Abstract
Partial nephrectomy (PN) is the recommended procedure over radical nephrectomy (RN) for patients with renal masses < 4 cm in diameter (Stage T1a). Patients with > 4 cm renal masses can also be treated with PN, but have a higher risk for positive surgical margins. Positive surgical margins (PSM), when present, are indicative of poor clinical outcomes. The current gold-standard histopathology method is not well-suited for the identification of PSM intra-operatively due to processing time and destructive nature. Here, video-rate structured illumination microscopy (VR-SIM) was investigated as a potential tool for PSM detection during PN. A clinical image atlas assembled from ex vivo renal biopsies provided diagnostically useful images of benign and malignant kidney, similar to permanent histopathology. VR-SIM was then used to image entire parenchymal margins of tumor resection covering up to >1,800× more margin surface area than standard histology. Aided by the image atlas, the study pathologist correctly classified all parenchymal margins as negative for PSM with VR-SIM, compared to standard post-operative pathology. The ability to evaluate large surgical margins in a short timeframe with VR-SIM may allow it to be used intra-operatively as a “safety net” for PSM detection, allowing more patients to undergo PN over RN.
Keywords: margin imaging, structured illumination microscopy, renal cell carcinoma, partial nephrectomy, cancer
Graphical abstract
Positive surgical margins (PSM) are rare in patients treated with partial nephrectomy (PN), but PSM potentially result in poor clinical outcomes. We demonstrate video-rate structured illumination microscopy (VR-SIM) as a potential tool to detect PSM intra-operatively during robotic partial nephrectomy. We demonstrated the ability to capture the full parenchymal margin surface from fresh uncut PN specimens with microscopic resolution within a 15–30-minute timeframe.
1. Introduction
Partial nephrectomy (PN) is the preferred surgical procedure in treating patients with pT1a renal cancer over radical nephrectomy (RN). Patients treated with PN have better renal, cardiovascular and survival outcomes than RN patients. Typically, pT1a patients have renal masses <4 cm in diameter which are localized to the kidney [1]. Even though positive surgical margins (PSM) after PN only occur in 2%–13% of patients [2–8], subsequently the potential for local cancer recurrence is higher and overall survival rate is lower in patients with PSMs [5, 9–11].
The current gold standard method to identify a PSM is permanent histopathology. Since permanent histopathology requires a lengthy fixation step, it does not aid the detection of PSM intra-operatively. To address this problem, frozen section analysis (FSA) is sometimes used for PSM detection [3, 6], primarily on high-risk patients [6]. However, FSA is labor intensive, time consuming and costly, which can prolong the ischemic times during PN. Several studies suggest that FSA results are not very accurate compared to post-operative pathology due to limited sampling [2, 3, 6, 7]. Therefore, the routine use of FSA during PN remains controversial and has not gained widespread traction or implementation into clinical practice as a standard of care at most institutions.
Ex vivo microscopy represents a potential alternative to FSA by utilizing optical sectioning which enables imaging of fresh tissue without fixation or destructive processing. Currently, multiple ex vivo microscopy methods are sufficient for imaging small fragments of excised tissue on the order of 1 cm2 or less [12–16], and some have been demonstrated for larger tissue resections on the order of 10 cm2 while maintaining subcellular lateral resolution [17]. However, surgical tumor resection surface areas in organs such as, for example the breast [18], prostate [19], and kidney (this paper) can greatly exceed 10 cm2. Therefore, very high throughput ex vivo microscopy methods are needed for real-time PSM detection in these organs with large tumor resection surfaces.
We have previously developed a video rate structured illumination microscope (VR-SIM) with the area-throughput and subcellular resolution necessary evaluate surgical margins intra-operatively [20]. We have demonstrated the utility of VR-SIM for rapid pathology imaging of small needle-core renal biopsies [21, 22], large prostate punch biopsies [21, 23], and radical prostatectomy margins [19]. In this work, we demonstrate the potential of VR-SIM as a practical and useful alternative to FSA for intra-operative microscopy of PN specimens to detect PSMs. We use VR-SIM to collect a thin optical section of the fresh uncut PN margin surfaces immediately after surgery. When imaged this way, VR-SIM has a dramatically higher area coverage of the margin than gold-standard histopathology. Importantly, the non-destructive nature of the technology does not compromise standard histopathology processing. The digital VR-SIM image is inherently capable of capturing the en face margin surface at the time of surgery, providing an ex vivo view that the gold-standard histopathology cannot provide. We demonstrate that imaging of the entire parenchymal surgical margin of fresh partial nephrectomy specimens at subcellular resolution is feasible, and provides useful images, which may find application in the operating room or the gross pathology laboratory.
