Abstract
Purpose
To use a fast three-dimensional rosette spectroscopic imaging (RSI) acquisition to quantitatively evaluate how spectral quality influences detection of the endogenous variation of gray and white matter metabolite differences in controls, and demonstrate how RSI can detect metabolic dysfunction in patients with neocortical abnormalities.
Methods
Data were acquired on a 3T MR scanner and 32-channel head coil, with RSI covering a 4 cm slab of fronto-parietal-temporal lobes. The influence of acquisition parameters and filtering on spectral quality and sensitivity to tissue composition was assessed by LCModel analysis, the Cramer-Rao lower bound (CRLB) and the standard errors from regression analyses. The optimized protocol was used to generate normative white and gray matter regressions and evaluate three patients with neocortical abnormalities.
Results
As a measure of the sensitivity to detect abnormalities, the standard errors of regression for Cr/NAA and Ch/NAA were significantly correlated with the CRLB values, R=0.89 and 0.92 respectively, both with p<0.001. The rosette acquisition with a duration of 9.6 min, produces a mean CRLB (%) over the entire slab of 4.6±2.6 and 5.8±2.3 for NAA and Cr respectively. This enables a Cr/NAA standard error of 0.08, i.e., detection sensitivity of 25% for a 50/50 mixed gray and white matter voxel. In healthy controls, the regression of Cr/NAA versus fraction gray matter in the cingulate differs from frontal and parietal regions.
Conclusions
Fast RSI acquisitions with regression analyses are able to identify metabolic differences across 4 cm slabs of the brain centrally and over the cortical periphery with high efficiency, generating results that are consistent with clinical findings.
Keywords: fast spectroscopic imaging, spectral quality, Rosette trajectories, RSI, CRLB, neocortical abnormalities, brain
Introduction
Fast encoding methods using echoplanar, spiral and rosette trajectories have made spectroscopic imaging clinically feasible with acquisition of multiple brain slices within 5–15 min. As is well known, interleaving spectral and spatial domain information via gradient readouts can accelerate acquisition times by an order of magnitude or more compared to conventional acquisitions (1–8). However, decreased scan times, increased gradient amplitudes and slew rates can also reduce spectral quality (9). Spectral quality is a complex parameter that integrates multiple factors including SNR, resonance overlap, linewidth (LW), line shape distortions due to eddy currents and baseline artifacts and is reflected in the Cramer Rao lower bound (CRLB) of the fitted spectrum. As discussed (10–13), the CRLB represents an estimate for the lowest standard deviation that the metabolite parameter can statistically have. However, while much work has been performed on the various methods of rapid spectroscopic imaging acquisition, there has been less experimental work evaluating the minimum acceptable mean CRLBs for detection of natural variation and pathology.
Since the concentrations of several key cerebral metabolites and their ratios are known to vary with gray matter content and regionally (13–16), the identification of metabolically abnormal voxels can be performed with confidence interval testing based on its tissue composition and regional classification (17,18). This confidence interval or regression approach is an alternative to the morphometric approach that uses non-linear co-registration of the subject’s brain into a common brain space (19,20). As discussed for anatomic studies (21), the morphometric approach provides voxel-to-voxel comparisons between balanced group sizes (control, patient), but with unbalanced groups (including single subject) comparisons, this approach can generate increased false positive errors (21). Improved voxel resolution (now available in large volume spectroscopic imaging of <=1cc) can make fidelity to individual subject anatomy important, and thus make pertinent the statistical assessment of each voxel without requiring co-registration into a common brain space. In this report, we examine how spectral quality affects the sensitivity for detection of gray-white tissue differences by the regression approach. This is done using a rosette spectroscopic imaging (RSI) acquisition, which is advantageous due to its high SNR sensitivity, flexibility in k-space trajectory design and reduced gradient demands (7,8). Use of a global inversion recovery (IR) to reduce extracerebral lipid contamination enables this acquisition to include coverage of the cortical periphery. The purpose of this study was to: 1) evaluate how variable spectral quality (as generated by the fast RSI) influences the detection of the endogenous variation of gray and white matter metabolism in controls and 2) demonstrate its use in the detection of metabolic dysfunction in patients with neocortical abnormalities.
The 3D RSI acquisition sequence will be made available through the C2P Siemens sharing mechanism; updates will be provided on Twitter @FastMrsi.
Methods
Subjects
To assess the relationship between spectral quality, CRLB and the standard error (SE) of the regressions, four healthy subjects were studied utilizing RSI acquisitions of three different durations (9.6, 6.0 and 4.8 min), with two shim strategies (whole brain and slab shimming), thus providing a range in SNR and linewidth. Separately, n=8 healthy volunteers (4F; mean age 44.1±4.8, age range 30–48) with no known history of brain disease were studied to establish control data. For this work, data from three patients with neocortical metabolic abnormalities are shown. This prospective study was approved by the Institutional Review Board and written informed consent was obtained from all subjects.
Pulse Sequence and Data Acquisition
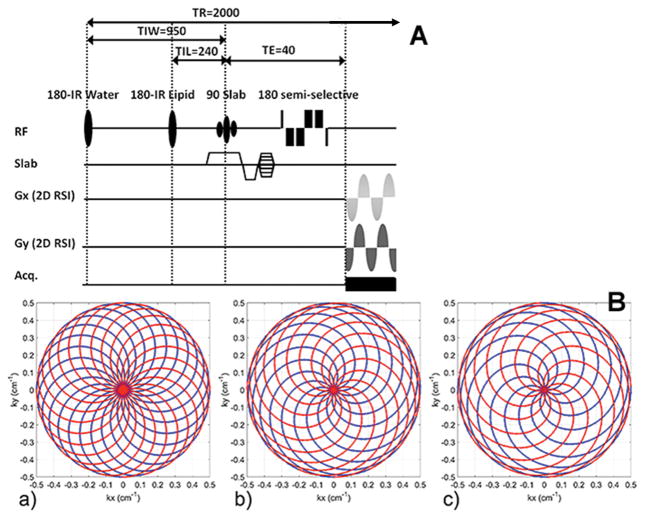
Data was collected on a Siemens (Erlangen, Germany) 3T Trio system with a 32 channel head coil. The pulse sequence (Fig. 1) uses a 40 mm slab selective excitation pulse and a numerically optimized semi-selective frequency refocusing pulse for water suppression. Additional water suppression is provided by a narrow band adiabatic inversion pulse and delay. A non-selective adiabatic inversion pulse with optimized inversion recovery time is used for lipid suppression. Lipid/water inversion delay and repetition times were: TIL/TIW/TR = 240/950/2000 ms. While the sequence enables short echo times (TEmin=18.2 ms), a moderate TE=40 ms was chosen to reduce macromolecular contributions to the baseline and reduce spectral overlap with amino acids. The spectroscopic image was generated using in-plane rosette encoding (two spatial, one spectral dimension) (7,8) and conventional phase encoding in the slab direction. The spectral bandwidth was SW=1250 Hz and readout time Tread=320ms. Sampling dwell time was 10μs.
Figure 1.
