Abstract
Indirect Acute Respiratory Distress Syndrome (iARDS) is caused by a non-pulmonary inflammatory process resulting from insults such as non-pulmonary sepsis. Neutrophils are thought to play a significant role in mediating ARDS, with the development of iARDS being characterized by dysregulation and recruitment of activated neutrophils into the lung. Recently, a novel mechanism of microbial killing by neutrophils was identified through the formation of neutrophil extracellular traps (NETs). NETs are comprised of large webs of decondensed chromatin released from activated neutrophils into the extracellular space; they are regulated by the enzyme PAD4 through mediation of chromatin decondensation via citrullination of target histones. Components of NETs have been implicated in ARDS. However, it is unknown if there is any pathological significance of NET formation in ARDS caused indirectly by non-pulmonary insult.
We subjected PAD4-/- mice and wildtype (WT) mice to a “2 hit” model of hypovolemic shock (fixed-pressure hemorrhage; Hem) followed by septic cecal ligation and puncture (CLP) insult (Hem/CLP). Mice were hemorrhaged and resuscitated; 24 hours post-Hem mice were then subjected to CLP. Overall, PAD4 deletion led to an improved survival as compared to WT mice. PAD4-/- mice displayed a marked decrease in neutrophil influx into the lung, as well decreased presence of pro-inflammatory mediators. PAD4-/- mice were also able to maintain baseline kidney function after Hem/CLP.
These data taken together suggest PAD4-mediated NET formation contributes to the mortality associated with Shock/Sepsis and may play a role in the pathobiology of end organ injury in response to combined hemorrhage plus sepsis.
Keywords: Neutrophils, hemorrhage, sepsis, inflammation, post translational modification
Introduction
Acute Respiratory Distress Syndrome (ARDS) is a type of non-cardiac respiratory failure characterized by bilateral alveolar infiltrates, decreased lung function, pulmonary edema, increased lung micro-vascular permeability, and influx/sequestration of activated neutrophils into lung interstitium and alveolar space (1–3). ARDS is associated with a mortality rate of about 40% and accounts for approximately 75,000 deaths per year in the United States (4, 5). ARDS can be caused by pulmonary (direct ARDS) or extra-pulmonary (indirect ARDS) insult. Direct ARDS (pneumonia, aspiration, and lung trauma) accounts for 57% of total cases, whereas indirect ARDS (iARDS), including extra-pulmonary sepsis and multisystem trauma, represents 43%, with approximately 25% of ARDS cases stemming from severe sepsis (2, 6). However, compared with direct ARDS, the pathophysiology of iARDS is much less well understood, possibly due to heterogeneity of the condition and the involvement of multiple systemic factors.
One of the key features of ARDS is the dysregulation and recruitment of activated neutrophils to the lung microvasculature, interstitium, and alveolar space (2). Excessive neutrophil activation and accumulation leads to increased Reactive Oxygen Species (ROS) production, and pro-inflammatory mediators along with a decrease in neutrophil apoptosis. Taken together, these changes lead to epithelial and endothelial cell damage (2, 7).
Neutrophil extracellular traps (NETs) are complex structures made of nuclear chromatin, histones, granular antimicrobial proteins, and some cytoplasmic proteins (8) that are capable of physically ensnaring bacteria and facilitating the interactions between bacteria and antimicrobial effectors, ultimately leading to enhanced bacterial killing. However, there has been increasing evidence that NETs may have detrimental consequences for the host. Increased NET formation has been linked to various disease states, such as autoimmune diseases as well as sepsis, which suggests that they contribute to excessive inflammation and tissue damage (9–11). Components of NETs including neutrophil derived circulating free DNA (CF-DNA/NETs) and circulating histones have been implicated in acute lung injury (12–14). Histones have been detected in the bronchoalveolar lavage fluid (BALF) and plasma of patients who developed ARDS after trauma and acid aspiration (14, 15), and have been implicated in direct alveolar lung damage in various animal models of lung injury (10).
The process of NET formation is mediated by the enzyme Peptidylarginine deiminase 4 (PAD4), which is essential for histone citrullination and subsequent chromatin decondensation. This histone citrullination is thought to promote NET formation in two ways: (1) by inducing chromatin decondensation and (2) by aiding in the release of chromosomal DNA (coated with antimicrobial molecules) into the extracellular space (16–18). These factors render PAD4 as a potential target for studying NET formation.
While NETs and their components have been observed in various models of direct acute lung injury, it is not understood how they function in a model of traumatic shock (hemorrhage) followed by septic insult, which is thought to more closely resemble the process of extra (indirect)-pulmonary ARDS encountered in many critically ill/injured patients.
We hypothesize that PAD4 catalyzed Histone 3 citrullination and the resultant formation of NETs contributes to systemic inflammation and by-stander tissue injury that leads to increased morbidity/mortality seen in the combined insults of hemorrhagic shock and sepsis insults (which induce acute lung injury as well as range of other organ injuries). Therefore, inhibition of PAD4 will decrease organ damage associated with iARDS and increase overall survival in a “2 hit” model of traumatic shock (fixed-pressure hemorrhage; Hem) followed by septic cecal ligation and puncture (CLP) insult (Hem/CLP).
To address this hypothesis, here we have subjected both PAD4-/- and WT mice to a two hit model of Hem/CLP and assessed for survival, cytokine analysis, and neutrophil infiltration in the lung and kidney. Taken together, our data suggests that PAD4/NET formation contributes to the mortality associated with shock/sepsis and also plays a role in the end organ injury seen in response to pathological processes regulating iARDS in the Hem/CLP model.
Material And Methods
Mice
C57BL/6 male mice (WT), ages 8-12 weeks, were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and used in all experiments. PAD4-deficient mice (PAD4-/-) were gifted by Dr. Kerri Mowen (Department of Pharmacology and Chemical Physiology, Scripps Research Institute, La Jolla, CA). LoxP sites were introduced into the introns flanking exons 9 and 10 of the PAD4 gene and PAD4 floxed mice were mated with CMV-Cre deleter mice to generate PAD4-deficient mice(19). PAD4-deficient mice are viable and have no gross anatomical abnormalities. All protocols carried out with animals were done according to NIH Guide for Animal Use and Care, and were approved by the Rhode Island Hospital Institutional animal care and use committee (AWC 0040-16).
Analysis of neutrophil effector functions
Neutrophils were isolated from naïve WT and PAD4-/- as previously described(20). Cells were then used for the following assays:
Respiratory burst capacity
Isolated blood neutrophils (3 × 105 cells/well) were placed on BSA coated 96-well plates with 2× Cytochrome C solution (Sigma Aldrich, Natick MA) and stimulated with phorbol myristate acetate (PMA). The plate was placed in a Microplate Bio Kinetics reader (BIOTEK Instruments, Winooski, VT) at 37°C and optical density readings at dual wavelengths of 550/630 nm were made every 2 min for 60 min using DeltaSoft3 software (BIOTEK Instruments). Neutrophil burst = 13.258 (a predetermined absorbance constant) *(OD at Xmin-OD at 0min)
Phagocytosis
Phagocytosis capabilities were measured using a phagocytosis kit that utilizes fluorescently labeled bacterial particles (ThermoFisher Scientific, Waltham, MA). Neutrophils (5 × 105 neutrophils/well) were plated and incubated at 37°C for 1 hr. The plates were aspirated and then 100 μL of the prepared fluorescent BioParticle suspension were added to the wells and incubated at 37°C for 2 hrs. After aspiration, 100 μL of prepared trypan blue suspension was added to all of the wells and incubated for 1 minute at room temperature. The trypan blue was removed and the microplate was placed in the fluorescence plate reader (FL×800, Bio-tek Instruments Inc., Winooski, VT) using ∼480 nm excitation, ∼520 nm emission and the appropriate sensitivity settings. Phagocytosis in response to the effector was then calculated.
NET formation
Thioglycolate elicited neutrophils were isolated and cultured on Poly-L-lysine coated plates for 4 hours at 37°C post 20nM PMA stimulation to induce NET production. 5μM of Sytox® green (Thermo Fischer Sci, Waltham, MA,) was added 10 minutes prior to imaging. Images were captured using a Nikon TE-2000U inverted microscope (Nikon, Melville, NY) coupled to an iXonEM + 897E back illuminated EMCCD camera (Andor, Belfast, UK). Bright field images were captured using NIS-Elements software (Nikon). A xenon lamp illuminated cells through a 33 mm ND4 filter and 20× Nikon Plan Apochromat objective using a Nikon B2-A long-pass emission filter set cube.