2. Materials and Methods
2.1 VR-SIM instrumentation
We have previously reported the development of VR-SIM for imaging large tissues stained with acridine orange [20] and its validation on prostate core biopsies [23] and prostate surgical margins [19]. The instrumentation is fully described in previous publications [19, 20, 23]. Briefly, it is composed of a custom SIM module connected via Thorlabs 30-mm cage system components to a modular automated epifluorescence microscopy platform (RAMM, Applied Scientific Instrumentation). The SIM module is comprised of LEDs for illumination, a polarizing beam splitter (Moxtek), and a spatial light modulator (SLM). Illumination was via a 470 nm LED (M470L2, Thorlabs) and a 630 nm LED (UHP-Mic-LED-630, Prizmatix) for multi-color illumination – only the 470 nm LED was used for acridine orange excitation in this work. The LEDs are combined with a dichroic beam combiner (Prizmatix) and projected onto a ferroelectric spatial light modulator (SLM, 3DM SXGA, Forth Dimension Displays) through a custom wire-grid polarizing beamsplitter (Moxtek), which was used to create the illumination patterns for SIM. The patterned illumination beam passed through a multiband excitation filter (FF01-390/482/532/640-25) and multiband dichroic beamsplitter (Di01-R405/488/532/635-25×36, Semrock) before being directly imaged onto the sample through a 10X, 0.45 NA Nikon Plan Apochromat objective lens. Fluorescence emission collected from the sample was passed via the dichroic beamsplitter through a multiband emission filter (FF01-446/510/581/703-25) before being imaged onto a sCMOS camera (Hamamatsu, Orca Flash 4.0 v2). The system was controlled via custom LabVIEW software (National Instruments) and custom triggering circuitry. The single-frame field-of-view was 1.33 mm × 1.33 mm at 1.3 μm lateral resolution (limited by the camera pixel size). While the axial response of SIM is tunable by the choice of pattern illumination frequencies, in this work we used a normalized pattern spatial frequency of ν = 0.02, which gave an experimentally-determined optical section thickness of 45 μm (defined as the half-width-half-max HWHM of the axial response to a planar fluorescent object) that matched well with theoretical predictions for incoherent SIM. This has been shown experimentally to provide over an order of magnitude increase in signal-to-background ratio (SBR) with high signal-to-noise ratio (SNR). In our particular optical configuration, it is possible experimentally to obtain a minimum optical section of 6 μm HWHM at a normalized spatial frequency of ν = 0.21 (6 pixel pitch on the SLM), although for thick specimens this can result in low SNR. Even thinner optical sections are attainable in theory up to an illumination pattern frequency of ν = 1, however our particular setup restricts the maximum illumination pattern frequency to ν = 0.21. For this study, each individual SIM frame of 2048 × 2048 pixels covering an area of 1.77 mm2 was collected in 30 ms.
2.2 Construction of a clinical VR-SIM renal imaging atlas
In order to identify PSMs on the surface of the PN specimens, it is necessary to accurately distinguish malignant tissue from benign renal tissue in the VR-SIM images. Therefore, we constructed an imaging atlas with benign and malignant features, serving as a reference standard for image evaluation. Six (6) core-needle biopsies were collected from de-identified ex vivo partial and radical nephrectomy specimens from 6 patients under an Institutional Review Board-approved tissue acquisition protocol. The biopsies were obtained using an 18-gauge core-needle biopsy gun (Bard Monopty, Bard, Tempe, AZ) and were taken directly from the renal specimens ex vivo. The tissues were stained with acridine orange (2%, Sigma Aldrich, A9231) diluted to 0.1% in phosphate buffered saline (PBS). The acridine orange solution was sprayed on the specimen and exposed for 30 seconds. The specimen was rinsed in PBS and placed on a standard glass slide. Specimens were then imaged using VR-SIM with a normalized pattern frequency of 0.02 for SIM and 0.1 s integration time. Then the biopsy was fixed in 10% formalin and processed for standard histopathology.
2.3 Imaging of partial nephrectomy specimens
We imaged the parenchymal margin surfaces from 17 patients providing informed consent under an Institutional Review Board-approved protocol from November 2014 to January 2016 who were undergoing robotic-assisted laparoscopic PN surgery for suspected kidney cancer at Tulane University Medical Center. The intact PN specimens obtained from robotic PN procedures were brought directly from the operating room to the nearby imaging lab within 10 minutes of excision. The imaging workflow, image mosaicking, and image processing are adapted from prior work with prostate margins [19]. Briefly, the imaging team was allowed to image the specimen for approximately 30 minutes before forwarding the specimen for standard-of-care histopathology processing. After acquisition but before staining, the specimen was rinsed with PBS to remove excess blood or fluid from the surface. The specimen was then blotted dry with lab tissue and 0.1% acridine orange in phosphate buffered saline (PBS) was sprayed on the surface and exposed for 30 seconds. Following the staining, the specimen was rinsed in a beaker containing PBS and again blotted dry. The specimen was then placed on a large glass slide in preparation for imaging without any cutting, compression, or other physical manipulation. For imaging, the stained specimen was positioned on a 50 × 75 × 1 mm glass slide on the VR-SIM stage above the imaging objective, and a mosaic image of the surface contacting the glass slide was collected (Figure 1 B). Similar to prior work [19], we used a normalized pattern frequency 0.018 for the best balance between background rejection/contrast/effective resolution and signal-to-noise ratio (SNR). The camera integration time was maintained at 10 ms (30 ms per SIM frame) for all the specimens, and the 475 nm illumination LED was kept to its highest current setting. Mosaics of the specimen were collected, flat-field corrected, and processed as multi-resolution tiled pyramidal TIFF or BigTIFF files using the same methods as prior work [19].