Pulse sequence (A) and rosette trajectory (B). (A) Slab selective moderate spin echo (TE 40ms) sequence using optimized semi-selective refocusing pulse supplemented with narrow band adiabatic inversion pulse to suppress water. Lipid suppression performed using a global inversion recovery with broadband adiabatic pulse. (B) Corresponding to the 9.6, 6.0 and 4.8min acquisitions (a,b,c), shown are the spatially interleaved rosette (kx,ky) trajectories using Nshots =24, 15, 12. For uniform k-space coverage, each shot is rotated by 2π/Nshots. Trajectory twist is ω2/ω1 = 1.00/1.85/2.13 (a, b, c), with each shot traversing the “outgoing” part (k=0 to kmax, shown in blue) during 400 μs -half of spectral dwell time, spDT (spDT=1/SW), and the “incoming” part in another 400 μs (kmax to k=0, shown in red).
To vary the SNR, three RSI trajectories were acquired, with equivalent FOVs (20 cm x 20 cm x 4.8 cm RL-AP-FH) and spatial resolution (20x20x12), for acquisitions of 9.6, 6.0 and 4.8 min. The nominal resolution was 0.4 cc and the effective voxel size was 1.25 cc (8,22). For the 9.6, 6.0 and 4.8 min acquisitions, the rosette trajectory used maximum gradient strengths, Gmax =4.6, 8.5, 9.8 mT/m and slew rates, Smax = 36, 80, 100 mT/m/ms respectively (Fig. 1). A water reference scan for phase correction and coil recombination was collected for each subject using the same rosette trajectory as for the metabolite acquisition, but without the water and lipid inversion pulses; TR=0.39 s and flip angle=15°. A 2D GRE scan, with 16 slices 3 mm thick and no gap, 128x128 matrix size, and same angulation and coverage/FOV as RSI, was also acquired for each subject.
To modulate the apparent linewidth, B0 shimming was performed either over the whole brain (extending from the most inferior extent of the temporal lobe to the vertex) or a 5.1 cm slab covering the 4.8 cm RSI slab. A non-iterative least squares B0 mapping algorithm ((23), Bolero, B0 loop encoded readout) with a five point B0 map was used. The standard deviation of the B0 field over the 5.1cm slab (σB0Global) decreased from 9.6±0.6 Hz to 6.5±0.8 Hz when using the targeted shim in place of the whole brain shim.
Image and spectral processing
For anatomical reference and segmentation, a 1mm isotropic T1-weighted MP2RAGE (24) scan was collected for each subject. The MP2RAGE was segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF), and further parcellated using Freesurfer (http://surfer.nmr.mgh.harvard.edu/) and co-registered with SPM (http://www.fil.ion.ucl.ac.uk/spm/) to the 2D GRE scan which covered the same space as the RSI scan. The GM, WM and CSF content of each RSI voxel was calculated by convolving the high resolution segmented and parcellated maps with the point spread function (psf) of the RSI acquisition and profile of the slab selective excitation pulse. To enable consideration of regional differences, the parcellated CSI voxels were classified as frontal, parietal, cingulate, thalamic, basal ganglia, temporal. Given that the CSI voxel size is much larger than the pixel size of the Freesurfer parcellated MP2RAGE image, and can include contributions from more than one parcel type, this classification was based on the majority fraction gray matter composition calculated from the MP2RAGE structural pixels; e.g., for a CSI voxel, if frontal GM/sum(all GM contributions)>50%, that CSI voxel is classified as frontal.
The metabolite (water-suppressed) and the water reference sets were processed simultaneously off-line with the same reconstruction parameters. As previously discussed, because useful spatial and spectral information is contained within the full signal bandwidth of FBW = γ · Gmax · FOV + SW (7,8) (40.4/73.6/84.7 kHz for the 9.6/6.0/4.8 min acquisitions), to remove noise introduced by the dt=10 μs sampling rate (which corresponds to a BW=100 kHz), the time-domain free induction decay data are Fourier transformed, a rectangular filter of FBW Hz is applied, followed by an inverse transform back to the time domain. Typical of large volume spectroscopic imaging studies that evaluate the entire intracranial slice(s), multiple avenues are needed for lipid and macromolecule suppression such as k-space extrapolation and lipid regularization (4,16,19,20). In this study we used a 50 Hz convolution difference for this purpose. A 2 Hz line broadening time-domain Gaussian filter, a 2D (kx,ky) Hamming and a 1D Hamming filter in kz-direction are also applied. The remainder of the processing is as previously described with a Kaiser-Bessel kernel width of W=4 (7,8,25), producing after channel recombination a reconstructed matrix of 32x32x16x512 (x, y, z, frequency). For each spatial location and channel, the zero-order phase and integral of the water peak were calculated using the water reference set and used for combining the channels of the water-suppressed RSI data set. The spectral data from the central 12 slices (avoiding chemical shift dispersion artifacts in outer slices) was automatically analyzed with LCModel (11, http://www.s-provencher.com/pages/lcmodel.shtml), which used 12 GAMMA-simulated metabolite functions (26) and default macromolecule components. To provide variance estimates for a given metabolite ratio, a combined CRLBest_ratio was calculated assuming well resolved spectra without covariance interaction:
| [1] |
where ci and ai are the CRLB and the amplitude for metabolite i, respectively (27). It should be noted that there are common assumptions implicit with the definition of a variance of a ratio including that the mean denominator parameter cannot be zero, and there is a relationship between the two parameters, both of which are true in healthy controls and in the large majority of patient volunteers.
Regression analyses and spectral filtering
Linear regression analyses were performed to determine the slope, intercept and standard error for Cr/NAA and Ch/NAA as a function of gray matter content, fGM = GM/(GM+WM). The ratio relative to NAA is used, given that most reports find that NAA is a more stable parameter between white and gray matter than either Cr (higher in normal gray matter) or Ch (higher in normal white matter, dependent on brain region) (28–30) and, in normal brain, the SNR for NAA is relatively higher, providing for a more reliable denominator. As a secondary analysis and to allow for possible regional (i.e., parcel) differences, regressions were individually calculated, e.g., over frontal and parietal regions. It should be noted that from the Freesurfer based parcellation, that if there is no dominant gray matter component (each parcel type contributed <50% gray matter), the classification of that pixel is left as generic gray matter and excluded from the parcel based regressions but included in the total gray matter regression.
To minimize the effects of zero-filling and psf overlap, every other pixel in all three directions (1/8 of all available pixels) were included in the regressions. To evaluate the effects of SNR and linewidth, regression analyses were performed using data from six acquisition strategies (3 durations x 2 shim methods) in 4 subjects. Pixels with greater than 40% tissue fraction (i.e., GM+WM; CSF is excluded) are initially included. Adding in the requirement that CRLB <20% (for all singlet metabolites) resulted in exclusion of 3.6±2.8% of all voxels with >40% tissue fraction, such that the two exclusion criteria for these acquisition conditions resulted in similar rates of voxel retention.
For the patient data, statistically significant increases in Ch/NAA and Cr/NAA were identified using the confidence interval (CI) of the regression from the healthy controls. The CI of the regression enables the statistical assessment of an additional datapoint with significance tα:
| [2] |
where ŷ is the predicted value for Cr/NAA or Ch/NAA, x is the gray matter content, x̄ is the mean gray matter content, t(α/2,n-2) is the t-statistic, n is the number of measures, Sxx = Σ(x − x̄)2 and MSe = Σ(y − Ŷ)2/(n − 2). For t(α=0.05,n>100 ), , i.e. ~4 times the size of the standard error of the regression . The test for significance for patient data, is done in each voxel: the measured metabolite ratio is compared to the control ŷ value corresponding to a fGM=x gray matter content for that voxel. If the difference between the measured ratio and ŷ is 1.96*SE or greater, the abnormality is significant at the p=0.05 level.
