P | Spearman correlation |
| rs2622849 | COL11A1 | 0.051 | T | 2.39E-07 | −1.071 | 0.291 | 0.063 |
| rs11025915 | NELL1 | 0.025 | A | 4.07E-06 | −1.306 | 0.003 | 0.176 |
| rs1986582 | – | 0.120 | G | 7.17E-06 | −0.629 | 0.421 | 0.048 |
| rs17047225 | TLL1 | 0.050 | C | 7.51E-06 | −0.815 | 0.964 | 0.003 |
| rs2668354 | SRGAP3 | 0.659 | A | 1.00E-05 | −0.471 | 0.307 | 0.061 |
| rs7514670 | – | 0.849 | A | 1.14E-05 | −0.552 | 0.734 | −0.020 |
| rs12127272 | – | 0.320 | G | 1.23E-05 | −0.453 | 0.481 | 0.042 |
| rs9931454 | – | 0.787 | C | 2.11E-05 | −0.495 | 0.824 | 0.013 |
| rs11122844 | – | 0.192 | C | 2.22E-05 | −0.501 | 0.729 | 0.021 |
| rs3849317 | – | 0.723 | C | 3.01E-05 | −0.425 | 0.938 | −0.005 |
| rs1151982 | – | 0.017 | T | 3.07E-05 | −1.476 | 0.163 | 0.082 |
| rs4766635 | – | 0.641 | A | 3.10E-05 | −0.421 | 0.561 | 0.035 |
| rs885339 | – | 0.247 | T | 3.34E-05 | −0.418 | 0.259 | 0.068 |
| rs7957299 | PPTC7 | 0.657 | C | 3.69E-05 | −0.371 | 0.702 | 0.023 |
| rs17000495 | MUC16 | 0.801 | G | 4.02E-05 | −0.445 | 0.182 | 0.079 |
| rs41478351 | MGC45800 | 0.101 | C | 4.20E-05 | −0.665 | 0.545 | 0.036 |
| rs16931403 | NFIB | 0.173 | G | 4.22E-05 | −0.457 | 0.024 | 0.136 |
| rs9344006 | – | 0.122 | G | 4.31E-05 | −0.524 | 0.785 | −0.016 |
| rs4843860 | IRF8 | 0.763 | A | 4.47E-05 | −0.426 | 0.022 | 0.135 |
| rs9352839 | – | 0.118 | A | 4.69E-05 | −0.532 | 0.846 | 0.012 |
| rs41430546 | – | 0.070 | G | 4.73E-05 | −0.754 | 0.219 | 0.073 |
| rs3850825 | ZNF496 | 0.240 | G | 5.01E-05 | −0.416 | 0.884 | 0.009 |
| rs6817370 | – | 0.865 | T | 5.11E-05 | −0.557 | 0.092 | 0.099 |
| rs9522086 | – | 0.191 | A | 5.49E-05 | −0.442 | 0.062 | 0.110 |
| rs2664109 | SRGAP3 | 0.642 | A | 5.99E-05 | −0.402 | 0.265 | 0.066 |
| rs1940080 | DLG2 | 0.501 | A | 6.00E-05 | −0.376 | 0.717 | 0.021 |
| rs7738642 | – | 0.130 | T | 6.26E-05 | −0.497 | 0.530 | −0.037 |
| rs3863992 | – | 0.171 | A | 8.04E-05 | −0.478 | 0.328 | 0.058 |
| rs11165740 | – | 0.360 | T | 8.38E-05 | −0.379 | 0.187 | 0.078 |
| rs948640 | – | 0.812 | C | 8.40E-05 | −0.454 | 0.946 | −0.004 |
| rs7522071 | ZNF496 | 0.229 | T | 8.41E-05 | −0.411 | 0.627 | −0.029 |
| rs1588571 | LOC100129666 | 0.062 | A | 8.53E-05 | −0.776 | 0.002 | 0.181 |
| rs13179406 | RNF130 | 0.890 | A | 8.54E-05 | −0.536 | 0.754 | 0.019 |
| rs987341 | – | 0.062 | T | 8.60E-05 | −0.758 | 0.784 | 0.016 |
| rs4937993 | NCAM1 | 0.520 | T | 8.75E-05 | −0.370 | 0.013 | 0.146 |
| rs2297694 | C9orf72 | 0.275 | G | 9.05E-05 | −0.413 | 0.208 | 0.075 |
| rs17079966 | RNF130 | 0.865 | G | 9.22E-05 | −0.494 | 0.834 | −0.012 |
| rs16839677 | OLFML2B | 0.076 | A | 9.26E-05 | −0.692 | 0.683 | −0.024 |
Abbreviations: SNP ID, single-nucleotide polymorphism identifier according to the dbSNP database; AUC, area under the plasma drug concentration−time curve
Adjusted for age, genetic ancestry, treatment risk, and BMD Z-score at diagnosis.