Figure 1.
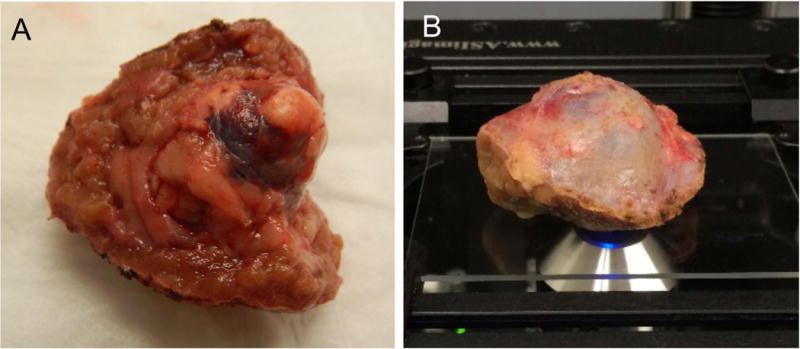
A) Photograph of a partial nephrectomy specimen with two parenchymal surfaces (face-up and face-right), B) Typical imaging arrangement with parenchymal surface face-down on the glass slide and capsular surface facing up.
Cases 1–3 were excluded from the study because they were used to optimize the imaging protocol. Case 4 was also excluded because two tumors were resected but only one specimen was imaged, and the identification of the imaged specimen was subsequently lost during the post-operative histologic processing. In Cases 7 and 9, two margin surfaces were imaged due to the geometry of the specimen. Specifically, in these cases the resected kidney specimens had more than one defined parenchymal surface, which resulted in multiple VR-SIM images for those specimens. Figure 1A shows an example of a gross specimen that had two parenchymal surfaces (Case 9), as well as a photograph of a kidney specimen prepared for imaging during a typical experiment. The two parenchymal sides in Case 9 were imaged separately, and two VR-SIM margin images were obtained.
2.4 Permanent histopathology processing and post-operative pathology results
At the conclusion of imaging, PN specimens were submitted to histology to undergo standard-of-care histology processing. At our institution, intact kidney margins are first covered with histology ink followed by specimen fixation in formalin for at least 24 hours prior to cutting. The PN specimen was sliced perpendicular to the parenchymal margin at approximately ~3 mm thick intervals. The rest of the specimen was then selectively sampled based on the relative location of the grossly-identified tumor. Then, all sections of tissue were placed in separate cassettes for formalin fixation and paraffin embedding. From each 3 mm-thick slice, a single 4-μm section was cut and mounted on a glass microscope slide, and stained with standard H&E. This is the standard procedure for all PN specimens at our institution. After processing, a pathologist reviewed the H&E slides in order to determine the margin status. This process resulted in an average of six individual microscope slides for pathologist review per specimen for the specimens analyzed in this study. Margin status and other pathologic details were obtained from the standard pathology report.
2.5 Pathologist review
One board certified pathologist co-author (ABS) reviewed the VR-SIM images of 18-gauge kidney biopsies as well as the corresponding H&E slides. The reviewer then assembled an atlas of VR-SIM images and corresponding H&E features. Using the images from the biopsy study as a reference, the pathologist then reviewed the VR-SIM images of each surgical case blindly, without knowledge of the final margin status. The reviewer had previous experience with reviewing VR-SIM images in other organ tissues stained with acridine orange. The study pathologist’s evaluation of margin status was compared against the final pathology report results.
2.5 Ethics statement
All experiments were conducted in accordance with procedures and guidelines outlined in study protocols approved by the Tulane University Biomedical Institutional Review Board. Informed consent was obtained from all patients included in the study.
3. Results
Table 1 contains a summary of the pathologic tumor stage, tumor Fuhrman nuclear grade, renal cancer type, size of the PN excised tissue, and number of histology H&E slides analyzed post-operatively. In this study there were two benign cases, eight pT1a cases, one pT1b case and one pT2a case. The Fuhrman nuclear grade ranged from 2 to 4. The number of H&E slides from each case ranged from 4 to 8. The greatest dimension of the PN specimen ranged from 2.9 cm to 8.8 cm, and the greatest dimension of the tumor ranged from 1.8 cm to 8.7 cm.