Results
SNR and LW
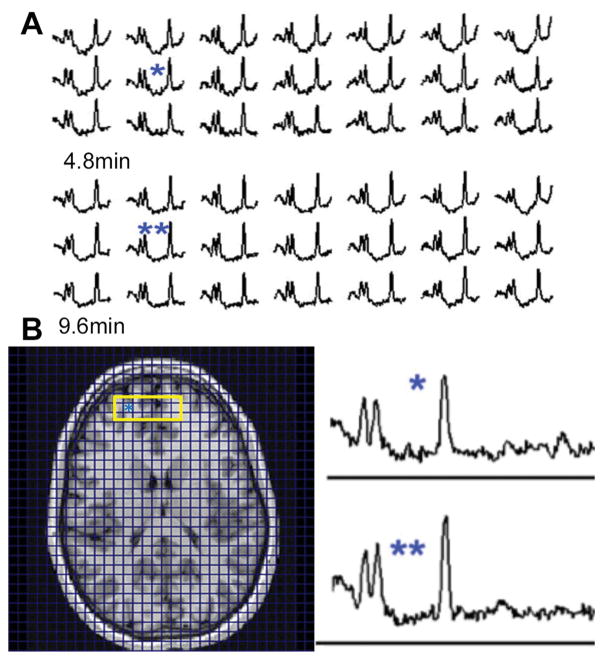
Fig. 2 presents data from a control subject, showing the greater SNR in the 9.6 min compared with the 4.8 min acquisition (over the group considering all voxels with >40% tissue fraction, SNR increased by 32±6%). From the group of n=4 volunteers, Table 1 summarizes the fractional variation in frontal lobe SNR and LW from the 4.8, 6.0, 9.6 min slab shimmed and 9.6 min whole brain shimmed acquisitions (data filtered on 40% tissue fraction inclusion criterion and 20% CRLB). A 40% tissue fraction criterion is used to enable inclusion of pixels with lower brain tissue content which is of significant interest for pathologies with neocortical and or periventricular involvement. The expected differences in SNR and LW are identified, i.e., the average linewidth decreases by 12% when slab shimming is used as opposed to whole brain shimming. Similarly, the SNR increases by 15–18% and 30–40% as the scan time is increased from 4.8 min to 6.0 min and to 9.6 min respectively, in agreement with the theoretical prediction of 26% and 41% based on acquisition duration. There is also a small but statistically significant decrease in linewidth, 6%, in the 9.6 min acquisitions in comparison to the 4.8 min scan, which likely results from greater residual eddy currents in the 4.8 min acquisitions where the Gmax and Smax are a factor of 2 and 3 higher respectively in comparison to the 9.6 min scan.
Figure 2.
Scout and spectra from a control volunteer. The yellow rectangle indicates the blocks of spectra shown from the 9.6 and 4.8 min acquisitions. A: spectra from anterior frontal lobe, insets show the corresponding 9.6 (**) and 4.8 (*) min LCM phased spectra. B: MP2RAGE image showing regions of extracted spectra, the asterisk indicates the location of spectra from (A).
Table 1.
Ratios of SNR and linewidth parameters between the variable spectral quality rosette datasets.
| Frontal lobe | NAA | Cr | Ch | LW |
|---|---|---|---|---|
| slab/whole brain shim | 1.01±0.02 | 1.01±0.02 | 1.01±0.01 | 0.88±0.04* |
| 9.6min/6.0min (slab shimmed) | 1.18±0.05* | 1.18±0.05* | 1.15±0.07* | 0.97±0.04 |
| 9.6min/4.8min (slab shimmed) | 1.40±0.08* | 1.40±0.10* | 1.37±0.10* | 0.94±0.03* |
| Total brain | ||||
| slab/whole brain shim | 0.99±0.01 | 0.99±0.02 | 1.00±0.02 | 0.95±0.05 |
| 9.6min/6.0min (slab shimmed) | 1.18±0.02* | 1.18±0.03* | 1.16±0.03* | 0.97±0.03 |
| 9.6min/4.8min (slab shimmed) | 1.32±0.07* | 1.31±0.09* | 1.30±0.08* | 0.96±0.02* |
Significance:
p<0.05 paired t-test different from 1.0.
To evaluate the extent to which the decreased SNR and increased LW affects the accuracy of quantitative analyses we evaluated the measured CRLBs as a function of the measured LW and SNR. As derived by Cavassila (10), the CRLB is a function of SNR and linewidth and for well isolated singlet resonances (Eq. [3], where σ refers to noise):
| [3] |
Fig. 3 displays a plot of the mean CRLB for NAA, Cr and Ch over all voxels for a given acquisition condition and subject as a function of the mean and , showing a highly statistically significant correlation R=0.95 (p<0.0001, slope 0.082±0.003). The mean Ch CRLB (%) values for the 9.6 min acquisitions in the frontal parcel (5.8±0.2) are similar to those reported by Sabati (4) for a 1.5 cc effective voxel size, across multiple vendors, 4.9–6.2. Thus, at this spatial resolution, acquisition time and acquisition conditions, SNR and LW are the dominant factors in determining the certainty of the spectral fits: with a 30% decline in SNR (reduced from a 9.6 min to 4.8 min acquisition), the mean CRLB rises by 25–30%.
Figure 3.
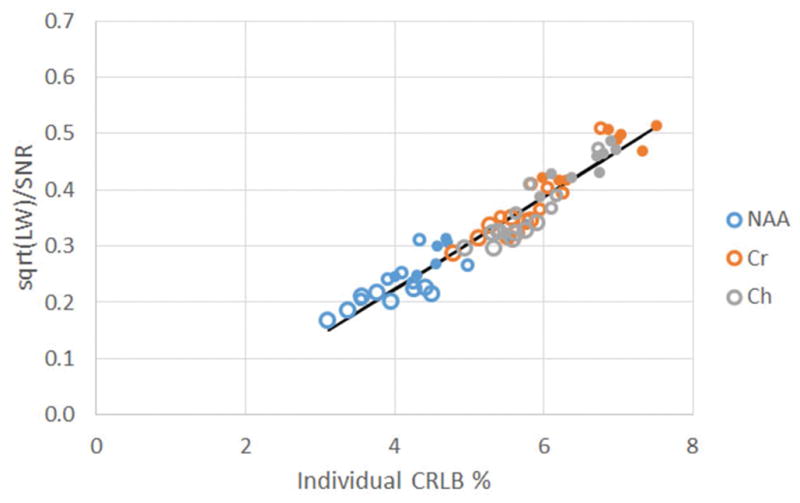
Based on the anticipated relationship between CRLB, SNR and linewidth (Eq. 3), the variable spectral quality datasets show a highly significant regression between the individual CRLB (x value) and (y value) for NAA, Cr or Ch averaged from the frontal parcel. Color indicates metabolite (blue NAA, red Cr, gray Ch); largest open circles are from 9.6 min, medium open circles 6.0 min, filled circles 4.8 min.