Hemorrhage (Hem) model
As previously described (21, 22), mice were anesthetized using an isofluorane vaporizer setup, restrained in supine position and catheters were inserted into both femoral arteries. When awake, as determined by a mean blood pressure of approximately 85 mm Hg, the mice were bled (0.8–1.0 mL) over a 5- to 10-min period to a mean blood pressure of 35 mm Hg (±5 mm Hg) and kept stable for 90 min. Immediately following hemorrhage mice were resuscitated through the catheter with Ringer's lactate at four times drawn blood volume. The incision was sutured closed and mice were then returned to their cages. Sham mice were anesthetized and both femoral arteries were ligated. Incisions were sutured closed and mice were returned to their cages.
Sepsis induced by cecal ligation and puncture (CLP)
Twenty-four hours after Hem, mice were anesthetized with isoflurane and a midline incision was made in the abdomen. The cecum was isolated and ligated at a point approximately 1 cm from the cecal tip, punctured twice with a 22-gauge needle, then gently squeezed to extrude a small amount of cecal contents from the perforation sites. Then the cecum was placed back into the peritoneal cavity and the incision was sutured closed in two layers. Mice were resuscitated with 1 mL Ringer's lactate by subcutaneous injection and then returned to their cages. In the sham mice, the cecum was exposed but neither ligated nor punctured (23).
Survival Study
Both WT and PAD4-/- mice were subjected to Hem/CLP or sham/sham procedure (n = 16-17/group), and were then returned to their cages, given access to food and water and assessed for morbidity/mortality for 14 days post procedure.
Sample Collection
Twenty-four hours post Hem/CLP or sham/sham procedures, mice were euthanized with either a Carbon Dioxide or isoflurane overdose. Blood was collected in heparinized tubes via cardiac puncture and centrifuged to obtain plasma. Bronchoalveolar lavage fluid (BALF) was collected as previously described (22). Briefly, the trachea was exposed via a midline incision and cannulated with a sterile polypropylene catheter. The lungs were lavaged with 0.6 ml saline, two times, for an average of 1 ml lavage fluid collected/lung. Lavage fluid was centrifuged at 1500 rpm for 10 min at 4°C, and the supernatant was tested for cytokine levels. The lung and kidney were also harvested for cytokine analysis as previously described (22). All samples were stored at -80°C until needed. Peritoneal fluid was collected by lavage with 1mL of PBS into the peritoneal cavity. 1×PBS was recollected for microbial burden assays. For the collection of peritoneal cells, 5mL of 1× PBS was injected and recollected via IP, and centrifuged at 10,000×g for 10 min. The cell pellet was then re-suspended for flow cytometry and western blot analysis. For lung and kidney histology, tissue was harvested and fixed in 10% formalin and paraffin embedded, and tissue sections were prepared as described previously (24, 25). Samples were then stained and examined by light microscopy.
Cytokine/chemokine analysis
Concentrations of interleukin (IL)-6, TNF-α, IL-10 (lung, kidney, BALF, and plasma), CXCL-1 (KC) and CXCL-2 (MIP-2) (lung and kidney tissue) were assessed via ELISA according to manufacturer's protocols (BD Bioscience, San Jose, CA) (26).
Myeloperoxidase (MPO) analysis
Mouse Myeloperoxidase (MPO) was measured using an enzyme-linked immunosorbent assay kit (ThermoFisher Scientific, Waltham, MA). Immunohistochemistry was also performed on formalin-fixed paraffin-embedded left lung tissue sections. Slides were deparaffinized and blocked with 5% goat serum for 1hr. Tissues were then probed with 1:200 MPO polyclonal antibody (PA-5-16672, ThermoFisher Scientific, Waltham, MA) overnight at 4°C in a humidified container. Detection was performed using a biotinylated goat anti-rabbit IgG secondary followed by avidin-biotin complex reagent and DAB colormetric substrate and a hematoxylin counterstain. Slides were mounted and analyzed at 20× using RGB, DIC N1 filter (0.33μM/pixel).
Flow cytometry
the left lobe of the lung underwent an enzymatic digestion, as described previously(24, 25), and cells collected from the peritoneum were processed and counted (1×106 cell/ml) for flow analysis. Antibodies used for analysis were purchased from eBioscience (San Diego, CA). (Anti-CD11b, 101212) (anti-F480 123110), or from Miltenyi Biotech (Auburn, CA) (anti-Ly6G, 130-102-227). Cell populations were determined using a MACSQuant Analyzer (Miltenyi Biotec Inc, San Diego, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR) (26).
Kidney dysfunction
To quantify blood urea nitrogen (BUN) levels in serum, a Urea assay kit (ab83362, Abcam, Cambridge, MA) was used. In brief, Urea acts as substrate with compounds in the presence of enzymes to form a product that reacts with the probe to generate color (ODmax=570nm). For Creatinine quantification, Creatinine Assay Kit (ab65340, Abcam, Cambridge, MA) was used to measure creatine concentration in serum (27). In the assay, creatinine is converted to creatine by creatininase, creatine is converted to sarcosine, which is specifically oxidized to produce a product which reacts with a probe to generate color (ODmax = 570 nm)
Evans Blue Dye (EBD) extravasation assay
Vascular permeability in the lungs and kidneys was assessed by measuring the EBD in the lung and kidney in ng/mg of tissue (28). Briefly, EBD (2.5 mg in 0.5 mL 0.9N saline) was administered intravenously by tail vein injection 15 minutes before euthanasia. The lungs and kidneys were harvested, weighed, and incubated in 2 mL of formamide for 24 h at 55°C. Following centrifugation, EBD was measured in supernatant, dual wavelenths 620/740 nm, compared with EBD standard curve, and normalized with total EBD in collected plasma.
Statistics
Results are expressed as Mean ± SEM. Statistical significance of the results presented were determined by one-way ANOVA (for multiple comparison) with All Pairwise Multiple Comparison Procedures (Holm-Sidak or Dunn's method), unpaired two-tailed Student's t-test (for normal distribution data), or log-rank test (for survival study) where appropriate. Statistical software used was Sigmaplot 11.0 (Systat Software, Inc., San Jose, CA).P ≤ 0.05 was used as a cutoff for significance.
Results
PAD4 deficiency does not affect other effector functions
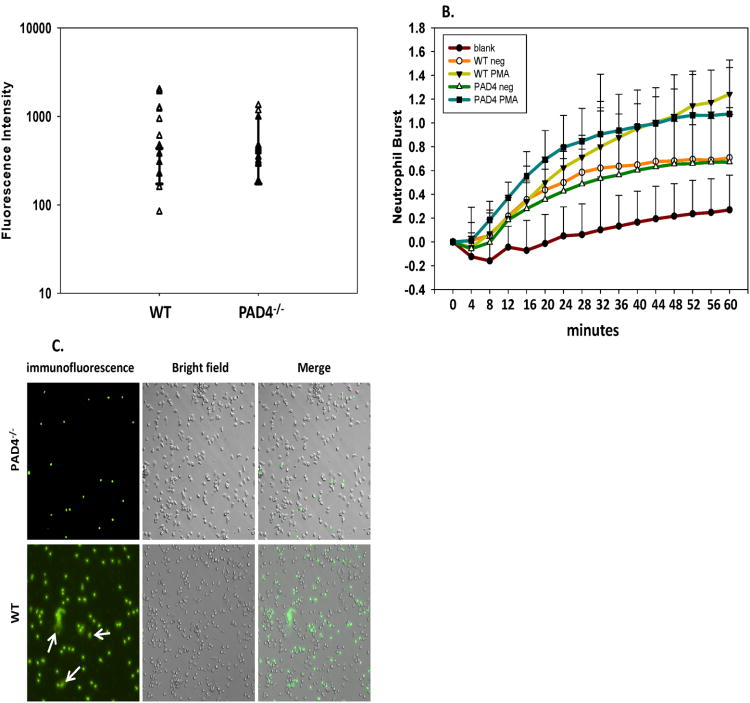
Firstly, we compared overall neutrophil effector functions in an ex-vivo setting. Neutrophils were isolated from naïve PAD4-/- and WT mice to compare effector functions and to ensure that the only difference was the ability to produce NETs. The ability of PAD4-/- neutrophils to phagocytize was equivalent to WT neutrophils (fig. 1A). When naïve PAD4–/– and WT neutrophils were stimulated with PMA, both groups were also capable of generating ROS over a time course of 60 min (fig. 1B). When isolated neutrophils were cultured on Poly-L-lysine for 4 hours post 20nM PMA stimulation, PAD4–/– neutrophils were unable to elicit NET formation; unlike WT neutrophils which produced NET structures after PMA stimulation (fig. 1C).
