Abstract
Asthma is a chronic inflammatory disease mediated by allergen-specific CD4 T cells which promote lung inflammation through recruitment of cellular effectors into the lung. A subset of lung T cells can persist as tissue-resident memory T cells (TRM) following infection and allergen induction, although the generation and role of TRM in asthma persistence and pathogenesis remain unclear. Here we used a mouse model of chronic exposure to intranasal house dust mite extract (HDM) to dissect how the lung TRM are generated and function in the persistence and pathogenesis of allergic airway disease. We demonstrate that both CD4+ and CD8+T cells infiltrate into the lung tissue during acute HDM exposure; however, only CD4+TRM, and not CD8+TRM persist longterm following cessation of HDM administration. Lung CD4+TRM cells are localized around airways and rapidly reactivated upon allergen re-exposure accompanied by the rapid induction of airway hyperresponsiveness independent of circulating T cells. Lung CD4+TRM activation to HDM challenge is also accompanied by increased recruitment and activation of dendritic cells in the lungs. Our results indicate that lung CD4+TRM can perpetuate allergen-specific sensitization and direct early inflammatory signals that promote rapid lung pathology, suggesting that targeting lung CD4+TRM could have therapeutic benefit in ameliorating recurrent asthma episodes.
INTRODUCTION
Asthma is a chronic inflammatory lung disease characterized by airway hyper-responsiveness, for which there is no cure. Asthma is triggered by the immune response to inhaled allergens which induces infiltration of effector T cells into the lung (1, 2). Type 2 helper T lymphocytes (Th2), and specific Th2 cytokines, including IL-4 and -5, are the major drivers of allergic asthma and promote airway inflammation, recruitment and activation of effector cells such as eosinophils (3–6) and mast cells (7), mucus production (8, 9) and increased airway hyperresponsiveness. Fibrosis and lung remodeling, observed in chronic disease (10), are also linked to Th2-mediated effects (11, 12). The mechanisms for the induction and chronicity of asthma are not known and providing insight into this process will allow the development of more targeted therapies to specifically inhibit the lung inflammatory response in this debilitating disease.
It has become increasingly clear that immune responses resident in the lung and other mucosal sites are critical to immune-mediated protection (13–16), and influence tissue inflammation and repair (17, 18). In mouse models of respiratory virus infection, we previously found that non-circulating tissue-resident memory CD4+ and CD8+ T cells were generated in the lung (designated lung TRM), potentially providing optimal protective responses to virus challenge, with minimal morbidity (19–21). Lung TRM can also be generated to intranasally-administered vaccines and to other respiratory pathogens (22–26), suggesting localized generation of lung TRM. The generation, persistence and functional role of memory T cells in asthma and chronic inflammatory lung disease is less clear. In mouse models of allergen sensitization, a previous study demonstrated generation of long-lived memory Th2 cells in response to ovalbumin sensitization (27), and a more recent study demonstrated the development of allergen-specific lung TRM in the more physiological model of house dust mite (HDM) allergen exposure (28). However, the role of lung TRM in perpetuating asthma chronicity is not well understood. Moreover, mechanisms by which lung TRM may promote an inflammatory response in the lung either through direct in situ activation and/or rapid recruitment and mobilization of immune effectors to the lung are not known.
In this study we report the biased generation and retention of lung CD4+TRM in allergic asthma from lung effector responses, while infiltrating effector CD8+T cells are not retained as resident lung populations. HDM-primed lung CD4+ TRM persist localized around airways following cessation of allergen exposure and exhibit rapid in situ reactivation upon secondary exposure to inhaled allergen, leading to airway hyper-responsiveness as a hallmark of chronic disease. This early, local reactivation of CD4+TRM is independent of circulating T cell responses, is characterized by increased production of IL-4, 5, and IL-17, and is associated with increased activation and recruitment of dendritic cells (DC), as a mechanism by which potent inflammatory responses and airway infiltration can derive from early in situ triggers. Together, our results demonstrate that persistence of lung CD4+TRM can potentiate longterm airway disease and that targeting this subset could be of therapeutic benefit in the treatment of chronic asthma.
MATERIALS AND METHODS
Mice
Female C57BL/6 mice (6–7 weeks of age) were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) and maintained under specific pathogen-free conditions at Columbia University Medical Center (CUMC). All animal procedures were conducted according to the NIH guidelines for the care and use of laboratory animals, and were approved by the Columbia University Institutional Animal Care and Use Committee (IACUC).
House dust mite sensitization and challenge
After sedation with isoflurane (5% induction, 2–3% maintenance dose), mice were administered house dust mite (HDM) extract (Dermatophagoides pteronyssinus; Greer Laboratories, Lenoir, NC) intranasally in PBS (40μg/25μl PBS) 5 times/week for 3 weeks as previously described (29) with control mice received PBS intranasally at the same time-points. For memory generation and secondary challenge, mice exposed to HDM for 3 wks were “rested” (no further manipulation) for 4–8 weeks, then challenged intranasally with 2 doses of HDM (40μg/25μl PBS) 24hrs apart. Airway hyper-responsiveness was measured 24 hours after the final HDM challenge.
Tissue harvesting and Flow cytometry
In vivo labeling of T cells with fluorescent antibodies was performed as previously described (Turner et al., 2014). Briefly, mice were administered, PE- or Alexa 647-conjugated anti-CD90.2 (anti-Thy1.2; clone 53-2.1, BioLegend), or PE-conjugated anti-CD45.2 antibody (clone 104, Biolegend), intravenously, and 7–10 minutes later lungs were perfused, dissected and digested in RPMI1640 medium with collagenase D, DNAse and Trypsin inhibitor for 1 hour at 37°C. Mediastinal lymph nodes and spleen were dissected and manually disrupted to generate single cell suspensions. Cell suspensions were stained with fluorescent-conjugated antibodies for CD4 (clones RM4-5, BD Biosciences, and GK1.5, eBioscience), CD8 (clone 53-6.7, BioLegend), CD11a (clone M17/4, BioLegend),CD11b (M1/70 eBioscience), CD11c (N418, eBioscience), CD25 (PC61.5, eBioscience), CD45 (30-F11, BioLegend), CD69 (clone H1.2F3, eBioscience), CD86 (GL-1, Biolegend), CD103 (clone 2E7, eBioscience) and Anti-Mouse MHC Class II (I-A/I-E) (clone M5/114.15.2, eBioscience) was performed according to manufacturers’ protocol. Stained cells were analyzed using the BD LSRII flow cytometer and flow-jo software (Treestar, Ashland, OR). Absolute cell numbers were determined by flow cytometry using CountBright Absolute Counting Beads (Invitrogen) according to manufacturer’s protocol.