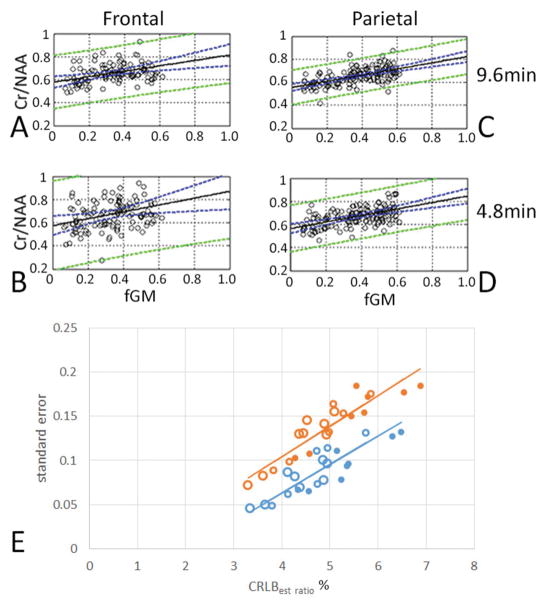
To evaluate the extent to which CRLB under these conditions affects the ability to detect differences in metabolite content, we carried out regression analyses of Cr/NAA and Ch/NAA versus fraction gray matter content for the different acquisition conditions. Displayed in Fig. 4A–D are the regressions for 4.8 and 9.6 min slab shimmed acquisitions for Cr/NAA and Ch/NAA from a single subject along with the 95% confidence intervals from frontal and parietal parcels (calculated from Eq. 2). The 95% confidence intervals for the 4.8 min acquisition are substantially increased in comparison to the 9.6 min acquisition. To examine how the CRLB influences the regressions we calculated SE of Cr/NAA and Ch/NAA with tissue gray matter from the multiple acquisition conditions. Fig. 4E shows that for both parameters determined from the frontal lobe parcel, there is a highly significant correlation between the SE with the calculated CRLBest_ratio (see Eq. 1). For the Cr/NAA regression of SE versus CRLBest_ratio: R=0.89, p<0.001, slope of 0.022. For the Ch/NAA regression of SE versus CRLBest_ratio: R=0.92, p<0.001, slope of 0.027. Thus, at this spatial resolution and acquisition duration, CRLB is the limiting factor, i.e. the longer scans with higher SNRs have smaller CRLBest_ratio, enabling a smaller SE and greater sensitivity to gray-white matter differences. With the LCM analysis performed using a single linewidth for all resonances, if both metabolites of a ratio A/B are of similar SNR (i.e., similar multiplicity, amplitude and coupling), Eq. 1 and 3 imply that the CRLBest ratio is . For Cr/NAA, Fig. 4E shows that changes in SNR and therefore CRLBest ratio are reflected in the SE, with a 4.8 min acquisition having a SE that is approximately 40% larger than a 9.6 min acquisition.
Figure 4.
The accuracy of the regression is better with the 9.6 min in comparison to 4.8 min acquisitions. Data from the frontal parcel (A,B) and parietal parcel (C,D) from a single subject are shown, showing the much tighter standard error (green lines) with the 9.6 min acquisitions (A,C). (E) In the frontal lobe, the CRLBest-ratio is significantly related with the standard error of regressions for both Cr/NAA (R= 0.89; p< 0.001; blue) and Ch/NAA (R= 0.92; p< 0.001; red) from n=4 volunteers. The large open symbols are 9.6 min; medium open symbols 6 min; filled symbols 4.8 min.
Additional spectral filtering
A common method to improve spectral quality and CRLB values is to impose additional inclusion criteria, e.g., a threshold linewidth (4) for inclusion for analysis. In principle this provides greater sensitivity for detection of abnormalities. However, this clearly restricts the extent of brain sampled to only those regions with better spectral quality while reducing the amount of brain actually evaluated. Table 2 summarizes the effect of decrementing the threshold linewidth used for inclusion on the fraction of retained voxels %rv (calculated from 40% tissue fraction inclusion) and SE over the various SNR and LW acquisitions from the frontal lobe. To balance both effects on SE and retained voxel numbers, Table 2 also shows the ratio Fi, 100*SEi /rv, for a given linewidth threshold i. Over this range of linewidth thresholds from 18 to 9 Hz, there is a progressive decline in %rv. With a 12 Hz threshold for inclusion, there is a loss of ~20% of frontal lobe voxels; at 9 Hz there is a loss of ~50% at which point all acquisitions become equal for %rv. With more aggressive thresholding for inclusion, the SE shows progressive decreases (improvement). Balancing the two effects, the ratio Fi shows a relatively flat behavior over 18 to 12 Hz but increases at 9 Hz, indicating that the improvement in SE is disproportionate to the loss in numbers of voxels. There are significant differences in the Fi between the 4.8 and 9.6 min slab shimmed values at 18 and 12 Hz. The effect of B0 shimming alone is seen in comparing the 9.6 min slab and 9.6 min whole brain shimmed studies. The 9.6 min slab shimmed acquisition using a 15 Hz threshold for inclusion gave the best %rv, similar SE, and thus the smallest Fi.
Table 2.
Different linewidth inclusion filtering on frontal lobe data: effects on % retained voxel (%rv), standard error of regression (SE) and their ratio F = 100* SE/rv. SL slab shimmed; WB whole brain shimmed.
| %rv1 | <18Hz | <15Hz | <12Hz | <9Hz |
|---|---|---|---|---|
| 4.8min SL | 92.4±3.9 | 85.3±7.4 | 73.4±10.3 | 48.5±10.9 |
| 6.0min SL | 96.2±2.6 | 90.2±5.0 | 76.8±8.5 | 52.9±11.1 |
| 9.6min SL | 96.5±2.7 | 91.6±4.9 | 80.7±8.9 | 54.3±11.4 |
| 9.6min WB | 92.6±4.1 | 84.4±7.6 | 70.0±6.4 | 45.7±9.3 |
| SE | ||||
| 4.8min SL | 0.087±0.018* | 0.081±0.012 | 0.074±0.010 | 0.064±0.009 |
| 6.0min SL | 0.080±0.027 | 0.073±0.02 | 0.063±0.015 | 0.055±0.014 |
| 9.6min SL | 0.065±0.015* | 0.063±0.014 | 0.054±0.006 | 0.048±0.005 |
| 9.6min WB | 0.071±0.017 | 0.063±0.010 | 0.054±0.005 | 0.045±0.006 |
| F = 100* SE/rv | ||||
| 4.8min SL | 9.5±2.2* | 9.7±2.1 | 10.5±2.5* | 14.5±5.8 |
| 6.0min SL | 8.4±3.0 | 8.2±2.6 | 8.4±3.7 | 11.6±5.9 |
| 9.6min SL | 6.8±1.6* | 6.9±1.7 | 6.9±1.3* | 9.5±3.1 |
| 9.6min WB | 7.7±2.1 | 7.6±1.8 | 7.8±1.2 | 10.4±2.9 |
%retained voxels referenced to voxel counts thresholded on 40% tissue fraction. Significance testing: paired t-tests between 4.8 and 9.6 min acquisitions,
two tailed p<0.05.
As a result, the application studies were performed using 9.6 min acquisitions with slab shimming, with >40% tissue fraction, <20% CRLB (NAA, Ch and Cr) and <15 Hz linewidth as voxel inclusion criteria. It should be noted that other avenues of filtering can be used, e.g., based on residual baseline flatness; nonetheless, recognition of voxel loss and regression accuracy need to be included in selection of such filters.
Regressions in different tissue parcels
To establish control values, data from the n=8 healthy subjects was used. Table 3 shows the pooled mean and standard deviation of the calculated slopes and intercepts. The mean CRLB (%) values for NAA, Cr and Ch were 4.6±2.5, 5.8±2.3 and 5.9±2.3. This analysis shows that the cingulate regression is significantly different (p<0.05) from the frontal regression as well as regressions using all brain pixels.