Across the genome, the strongest association of a SNP with BMD Z-score changes from diagnosis to week 120 was observed for a SNP in the COL11A1 gene (rs2622849) in chromosome 1p21.1 (Fig. 2B). The T allele of this intronic SNP was associated with a significant decrease in BMD Z-score (coefficient of −1.071; P = 2.39 × 10−7) (Table 5). Similarly, the A allele of the NELL1 SNP (rs11025915) (Fig. 2C) was associated with a decrease in BMD Z-score (coefficient of −1.306; P = 4.07 × 10−6).
As the dexamethasone AUC was negatively associated with BMD Z-score changes, we evaluated the associations of 38 germline SNP genotypes that are significant for a decrease in BMD Z-score from diagnosis to week 120 with larger dexamethasone AUCs. Five SNPs (13.2%) had P-values of less than 0.05 (Table 5), including NELL1 (the A allele of rs11025915, P = 0.003) and NFIB (the G allele of rs16931403, P = 0.024).
DISCUSSION
We showed that BMD in patients with ALL treated without cranial irradiation significantly decreased by week 120 of continuation therapy and partly recovered by 2 years off therapy. The BMD in most patients with a low BMD Z-score (<−1.5) at diagnosis remained low at week 120 and 2 years off therapy. BMD Z-scores significantly decreased from diagnosis to week 120 in older patients and in those with higher BMD Z-scores at diagnosis, greater dexamethasone exposure, and/or SNPs identified by a GWAS.
The median BMD Z-score at diagnosis in our patients (0.06) was comparable to that in the general population, but the scores ranged from −3.27 to 3.56. As a result of leukemia cell proliferation in the bone marrow and cytokine-mediated osteoclast activity, children with ALL can present with low BMD Z-scores at diagnosis,4 and low Z-scores at diagnosis are associated with persistently low BMD Z-scores during and after therapy. BMD Z-scores at diagnosis were significantly higher in patients aged 10 years or older at diagnosis; however, these patients are considered to have a higher leukemia risk and typically receive higher and more intense doses of chemotherapeutic agents that disturb bone mineralization, resulting in a greater decline in BMD over the course of therapy. Among the clinical characteristics, a decline in height Z-score from diagnosis to week 120 was significantly associated with a decline in BMD Z-score in the same period. BMD and height increase significantly during puberty,2, 19 and normal accruals might have been compromised and/or delayed during chemotherapy, causing significantly more patients in this age group to have a BMD Z-score of less than −1.5 at week 120. The associations of older age and low BMD Z-score at diagnosis with lower BMD during continuation therapy have been described.20
A larger dexamethasone AUC, a known factor for bone morbidity,12 was significantly associated with a decrease in BMD Z-score during therapy. Although glucocorticoids play important roles in BMD deficits, other chemotherapeutic agents can contribute to such deficits. Low albumin and high triglyceride and total cholesterol levels in week 7 of continuation were significantly associated with a decrease in BMD Z-score, although these factors did not remain significant with the AIC and/or in multivariable analysis. Lower albumin levels and older age are associated with a larger dexamethasone AUC.12 Hypoalbuminemia is caused by the administration of asparaginase, which is more frequently used in older patients. Hypertriglyceridemia and hypercholesterolemia, which occur frequently during ALL therapy, are associated with asparaginase activity because asparaginase can inhibit lipoprotein lipase activities.12 Thus, asparaginase may indirectly cause a decline in BMD by increasing the dexamethasone AUC. Methotrexate reportedly reduces the proliferation of pre-osteoblasts and osteoblasts, increases the proliferation of osteoclasts,21 and contributes to calcium imbalance by interfering with renal function.22 However, the methotrexate AUC did not remain significant in the multivariable analysis.