Cytokine assays
CD4+CD44+CD62L− memory T cells protected from i.v. anti-CD90.2 staining as above were sorted from lungs of HDM memory mice using BD Influx Cell Sorter. The resultant puritifed lung CD4+TRM were co-cultured with an equal number of CD3− splenocytes in the presence of either BSA or HDM (100 μg/mL) for 24 hours in complete click’s media in a 96-well U-bottom plate. Supernatants were analyzed for cytokine content using the Legendplex Mouse Th Cytokine kit and a BD LSRII Flow Cytometer. Data were further analyzed using Legendplex v8.0 software (Biolegend).
In vivo Airway hyper-responsiveness (Flexivent)
After sedation with pentobarbitol 75 mg/kg, the neck was dissected, and the trachea was cannulated with an 18-gauge beveled tracheal tube. The mouse was then placed on a mouse ventilator (flexiVent, SCIREQ, Montreal, Quebec, Canada) with a tidal volume of 8 ml/kg and frequency of 150 breaths/min. After 3–5mins equilibration on the ventilator and maintaining the mice at 37°C with a homeothermic blanket (Homeothermic Blanket System, Harvard Apparatus, Holliston, Massachusetts, USA), mice were given succinylcholine 0.5mg every 14 minutes by i.p. injection; a graded MCh intranasal challenge was initiated, and airway resistance was measured using the flexiVent system.
Bronchoalveolar lavage and differential cell count
esuspended in PBS. Total cell concentrations were counted with a hemocytometer. Cytospin preparations were stained with Quick-Diff (Imeb Inc.) and cells were analyzed for differential counts using morphological criteria.
Sample preparation for confocal microscopy
Mice were sacrificed and the lungs were perfused, inflated with agarose and sectioned using a Leica VT 1000s vibratome as previously described (19). Tissue slices were first treated with endogenous streptavidin/biotin-blocking reagents (Life Technologies) then stained with biotin conjugated anti-collagen I mAb (Rockland Immunochemicals Inc, Gilbertsville PA) followed by streptavidin conjugated Dylight 488 (Abcam, Cambridge MA). Lung sections were then stained with fluorescein-labeled lectin from the Cry-Baby Tree, Erythrina crystagalli (Vector Labs), AF647-conjugated or Pacific Blue-conjugated anti-CD4 mAb (clone RM4-4) and PE-conjugated anti B220. Images were acquired and processed using a Zeiss LSM 700 Laser Scanning Microscope and software (Thornwood, NY).
T cell depletion
For peripheral T cell depletion, mice were treated with or anti-Thy1 mAb, clone: T24/31 (Bio X cell, West Lebanon, NH) or isotype control IgG2b (50μg per mouse) daily for three days prior to a one day following HDM challenge.
RESULTS
Biased Generation of Lung CD4 TRM in a mouse model of chronic allergic asthma
To define the role of lung TRM in the pathogenesis of allergic asthma we employed a physiological mouse model of chronic HDM exposure (30), involving daily intranasal (i.n.) challenge of HDM over 3 weeks. This protocol of repeated HDM exposure results in severe symptoms that mimic chronic asthma in humans including increased airway hyperresponsiveness to methacholine, severe eosinophilic inflammation and increased expression of Th2-associated cytokines (30).
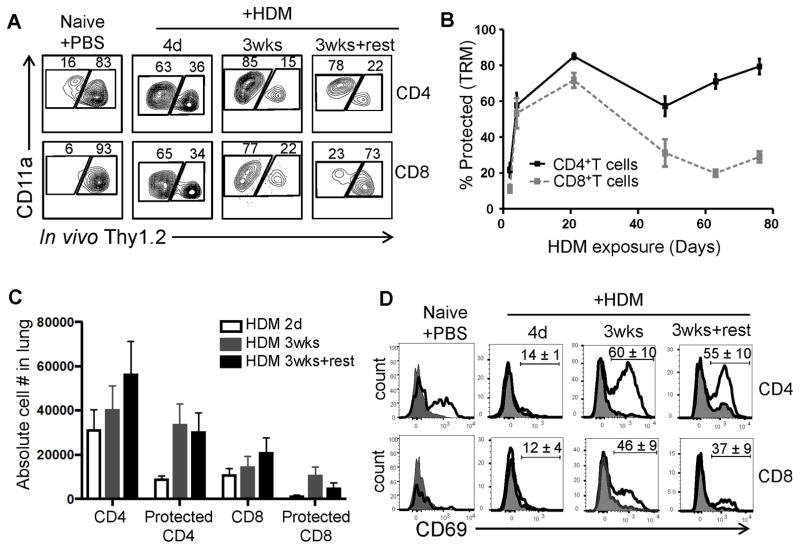
To assess whether HDM exposure generated lung TRM, we used an in vivo, antibody-labeling assay involving intravenous injection of mice with fluorescently labeled anti-Thy1.2 mAb (i.v. Ab) prior to tissue harvest. With this approach, T cells resident within lung tissue are protected from i.v. Ab labeling, while circulating cells in the vasculature bind fluorescent Ab (19, 31). In PBS-treated mice, the majority of lung CD4 and CD8 T cells are labeled with i.v. Ab (>80% CD4+ and >90% CD8+, Fig. 1A, left) confirming that lungs of naïve mice contain predominantly circulating T cells which are not retained in the tissue (19). By contrast, following HDM exposure, the majority of CD4+ and CD8+ T cells in the lung are protected from i.v. Ab labeling, as early as 4 days of HDM administration (given each day) with increasing frequencies after 3 weeks of daily HDM exposure (Fig. 1A, B). These results indicate that acute and continuous exposure to HDM alters the composition of lung T cells from mostly circulating to predominantly in the tissue.