Table 3.
Regression statistics from parcellated regions.
| Cr/NAA | Ch/NAA | |||||
|---|---|---|---|---|---|---|
| Slope: mean±SEM | Intercept: mean±SEM | Std error | Slope, mean±SEM | Intercept: mean±SEM | Std error | |
| Frontal | 0.16±0.01* | 0.55±0.01 | 0.07 | −0.28±0.03 | 0.75±0.01 | 0.13 |
| Parietal | 0.20±0.01* | 0.55±0.01 | 0.06 | −0.34±0.02 | 0.70±0.01 | 0.08 |
| Cingulate | 0.39±0.03* | 0.51±0.01 | 0.07 | NS | ||
| Total | 0.21±0.01* | 0.55±0.004 | 0.08 | −0.32±0.02 | 0.75±0.01 | 0.14 |
N=8 controls, age range 30–48yo;
p<0.05 significantly different between cingulate with frontal, parietal and total parcels
Sensitivity to pathology
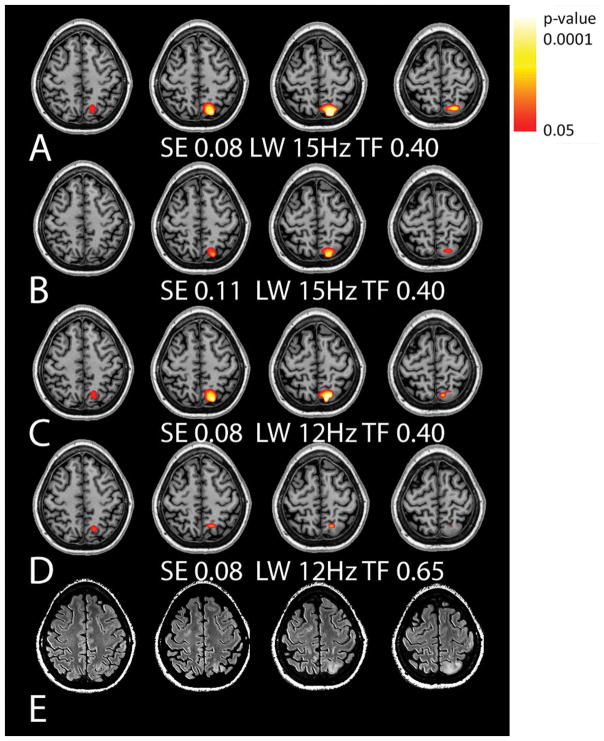
To demonstrate the sensitivity of the 3D RSI and the effects of spectral quality to detect pathology, three patients with neocortical abnormalities are presented in Fig. 5–7. These analyses were performed using overall gray matter regressions based on the control data as defined (Table 3) and show the consequence of variable filtering (tissue fraction or linewidth), or a larger or smaller SE of regression. Two values of SE are considered: the SE experimentally determined from the 9.6 min acquisition from the age-matched control group and an SE determined from Fig. 4E based on a lower SNR expected from a 4.8 min acquisition. For Cr/NAA the SE is 0.08 and 0.11 respectively; for Ch/NAA the SE are 0.14 and 0.18.
Figure 5.
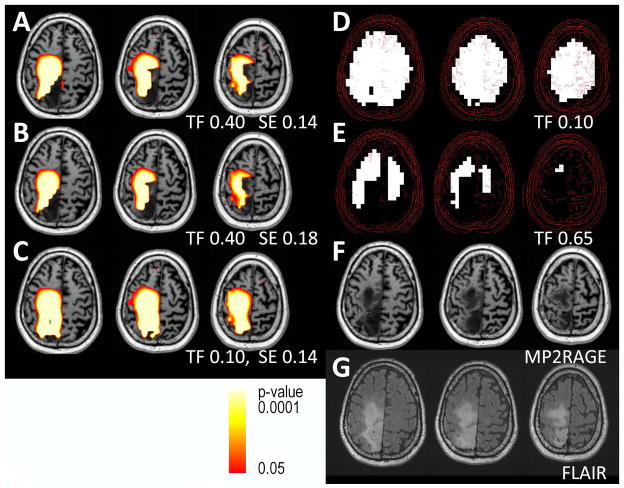
Data from a patient with a parietal lesion of unknown pathology, either gliosis or tumor. The MRSI data for this patient showed no significant abnormalities for Ch/NAA. Shown are Cr/NAA t-maps (A–D) with varying levels of filtering and the corresponding FLAIR images (E). The use of a 0.08 standard error of regression for Cr/NAA with tissue gray matter generated a larger area of metabolic dysfunction than a 0.11 standard error (A,B). Filtering with a narrower linewidth (C) and larger total brain inclusion (D) criterion both result in reducing the observed volume of abnormality in comparison to (A, B).
Figure 7.
Data from an astrocytoma patient with recurrent tumor is shown. For clarity, every other slice from the superior 2 cm is shown. There is widespread Ch/NAA and Cr/NAA (not shown) abnormality. (A,B) Using a standard error of regression at 0.14 for Ch/NAA shows the more extensive abnormalities in comparison to a standard error of 0.18. (C) Reducing the total brain filter criterion to 10% does not introduce erroneous voxels, while the lesion is more consistently detected. Based on the MP2RAGE image alone, there is mis-segmentation of the right central parietal tumor region as CSF; to include these areas in this MRSI analysis, the tissue classification of these tumor regions was assigned to 100% white matter; the severity of Ch/NAA abnormality in this region remains. (D,E) demonstrate the loss of pixels when filtered at a total brain of 10 vs. 65%. The anatomy is shown with the MP2RAGE and FLAIR images (F,G).
Subject #1 is a 33 year old female with a history of childhood epilepsy and meningitis who was referred to assess whether the FLAIR abnormality in the left parietal lobe was due to gliosis or potentially a low grade glioma. The identification of significantly increased Cr/NAA but normal Ch/NAA in this neocortical region (Fig. 5, the Ch/NAA color statistical overlay is not shown since p>0.05 for all pixels) strongly supported gliosis as opposed to a low-grade tumor, which was consistent with clinical follow-up. As evaluated with varying levels of filtering, Fig. 5A–D shows that the extent of abnormality is most thoroughly identified with a standard error of the regression being 0.08 (measured from 9.6 min acquisition) rather than 0.11 (measured from 4.8 min acquisition). With a linewidth inclusion criteria of 15 Hz, more of the lesion is included in comparison to the 12 Hz filter (Fig. 5C).