Table 1.
Case ID, tumor stage, Fuhrman nuclear Grade, renal cancer cell type, size of the excised partial nephrectomy (PN) specimen, size of the tumor, and the number of slides from the post-operative pathology reports for each cases.
| Case ID | Tumor Stage | Fuhrman Nuclear Grade | Renal Tumor Type | Size of PN specimen (cm × cm × cm) | Size of Tumor (cm × cm × cm) | # H&E slides |
|---|---|---|---|---|---|---|
| 5 | pT1a | 3/4 | Papillary | 6.2 × 5.0 × 4.9 | 3.9 × 3.5 × 3.5 | 8 |
| 6 | pT1b | 2/3 | Clear cell | 7.5 × 6.0 × 5.1 | 5.1 × 3.1 × 3.0 | 8 |
| 7 | pT1a | N/A | Chromophobe | 3.4 × 3.1 × 2.3 | 2.4 × 1.5 × 1.4 | 4 |
| 8 | pT1a | 2/4 | Clear cell | 4.5 × 4.1 × 2.3 | 2.1 × 2.1 × 1.9 | 6 |
| 9 | pT1a | 2/4 | Clear cell | 5.3 × 4.4 × 3.9 | 3.7 × 3.4 × 2.5 | 7 |
| 10 | pT1a | 3–4/4 | Clear cell and acidophilic | 3.5 × 3.0 × 2.2 | 2.0 × 1.6 × 1.4 | 7 |
| 11 | N/A | N/A | Oncocytoma | 4.2 × 3.4 × 2.4 | 1.9 × 1.5 × 2.4 | 6 |
| 12 | pT1a | 3/4 | Papillary | 4.6 × 4.0 × 3.1 | 3.5 × 2.5 × 1.7 | 6 |
| 13 | pT1a | 2/4 | Clear cell | 5.8 × 3.5 × 2.3 | 2.1 × 1.6 × 2.5 | 8 |
| 14 | pT1a | 2/3 | Clear cell | 2.9 × 2.4 × 2.7 | 2.5 × 2.4 × 1.1 | 5 |
| 15 | pT1a | 2/4 | Clear cell | 3.5 × 2.4 × 2.3 | 1.8 × 1.5 × 1.2 | 4 |
| 16 | pT2a | 2/4 | Papillary | 8.8 × 6.6 × 5.5 | 8.7 × 6.5 × 5.3 | 6 |
| 17 | N/A | N/A | Angiomyolipoma | 3.3 × 2.2 × 1.3 | 2.1 × 1.5 × 1.5 | 8 |
Table 2 contains a summary of the total VR-SIM imaged area and the total pixel counts of each VR-SIM image from each of the 13 cases analyzed in this study. In cases 7 and 9 multiple margins were imaged due to the geometric shape of the excised PN specimen. The total tissue image areas and pixel counts for each case were calculated based on thresholding the VR-SIM images to eliminate from the area calculation areas of the image not containing tissue. One uniform threshold was set for all the images in this study. The imaged area ranged from 0.41 to 17.93 cm2 in these 13 cases, and the image sizes (pixel counts) ranged from 0.10 to 4.24 gigapixels.
Table 2.
Case ID, number of VR-SIM margins imaged, total VR-SIM imaged area, and total pixels from VR-SIM imaging for each cases.
| Case ID | # VR-SIM margin surfaces imaged | VR-SIM Tissue Image Area (cm2) | Total image pixels (×109) |
|---|---|---|---|
| 5 | 1 | 4.29 | 1.02 |
| 6 | 1 | 17.93 | 4.24 |
| 7 | 2 | 7.06 | 1.67 |
| 8 | 1 | 4.59 | 1.09 |
| 9 | 2 | 15.19 | 3.60 |
| 10 | 1 | 3.25 | 0.77 |
| 11 | 1 | 4.37 | 1.03 |
| 12 | 1 | 4.35 | 1.03 |
| 13 | 1 | 3.50 | 0.83 |
| 14 | 1 | 0.41 | 0.10 |
| 15 | 1 | 2.68 | 0.63 |
| 16 | 1 | 12.23 | 2.89 |
| 17 | 1 | 1.66 | 0.39 |
In this study, we chose acridine orange as the contrast agent because it has a short topical incubation time and requires a short image acquisition time due to its brightness, which is advantageous for scanning of larger tissues and has been shown sufficient to identify normal and cancerous features in previous studies in other organs [19, 23]. In addition, at the concentrations we employ it serves as a useful general stain which broadly highlights tissue microanatomy in addition to nuclear contrast. In this study, we first conducted a review of VR-SIM images of 18-gauge kidney biopsies with the study pathologist (ABS) to generate an image atlas, composed of normal and malignant tissue types expected to be observed in the surgical margin images. The imaging atlas was composed from 5 biopsies, 3 of which were malignant and 2 of which were benign. A sixth biopsy was imaged to demonstrate the improvement in contrast that is achieved with SIM over standard widefield imaging. Figure 2 shows an entire kidney biopsy imaged with standard widefield (Figure 2A) and VR-SIM (Figure 2B), along with VR-SIM and H&E pairs for the common features that were observed in the biopsy study. The pathologist was able to identify important structures such as glomeruli (Figure 2C,D), renal tubules (Figure 2E,F), and adipose tissue (Figure 2G,H) in the benign kidney tissues. Malignant areas on VR-SIM imaging were distinguished as hyper-cellular areas with disruption of normal kidney organization (Figure 2I,J). The study pathologist then used these VR-SIM:H&E image pairs as references when reviewing the VR-SIM images of the margins.