Figure 1. House dust mite sensitization preferentially induces CD4+TRM in the lung.
(A) Flow cytometric analysis of lung T cells labeled (right gate in flow cytometry plots) or protected (left gate) from intravenously (i.v.) administered fluorescent anti-Thy1.2 antibody following house dust mite (HDM) sensitization. (B) Frequency of lung CD4+ and CD8+TRM defined by the % protected CD4+ or CD8+ T cells during and following HDM treatment (mean ± SEM; n = 4–15 mice per group; compiled from 4 independent experiments). (C) Absolute numbers of CD4+, CD8+, protected CD4+ and protected CD8+T cells in the lung (n= 3–4 mice per group compiled from 3 independent experiment). (D) CD69 expression by labeled (shaded histogram) and protected (black line) lung CD4+ (top row) and CD8+ (bottom row) T cells. Numbers indicate the percent CD69+ (mean±SEM) among protected T cells (n =3–4 mice per group; representative of 3 independent experiments).
The persistence of these lung tissue T cells as TRM was further assessed by stopping further HDM exposure in mice “rested” for 4–8 weeks following the last HDM administration (HDM-memory mice). Interestingly, while CD4+T cells maintained their distribution within the protected fraction after i.v. Ab labeling, lung CD8+T cells were predominantly labeled in HDM-memory mice (Fig. 1A, B). We assessed absolute lung T cell counts to determine whether these distinct proportions of protected lung T cells were due to differential generation of lung CD4+ and CD8+TRM following HDM exposure. While HDM exposure triggered increased numbers of CD4+ and CD8+T cells to the lungs which were predominantly distributed in protected fraction, CD4+T cells were recruited in greater numbers, and persisted in HDM memory mice, while numbers of protected lung CD8+T cells were reduced significantly (Fig. 1C). Moreover, lung CD4+T cells in HDM memory mice exhibited upregulated CD69 expression, a phenotypic hallmark of TRM (21, 32), while CD8+ T cells were largely CD69-negative, even after acute HDM exposure (Fig. 1D). Taken together, these results show that in contrast to respiratory virus infection, which generates both CD4+ and CD8+TRM in the lung (19, 33), HDM exposure specifically primes for establishment of lung CD4+TRM.
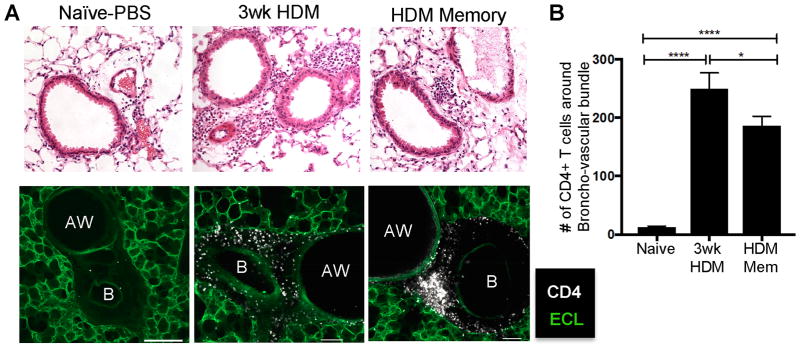
We previously showed that lung TRM generated from influenza infection were localized to specific niches around airways (19). Here, we assessed whether the TRM phenotype CD4+T cells generated following HDM exposure were localized in similar niches. Overall, there was extensive mononuclear cell infiltration in the lungs of mice exposed to HDM compared to lungs of naïve mice, which was concentrated around airways by H&E staining (Fig. 2A, top row). By confocal microscopy, lung CD4+T cells were found to accumulate in clusters around the major airways and within bronchovascular bundles in lungs after 3 weeks of acute HDM exposure and were similarly localized in these regions following cessation of HDM exposure in HDM-memory mice (Fig. 2A, lower panel). The numbers of CD4+T cells clustering about the bronchovascular bundles was consistently increased in HDM-sensitized mice after both 3 weeks and longer times after cessation of sensitization (Fig. 2B). The cells that localize around the blood vessels appear to be within the tunica media of the walls of the blood vessels, which appear to be enlarged (Fig. 2, lower). By contrast, circulating CD4+T cells (in vivo labeled) were located within the capillaries of alveolar walls and appeared as punctate dots distributed across the lung (Supplemental Fig. 1). Thus, CD4+TRM generated from HDM exposure persist around airways, suggesting they are poised for early response to inhaled allergens.
Figure 2. Allergen-primed lung CD4+TRM localize around airways.
(A) Upper: H&E stained lung slices from PBS sensitized, HDM sensitized and HDM memory mice (upper panels). Lower: Confocal microscope images of lung sections stained with Fluorescein labeled ECL (green) and anti-CD4 (white) and their association with the lung airway (AW) and blood vessel (B). Images are representative of 8 mice from 5 independent experiments. Scale bar represents 100μm. (B) Number of CD4 T cells (mean ± SEM) surrounding broncho-vascular bundles for each group (N= 6–11 bronchovascular bundles per group compiled from 5 independent experiments)(* p<0.1. **** p<0.0001, 1-way ANOVA).