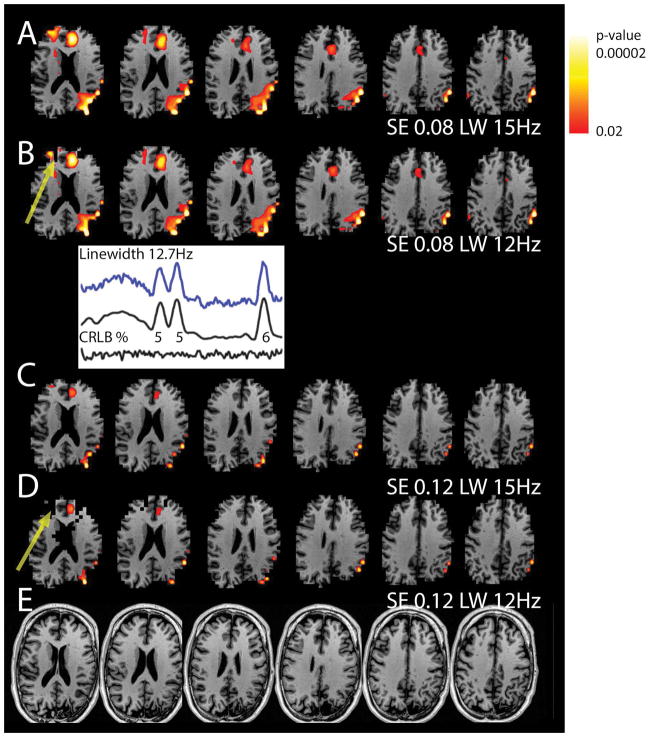
Subject #2 is a 42 year old male with previous left parietal and temporal lobe surgeries and was referred for recurrent seizures. In Fig. 6, the anatomical (MP2RAGE) pixels are not displayed if the spectroscopic pixel is excluded by the applied thresholds. The extensive regions of parietal abnormality (Cr/NAA) are consistent with his clinical history and are most completely identified with the optimized spectral filtering parameters (SE 0.08, LW 15 Hz, in comparison to SE 0.11, LW 12 Hz; Fig. 6A). There are also substantial bifrontal Cr/NAA abnormalities which are less consistently assessed with the 12 Hz vs 15 Hz linewidth filtering (Fig. 6B,D, arrows). Based on other clinical findings, this patient underwent additional surgery to resect the left medial temporal lobe. However, his seizures persisted very shortly after surgery, consistent with his clinical data, seizure behavior and the widespread dysfunctional network identified in the MRSI data.
Figure 6.
Data from an epilepsy patient with a history of previous temporal-parietal lobe surgery, referred for recurrent seizures. Shown are Cr/NAA abnormality overlay maps on MP2RAGE structural images. If a given pixel is omitted via filtering, the MP2RAGE anatomy is excluded in the image. (A,B) the linewidth filtering shows a more complete distribution of the abnormalities for the 15 Hz filtering, with the 12 Hz filtering reducing the number of anterior pixels. The spectra shown is taken from the region identified by the arrow: included when filtered at 15Hz, but excluded when filtered at 12Hz. (C,D) Using a larger standard error of regression reduces the volume and intensity of abnormality at both 15 and 12 Hz linewidth filtering. The yellow arrows indicate the regions of omitted pixels due to the linewidth filters. (E) MP2RAGE structural underlays.
Subject #3 is a 46 year old male with a history of a right parietal-occipital astrocytoma who showed possible tumor progression and was referred to assess whether the interval change (seen in FLAIR and contrast enhancement in the slices shown) was due to tumor progression or treatment effect (Fig. 7). Consistent with subsequent PET and biopsy data, the MRSI shows widespread abnormalities in Ch/NAA and Cr/NAA (data not shown) with a central to neocortical gradient of abnormality. The increase in Ch/NAA in tumors is hypothesized given this pathology’s known increase in cellularity and membrane turnover (31). Using a tissue fraction inclusion of 40%, 15 Hz linewidth filter and standard error of 0.14 (9.6 min acquisition) vs 0.18 (anticipated 4.8 min acquisition), the margins of the lesion is more definitively identified with the 0.14 standard error. As is common with tumor based lesions, the anatomic tissue characterization can be substantially distorted: based on the T1W-MP2RAGE, the Freesurfer tissue segmentation classified much of the central right parietal lobe as CSF. This mis-segmentation can be readily improved by including FLAIR signal contrast in the analysis; however for this example we use the MP2RAGE data to show the tissue fraction threshold set at 40% and 10% with unchanged linewidth filtering at 15 Hz (Fig. 7A–C). Even with the minimal 10% tissue filtering, the spectral quality did not substantially deteriorate; however Fig. 7D, E demonstrate the loss of pixels when filtered at a total brain of 10% vs. 65%. It is also clear that such mis-segmentation can cause problems for the gray-white matter regression. In such cases, the most expedient approach in the MRSI analysis of Ch/NAA is to classify pathologic voxels as 100% white matter. Since this parameter typically increases in white matter and in pathology, the significance testing for such voxels would therefore be based on comparing its value with that encountered in the highest normal condition and thus maintains the accuracy of the analysis albeit at a small drop in sensitivity. To be consistent with this approach, when assessing Cr/NAA, voxels of pathologic or undefined tissue type would be assigned a composition of 100% gray matter. Finally, it has been suggested that in many patients, the contralateral loci may serve as a “control” comparison. While this may be feasible for a few subjects (e.g., patient #1 above), it would be misleading for subjects #2 and 3, as aggressive brain tumors or treatment effects commonly involve both sides of the brain, and in chronic epilepsy, the distribution of dysfunction can be bilateral.
Discussion
Fast spectroscopic imaging with the rosette trajectory
In many diagnostic clinical applications, MRSI is performed as an additional imaging measure to resolve a spatially localized question for which anatomical imaging is not definitive. Thus the need for whole brain spectroscopic imaging studies is variable, and commonly dependent on the disease pathology and patient. In many cases, the target regions are commonly known, either in advance or identifiable on anatomical images. There are several advantages for a study that evaluates only the clinically relevant regions. By reducing coverage in the head-foot direction, shorter minimum scan times can be used with smaller numbers of encoding steps for equivalent slice thickness, and B0 homogeneity can be improved by reducing the target ROI for shimming. Thus for MRSI to maximize spectral quality over the entire ROI over a limited time, a limited coverage study is advantageous for 3D acquisitions where a true multi-slice acquisition with dynamic shim updating (32) is not possible or sufficient. The rosette trajectories provide flexibility in achieving the desired spatial resolution and spectral bandwidth for an MRSI acquisition in a reduced scan duration, with decreased gradient demands and superior sensitivity (8). As well known (28), lipid suppression based on a global IR pulse reduces the available metabolite SNR, here calculated (for TR/TIL=2000/240 ms) to result in 53 to 61% of signal relative to a non-suppressed acquisition for the typical T1 range of values of 1.1–1.8 seconds (which include the white and gray matter metabolite T1s at 3T, (33)). There are several ways to avoid use of the IR, e.g., through spectral-spatial pulse designed to excite the target ROI (34), parallel transmission for targeted lipid suppression (35) or acquisition of a high-resolution lipid mask for regularization of the lipid signal (36).
Spectral quality, CRLB and SNR
For spectroscopic imaging studies with effective voxel sizes of ~1 cc or less, variations in voxel gray matter content can significantly alter metabolite levels and ratios. Thus for regression based analyses, the size of the standard error of the regression determines the ability to detect the differences between white and gray matter and to detect pathological changes. The present data show that: 1) for resolutions ~1 cc and 5–10 min acquisitions, the mean CRLB is linearly dependent on and the inverse of SNR, consistent with Eq. 3; 2) restricted target acquisition and shimming improves voxel loss due to poor linewidth and 3) the CRLBest_ratio % in the 4–7 range linearly correlates with the SE and confidence interval (CI). For Cr/NAA, a 9.6 min acquisition has a SE=0.08 while the 4.8 min acquisition has SE=0.11; for the latter acquisition, the larger SE means that (for a 50/50 mixed gray-white matter voxel) a 40% larger change in Cr/NAA is required for detection of pathology.