Figure 2.
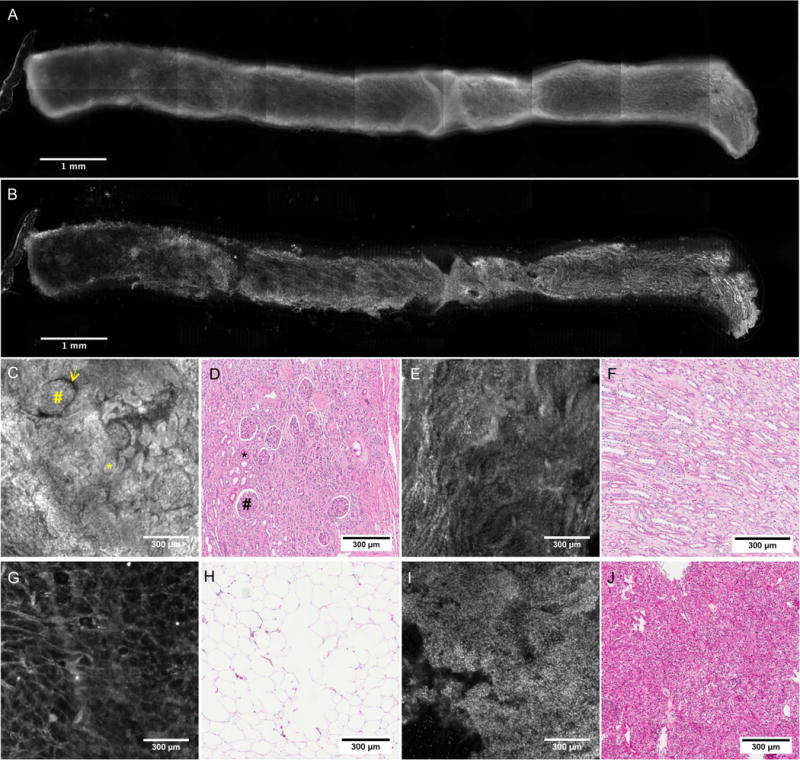
A) Widefield and B) VR-SIM image of an intact kidney biopsy stained with acridine orange. C–J) VR-SIM and corresponding H&E sections for common features observed in renal biopsies. C) VR-SIM image and D) H&E section depicting glomeruli (#), Bowman’s space (yellow arrow), and tubules in cross-section (*). E) VR-SIM image and F) H&E section depicting renal tubules in longitudinal section. G) VR-SIM image and H) H&E section of adipose tissue. I) VR-SIM image and J) H&E section of renal carcinoma.
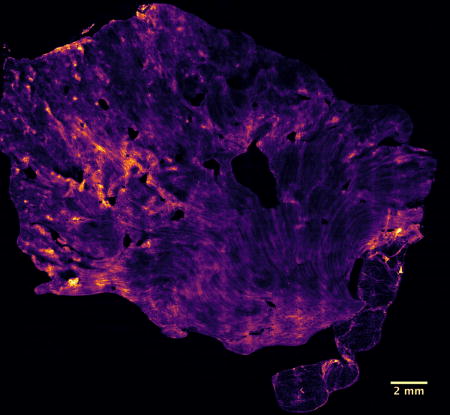
Figure 3 shows a full VR-SIM image of one of the parenchymal tumor resection margins from Case 7 and associated detailed zooms, demonstrating the multi-scale nature of the acquired images. In the large macro-scale image, the gross macro-scale architecture of the kidney, progressing from the renal sinus to the renal medulla to the renal cortex, is well demonstrated. As observed in the zoomed images, regions of adipose in the renal sinus (Figure 3A), tubules and collecting ducts in the renal medulla (Figure 3B), and convoluted tubules and glomeruli (Figure 3C), the latter observed in the larger macro-scale view as bright ‘dots’, in the renal cortex, are clearly identified. The VR-SIM image provides the necessary contrast and resolution for identifying these important features.
Figure 3.
VR-SIM image of parenchymal margin from Case 7, with zooms of tubules and collecting ducts in the renal medulla (A), hilar adipose tissue in the renal sinus (B) and glomeruli (*) in the renal cortex (C).