Secondary challenge with HDM results in rapid asthma response and immune cell infiltration into airways
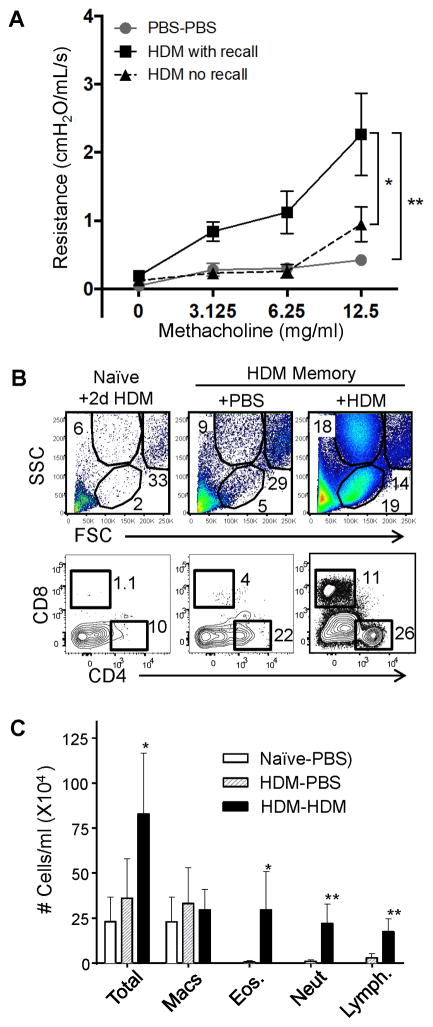
We assessed whether the persistence of lung CD4+TRM was associated with a secondary response to HDM challenge, manifested by rapid induction of airway hyperresponsiveness following short-term HDM exposure. For assessing secondary responses, we used a 2-day (2d) HDM challenge that does not trigger symptoms in naïve mice (30). We observed an increase in airway hyper-responsiveness in HDM memory mice after 2d challenge with HDM compared to PBS-challenge and HDM challenge of naïve mice (Fig. 3A), indicating that pathological symptoms of asthma occur with the kinetics of a secondary response in HDM-memory mice. Whether this asthmatic response was associated with an increase in immune cell infiltrates into the airways characteristic of allergic asthma was further examined. Overall, there was a substantial increase in the total immune cell infiltrate in the BAL during the secondary HDM challenge with a significant increase in lymphocytes, eosinophils and neutrophils (Fig. 3B, C), characteristic of an acute asthmatic response (34). While the T cells present in the BAL of HDM-memory mice challenged with PBS were predominantly CD4+T cells with negligible CD8+T cells (consistent with biased persistence of CD4+TRM), there was an increase in both CD4+ and CD8+ T cells in the airways of HDM-memory mice following 2d HDM challenge (Fig. 3B). Together, these results show a rapid infiltration of innate and adaptive lymphocytes into the airways in mice with persisting lung CD4+TRM generated from previous HDM exposure.
Figure 3. Increased airway hyper-responsiveness and rapid influx of granulocytes and lymphocytes into the airways following re-exposure to allergen.
(A) Airway hyper-responsiveness following methacholine challenge of mouse groups previously exposed to PBS or HDM for 3 weeks (“HDM memory”), then rested and challenged with PBS or HDM after 2 days (2d) (n= 4–11 mice per group compiled from 3 independent experiments) (* p<0.01, ** p<0.01, 2-way ANOVA). (B) Frequency of lymphocytes (low FSC, low SSC) and granulocytes (low FSC, high SSC) in the BAL of HDM memory mice following 2d HDM challenge (upper). Representative flow cytometry analysis (lower) of CD4+ and CD8+T cells in the BAL, representative of 4 independent experiments (C) Cellular content of the BAL in naïve and HDM memory mice following 2d HDM challenge as in (A). Graph shows the numbers (mean±SEM) of indicated cell types within the BAL of naïve and HDM memory mice following 2d PBS or HDM challenge (n= 4–8 mice per group compiled from 2 independent experiments).
Lung CD4 TRM are activated in situ and mediate secondary HDM responses
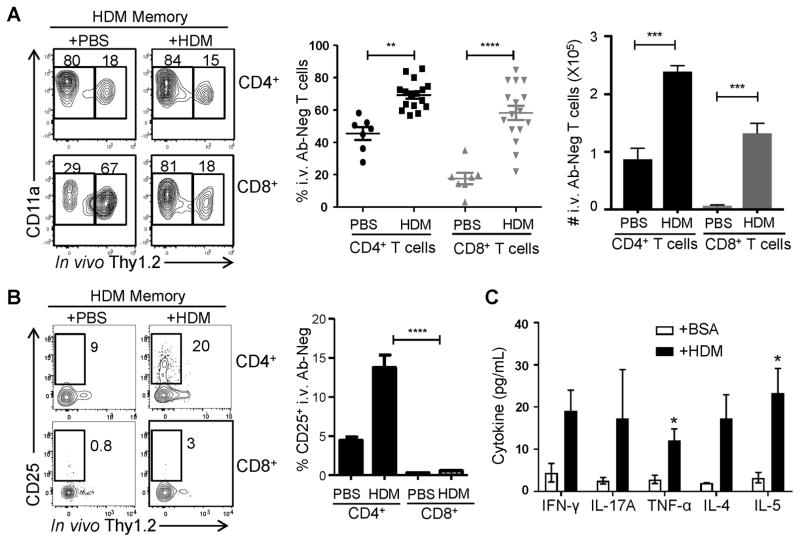
We examined whether lung CD4+TRM mediated secondary responses to allergen rechallenge in vivo and their functional responses to HDM stimulation in vitro. In vivo, we examined whether circulating or resident lung T cells were responding to HDM challenge. In naïve mice, few lung TRM are present (Fig. 1A, (19)) and a 2d HDM challenge did not result in significant lung TRM generation or lung T cell activation as assessed by upregulation of the activation marker CD25 (Supplemental Fig. 2). By contrast, 2d HDM challenge of HDM-memory mice triggered measurable lung T cell responses, including a significant increase in the frequency and absolute numbers of memory (CD11ahi) CD4+ and CD8+ T cells protected from i.v. Ab (Fig 4A). Moreover, in HDM-challenged HDM-memory mice, there was early upregulation of CD25 expression exclusively by CD4+TRM (protected) but not circulating (labeled) lung CD4+T cells, or by protected or labeled CD8+T cells (Fig. 4B). These results provide evidence for rapid in situ activation of lung CD4+TRM by HDM.