Figure 1. PAD4 gene deficiency effects NET formation but does not inhibit other neutrophil effector functions.
Neutrophils from naïve C57BL/6 (WT) and PAD4-/- mice were analyzed for other anti-microbial functions ex-vivo. Phagocytosis capabilities were comparable in WT and PAD4-/- mice (A). ROS generation after stimulation with PMA was increased in WT and PAD4-/- mice (B). Neutrophils were isolated via thioglycolate injection and were cultured on Poly-L-lysine coated plates for 4 hours post 20nM PMA stimulation to elicit NET production. NETs were visualized via immunofluorescence with Sytox green (C). PAD4-/- neutrophils displayed a deficiency in producing NETs, while WT neutrophils were able to produce NET structures after PMA stimulation. n=3 independent experiments/samples run in triplicate
PAD4 deficiency reduces mortality and bacterial burden after Hem/CLP
To better understand the role NETs play in shock/sepsis associated mortality, we performed Hem/CLP on WT and PAD4-/- mice, and observed survival over a 14 day time period. Overall, PAD4-/- mice displayed improved survival with an overall survival rate of 94% as compared to WT mice which had a 59% survival rate after 14 days post Hem/CLP (fig. 2). Animals undergoing sham/sham surgery (n=16/group) in both the WT and PAD4-/- had a 100% survival rate (data not shown).
Figure 2. PAD4-deficient mice display improved survival in Hem/CLP model of iARDS.
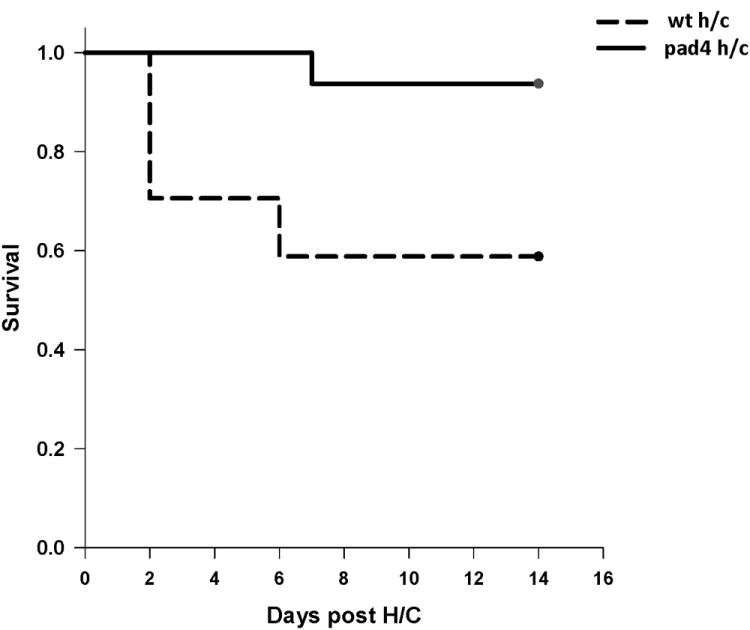
Mice were subjected to hemorrhage and subsequent septic insult and then monitored daily for 14 days. PAD4-/- mice had 90% survival at 14 days compared to the 50% survival in WT mice. Sham animals from both groups had a 100% survival rate. log-rank test. p=0.013. (n=6 sham/sham (not shown); n=16-17 Hem/CLP per group)
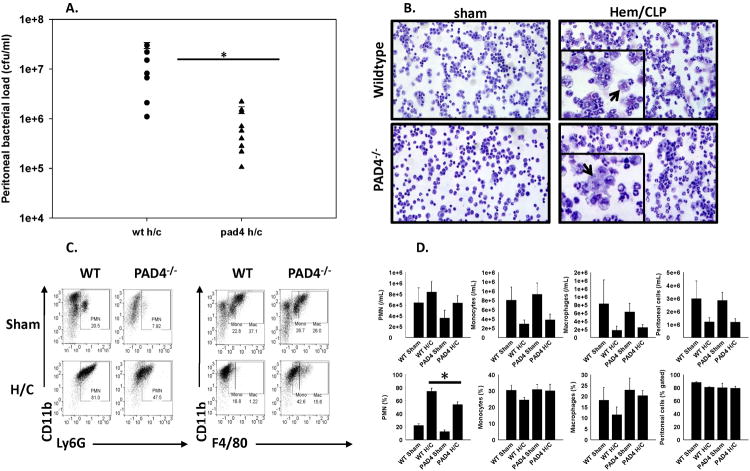
Previous studies have shown that PAD4 plays an important role in neutrophil antibacterial function mediated by NETs to effectively contain various pathogens (8, 18). Therefore, we hypothesized that PAD4–/– mice would be more susceptible to an overwhelming bacterial burden in the peritoneal cavity, due to a reduced ability to capture, contain and eliminate bacteria via NETosis. Surprisingly, the bacterial load in the peritoneum 24 hours after CLP (48 hours post Hem), was significantly lower in the PAD4-/- mice than in the WT (fig. 3A), both WT and PAD4-/- sham/sham groups) had no bacterial growth, suggesting that PAD4-/- mice had enhanced bacterial clearance capabilities compared to WT mice after Hem/CLP. In an attempt to delineate the enhanced ability of PAD4-/- mice to clear pathogens, we analyzed the cell populations within the peritoneal cavity 24 hrs after Hem/CLP. When assessing the morphology of cells collected from the peritoneum with Giemsa stain, morphological differences were noted in macrophages between the two groups. While WT macrophages appear granulated, PAD4-/- peritoneal macrophages appear to have a smoother morphology (fig. 3B). The abundance of neutrophils, monocytes, and macrophages in the peritoneum was measured by flow cytometry based on Ly6G, CD11b, and F4/80 staining. Neutrophils were defined as Ly6G+CD11b+, monocytes as F4/80midCD11b+, and macrophages as F4/80hiCD11b+(fig. 3 C-D). In absolute number, there was no difference in neutrophils, monocytes, and macrophages between WT and PAD4-/- mice after Hem/CLP. It was noted that the percentage of neutrophils was decreased in PAD4-/- mice. Although no significant difference was found, there seems to be a trend toward increased frequency of monocytes and macrophages in the peritoneal cavity of PAD4-/- mice after Hem/CLP.
Figure 3. PAD4-/- mice exhibit enhanced bacterial clearance at site of infection after Hem/CLP.
Twenty four hours after Hem/CLP, peritoneal cells were collected and assessed for neutrophil/macrophage recruitment as well as antimicrobial function. Lavage contents collected from the peritoneum 24 hrs post Hem/CLP were placed on blood agar plates for 24 hrs at 37°C. PAD4-/- mice had significantly reduced colony formations compared to WT mice. Sham animals from both groups had no colony formations (A). Cytospins of peritoneal cells were visualized with Giemsa 20× using RGB, DIC N1 filter (0.33μM/pixel). Morphological differences were noted between macrophage populations with WT Hem/CLP peritoneal macrophages appearing more granulated compared to the peritoneal macrophages from the PAD4-/- Hem/CLP mice. Cell morphology was similar in both sham groups (B). The abundance of different leukocyte populations in the peritoneum was measured by flow cytometry based on Ly6G, CD11b, and F4/80 staining (C-D). In absolute number, there was no difference in neutrophils, monocytes, and macrophages between WT and PAD4-/- mice after Hem/CLP. The percentage of neutrophils was decreased in PAD4-/- mice, and, though no significant difference was found, there seems to be a trend toward increased frequency of monocytes and macrophages in PAD4-/- mice after Hem/CLP. One way ANOVA, #p≤0.05 sham vs. Hem/CLP; * p≤0.05 WT Hem/CLP vs PAD4-/- Hem/CLP. n=7-8/group
In addition to assessing cell populations locally in the peritoneum, overall circulating leukocyte numbers were assessed via Complete Blood Count (CBC) analysis 24 hrs post Hem/CLP. There were no overt differences in overall White Blood Counts (WBC) (fig.4A) or in circulating leukocyte populations (fig.4B-F) between groups after Hem/CLP. Instead there was a fair amount of variability within each group, with sham groups displaying even greater variability in leukocyte counts than their Hem/CLP counterparts.
Figure 4. Circulating neutrophil counts are equivalent between PAD4-/- and WT mice after Hem/CLP.