In the GWAS, the 2 SNPs most associated with BMD Z-score change from diagnosis to week 120 were those in the COL11A1 and NFIB genes, which are important in osteogenesis and bone mineralization. COL11A1 encodes the alpha-1 chain of type XI collagen, which is composed of 3 alpha-chain products from COL11A1, COL11A2, and COL2A1.23 The phenotypes of type XI collagenopathies caused by COL11A1 mutations in humans are heterogeneous and are associated not only with syndromes characterized by myopia, midfacial retrusion, and micrognathia but also with skeletal dysplasia. NELL1 is a secreted protein whose expression promotes osteoblast cell differentiation and terminal mineralization and inhibits osteoclast-induced bone resorption.24 NELL-1 overexpression results in craniosynostosis, whereas decreased NELL-1 expression leads to skeletal undermineralization. Administration of recombinant NELL-1 improves BMD in rodents. NELL-1 and NFIB are associated with larger dexamethasone AUCs. NFIB is a cellular transcription factor that plays critical roles in osteoblast proliferation, differentiation, and survival and that regulates cartilage development.25 Nfib and the glucocorticoid receptor gene Nr3c1 co-regulate genes related to lung maturation,26 and NFIB and glucocorticoid receptor are critically involved in the development of milk-secreting alveolar epithelium in the mammary gland.27 Such interactions may alter dexamethasone pharmacokinetics during chemotherapy.
In our study, BMDs partly recovered after patients completed therapy. This result must be interpreted cautiously, as patients who underwent a QCT examination at 2 years off therapy had lower BMD Z-scores at diagnosis and week 120 when compared to those who did not undergo a QCT examination, although there were no differences in other clinical characteristics. However, among patients who were evaluated by QCT at 2 years off therapy, the median BMD Z-score at 2 years off therapy (−0.72) was still lower than that at diagnosis (−0.05) and showed only partial improvement from that at week 120 (−1.13). Survivors treated without cranial irradiation have better BMD recovery after chemotherapy when compared to those who undergo irradiation.3, 28 We evaluated clinical factors associated with changes in BMD Z-score from week 120 to 2 years off therapy. Importantly, at 2 years off therapy, the BMD Z-scores increased by a significantly greater extent in older patients than in younger patients, who did not lose BMD during therapy or gain it after therapy as did older patients. Although there was no significant difference between the age groups, the median BMD Z-score in younger patients was −0.79, compared with −0.26 in older patients. Patients with low BMD Z-scores at diagnosis had low BMD Z-scores not only at week 120 but also at 2 years off therapy. Younger patients with low BMD Z-scores at diagnosis may require long-term follow-up, and it needs to be determined whether they experience a typical physiologic BMD increase after the age of 10 years. In addition, patients with BMD Z-scores of less than −1.5 at 2 years off therapy require close observation and/or intervention.
Interventions to prevent bone loss during ALL therapy may include dietary counseling/supplementation to ensure adequate vitamin D and calcium intake,13 dexamethasone dose adjustment, and/or weight-bearing exercise.29 However, persuading children and families to adhere to dietary modifications may be particularly challenging during ALL treatment. For adolescent and young adult survivors of ALL, supplementation with cholecalciferol and calcium conferred no additional benefit.13 Pharmacokinetics-based adjustment of the dexamethasone doses may compromise the desired anti-leukemia response. Weight-bearing exercise at the frequency and intensity necessary for optimal bone growth is difficult during ALL therapy and is discouraged for patients with osteonecrosis (another chemotherapy-related bone toxicity in ALL). However, it has been reported that low-magnitude, high-frequency mechanical stimulation (LMS) improves or prevents BMD loss in postmenopausal women,30 patients with dystrophinopathies,31 and children who have undergone cancer therapy.32 As the patients need only stand on a vibrating platform for 10 min twice each day, the use of LMS to counter BMD loss during cancer therapy should be evaluated, and we are performing a placebo-controlled randomized study in patients with ALL (NCT03117751). The efficacy of pharmacologic agents such as bisphosphonate and receptor activator of NF-κB ligand (RANKL) inhibitor has been reported in pilot trials,33–35 and these agents may be beneficial if a successful treatment approach is determined in the context of ALL therapy.