Figure 4. Local reactivation of CD4+TRM after allergen challenge.
(A) HDM memory mice were challenged with PBS or HDM and after two days, the proportion of labeled and protected CD4+ and CD8+T cells within the lung was determined by in vivo Ab labeling as in Fig. 1. Graph (middle) shows the mean frequencies (±SEM) of protected CD4+ and CD8+T cells (TRM, n= 7–16 mice per group compiled from 3 independent experiments) (** p<0.01. **** p<0.0001, 1-way ANOVA). Graph (right) shows the mean numbers (±SEM) of protected CD4+ and CD8+T cells (TRM, n= 3–4mice per group) (*** p<0.001, 1-way ANOVA) (B) CD25 upregulation by lung TRM following 2d PBS or HDM challenge shown in representative flow cytometry plots (left), and as %CD25+TRM (mean±SEM) compiled from 3 independent experiments (n= 9–13 mice per group). C. Production of pro-inflammatory (IFN-γ, TNF-α, IL-17) and Th2-like (IL-4, 5) cytokines by sorted lung CD4+TRM cells after 24 hours of stimulation by either BSA or HDM extract (100μg/mL) with cytokines in supernatants (pg/ml, mean±SEM compiled from three experiments) measured using an antibody bead-array assay (see methods). *. P<0.05; p=0.051 for IL-4 and IFN-γ.
The ability of lung CD4+TRM generated from HDM sensitization to mediate functional HDM-specific secondary responses was assessed by ex vivo stimulation of sorted i.v. Ab- protected lung CD4+ T cells from HDM-memory mice with HDM or control BSA. We measured the production of multiple pro-inflammatory and Th2-like cytokines in supernatants after 24hrs of stimulation. Notably, lung CD4+TRM produced multiple cytokines, including Th2-like cytokines IL-5 and IL-4, the Th17-cytokine IL-17, and Th1-like cytokines IFN-γ and TNF-α, in response to HDM stimulation, but not in response to stimulation with an irrelevant antigen (Fig. 4D). Production of these cytokines exhibited an HDM dose-dependent response (data not shown) further establishing antigen-specificity. Together, these results indicate that HDM-specific lung CD4+TRM comprise a multi-functional population, producing Th2- like and pro-inflammatory cytokines in response to allergen re-challenge.
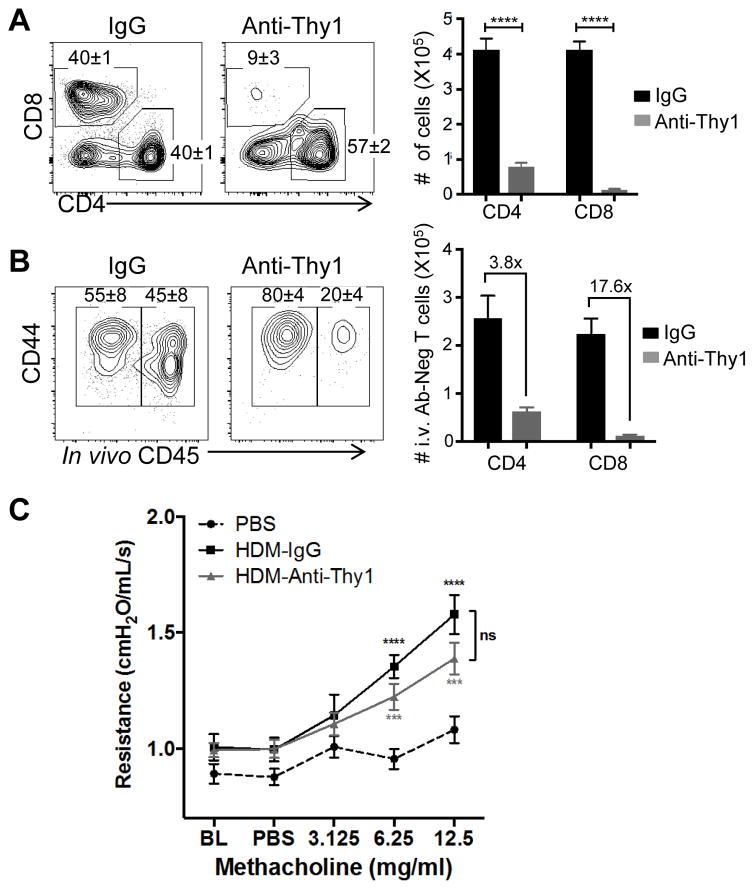
Lung CD4+TRM-mediated airway hyperreponsiveness persists in the presence of peripheral T cell depletion
To determine whether lung TRM were sufficient to trigger secondary HDM responses and airway hyporesponsiveness, we treated HDM-memory mice prior and following 2d HDM-challenge with a pan anti-T cell antibody shown previously to primarily deplete circulating T cells (35, 36). Treatment of HDM-memory mice with anti-Thy1 antibodies depleted 75% of lung CD4+ T cells and >95% of lung CD8+T cells (Fig. 5A). The majority of residual CD4+T cells in the lung following treatment were TRM, as indicated by their protection from i.v. Ab labeling (Fig. 5B, left), while nearly all lung CD8+T cells were depleted (Fig. 5B, right). Importantly, increased airway hyperresponsiveness to HDM challenge in HDM memory compared to naïve mice was observed comparably in both control IgG- and anti-Thy1-treated mice as shown by (Fig. 5C). Together, these results indicate that the asthma response mediated by HDM-memory mice was not inhibited by peripheral T cell depletion, suggesting that lung CD4+TRM are sufficient to trigger lung-localized secondary responses and asthmatic symptoms.