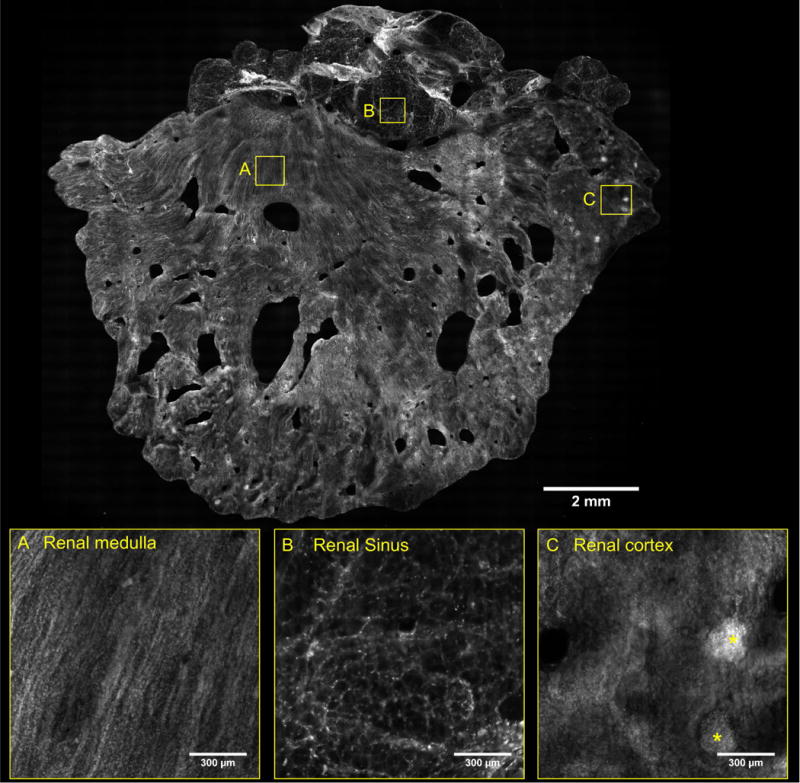
Figure 4 contains images of additional features observed in the VR-SIM images of the renal parenchymal surgical margins. In addition to the prior examples of collecting ducts and tubules and renal corpuscles, examples of medullary rays and adjacent cortical labyrinth are shown in Figure 4A. Examples of features not unique to the kidney such as small arterioles and venules (Figure 4B), larger arteries (Figure 4C), and peripheral nerves with adjacent nutrient vessels (Figure 4D), were also apparent and were consistent with the same features observed in prior work in the prostate [19].
Figure 4.
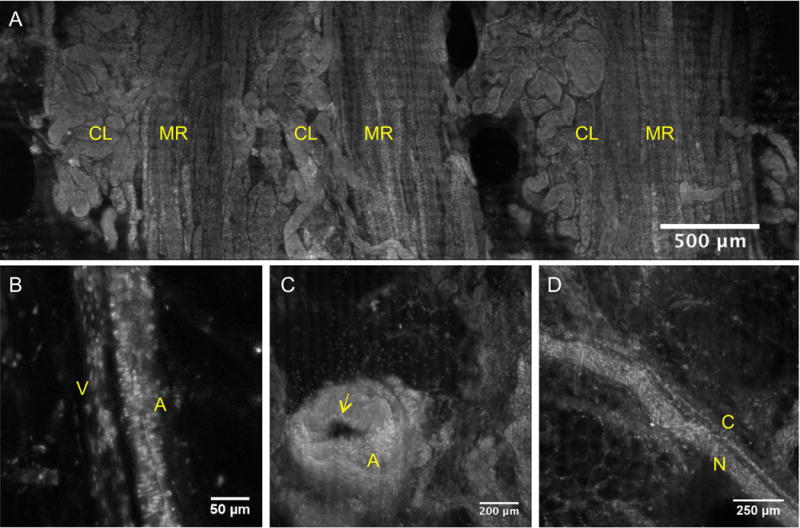
VR-SIM images of tissue landmarks and structures commonly observed in the surgical margin images. A) Medullary rays (MR) marked by vertically ascending tubules and cortical labyrinth (CL) marked by intervening convoluted tubules. B) Arteriole (A) differentiated from an adjacent venule (V) by horizontally-oriented smooth muscle nuclei giving a ‘banded’ appearance and larger width, that the venule lacks. C) Artery (A) with a clear lumen and layers of wavy smooth muscle cell nuclei constituting the vessel wall, with apparent elastic lamina (yellow arrow) bordering the lumen. D) Peripheral nerve (N) and an associated small nutrient microvessel (C).