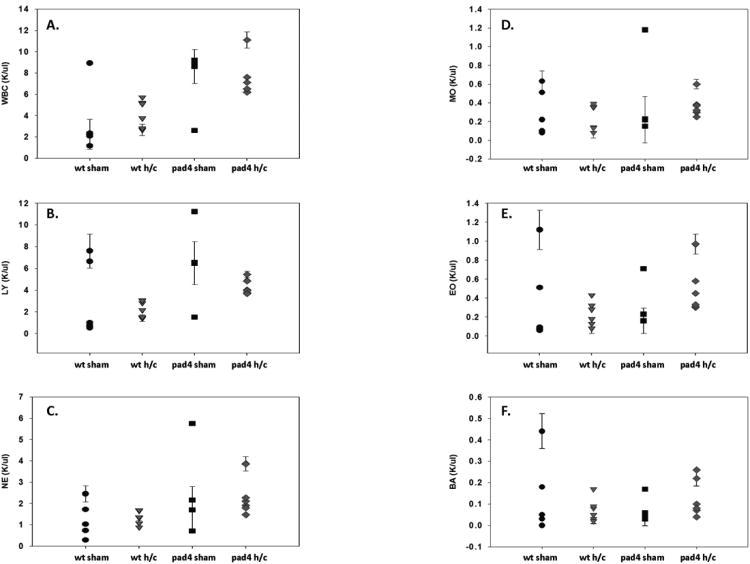
Whole blood from WT and PAD4-/- mice was analyzed for circulating leukocyte cell totals after Hem/CLP. Total white blood count (WBC) (A), lymphocytes (B), neutrophils (C), monocytes (D), eosinophils (E), and basophils (F) were analyzed. Overall, there was a great deal of variability in WT and PAD4-/- sham groups. While leukocyte counts were more consistent after Hem/CLP, numbers were comparable between both groups with no significant differences noticed. n=6-9/group.
Effects of PAD4 on inflammation in mice after Hem/CLP
Next we sought to determine if alterations in the inflammatory response after Hem/CLP contributed to increased survival of PAD4-/- mice, since extracellular histones and NETs have been implicated in causing excessive inflammation and tissue damage (11, 29, 30). We measured IL-6 and TNF-α levels as markers of systemic inflammation (i.e. in the blood) and of tissue inflammation (i.e. in the lung). We also assessed IL-10 levels, given its role in anti-inflammation as an immune response to shock (1, 31). PAD4-/- lung homogenates displayed a significant reduction of pro-inflammatory cytokine IL-6 and TNF-α levels (fig. 5A-C) compared to WT mice after Hem/CLP. BALF IL-6 levels in PAD4-/- mice were also attenuated, whereas in WT mice there was a significant increase detected after Hem/CLP (fig. 5D-F). We also assessed inflammation in the kidneys as we noted that these organs in PAD4-/- mice appeared different (less mottled, better color) upon a gross necropsy. There was no difference in IL-6 levels across all groups. PAD4-/- kidney TNF-α levels were higher compared to WT after Hem/CLP, but there was no difference in TNF-α levels detected among PAD4-/- Hem/CLP versus sham mice (fig. 5G-I). Anti-inflammatory cytokine IL-10 levels in WT mice were significantly increased in the lung (fig. 5C) but were decreased in the kidney (fig. 5I). In PAD4-/- mice, the opposite effect was noted with decreased levels measured in the lung and increased levels in the kidney compared to WT after Hem/CLP, albeit comparable to PAD4-/- kidney sham mouse levels. In blood, there were no significant differences between the two groups with WT and the PAD4-/- displaying significant increases in systemic IL-6 levels (data not shown). Overall, PAD4-/- mice showed evidence of a decreased pro-inflammatory cytokine response in the lung after Hem/CLP, with minimal differences detected within the kidney.
Figure 5. PAD4-/- results in decreased pro-inflammatory and anti-inflammatory cytokine levels in the lungs of Hem/CLP mice.
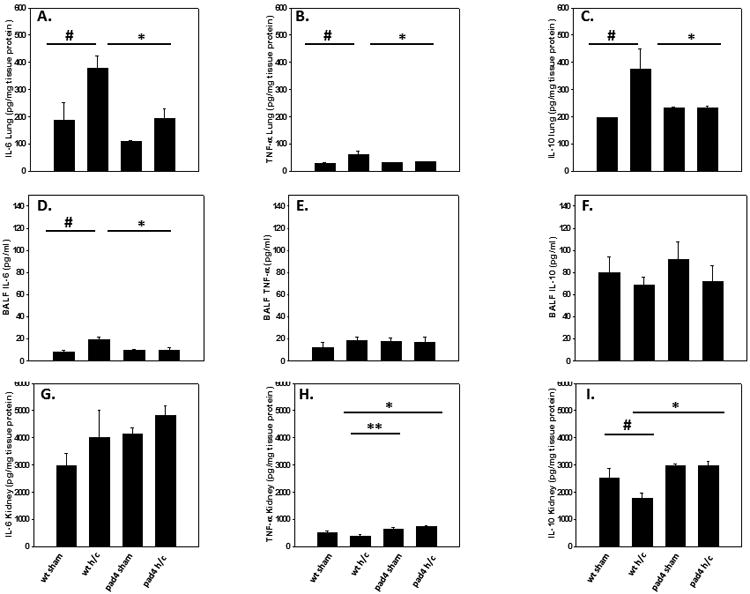
Pro-inflammatory and anti-inflammatory cytokine levels were assessed in lung tissue, the bronchial-aveolar lavage fluid (BALF) and kidney tissue of WT and PAD4-/- mice. Within the lung tissue, there were significant decreases in IL-6 (A), TNF-α (B) and IL-10 levels (C) in PAD4-/- after Hem/CLP. In the BALF, PAD4-/- displayed no increase in IL-6 levels, unlike the WT mice (D), with no differences detected in TNF-α (E) and IL-10 levels (F) between groups. In the kidney, there were no differences in IL-6 levels (G). PAD4-/- kidney TNF-α levels were higher compared to WT after hem/CLP (H), however, TNF-α levels between PAD4-/- sham and PAD4-/- Hem/CLP was unchanged. IL-10 levels in WT mice were decreased in the kidney compared to WT sham levels after Hem/CLP (I), while In PAD4-/-, IL-10 levels in the kidney were unchanged between sham and Hem/CLP animals. One Way ANOVA, #p≤0.05 sham vs. Hem/CLP; * p≤0.05 WT Hem/CLP vs PAD4-/- Hem/CLP; ** p≤0.05 WT Hem/CLP vs PAD4-/- sham. Lung tissue n=5-7/group, BALF n=6-9/group, Kidney tissue n=5-9/group
Effects of PAD4 on organ injury indices after Hem/CLP
The Lung
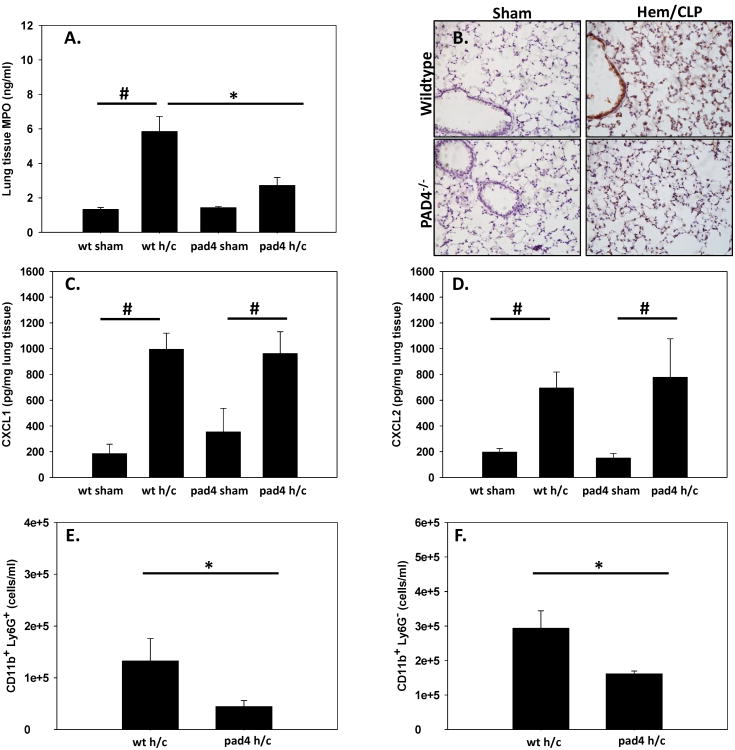
One of the hallmarks of indirect ARDS is the sequestration and activation of neutrophils in the lung (1–3). MPO activity as an index of neutrophil influx was assessed in lung tissue lysates and in histological samples. Previous studies from our lab have shown that MPO activity is increased significantly in the lungs after Hem/CLP (21). PAD4-/- mice had attenuated levels of MPO in the lungs after Hem/CLP that was comparable to sham levels, whereas WT Hem/CLP mice had significant increases in MPO (fig. 6A-B). To the extent that the reduction in MPO as an index of neutrophil influx was related to altered chemokine mediated chemoattraction, we looked at chemoattractant levels of CXCL-1 and CXCL-2 within the lung. There was no difference between Hem/CLP WT and PAD4-/- mice, with both groups displaying elevated chemokine levels (fig. 6C-D). While there was no reduction in chemoattractant levels, the PAD4-/- mouse exhibited significant decreases in CD11b+ Ly6G+ cells (neutrophils) as well as CD11b+ Ly6G-(myeloid cell types) cell populations within the lung (fig. 6E-F). Overall, this data demonstrates that PAD4-/- mice had a decrease in MPO activity in the lung as well as decreased neutrophil infiltration into the lung after Hem/CLP.