Figure 5. Lung CD4+TRM persist and mediate airway hyporesponsiveness to HDM challenge in the presence of peripheral T cell depletion.
HDM memory mice were treated with anti-Thy1 T cell-depleting antibody or control IgG prior to 2d challenge with HDM as in Fig. 4. (A) Representative flow cytometry plots (left) show frequency (mean±SEM) of lung CD4+ and CD8+ T cells gated on CD3+ cells. Graphs (right) show absolute numbers (mean±SEM) of lung CD4+ and CD8+T cells from control IgG- and anti-Thy1-treated mice. (B) Representative flow cytometry plots (left) showing frequency (mean±SEM) of lung CD4+T cells labeled and protected from i.v. Ab staining in control IgG- and anti-Thy1-treated mice. Graphs (right) show absolute cell numbers of i.v. Ab negative (i.e. protected) lung CD4+ and CD8+ T cells (mean±SEM from n= 4 mice per group). The mean fold-change in absolute cell counts between IgG- and anti-Thy1-treated mice is shown on the graph. (C) Airway hyper-responsiveness following methacholine challenge of naïve (PBS treated), IgG- and Anti-Thy1-treated HDM memory mice after 2d HDM challenge (n= 4–8 mice per group). (Means ±SEM, ns-not significant *** p<0.001, **** p<0.0001, 2-way ANOVA).
Mobilization of lung DC in secondary HDM responses
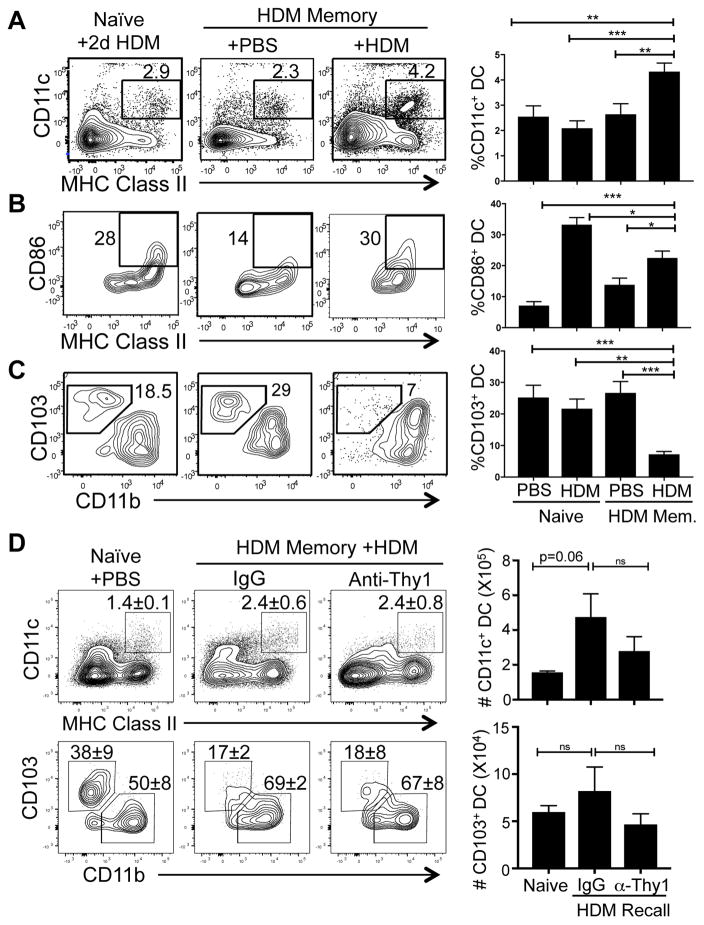
Given the ability of lung-localized CD4+T cells to respond to HDM challenge and trigger asthma, we hypothesized that lung CD4+TRM may mobilize a lung response through local activation of innate cells. To determine the effect of local reactivation of lung CD4+TRM on antigen presenting cells we analyzed the recruitment and activation of CD11c+ dendritic cells (DCs) in the lungs following 2d HDM challenge of HDM memory mice. There was an increased frequency and absolute number of CD11c+MHChi DCs in the lung in HDM-memory mice challenged with HDM compared to PBS, which had similar DC frequencies as PBS- or HDM-treated naive mice (Fig. 6A, right, Supplemental Fig. 3). Lung DCs in HDM-challenged compared to PBS-treated HDM-memory mice also exhibit increased upregulation of CD86 (Fig. 6B). The DCs present in naïve mice treated with HDM also exhibited CD86 expression, although much fewer DCs were present in the lung compared to HDM-memory mice (Fig. 6A).
Figure 6. CD4+TRM-mediated recall response to HDM alters lung dendritic cell (DC) populations.
(A–C) DC frequency and phenotype in the lungs of naïve and HDM memory mice challenged with PBS or HDM shown as representative flow cytometry plots (left) and graphs of compiled frequencies (right, mean±SEM) from 3 independent experiments (n=7–10 mice per group). (A) DC frequency as defined by CD11c+MHChi expression. (B) Expression of CD86 by CD11c+MHChi DC. (C) Frequency of CD103+CD11blo DC gated on CD11c+ DC. (* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001). (D) HDM memory mice treated with IgG or anti-Thy1 received a 2d challenge with HDM as in Fig. 5. Frequency (left, shown as representative flow cytometry plots, mean ± SEM) and absolute numbers (right, mean ± SEM) of CD11c+MHChi DCs (upper) and CD103+CD11blo DC (lower) in the lungs of naïve and HDM memory mice challenged with PBS or HDM (* p<0.05) (n=6 mice per group). Upper flow plots gated on lymphocytes, lower plots gated on CD11c+ MHC class IIhi cells.