None of the SNPs that we evaluated reached genome-wide significance (<5 × 10−8), probably because of the limited sample size; therefore, a further study including a replication cohort is required to confirm our findings. However, this is the first GWAS to investigate genetic risk factors for BMD changes in patients with ALL, and 2 SNPs have been found to play critical roles in bone development. Bone density can be evaluated by either QCT or dual X-ray absorptiometry (DXA). Both approaches have strengths and weaknesses, although DXA is more widely used.36, 37 DXA can assess the BMD of the whole body or individual areas and define BMD Z-scores by race-matched references in addition to age and sex. However, DXA measures a 2-dimensional area, with the volumetric result being generated by calculation, and this may lead to an underestimation of BMD in smaller bones. The BMD determination by QCT is volumetric and independent of bone size, and it is not hampered by longitudinal bone growth. Furthermore, the QCT software can correct for the curves in patients with scoliosis. In this study, we presented the trabecular BMD because trabecular bone is much more metabolically active than cortical bone and is considered a more sensitive indicator of skeletal metabolism and response to stresses and interventions.38 Because the QCT reference data are based on the white population14 and the BMD Z-scores in black patients tended to be higher than those in white patients,39 we adjusted the data for BMD Z-score changes and categories with respect to race. As baseline BMD Z-scores were significantly associated with subsequent values, we also adjusted these data to account for baseline values. Although we did not collect bone-fracture data prospectively, low BMD measured by QCT or DXA is associated with future fracture risk.20, 40, 41
In conclusion, BMD Z-scores decrease significantly in children receiving contemporary therapy for ALL without cranial irradiation, and in many cases the scores do not fully recover by 2 years off therapy. Early recognition of BMD changes may facilitate developing strategies to optimize bone health, especially in patients with risk factors identified in this study.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by grants CA21765, CA02394, and P50 GM115279 from the National Institutes of Health and by ALSAC.
We thank Keith A. Laycock, PhD, ELS, (St. Jude Children’s Research Hospital) for editorial assistance.
Footnotes
Conflict of interest disclosures: The authors have declared no conflicts of interest.
Author contributions
Study concept: Hiroto Inaba, Sue C. Kaste
Study design: Hiroto Inaba, Sue C. Kaste
Data acquisition: Hiroto Inaba, Xueyuan Cao, Alice Han, John C. Panetta, Cheng Cheng, Ching-Hon Pui, Mary V. Relling, Sue C. Kaste
Quality control of data and algorithms: Hiroto Inaba, Xueyuan Cao, Sue C. Kaste
Data analysis and interpretation: Hiroto Inaba, Xueyuan Cao, John C. Panetta, Kirsten Ness, Monika L. Metzger, Jeffrey E. Rubnitz, Raul C. Ribeiro, John T. Sandlund, Sima Jeha, Cheng Cheng, Ching-Hon Pui, Mary V. Relling, Sue C. Kaste
Statistical analysis: Xueyuan Cao, Cheng Cheng
Manuscript preparation: Hiroto Inaba, Xueyuan Cao, Alice Han, Sue C. Kaste
Manuscript editing: All authors
Manuscript review: All authors
References
- 1.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–1955. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostoufi-Moab S, Halton J. Bone morbidity in childhood leukemia: epidemiology, mechanisms, diagnosis, and treatment. Curr Osteoporos Rep. 2014;12:300–312. doi: 10.1007/s11914-014-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Sluis IM, van den Heuvel-Eibrink MM, Hählen K, Krenning EP, de Muinck Keizer-Schrama SM. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr. 2002;141:204–210. doi: 10.1067/mpd.2002.125728. [DOI] [PubMed] [Google Scholar]
- 5.Halton JM, Atkinson SA, Fraher L, et al. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J Bone Miner Res. 1996;11:1774–1783. doi: 10.1002/jbmr.5650111122. [DOI] [PubMed] [Google Scholar]