Figure 6. Neutrophil influx and MPO activity is decreased in PAD4-/- mouse lung tissue following Hem/CLP.
Lung tissue was assessed for neutrophil infiltration and activation. MPO levels were significantly decreased in PAD4-/- compared to WT mice after Hem/CLP when analyzed via ELISA (A) as well as histologically (B). Chemoattractants CXCL-1 (C) and CXCL-2 (D) were up-regulated in homogenized WT and PAD4-/- lung tissue after Hem/CLP. While no difference in chemoattractant levels, overall neutrophils (as defined as Ly6G+ CD11B+ cells) were significantly decreased in PAD4-/- lung homogenates after Hem/CLP via flow cytometry (E) and also displayed a significant decrease in Ly6G- CD11B+ cells (F). One way ANOVA, #p≤0.05 sham vs. Hem/CLP; * p≤0.05 WT Hem/CLP vs PAD4-/- Hem/CLP. n=6-9/group
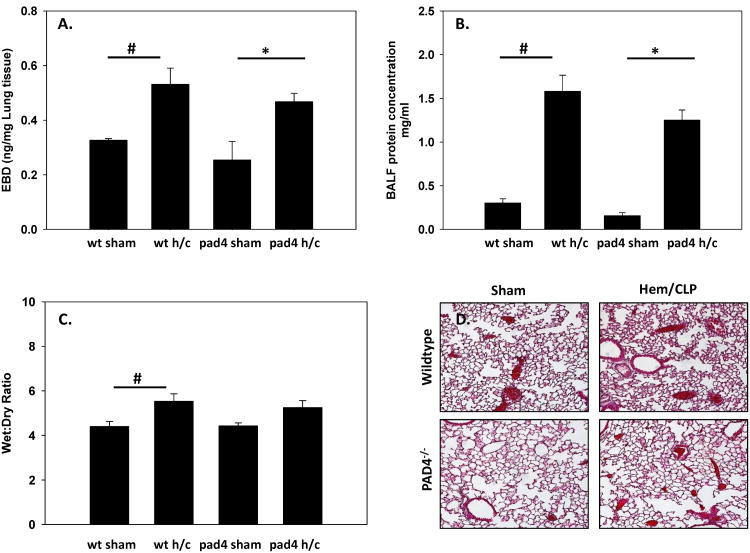
Loss of vascular integrity after a shock/sepsis leads to increased permeability; sequelae include organ edema, tissue inflammatory cell infiltrates, and an increase in tissue/vascular protein leak (1, 32, 33). NET formation has been shown to contribute to the pathogenesis of numerous vascular disorders and recently has been implicated in the destabilization of intracellular cell junctions and subsequent increased endothelial cell permeability (34). In this respect, pulmonary vascular permeability was assessed using Evans Blue Dye extravasation assay. There was no significant difference in vascular leakage between WT and PAD4-/- Hem/CLP mice, with both groups displaying a similar rise in lung permeability (fig. 7A) when compared to sham groups. This corresponded with an increase in protein concentrations in BALF in both WT and PAD4-/- Hem/CLP mice (fig. 7B). Lung edema was determined using wet:dry ratios (7C). WT mice measured a significant increase in wet:dry ratios compared to the sham group, while the PAD4-/- mice did not. However, both WT and PAD4-/- lungs had increased wet:dry ratios after 24hrs Hem/CLP with no significant differences noted between the experimental groups. This suggests that lungs from both groups were experiencing some extent of edema. Sections of the left lobe were then analyzed with aHaemotoxylin and Eosin (H&E) stain to look for more evidence of a possible difference in lung injury between groups. This Histologic analysis showed that WT mice displayed an increase in interstitial thickening and increased alveolar congestion (fig. 7D) after Hem/CLP. While there was also evidence of lung injury in the PAD4-/- mice, it was attenuated compared to the WT mice. Overall, this data suggests that PAD4 gene deletion leads to a decrease in MPO and inflammatory cytokine activity, but there are minimal changes in other indices of lung injury.
Figure 7. Indices of Lung Injury after Hem/CLP.
Different parameters of lung injury were assessed in both WT and PAD4-/- after Hem/CLP to gauge lung pathology. Vascular permeability was assessed using Evans Blue Dye extravasation assay 24 hrs after Hem/CLP. No difference in vascular leakage between WT and PAD4-/- mice was detected after Hem/CLP, with both groups exhibiting a similar rise in lung permeability (A). This was consistent with an increase in protein concentrations seen in the BALF in WT and PAD4-/- mice (B). Lung wet:dry ratios showed an increase in lung edema compared to sham mice in both groups (C.) Histological analysis of the left lung lobe via H&E staining displays an increase in interstitial thickening and alveolar congestion in the WT lung after Hem/CLP. This is mitigated in the PAD4-/- lung (D). One Way ANOVA, #p≤0.05 sham vs. Hem/CLP; * p≤0.05 WT Hem/CLP vs PAD4-/- Hem/CLP. Lung samples n=3-8/group, BALF n=3-6/group
The Kidney
In various experimental studies, it has been shown that acute lung injury correlated with altered kidney function (35–37). In addition, PAD4 has been associated with kidney inflammation and dysfunction during renal failure (38). Therefore, we delved into kidney injury and function after Hem/CLP in PAD4-/- mice. First, we assessed MPO levels in the kidney as we did with the lung to look at neutrophil activation within the kidney. There was no difference in MPO levels between WT and PAD4-/- mice, with both groups displaying MPO levels comparable to sham levels after Hem/CLP (fig. 8A). Similarly, when measuring neutrophil chemoattractants, CXCL-1 levels were unchanged in the kidney across all groups (fig. 8B). Baseline CXCL-2 levels were significantly higher in the sham PAD4-/- kidneys as compared to WT (fig. 8C). Subsequently, after Hem/CLP, CXCL-2 levels in the PAD4-/- were decreased to comparable WT levels (fig. 8C).
Figure 8. MPO levels are not altered in the kidneys of WT compared PAD4-/- mice following Hem/CLP.