It was previously shown in an infection model that the lung contains two major types of DC subsets exhibiting CD103hi and CD11bhi phenotypes, respectively, which both have the capacity to migrate to tissue-draining lymph nodes and present antigens to T cells (37). We asked whether alterations in lung DC subsets was associated with HDM challenge of naïve or HDM-memory mice. The frequency of CD103hi DC in the lungs of naïve mice treated with PBS or HDM and HDM memory mice treated with PBS was consistently 20–30% with CD11bhi subset predominating (Fig 6C). In HDM-memory mice challenged with HDM, the frequency of CD103hi DC was significantly decreased (Fig. 6C) resulting in a higher ratio of CD11bhi/CD103hi DC in the lung. We observed similar changes in the composition of these DC subsets in the lungs of HDM memory mice treated with Anti-Thy1 mAb (Fig. 6D). Interestingly, the numbers of CD103hi DC did not change significantly despite their reduced frequencies (Fig. 6D), indicating and increase in the CD11bhi DC population perhaps due to recruitment from the blood. These results suggest that lung CD4+TRM cells coordinate rapid lung inflammation through DC activation and recruitment.
DISCUSSION
Tissue resident memory T cells have been studied for their role in protecting from site-specific pathogens, while their presence and function in the context of chronic inflammation and antigen exposure is not well understood. In this study we provide evidence for biased generation and longterm maintenance of lung CD4+TRM in response to chronic exposure to HDM, a major allergen. These lung CD4+TRM undergo site-specific reactivation at rapid times after allergen challenge, and are sufficient to trigger lung inflammation and the asthma response, without significant participation of circulating T cell populations. We further demonstrate that CD4+TRM reactivation triggers rapid mobilization and activation of lung DC populations, with loss of migratory DC. Our results indicate that longterm allergen sensitization in the lung can be maintained through CD4+TRM, and suggest that targeting TRM in situ has potential to reduce the chronicity of lung allergic responses.
Our results show that during the acute and ongoing response to HDM, both CD4+ and CD8+T cells are extensively recruited to the lung tissues; however, only CD4+T cells are maintained as TRM cells. In contrast, the increased numbers and frequencies of CD8 T cells in the lung are greatly reduced after cessation of HDM treatment, suggesting that they either die and/or migrate out of the lung in the absence of chronic HDM exposure. While there are known MHC-I restricted epitopes within HDM (38, 39), it is not clear whether CD8+T cells present in the lung following HDM exposure are specifically primed, or non-specifically recruited due to inflammatory signals in the lung. By contrast, a proportion of lung CD4+TRM are HDM-specific based on functional recall shown here, consistent with HDM-tetramer-specific CD4+TRM demonstrated in a previous study (28). Polyclonal HDM-specific responses by CD4+TRM identified here produce both Th2-like, as typically associated with asthma as well as IL-17, which has more recently been associated with severe asthma responses children (40, 41). CD4+TRM-mediated asthma in this model may therefore recapitulate more severe disease as observed in humans.
Local reactivation of TRM populations are important for protection against tissue-tropic infections, including influenza virus infection in the lungs, and herpes virus infection in the skin and genital mucosa, and listeria infection in the intestines (35, 36, 42, 43). TRM-derived cytokines can induce the mobilization of immune cells into tissues, enhance the innate immune response by macrophages and NK cells and also promote infiltration of CD8+T and B cells (35, 43, 44). In the context of allergen challenge, we found that local reactivation of CD4+TRM was associated with increased recruitment of CD4+ and CD8+T cells, granulocytes (eosinophils) and CD11c+ DC into the lung, as well as DC activation, which is essential for induction of asthma in mouse models (45). There was also an increase in CD11bhi DC in the lung associated with HDM-specific CD4+TRM recall. As these recruitment/activation events occurred without the participation of circulating T cells, we propose that TRM direct lung inflammation directly from their resident niche in the tissues through promoting rapid DC activation and recruitment in the lung.
Our results suggest that lung CD4+TRM contribute to the chronic symptoms of allergic asthma through recurrent activation and effector functions upon exposure to inhaled allergens. HDM sensitization in this model required repeated exposure to inhaled HDM over three weeks, and contrasts earlier models of repeated inhalation of ovalbumin protein leading to antigen-non-responsiveness (46, 47). In HDM-sensitized mice, allergen re-exposure leads to the rapid appearance of the characteristic asthma symptoms including airway hyper-responsiveness and airway eosinophilic infiltration. This secondary asthmatic response following cessation of allergen exposure closely mimics the hallmarks of human asthma that persists for decades of life There is evidence in human asthma studies of local reactivation of T cells in the lung following allergen re-exposure (48), indicating that this model of HDM TRM generation and reactivation can be relevant to investigate mechanisms for allergen recall that occur clinically. Importantly, targeting memory CD4 TRM in the lung for depletion or inhibition could potentially remove the critical source of initial inflammatory signals that recruit other effector cells to the lung, and reduce disease persistence.
Supplementary Material
Acknowledgments
The authors would like to thank Tina Zelonina for the excellent technical help, and Ms. Rebecca Guyer for help with the mouse colony.