Table 3 contains the results of blinded pathologist review of VR-SIM images against standard post-operative histopathology. All of the surgical margins were identified as negative for exposed tumor in both VR-SIM reviews and post-operative pathology reviews. The lack of cancer-positive surgical margins either on permanent histopathology or VR-SIM in this study precluded a computation of sensitivity and specificity, however we achieved 100% negative predictive value (NPV).
Table 3.
Confusion matrix for VR-SIM against standard post-operative histopathology.
| VR-SIM | |||
|---|---|---|---|
| Negative | Positive | ||
| Histopathology | Negative | 13 | 0 |
| Positive | 0 | 0 | |
4. Discussion
We recently completed a validation study of prostate margin evaluation using VR-SIM against gold-standard post-operative pathology [19]. In that study we utilized VR-SIM to image the margin surfaces of intact prostatectomies and were able to identify cancerous glands at the margin based on pathologist review. In this study we extended that approach for detection of PSMs for renal cancer management. We fully imaged surgical margins from 13 patients at microscopic resolution in 30 minutes or less including staining and processing time. The imaging time may be further reduced with a faster mechanical scan stage, which can potentially reduce the time to less than 15 minutes. We were able to produce images that have excellent correlation with standard histology images using a single topical fluorescent contrast agent, acridine orange. Malignant renal tumors were identified by the study pathologist on VR-SIM images of an 18-gauge kidney biopsy, indicating the potential for a PSM to be caught by a pathologist at initial SIM review. PN is offered to patients with stage pT1b (> 4cm renal mass) if technically possible, but this option is limited in more advanced stage renal cancer patients. However, with the right combination of location, size, and surgeon experience, PN can be just as effective oncologically as RN in larger tumors (> 4cm) with the added benefit of preserving and optimizing renal function. Several recent studies have shown that PN can be successful in renal masses with diameters larger than 4 cm, however, the PSM rate of PN in >4 cm masses is higher than <4 cm masses [5, 24].
We demonstrated that VR-SIM is a feasible and practical tool for intraoperative kidney margin assessment. In this study, all the cases had negative surgical margins on permanent histological review, and no areas suspicious for tumor were identified in the VR-SIM images. The positive margin rate in PN is very low at our institution, which correlates well with the current literature [2–4, 6–8]. However, in larger tumors and in cases with high surgical suspicion of PSM, VR-SIM may be of high clinical value for the immediate, non-destructive screening of these specimens and guidance for intra-operative decision making. Importantly, the staining and imaging process does not have any deleterious effects on downstream histological processing, therefore not compromising standard-of-care definitive diagnostic and grading procedures.
Another advantage of VR-SIM is that it samples a significantly higher area of margin surface compared to gold standard histopathology methods. In this study, each case resulted in 4 to 8 H&E slides under standard-of-care protocols depending on the size of the specimen (Table 3). A standard pathology processed slide typically contains a 4 micron-thick section of a 3 mm thick slice of the margin, which only allows the pathologist to examine 0.0012 cm2 area of the margin surface area per slide. Therefore, in each case, standard pathology only evaluates a 0.0048–0.0096 cm2 area of the margin. From Table 2, case 14 has the lowest VR-SIM imaged area (0.41 cm2) which is 68.33× higher than gold-standard histopathology (5 H&E slides, 0.006 cm2). This advantage is even higher for larger specimens, for example Case 6, which had an imaged tissue area of 17.9 cm2 compared to the corresponding H&E margin surface area (8 slides, 0.0096 cm2) – an improvement of over 1,800× in terms of sampled image area. Due to the inherently higher surface sampling of VR-SIM compared to standard histology processing, VR-SIM would allow for the pathologist to more thoroughly examine the entire surgical margin in its fresh ex-vivo state. This is clearly demonstrated in Figure 3, where the complete margin landscape and normal renal structures are easily identified on the intact specimen. Areas of the margin where tumor was inadvertently cut across would be expected to be easily identified as a clear disruption in the normal expected architecture of the renal tissue, with the reviewing pathologist being able to zoom in to observe the tissue at the cellular level for confirmation. It may also be possible to use computer-automated strategies (for instance based on image texture or more advanced approaches) to automatically highlight areas of interest for pathologist review, decreasing the time needed to review the images in a timely manner. The use of this type of imaging of fresh surgical specimens may be useful both for intra-operative screening for the presence of PSMs to guide additional immediate resection, as well as to guide the selection of appropriate areas to sample for permanent histopathology in the gross pathology laboratory.
This study is limited by the small sample size and the lack of histologically-confirmed positive surgical margins, precluding a full estimate of the accuracy of full-margin-surface VR-SIM imaging for PSM detection. However, it confirms that the approach is feasible, and encouraging imaging results from malignant renal biopsies suggest a potential role for VR-SIM in the operating room as an intra-operative tumor margin screening tool, or in the pathology lab as a useful tool for directed tissue sampling for permanent histopathology. For future work, we will continue to collect further margin data and samples from PN cases. In particular, we will focus on using VR-SIM to image cases with renal masses larger than 4 cm, since the PSM rate is expected to be higher in PN cases with masses >4 cm in diameter. With VR-SIM as an intra-operative “safety net,” and inherently more thorough tool in evaluating surgical margins, more patients may choose PN over RN, optimizing the safety and overall benefits to quality of life afforded to those undergoing the less morbid procedure of partial nephrectomy.