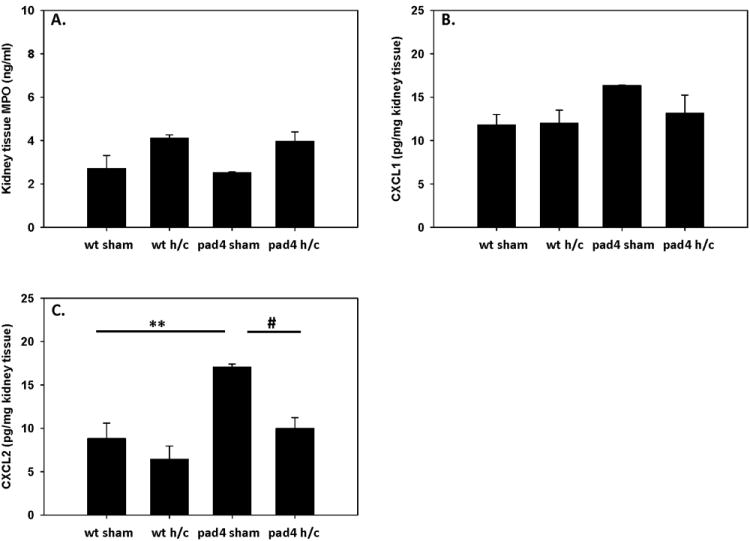
Homogenized kidney tissue was assessed for MPO activity after Hem/CLP. There were no significant changes detected in MPO levels between WT and PAD4-/- groups (A). CXCL-1 levels were unchanged in the kidney across all groups (B), while CXCL-2 levels were significantly higher in the sham PAD4-/- kidneys as compared to WT (C). However, after Hem/CLP, CXCL2 levels in the PAD4-/- were decreased to comparable WT levels. One way ANOVA, #p≤0.05 sham vs. Hem/CLP; **p≤0.05 WT sham vs PAD4-/- sham. n=6-9/group
To further investigate the role of PAD4 gene deficiency on kidney function after Hem/CLP, we measured BUN and creatinine levels in the plasma of WT and PAD4-/- mice. BUN levels were significantly increased in the WT mice, but were attenuated in the PAD4-/- mice (fig. 9A). Creatinine levels in both groups were comparable to sham levels (fig. 9B). These BUN and Creatinine levels were used to calculate BUN: Creatinine ratios (BUN:Cr) as a measurement of pre-renal injury. WT mice displayed a significant increase in their BUN:Crs ratio suggesting that WT mice were in pre-renal failure, which can be caused by a decreased effective arterial blood volume, whereas PAD4-/- remained at sham levels (fig. 9C). Kidney vascular permeability was then assessed using EBD. PAD4-/- mice displayed a significant decrease in vascular leakage as compared to WT mice after Hem/CLP as measured by a significant decline of EBD recovered from the kidneys (fig. 9D). Together this data suggest that PAD4-/- kidneys are better able to maintain normal kidney function than WT mice in the face of Hem/CLP.
Figure 9. PAD4-/- mice maintain kidney function after Hem/CLP.
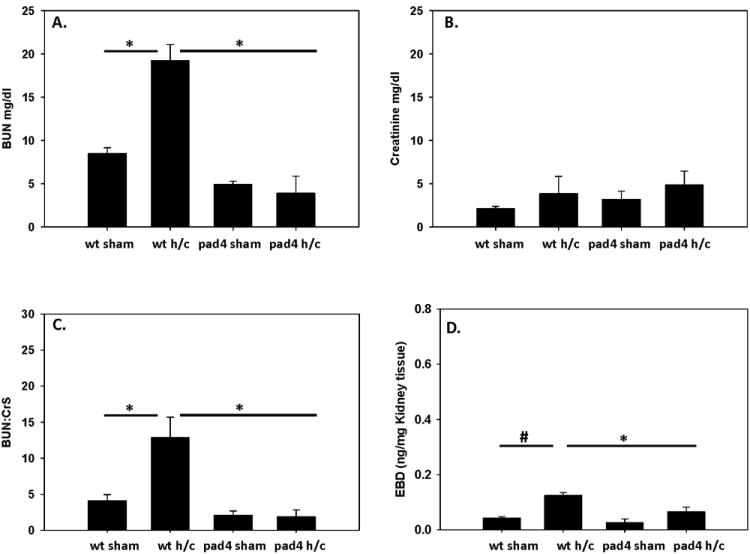
Indices of kidney function was measured in WT and PAD4-/- mice after Hem/CLP. BUN and Creatinine levels were measured in plasma as a measurement of renal function. BUN levels were comparable to sham levels in the PAD4-/- mice whereas WT had significantly increased levels after Hem/CLP (A) while creatinine levels in both groups were similar to their correspondent sham levels (B). When calculating BUN:Cr ratio WT mice displayed a significant increase in BUN:Cr ratios, whereas PAD4-/- remained at sham levels (C). Vascular permeability was assessed using Evans Blue Dye extravasation assay 24 hrs after Hem/CLP. Kidney permeability was significantly decreased in the PAD4-/- after Hem/CLP as measured by a significant decline of Evans Blue Dye recovered from the kidneys (D). One Way ANOVA, #p≤0.05 sham vs. Hem/CLP; * p≤0.05 WT Hem/CLP vs PAD4-/- Hem/CLP. n=6-9/group BUN and Cr assays n=3-6/group EBD assay.
Discussion
PAD4 activity is an essential component in histone citrullination and subsequent chromatin decondensation, which are critical steps needed for NET formation (18, 39). Conversely, PAD4 activity and histone citrullination has also been shown to be a contributor of various disease states and serves as a mediator of many inflammatory signals that prompt the neutrophils' response to infection (10, 29, 30, 40, 41). These effects have been linked to tissue damage as well as increased mortality.
Components of NETs, such as circulating free DNA (CF-DNA) and circulating histones have been implicated in acute lung injury and ARDS (12–14). Infused circulating histones have been shown to lead to alveolar capillary obstruction, histone toxicity in lung tissue and activation of the coagulation cascade. These factors contribute to lung injury as well as multiple organ failure (MOF) (14). Extracellular histones have been implicated in lung damage and direct alveolar damage in various animal models of lung injury (10). On the other hand, depletion or neutralization of neutrophils in mouse models leads to a decrease in circulating histones and reduced lung injury (42, 43).
In a murine CLP model of sepsis, PAD4-/- mice seemingly had no real survival advantage over there PAD4+/+ counterparts (44). In our model of Hem/CLP we found that PAD4 gene deficiency provides an increase in survival over a 14 day period. While we cannot exclusively give credit to the improved outcome in the PAD4–/– mice to lack of NETs, our data is in line with survival data described in sepsis models that use chemical inhibition of PAD, which imparts an increase in survival after CLP (45–47).
PAD4-/- mice have been shown to be susceptible to bacterial infections and their neutrophils display a significant reduction in bacterial killing in vitro (18). However, this is in contrast to our results, which found PAD4-/- mice had enhanced bacterial clearance in the peritoneum after shock/septic insult. Studies looking at the bactericidal ability of NETs support the idea that bacterial entrapment rather than bacterial killing is the primary function of NETs in infection (48, 49). The neutrophil count in the peritoneum was unchanged between WT and PAD4-/- mice after Hem/CLP, but proportionally neutrophils were less representative in PAD4-/- peritoneal lavages. Therefore, a lack of NETs may not necessarily mean a reduction in bacterial killing, and the increased peritoneal bacterial clearance seen in PAD4-/- mice after Hem/CLP may be the result of other consequences of PAD4 gene deficiency, such as a reduction in immune dysfunction. To try and explain this increase in bacterial clearance we looked at other cell types, macrophages and monocytes, that also play a role in bacterial killing in polymicrobial sepsis (26, 50, 51). Monocytes are recruited to sites of infection and differentiated to macrophages. When differentiation of monocytes into macrophages occurs, the mRNA expression of PAD4 is lost. However, PAD4 protein levels remain unchanged, suggesting that the PAD4 protein is not rapidly degraded (52, 53). Hence, the loss of PAD4 may alter the function of these cells. Flow data analysis of peritoneal lavages after Hem/CLP suggests that there is a trend toward an increase in monocytes and macrophage recruitment to the site of infection in PAD4-/- mice, although not significant. In addition, these mice display an altered macrophage morphology with smoother, less granulated macrophages, which could suggest a lessened dysregulation/apoptosis of macrophages which can be seen after polymicrobial insult (50, 54, 55). Taken together, our data suggest that PAD4 gene deletion may affect other cell types that might be contributing toward the increased bacterial clearance and enhanced survivability during Hem/CLP independent of NETosis.
NETs can lead to a dysregulation/accumulation of pro-inflammatory mediators within tissues as well as the vasculature, leading to tissue damage and organ failure (9–11). In various animal models of lung injury, extracellular histones have been found to be a major contributor of pro-inflammatory cytokines in ARDS leading to lung injury and directly cause alveolar damage (10). After Hem/CLP, PAD4-/- mice displayed significant decreases in TNF-α in lung tissue and IL-6 in both lung tissue and BALF. PAD4-/- also led to a decrease in IL-10 production in the lung. The reduction in cytokine levels in the lung of Hem/CLP PAD4-/- mice may suggest that the lack of NET formation reduces the inflammatory mediators that are caused by extracellular histones and NETs.