Footnotes
supported by NIH HL116136 awarded to D.L.F. and a Parker Frances family Foundation grant awarded to D.L.T. Research performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under award S10RR027050. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Gonzalez MC, Diaz P, Galleguillos FR, Ancic P, Cromwell O, Kay AB. Allergen-induced recruitment of bronchoalveolar helper (OKT4) and suppressor (OKT8) T-cells in asthma. Relative increases in OKT8 cells in single early responders compared with those in late-phase responders. Am Rev Respir Dis. 1987;136:600–604. doi: 10.1164/ajrccm/136.3.600. [DOI] [PubMed] [Google Scholar]
- 2.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 3.Crosby JR, Shen HH, Borchers MT, Justice JP, Ansay T, Lee JJ, Lee NA. Ectopic expression of IL-5 identifies an additional CD4(+) T cell mechanism of airway eosinophil recruitment. Am J Physiol Lung Cell Mol Physiol. 2002;282:L99–108. doi: 10.1152/ajplung.2002.282.1.L99. [DOI] [PubMed] [Google Scholar]
- 4.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 5.Teran LM. Chemokines and IL-5: major players of eosinophil recruitment in asthma. Clin Exp Allergy. 1999;29:287–290. doi: 10.1046/j.1365-2222.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 6.Sur S, Kita H, Gleich GJ, Chenier TC, Hunt LW. Eosinophil recruitment is associated with IL-5, but not with RANTES, twenty-four hours after allergen challenge. J Allergy Clin Immunol. 1996;97:1272–1278. doi: 10.1016/s0091-6749(96)70195-1. [DOI] [PubMed] [Google Scholar]
- 7.Olsson N, Taub DD, Nilsson G. Regulation of mast cell migration by T and T cytokines: identification of tumour necrosis factor-alpha and interleukin-4 as mast cell chemotaxins. Scand J Immunol. 2004;59:267–272. doi: 10.1111/j.0300-9475.2004.01397.x. [DOI] [PubMed] [Google Scholar]
- 8.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 9.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos-Barbon D, Presley JF, Hamid QA, Fixman ED, Martin JG. Antigen-specific CD4+ T cells drive airway smooth muscle remodeling in experimental asthma. J Clin Invest. 2005;115:1580–1589. doi: 10.1172/JCI19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 13.Zens KD, Farber DL. Memory CD4 T cells in influenza. Curr Top Microbiol Immunol. 2015;386:399–421. doi: 10.1007/82_2014_401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner DL, Farber DL. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol. 2014;5:331. doi: 10.3389/fimmu.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 16.Carbone FR. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. J Immunol. 2015;195:17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 17.Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, Umemura M, Kyusai A, Tomura M, Nakayama T, Woodland DL, Kohlmeier JE, Miyazawa M. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med. 2016;213:3057–3073. doi: 10.1084/jem.20160938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84:9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim BS, Choi Y, Cheon IS, Song MK. Sublingual delivery of vaccines for the induction of mucosal immunity. Immune Netw. 2013;13:81–85. doi: 10.4110/in.2013.13.3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen D, Mortensen R, Rosenkrands I, Dietrich J, Andersen P. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.28. [DOI] [PubMed] [Google Scholar]
- 24.Orr MT, Beebe EA, Hudson TE, Argilla D, Huang PW, Reese VA, Fox CB, Reed SG, Coler RN. Mucosal delivery switches the response to an adjuvanted tuberculosis vaccine from systemic TH1 to tissue-resident TH17 responses without impacting the protective efficacy. Vaccine. 2015;33:6570–6578. doi: 10.1016/j.vaccine.2015.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1:e85832. doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mojtabavi N, Dekan G, Stingl G, Epstein MM. Long-lived Th2 memory in experimental allergic asthma. J Immunol. 2002;169:4788–4796. doi: 10.4049/jimmunol.169.9.4788. [DOI] [PubMed] [Google Scholar]
- 28.Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, Keitany GJ, Garza EN, Fraser KA, Moon JJ, Altemeier WA, Masopust D, Pepper M. Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity. 2016;44:155–166. doi: 10.1016/j.immuni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldklang MP, Perez-Zoghbi JF, Trischler J, Nkyimbeng T, Zakharov SI, Shiomi T, Zelonina T, Marks AR, D’Armiento JM, Marx SO. Treatment of experimental asthma using a single small molecule with anti-inflammatory and BK channel-activating properties. FASEB J. 2013;27:4975–4986. doi: 10.1096/fj.13-235176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169:378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 31.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schenkel JM, Masopust D. Tissue-Resident Memory T Cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niu N, Le Goff MK, Li F, Rahman M, Homer RJ, Cohn L. A novel pathway that regulates inflammatory disease in the respiratory tract. J Immunol. 2007;178:3846–3855. doi: 10.4049/jimmunol.178.6.3846. [DOI] [PubMed] [Google Scholar]
- 35.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8 T cells. Nat Immunol. 2013:14. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris SJ, Roth JF, Savage N, Woodrow SA, Hemingway IK, Hoyne GF, Lamb JR, Layton GT. Prediction of murine MHC class I epitopes in a major house dust mite allergen and induction of T1-type CD8+ T cell responses. Int Immunol. 1997;9:273–280. doi: 10.1093/intimm/9.2.273. [DOI] [PubMed] [Google Scholar]
- 39.Draghi M, Jarman ER, Grifantini R, Galli-Stampino L, Lamb JR, Valiante NM, Grandi G. Different profile of CD8+ effector T cells induced in Der p 1-allergic and naive mice by DNA vaccination. Eur J Immunol. 2002;32:3720–3728. doi: 10.1002/1521-4141(200212)32:12<3720::AID-IMMU3720>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 40.Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190:1094–1101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 41.Seys SF, Grabowski M, Adriaensen W, Decraene A, Dilissen E, Vanoirbeek JA, Dupont LJ, Ceuppens JL, Bullens DM. Sputum cytokine mapping reveals an ‘IL-5, IL-17A, IL-25-high’ pattern associated with poorly controlled asthma. Clin Exp Allergy. 2013;43:1009–1017. doi: 10.1111/cea.12125. [DOI] [PubMed] [Google Scholar]
- 42.Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Maree AF, Zal T, de Boer RJ, Haanen JB, Schumacher TN. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A. 2012;109:19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 45.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163:2592–2600. [PubMed] [Google Scholar]
- 47.Seymour BW, Gershwin LJ, Coffman RL. Aerosol-induced immunoglobulin (Ig)-E unresponsiveness to ovalbumin does not require CD8+ or T cell receptor (TCR)-gamma/delta+ T cells or interferon (IFN)-gamma in a murine model of allergen sensitization. J Exp Med. 1998;187:721–731. doi: 10.1084/jem.187.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yurovsky VV, Weersink EJ, Meltzer SS, Moore WC, Postma DS, Bleecker ER, White B. T-Cell repertoire in the blood and lungs of atopic asthmatics before and after ragweed challenge. Am J Respir Cell Mol Biol. 1998;18:370–383. doi: 10.1165/ajrcmb.18.3.2935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.