The present data show that the sensitivity for detecting abnormalities, quantified by the SE parameter, is dependent on CRLB and small differences in CRLB are important. Given the CRLB’s dependence with SNR and linewidth, changes in acquisition settings such voxel size, acquisition time and shimming will obviously affect the SE. The 9.6 min RSI acquisition with localized shimming typically achieved CRLBest_ratio of ~4.8%, producing a desirable standard error of 0.08, which is good for a sensitivity to 25% changes in the value of Cr/NAA. For different sensitivity goals, Figures 3 and 4 can be used to estimate the needed mean CRLB (and SNR, linewidths) to achieve a given standard error of regression. For example, for equivalent voxel size and linewidth, the slope from Fig. 4, 0.032± 0.0035 for Cr/NAA (0.035±0.0034, Ch/NAA), indicates that a 1 unit increase in CRLBest ratio results in a 0.03 or 38% increase in SE. While these findings have been established with the consistent voxel size of 0.4 cc nominal, the extrapolation of this to other voxel sizes is applicable if the SNR efficiency per scan is maintained. However it should be noted that with different voxel sizes, the variable dynamic range of fraction gray matter content (which influences regression accuracy) will likely change together with the regression statistics.
It should be recognized that under conditions with poor eddy current performance or motion due to poor patient compliance, LCModel processing may be inadequate and the CRLB analysis can fail. This means that the rosette acquisition is particularly suited for spectroscopic imaging with its minimal gradient demands (9). Furthermore, patient compliance may be better with the lower acoustic noise which result from the rosette with its decreased acquisition gradients and slew rates, given that high gradient amplitudes and fast switching rates can generate potentially high levels of acoustic noise (37). The significance seen in the relationships between SE, CRLB and SNR (Fig. 3 and 4) argues strongly for the adequacy of the applied LCModel in this acquisition.
The CRLB and resulting regression statistics can be partially improved through the use of additional voxel inclusion criteria such as more restrictive tissue fraction (GM+WM) and linewidth requirements. As shown in all of the patient examples tissue fraction can be a significant factor that needs to be considered as a function of study target. For linewidth criteria, we find that aggressive filtering of 9 Hz results in greater pixel loss without proportional improvement in SE. However down to 12 Hz, the F = 100*SE/rv is relatively flat i.e., for a given acquisition duration, SE improves with declining voxel numbers. In these studies where accurate evaluation of the neocortical ribbon is important, we opt for high voxel retention, and thus use 15 Hz filtering which in the frontal lobe, results in ~90% retention for pixels with >40% tissue fraction. With voxel retention as a priority, it is clear that overall spectral quality needs to be high such that aggressive filtering is not needed to generate a small SE. Our patient examples (Fig. 5–7) demonstrate that the regression statistics (as determined from the 9.6 min acquisition with SE values of 0.08 and 0.14 Cr/NAA, Ch/NAA respectively), are sensitive to the metabolic pathology in epilepsy and tumor patients.
Sensitivity to tissue differences and pathology
Gray-white matter differences are a known source of natural variation that, via regression analyses, define a sensitivity target for the detection of metabolic abnormalities. This regression analysis approach allows each voxel to be statistically considered without requiring co-registration into a common brain space, which is important for disorders that have distorted anatomy or pathology. While the accuracy of this approach requires accurate tissue segmentation, in cases of abnormal tissue segmentation (which is common in tumor pathologies, e.g., Fig. 7), a trade-off between sensitivity and accuracy of pathology can be achieved by appropriately assigning the tissue type. It should be noted that this paper does not explicitly compare the regression with the morphometric approach; for example at its simplest (based solely on gray-white-CSF segmentation), this regression approach does not make use of neighborhood information and does not apply methods for multiple comparisons corrections (i.e., false discovery rate). However, given the observations that these metabolites vary depending on brain region as shown in Table 3, with acceptable tissue parcellation data (recognizing that in cases of substantially distorted anatomy, parcellation will be inaccurate), the regression approach can incorporate neighborhood information. By using the appropriate regression parameters for a given voxel parcel (e.g., frontal, cingulate parcel), the confidence interval accuracy for that voxel will improve. Since the parcellation data are ultimately returned into native space, the regression approach will still generally retain all individual anatomical detail. Finally, while the morphometric analysis enables the correction for multiple comparisons, this could be addressed in the present approach by setting the threshold p-values more aggressively.
Conclusions
With the restricted brain coverage 9.6 min acquisition, the rosette spectroscopic imaging acquisition results in high consistency of voxel retention given the relatively mild applied tissue fraction and linewidth filtering. This rapid acquisition is able to identify metabolic abnormalities across 4 cm slabs of the brain both centrally and over the cortical periphery with high efficiency, providing results that are consistent with individual clinical details and outcomes. Not surprisingly, these data show that for accuracy in detection of pathology, the standard error of regression is dependent on the CRLB and spectral quality, which in turn, are well correlated with SNR.
Acknowledgments
Funding source: We gratefully acknowledge support from NIH EB011639, NS090417, NS081772, and T. Kober PhD and B. Marechal PhD at Siemens Medical Systems for the MP2RAGE WIP.
References
- 1.Mansfield P. Spatial mapping of the chemical shift in NMR. Magn Reson Med. 1984;1:370–386. doi: 10.1002/mrm.1910010308. [DOI] [PubMed] [Google Scholar]
- 2.Posse S, DeCarli C, Le Bihan D. Three-dimensional echo-planar MR spectroscopic imaging at short echo times in the human brain. Radiology. 1994;192:733–738. doi: 10.1148/radiology.192.3.8058941. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham CH, Vigneron DB, Chen AP, Xu D, Nelson SJ, Hurd RE, Kelley DA, Pauly JM. Design of flyback echo-planar readout gradients for magnetic resonance spectroscopic imaging. Magn Reson Med. 2005;54:1286–1289. doi: 10.1002/mrm.20663. [DOI] [PubMed] [Google Scholar]
- 4.Sabati M, Sheriff S, Gu M, Wei J, Zhu H, Barker PB, Spielman DM, Alger JR, Maudsley AA. Multivendor implementation and comparison of volumetric whole-brain echo-planar MR spectroscopic imaging. Magn Reson Med. 2015;74:1209–1220. doi: 10.1002/mrm.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adalsteinsson E, Irarrazabal P, Topp S, Meyer C, Macovski A, Spielman DM. Volumetric spectroscopic imaging with spiral-based k-space trajectories. Magn Reson Med. 1998;39:889–898. doi: 10.1002/mrm.1910390606. [DOI] [PubMed] [Google Scholar]