One of the key features of ARDS is the dysregulation and recruitment of activated neutrophils to the lung microvasculature, interstitium, and alveolar space (1–3). This excessive neutrophil activation and accumulation leads to increased ROS production, increased pro-inflammatory mediators, and decreased PMN apoptosis. These changes culminate in epithelial and endothelial cell damage (2, 7). PAD4 gene deletion does not seem to play a role in the increased lung micro-vascular permeability seen in iARDs, However, to our knowledge, our results show for the first time that PAD4-/- leads to a reduction of neutrophil sequestration in the lung after a shock/septic insult resulting in reductions in MPO activity within the lung. In addition to decreased neutrophil numbers, PAD4-/- mice also display a decrease in CD11b+ Ly6G- myeloid cells in the lung. This could be of interest, as PAD4 is also foundin granulocytes and monocytes (52, 56–59), which also play a role in the innate immune response to infection. Nonetheless, it is unclear the exact mechanism in which PAD4 alters neutrophil recruitment to the lung. There was significant increases in CXCL-1 and CXCL-2 in both PAD4-/- as well as WT mice, suggesting no alterations in chemotactic signaling. Our mouse model of hemorrhagic shock induces neutrophil “priming”, which predisposes the neutrophil to inflammatory stimuli that leads to an altered response after a subsequent secondary challenge, CLP, (1, 60, 61). It is possible that this “priming” event is altered by PAD4 deficiency, leading to a decrease of neutrophil sequestration into the lung, and should be further explored.
Critically ill patients who suffer from sepsis are not only at risk of developing ARDS (the most common organ injury noted in the critically ill patient), but also other organ system dysfunctions (62, 63). In this respect; 35% of patients with ARDS also develop acute kidney injury (AKI), and the development of this secondary insult is associated with dramatically increased patient mortality (64, 65). In this respect, many of the pathways that are implicated in the development of ARDS are also thought to play a role in the development of acute kidney injury (65). It is this close association that makes the kidney an organ of interest when studying the MOF associated with sepsis induced iARDS. When analyzing kidney function after Hem/CLP, there were minimal changes in cytokine and chemokines production between groups, however, vascular leakage was significantly decreased in the PAD4-/- mice as compared to the WT mice. Additionally, while WT mice displayed significant increases in BUN levels after Hem/CLP, there was no statistically significant change in BUN levels between PAD4-/- sham and Hem/CLP mice. All four mice groups maintained normal creatinine levels. However, when BUN:Cr were calculated, PAD4-/- mice maintained normal ratios where WT Hem/CLP mice had a significantly increased ratios. Urea levels are disproportionately increased compared to the Cr in WT mice, suggesting volume depletion/dehydration/hemorrhage which we would expect to see after Hem/CLP. However, Urea levels comparable to sham mice in the PAD4-/- group would suggest that their kidneys were better able to tolerate the hemorrhagic shock. It is important to note that PAD4 specifically has been implicated in the damage seen in renal ischemia reperfusion (I/R) injury by increasing renal tubular inflammatory responses and neutrophil infiltration into the kidney (38). In addition, PAD4 deficient mice have been reported to display a reduction in renal tubular necrosis, inflammation, and apoptosis, as well as decreases in neutrophil infiltration after I/R in the kidneys and liver via immunohistochemistry (66), suggesting that PAD4 may play a role in increasing the renal tubular inflammatory response and neutrophil infiltration after renal I/R injury. It is unclear if the maintenance of kidney function and integrity, (as assessed by decreased vascular permeability after Hem/CLP), in PAD4-/- mice can be attributed to lack of NET formation, as MPO and chemokine levels were similar between groups. However, as MOF is progressive through the course of disease, it would be interesting to investigate neutrophil recruitment and NET formation within the kidney to detect any differences that might occur later in the course of the disease state caused by Hem/CLP.
In conclusion, our study demonstrated for the first time that inhibition of PAD4 through gene deletion and subsequent NET formation leads to a reduction of sequestered neutrophils to the lung and reductions in MPO as well as pro-inflammatory cytokine signaling within the lung in a mouse iARDS model induced by Hem/CLP. Additionally, PAD4-/- mice maintain baseline kidney function, which decreases the chances of renal failure. Taken together, these alterations in the immune responses may be attributed to the overall increase in survival seen in PAD4-/- mice after Hem/CLP. While these results seen in the PAD4-/- mice cannot be definitively attributed to inhibition of NET formation, it is a step in the right direction to study the effect of NETs on the immune response to shock/sepsis. While PAD4 specific inhibitors are under development (67), the use of Cl-amidine, which has been used extensively to study NET formation in various disease sates, including sepsis,(45, 68, 69)can be utilized to further study and understand the role of NET formation in the critically ill patient.
Acknowledgments
This work was funded by NIH T32 HL134625 (B.M.B.), R01-GM46354 & R35 GM118097 (A.A.), and R01-GM066194 (J.S.R).
The authors of this manuscript would like to thank Dr. Kerri Mowen (Scripps Research Institute, La Jolla, CA) for gifting the PAD4-/- mice used in these studies
References
- 1.Lomas-Neira J, Chung CS, Perl M, Gregory S, Biffl W, Ayala A. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Am J Physiol Lung Cell Mol Physiol. 2006;290:L51–8. doi: 10.1152/ajplung.00028.2005. [DOI] [PubMed] [Google Scholar]
- 2.Perl M, Lomas-Neira J, Venet F, Chung CS, Ayala A. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med. 2011;5:115–26. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venet F, Chung CS, Huang X, Lomas-Neira J, Chen Y, Ayala A. Lymphocytes in the development of lung inflammation: a role for regulatory CD4+ T cells in indirect pulmonary lung injury. J Immunol. 2009;183:3472–80. doi: 10.4049/jimmunol.0804119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–64. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 5.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 6.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 7.Perl M, Lomas-Neira J, Chung CS, Ayala A. Epithelial cell apoptosis and neutrophil recruitment in acute lung injury-a unifying hypothesis? What we have learned from small interfering RNAs. Mol Med. 14:465–75. doi: 10.2119/2008-00011.Perl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 9.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis. 2012;25:321–7. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margraf S, Lögters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008;30:352–8. doi: 10.1097/SHK.0b013e31816a6bb1. [DOI] [PubMed] [Google Scholar]
- 14.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W, Wang G, Toh CH. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–9. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Wen Z, Guan L, Jiang P, Gu T, Zhao J, Lv X, Wen T. Extracellular histones play an inflammatory role in acid aspiration-induced acute respiratory distress syndrome. Anesthesiology. 2015;122:127–39. doi: 10.1097/ALN.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 16.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–13. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–62. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6:e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lomas JL, Chung CS, Grutkoski PS, LeBlanc BW, Lavigne L, Reichner J, Gregory SH, Doughty LA, Cioffi WG, Ayala A. Differential effects of macrophage inflammatory chemokine-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: assessment by adoptive cells transfer in mice. Shock. 2003;19:358–65. doi: 10.1097/00024382-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Lomas-Neira J, Chung CS, Grutkoski PS, Dunican A, Simms HH, Cioffi WG, Ayala A. Divergent roles of murine neutrophil chemokines in hemorrhage induced priming for acute lung injury. Cytokine. 2005;31:169–79. doi: 10.1016/j.cyto.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Cheng T, Bai J, Chung CS, Chen Y, Biron BM, Ayala A. Enhanced Innate Inflammation Induced by Anti-BTLA Antibody in Dual Insult Model of Hemorrhagic Shock/Sepsis. Shock. 2016;45:40–9. doi: 10.1097/SHK.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai J, Tang L, Lomas-Neira J, Chen Y, McLeish KR, Uriarte SM, Chung CS, Ayala A. TAT-SNAP-23 treatment inhibits the priming of neutrophil functions contributing to shock and/or sepsis-induced extra-pulmonary acute lung injury. Innate Immun. 2015;21:42–54. doi: 10.1177/1753425913516524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang L, Bai J, Chung CS, Lomas-Neira J, Chen Y, Huang X, Ayala A. Programmed cell death receptor ligand 1 modulates the regulatory T cells' capacity to repress shock/sepsis-induced indirect acute lung injury by recruiting phosphatase SRC homology region 2 domain-containing phosphatase 1. Shock. 2015;43:47–54. [Google Scholar]