- 6.Strauss AJ, Su JT, Dalton VM, Gelber RD, Sallan SE, Silverman LB. Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol. 2001;19:3066–3072. doi: 10.1200/JCO.2001.19.12.3066. [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.te Winkel ML, van Beek RD, de Muinck Keizer-Schrama SM, et al. Pharmacogenetic risk factors for altered bone mineral density and body composition in pediatric acute lymphoblastic leukemia. Haematologica. 2010;95:752–759. doi: 10.3324/haematol.2009.016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.te Winkel ML, de Muinck Keizer-Schrama SM, de Jonge R, et al. Germline variation in the MTHFR and MTRR genes determines the nadir of bone density in pediatric acute lymphoblastic leukemia: a prospective study. Bone. 2011;48:571–577. doi: 10.1016/j.bone.2010.10.163. [DOI] [PubMed] [Google Scholar]
- 10.Jones TS, Kaste SC, Liu W, et al. CRHR1 polymorphisms predict bone density in survivors of acute lymphoblastic leukemia. J Clin Oncol. 2008;26:3031–3037. doi: 10.1200/JCO.2007.14.6399. [DOI] [PubMed] [Google Scholar]
- 11.Park HW, Tse S, Yang W, et al. A genetic factor associated with low final bone mineral density in children after a long-term glucocorticoids treatment. Pharmacogenomics J. 2017;17:180–185. doi: 10.1038/tpj.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawedia JD, Kaste SC, Pei D, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117:2340–2347. doi: 10.1182/blood-2010-10-311969. quiz 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaste SC, Qi A, Smith K, et al. Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2014;61:885–893. doi: 10.1002/pbc.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilsanz V, Gibbens DT, Roe TF, et al. Vertebral bone density in children: effect of puberty. Radiology. 1988;166:847–850. doi: 10.1148/radiology.166.3.3340782. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Panetta JC, Cai X, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26:1932–1939. doi: 10.1200/JCO.2007.13.8404. [DOI] [PubMed] [Google Scholar]
- 16.Mikkelsen TS, Sparreboom A, Cheng C, et al. Shortening infusion time for high-dose methotrexate alters antileukemic effects: a randomized prospective clinical trial. J Clin Oncol. 2011;29:1771–1778. doi: 10.1200/JCO.2010.32.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauley JL, Panetta JC, Crews KR, et al. Between-course targeting of methotrexate exposure using pharmacokinetically guided dosage adjustments. Cancer Chemother Pharmacol. 2013;72:369–378. doi: 10.1007/s00280-013-2206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng C, Pounds SB, Boyett JM, Pei D, Kuo ML, Roussel MF. Statistical significance threshold criteria for analysis of microarray gene expression data. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1064. Article36. [DOI] [PubMed] [Google Scholar]
- 19.Naka H, Iki M, Morita A, Ikeda Y. Effects of pubertal development, height, weight, and grip strength on the bone mineral density of the lumbar spine and hip in peripubertal Japanese children: Kyoto kids increase density in the skeleton study (Kyoto KIDS study) J Bone Miner Metab. 2005;23:463–469. doi: 10.1007/s00774-005-0629-0. [DOI] [PubMed] [Google Scholar]
- 20.Rayar MS, Nayiager T, Webber CE, Barr RD, Athale UH. Predictors of bony morbidity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59:77–82. doi: 10.1002/pbc.24040. [DOI] [PubMed] [Google Scholar]
- 21.Xian CJ, Cool JC, Scherer MA, et al. Cellular mechanisms for methotrexate chemotherapy-induced bone growth defects. Bone. 2007;41:842–850. doi: 10.1016/j.bone.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 22.van Leeuwen BL, Kamps WA, Jansen HW, Hoekstra HJ. The effect of chemotherapy on the growing skeleton. Cancer Treat Rev. 2000;26:363–376. doi: 10.1053/ctrv.2000.0180. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Lacerda DA, Warman ML, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995;80:423–430. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- 24.James AW, Shen J, Zhang X, et al. NELL-1 in the treatment of osteoporotic bone loss. Nat Commun. 