- 6.Andronesi OC, Gagoski BA, Sorensen AG. Neurologic 3D MR spectroscopic imaging with low-power adiabatic pulses and fast spiral acquisition. Radiology. 2012;262:647–661. doi: 10.1148/radiol.11110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schirda CV, Tanase C, Boada FE. Rosette spectroscopic imaging: Optimal parameters for alias-free, high sensitivity spectroscopic imaging. J Magn Reson Imaging. 2009;29:1375–1385. doi: 10.1002/jmri.21760. [DOI] [PubMed] [Google Scholar]
- 8.Schirda CV, Zhao T, Andronesi OC, Lee Y, Pan JW, Mountz JM, Hetherington HP, Boada FE. In vivo brain rosette spectroscopic imaging (RSI) with LASER excitation, constant gradient strength readout, and automated LCModel quantification for all voxels. Magn Reson Med. 2016;76:380–390. doi: 10.1002/mrm.25896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DH, Spielman DM. Reducing gradient imperfections for spiral magnetic resonance spectroscopic imaging. Magn Reson Med. 2006;56:198–203. doi: 10.1002/mrm.20928. [DOI] [PubMed] [Google Scholar]
- 10.Cavassila S, Deval S, Huegen C, Van Ormondt D, Graveron-Demilly D. Cramer-Rao bound expressions for parametric estimation of overlapping peaks: influence of prior knowledge. J Magn Reson. 2000;143:311–320. doi: 10.1006/jmre.1999.2002. [DOI] [PubMed] [Google Scholar]
- 11.Provencher SW. Automatic quantitation of localized in vivo1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 12.Jiru F, Skoch A, Klose U, Grodd W, Hajek M. Error images for spectroscopic imaging by LCModel using Cramer–Rao bounds. MAGMA. 2006;19:1–14. doi: 10.1007/s10334-005-0018-7. [DOI] [PubMed] [Google Scholar]
- 13.Kreis R. The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magn Reson Med. 2016;75:15–18. doi: 10.1002/mrm.25568. [DOI] [PubMed] [Google Scholar]
- 14.Hetherington HP, Mason GF, Pan JW, Ponder SL, Vaughan JT, Twieg DB, Pohost GM. Evaluation of cerebral gray and white matter metabolite differences by spectroscopic imaging at 4. 1 T. Magn Reson Med. 1994;32:565–571. doi: 10.1002/mrm.1910320504. [DOI] [PubMed] [Google Scholar]
- 15.López-Villegas D, Kimura H, Tunlayadechanont S, Lenkinski RE. High spatial resolution MRI and proton MRS of human frontal cortex. NMR Biomed. 1996;9:297–304. doi: 10.1002/(SICI)1099-1492(199610)9:7<297::AID-NBM433>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner M. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetherington HP, Pan JW, Mason GF, Adams D, Vaughn MJ, Twieg DB, Pohost GM. Quantitative 1H spectroscopic imaging of human brain at 4. 1 T using image segmentation. Magn Reson Med. 1996;36:21–29. doi: 10.1002/mrm.1910360106. [DOI] [PubMed] [Google Scholar]
- 18.Chu WJ, Kuzniecky RI, Hugg JW, Abou-Khalil B, Gilliam F, Faught E, Hetherington HP. Statistically driven identification of focal metabolic abnormalities in temporal lobe epilepsy with corrections for tissue heterogeneity using 1H spectroscopic imaging. Magn Reson Med. 2000;43:359–367. doi: 10.1002/(sici)1522-2594(200003)43:3<359::aid-mrm7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Maudsley A, Darkazanli A, Alger J, Hall L, Schuff N, Studholme C, Yu Y, Ebel A, Frew A, Goldgof D. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006;19:492–503. doi: 10.1002/nbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donadieu M, Le Fur Y, Lecocq A, Maudsley AA, Gherib S, Soulier E, Confort-Gouny S, Pariollaud F, Ranjeva MP, Pelletier J. Metabolic voxel-based analysis of the complete human brain using fast 3D-MRSI: Proof of concept in multiple sclerosis. J Magn Reson Imaging. 2016;44:411–419. doi: 10.1002/jmri.25139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmond C, Ashburner J, Vargha-Khadem F, Connelly A, Gadian D, Friston K. Distributional assumptions in voxel-based morphometry. Neuroimage. 2002;17:1027–1030. [PubMed] [Google Scholar]
- 22.Zierhut ML, Ozturk-Isik E, Chen AP, Park I, Vigneron DB, Nelson SJ. 1H spectroscopic imaging of human brain at 3 Tesla: Comparison of fast three-dimensional magnetic resonance spectroscopic imaging techniques. J Magn Reson Imaging. 2009;30:473–480. doi: 10.1002/jmri.21834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan JW, Lo KM, Hetherington HP. Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magn Reson Med. 2012;68:1007–1017. doi: 10.1002/mrm.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele P-F, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T 1-mapping at high field. Neuroimage. 2010;49:1271–1281. doi: 10.1016/j.neuroimage.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for Fourier inversion using gridding (computerised tomography application) IEEE Trans Med Imaging. 1991;10:473–478. doi: 10.1109/42.97598. [DOI] [PubMed] [Google Scholar]
- 26.Smith S, Levante T, Meier BH, Ernst RR. Computer simulations in magnetic resonance. An object-oriented programming approach. J Magn Reson Ser A. 1994;106:75–105. [Google Scholar]
- 27.Frishman F. A Modern Course on Statistical Distributions in Scientific Work. Springer; 1975. On the arithmetic means and variances of products and ratios of random variables; pp. 401–406. [Google Scholar]
- 28.Hetherington HP, Pan JW, Mason GF, Ponder SL, Twieg DB, Deutsch G, Mountz J, Pohost GM. 2D 1H spectroscopic imaging of the human brain at 4. 1 T. Magn Reson Med. 1994;32:530–534. doi: 10.1002/mrm.1910320417. [DOI] [PubMed] [Google Scholar]
- 29.Wiedermann D, Schuff N, Matson GB, Soher BJ, Du AT, Maudsley AA, Weiner MW. Short echo time multislice proton magnetic resonance spectroscopic imaging in human brain: metabolite distributions and reliability. Magn Reson Imaging. 2001;19:1073–1080. doi: 10.1016/s0730-725x(01)00441-6. [DOI] [PubMed] [Google Scholar]
- 30.Wiebenga OT, Klauser AM, Nagtegaal GJ, Schoonheim MM, Barkhof F, Geurts JJ, Pouwels PJ. Longitudinal absolute metabolite quantification of white and gray matter regions in healthy controls using proton MR spectroscopic imaging. NMR Biomed. 2014;27:304–311. doi: 10.1002/nbm.3063. [DOI] [PubMed] [Google Scholar]
- 31.Rapalino O, Ratai E. Multiparametric Imaging Analysis: Magnetic Resonance Spectroscopy. Magnetic resonance imaging clinics of North America. 2016;24:671–686. doi: 10.1016/j.mric.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Koch KM, McIntyre S, Nixon TW, Rothman DL, de Graaf RA. Dynamic shim updating on the human brain. J Magn Reson. 2006;180:286–296. doi: 10.1016/j.jmr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Ethofer T, Mader I, Seeger U, Helms G, Erb M, Grodd W, Ludolph A, Klose U. Comparison of longitudinal metabolite relaxation times in different regions of the human brain at 1. 5 and 3 Tesla. Magn Reson Med. 2003;50:1296–1301. doi: 10.1002/mrm.10640. [DOI] [PubMed] [Google Scholar]
- 34.Xu D, King KF, Zhu Y, McKinnon GC, Liang ZP. A noniterative method to design large-tip-angle multidimensional spatially-selective radio frequency pulses for parallel transmission. Magn Reson Med. 2007;58:326–334. doi: 10.1002/mrm.21314. [DOI] [PubMed] [Google Scholar]
- 35.Hetherington HP, Avdievich NI, Kuznetsov AM, Pan JW. RF shimming for spectroscopic localization in the human brain at 7 T. Magn Reson Med. 2010;63:9–19. doi: 10.1002/mrm.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilgic B, Chatnuntawech I, Fan AP, Setsompop K, Cauley SF, Wald LL, Adalsteinsson E. Fast image reconstruction with L2-regularization. J Magn Reson Imaging. 2014;40:181–191. doi: 10.1002/jmri.24365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McJury M, PhD, Frank G. Auditory noise associated with MR procedures: a review. J Magn Reson Imaging. 2000;12:37–45. doi: 10.1002/1522-2586(200007)12:1<37::aid-jmri5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]