- 25.Monaghan SF, Thakkar RK, Heffernan DS, Huang X, Chung CS, Lomas-Neira J, Cioffi WG, Ayala A. Mechanisms of indirect acute lung injury: a novel role for the coinhibitory receptor, programmed death-1. Ann Surg. 2012;255:158–64. doi: 10.1097/SLA.0b013e31823433ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol. 2012;92:593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchins NA, Chung CS, Borgerding JN, Ayala CA, Ayala A. Kupffer cells protect liver sinusoidal endothelial cells from Fas-dependent apoptosis in sepsis by down-regulating gp130. Am J Pathol. 2013;182:742–54. doi: 10.1016/j.ajpath.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raptopoulou A, Sidiropoulos P, Katsouraki M, Boumpas DT. Anti-citrulline antibodies in the diagnosis and prognosis of rheumatoid arthritis: evolving concepts. Crit Rev Clin Lab Sci. 2007;44:339–63. doi: 10.1080/10408360701295623. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta. 2013;1829:1126–35. doi: 10.1016/j.bbagrm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latifi SQ, O'Riordan MA, Levine AD. Interleukin-10 controls the onset of irreversible septic shock. Infect Immun. 2002;70:4441–6. doi: 10.1128/IAI.70.8.4441-4446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lomas-Neira J, Venet F, Chung CS, Thakkar R, Heffernan D, Ayala A. Neutrophil-endothelial interactions mediate angiopoietin-2-associated pulmonary endothelial cell dysfunction in indirect acute lung injury in mice. Am J Respir Cell Mol Biol. 2014;50:193–200. doi: 10.1165/rcmb.2013-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lomas-Neira JL, Heffernan DS, Ayala A, Monaghan SF. BLOCKADE OF ENDOTHELIAL GROWTH FACTOR, ANGIOPOIETIN-2, REDUCES INDICES OF ARDS AND MORTALITY IN MICE RESULTING FROM THE DUAL-INSULTS OF HEMORRHAGIC SHOCK AND SEPSIS. Shock. 2016;45:157–65. doi: 10.1097/SHK.0000000000000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldabbous L, Abdul-Salam V, Mc Kinnon T, Duluc L, Pepke-Zaba J, Southwood M, Ainscough AJ, Hadinnapola C, Wilkins MR, Toshner M, Wojciak-Stothard B. Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2016;36:2078–87. doi: 10.1161/ATVBAHA.116.307634. [DOI] [PubMed] [Google Scholar]
- 35.Darmon M, Clec'h C, Adrie C, Argaud L, Allaouchiche B, Azoulay E, Bouadma L, Garrouste-Orgeas M, Haouache H, Schwebel C, Goldgran-Toledano D, Khallel H, Dumenil AS, Jamali S, Souweine B, Zeni F, Cohen Y, Timsit JF. Acute Respiratory Distress Syndrome and Risk of AKI among Critically Ill Patients. Clin J Am Soc Nephrol. 2014;9:1347–1353. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuiper JW, Groeneveld ABJ, Slutsky AS, Plötz FB. Mechanical ventilation and acute renal failure. Crit Care Med. 2005;33:1408–15. doi: 10.1097/01.ccm.0000165808.30416.ef. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: the role of the kidney. Curr Opin Crit Care. 2009;15:481–487. doi: 10.1097/MCC.0b013e328332f69e. [DOI] [PubMed] [Google Scholar]
- 38.Ham A, Rabadi M, Kim M, Brown KM, Ma Z, D'Agati V, Lee HT. Peptidyl arginine deiminase-4 activation exacerbates kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;307:F1052–62. doi: 10.1152/ajprenal.00243.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leshner M, Wang S, Lewis C, Zheng H, Chen XA, Santy L, Wang Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight JS, Zhao W, Luo W, Subramanian V, O'Dell AA, Yalavarthi S, Hodgin JB, Eitzman DT, Thompson PR, Kaplan MJ. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123:2981–93. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, Davis T, Matesic LE, Thompson PR, Hofseth LJ. Suppression of colitis in mice by Cl-amidine: a novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G929–38. doi: 10.1152/ajpgi.00435.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Guan L, Yu J, Zhao Z, Mao L, Li S, Zhao J. Pulmonary endothelial activation caused by extracellular histones contributes to neutrophil activation in acute respiratory distress syndrome. Respir Res. 2016;17:155. doi: 10.1186/s12931-016-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV, Standiford TJ, Ward PA. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013;27:5010–21. doi: 10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, Wang Y, Wagner DD. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125:1948–56. doi: 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biron BM, Chung CS, O'Brien XM, Chen Y, Reichner JS, Ayala A. Cl-Amidine Prevents Histone 3 Citrullination and Neutrophil Extracellular Trap Formation, and Improves Survival in a Murine Sepsis Model. J Innate Immun. 2017;9:22–32. doi: 10.1159/000448808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Liu Z, Liu B, Zhao T, Chong W, Wang Y, Alam HB. Citrullinated histone H3: A novel target for the treatment of sepsis. Surgery. 2014 doi: 10.1016/j.surg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao T, Pan B, Alam HB, Liu B, Bronson RT, Deng Q, Wu E, Li Y. Protective effect of Cl-amidine against CLP-induced lethal septic shock in mice. Sci Rep. 2016;6:36696. doi: 10.1038/srep36696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolaczkowska E, Jenne CN, Surewaard BGJ, Thanabalasuriar A, Lee WY, Sanz MJ, Mowen K, Opdenakker G, Kubes P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. doi: 10.1038/ncomms7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Brien XM, Biron BM, Reichner JS. Consequences of extracellular trap formation in sepsis. Curr Opin Hematol. 2017;24:66–71. doi: 10.1097/MOH.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–8. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ayala A, Perrin MM, Kisala JM, Ertel W, Chaudry IH. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophages to release inflammatory mediators (interleukins-1 and -6 and tumor necrosis factor) Circ Shock. 1992;36:191–9. [PubMed] [Google Scholar]
- 52.Vossenaar ER, Radstake TRD, van der Heijden A, van Mansum MAM, Dieteren C, de Rooij DJ, Barrera P, Zendman AJW, van Venrooij WJ. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63:373–81. doi: 10.1136/ard.2003.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vossenaar ER, Zendman AJW, van Venrooij WJ, Pruijn GJM. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–18. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 54.Ayala A, Urbanich MA, Herdon CD, Chaudry IH. Is sepsis-induced apoptosis associated with macrophage dysfunction? J Trauma. 1996;40:568–73–4. doi: 10.1097/00005373-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6(1):S27–38. [PubMed] [Google Scholar]
- 56.Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol. 2004;11:777–83. doi: 10.1038/nsmb799. [DOI] [PubMed] [Google Scholar]
- 57.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277:49562–8. doi: 10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- 58.Asaga H, Nakashima K, Senshu T, Ishigami A, Yamada M. Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils. J Leukoc Biol. 2001;70:46–51. [PubMed] [Google Scholar]
- 59.Liu YL, Chiang YH, Liu GY, Hung HC. Functional role of dimerization of human peptidylarginine deiminase 4 (PAD4) PLoS One. 2011;6:e21314. doi: 10.1371/journal.pone.0021314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lomas-Niera JL, Perl M, Chung CS, Ayala A. Shock and hemorrhage: an overview of animal models. Shock. 2005;24(1):33–9. doi: 10.1097/01.shk.0000191411.48719.ab. [DOI] [PubMed] [Google Scholar]
- 61.Ayala A, Chung CS, Lomas JL, Song GY, Doughty LA, Gregory SH, Cioffi WG, LeBlanc BW, Reichner J, Simms HH, Grutkoski PS. Shock-induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Am J Pathol. 2002;161:2283–94. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu KD, Matthay MA. Advances in critical care for the nephrologist: acute lung injury/ARDS. Clin J Am Soc Nephrol. 2008;3:578–86. doi: 10.2215/CJN.01630407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB, Wheeler A, Korpak A, Thompson BT, Chertow GM, Matthay MA and National Heart, Lung, and Blood Institute ARDS Network Clinical Trials Group. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35:2755–61. [PMC free article] [PubMed] [Google Scholar]
- 66.Rabadi M, Kim M, D'Agati V, Lee HT. Peptidyl arginine deiminase-4-deficient mice are protected against kidney and liver injury after renal ischemia and reperfusion. Am J Physiol Renal Physiol. 2016;311:F437–49. doi: 10.1152/ajprenal.00254.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, Bicker KL, Bingham RP, Campbell M, Chen YH, Chung CW, Craggs PD, Davis RP, Eberhard D, Joberty G, Lind KE, Locke K, Maller C, Martinod K, Patten C, Polyakova O, Rise CE, Rüdiger M, Sheppard RJ, Slade DJ, Thomas P, Thorpe J, Yao G, Drewes G, Wagner DD, Thompson PR, Prinjha RK, Wilson DM. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11:189–91. doi: 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, Davis T, Matesic LE, Thompson PR, Hofseth LJ. Suppression of colitis in mice by Cl-amidine: a novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G929–38. doi: 10.1152/ajpgi.00435.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slack JL, Causey CP, Thompson PR. Protein arginine deiminase 4: a target for an epigenetic cancer therapy. Cell Mol Life Sci. 2011;68:709–20. doi: 10.1007/s00018-010-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]