2015;6:7362. doi: 10.1038/ncomms8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Casellas LA, Wang X, Howard KD, Rehage MW, Strong DD, Linkhart TA. Nuclear factor I transcription factors regulate IGF binding protein 5 gene transcription in human osteoblasts. Biochim Biophys Acta. 2009;1789:78–87. doi: 10.1016/j.bbagrm.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lajoie M, Hsu YC, Gronostajski RM, Bailey TL. An overlapping set of genes is regulated by both NFIB and the glucocorticoid receptor during lung maturation. BMC Genomics. 2014;15:231. doi: 10.1186/1471-2164-15-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin HY, Willi M, Yoo KH, et al. Hierarchy within the mammary STAT5-driven Wap super-enhancer. Nat Genet. 2016;48:904–911. doi: 10.1038/ng.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurney JG, Kaste SC, Liu W, et al. Bone mineral density among long-term survivors of childhood acute lymphoblastic leukemia: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2014;61:1270–1276. doi: 10.1002/pbc.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman A, te Winkel ML, van Beek RD, et al. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53:64–71. doi: 10.1002/pbc.21942. [DOI] [PubMed] [Google Scholar]
- 30.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 31.Petryk A, Polgreen LE, Grames M, Lowe DA, Hodges JS, Karachunski P. Feasibility and tolerability of whole-body, low-intensity vibration and its effects on muscle function and bone in patients with dystrophinopathies: a pilot study. Muscle Nerve. 2017;55:875–883. doi: 10.1002/mus.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogil RJ, Kaste SC, Ferry RJ, Jr, et al. Effect of low-magnitude, high-frequency mechanical stimulation on BMD among young childhood cancer survivors: a randomized clinical trial. JAMA Oncol. 2016;2:908–914. doi: 10.1001/jamaoncol.2015.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barr RD, Guo CY, Wiernikowski J, Webber C, Wright M, Atkinson S. Osteopenia in children with acute lymphoblastic leukemia: a pilot study of amelioration with pamidronate. Med Pediatr Oncol. 2002;39:44–46. doi: 10.1002/mpo.10057. [DOI] [PubMed] [Google Scholar]
- 34.Wiernikowski JT, Barr RD, Webber C, Guo CY, Wright M, Atkinson SA. Alendronate for steroid-induced osteopenia in children with acute lymphoblastic leukaemia or non-Hodgkin’s lymphoma: results of a pilot study. J Oncol Pharm Pract. 2005;11:51–56. doi: 10.1191/1078155205jp145oa. [DOI] [PubMed] [Google Scholar]
- 35.Mok CC, Ho LY, Ma KM. Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone. 2015;75:222–228. doi: 10.1016/j.bone.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Leonard MB. Assessment of bone health in children and adolescents with cancer: promises and pitfalls of current techniques. Med Pediatr Oncol. 2003;41:198–207. doi: 10.1002/mpo.10337. [DOI] [PubMed] [Google Scholar]
- 37.Kaste SC, Tong X, Hendrick JM, et al. QCT versus DXA in 320 survivors of childhood cancer: association of BMD with fracture history. Pediatr Blood Cancer. 2006;47:936–943. doi: 10.1002/pbc.20854. [DOI] [PubMed] [Google Scholar]
- 38.Cann CE, Genant HK, Young DR. Comparison of vertebral and peripheral mineral losses in disuse osteoporosis in monkeys. Radiology. 1980;134:525–529. doi: 10.1148/radiology.134.2.6766220. [DOI] [PubMed] [Google Scholar]
- 39.Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG. Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med. 1991;325:1597–1600. doi: 10.1056/NEJM199112053252302. [DOI] [PubMed] [Google Scholar]
- 40.Cann CE, Genant HK, Kolb FO, Ettinger B. Quantitative computed tomography for prediction of vertebral fracture risk. Bone. 1985;6:1–7. doi: 10.1016/8756-3282(85)90399-0. [DOI] [PubMed] [Google Scholar]
- 41.te Winkel ML, Pieters R, Hop WC, et al. Bone mineral density at diagnosis determines fracture rate in children with acute lymphoblastic leukemia treated according to the DCOG-ALL9 protocol. Bone. 2014;59:223–228. doi: 10.1016/j.bone.2013.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.