Acknowledgments
This work was supported by grants from the NIH/NCI (grant 1R21CA159936 to J.Q.B.) and the NCI Innovative Molecular Analysis Technologies (IMAT) program (grant 1R33CA196457 to J.Q.B.), and an institutional grant from Tulane University. D.B.T. was supported by a National Science Foundation IGERT grant to Tulane University (DGE-1144646). This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–9. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Breda A, Stepanian SV, Liao J, Lam JS, Guazzoni G, Stifelman M, et al. Positive margins in laparoscopic partial nephrectomy in 855 cases: a multi-institutional survey from the United States and Europe. J Urol. 2007;178:47–50. doi: 10.1016/j.juro.2007.03.045. discussion. [DOI] [PubMed] [Google Scholar]
- 3.Eroglu M, Unsal A, Bakirtas H, Tekdogan U, Ataoglu O, Balbay MD. Routine frozen-section biopsy from the surgical bed should be performed during nephron-sparing surgery for renal cell carcinoma. Scand J Urol Nephrol. 2005;39:222–5. doi: 10.1080/00365590510007757. [DOI] [PubMed] [Google Scholar]
- 4.Khalifeh A, Kaouk JH, Bhayani S, Rogers C, Stifelman M, Tanagho YS, et al. Positive surgical margins in robot-assisted partial nephrectomy: a multi-institutional analysis of oncologic outcomes (leave no tumor behind) J Urol. 2013;190:1674–9. doi: 10.1016/j.juro.2013.05.110. [DOI] [PubMed] [Google Scholar]
- 5.Kim DK, Kim LH, Raheem AA, Shin TY, Alabdulaali I, Yoon YE, et al. Comparison of Trifecta and Pentafecta Outcomes between T1a and T1b Renal Masses following Robot-Assisted Partial Nephrectomy (RAPN) with Minimum One Year Follow Up: Can RAPN for T1b Renal Masses Be Feasible? PLoS ONE. 2016;11:e0151738. doi: 10.1371/journal.pone.0151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubinski DJ, Clark PE, Assimos DG, Hall MC. Utility of frozen section analysis of resection margins during partial nephrectomy. Urology. 2004;64:31–4. doi: 10.1016/j.urology.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Permpongkosol S, Colombo JR, Jr, Gill IS, Kavoussi LR. Positive surgical parenchymal margin after laparoscopic partial nephrectomy for renal cell carcinoma: oncological outcomes. J Urol. 2006;176:2401–4. doi: 10.1016/j.juro.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Zigeuner R, Quehenberger F, Pummer K, Petritsch P, Hubmer G. Long-term results of nephron-sparing surgery for renal cell carcinoma in 114 patients: risk factors for progressive disease. Bju Int. 2003;92:567–71. doi: 10.1046/j.1464-410x.2003.04414.x. [DOI] [PubMed] [Google Scholar]
- 9.Ani I, Finelli A, Alibhai SM, Timilshina N, Fleshner N, Abouassaly R. Prevalence and impact on survival of positive surgical margins in partial nephrectomy for renal cell carcinoma: a population-based study. Bju Int. 2013;111:E300–5. doi: 10.1111/j.1464-410X.2012.11675.x. [DOI] [PubMed] [Google Scholar]
- 10.Maurice MJ, Zhu H, Kim SP, Abouassaly R. Reexamining the Association Between Positive Surgical Margins and Survival After Partial Nephrectomy in a Large American Cohort. J Endourol. 2016;30:698–703. doi: 10.1089/end.2016.0031. [DOI] [PubMed] [Google Scholar]
- 11.Pierorazio PM, Johnson MH, Patel HD, Sozio SM, Sharma R, Iyoha E, et al. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-Analysis. J Urol. 2016;196:989–99. doi: 10.1016/j.juro.2016.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gareau DS, Li YB, Huang B, Eastman Z, Nehal KS, Rajadhyaksha M. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. Journal of Biomedical Optics. 2008;13:054001. doi: 10.1117/1.2981828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain M, Robinson BD, Aggarwal A, Shevchuk MM, Scherr DS, Mukherjee S. Multiphoton microscopy for rapid histopathological evaluation of kidney tumours. Bju Int. 2016;118:118–26. doi: 10.1111/bju.13377. [DOI] [PubMed] [Google Scholar]
- 14.Lee HC, Zhou C, Cohen DW, Mondelblatt AE, Wang Y, Aguirre AD, et al. Integrated optical coherence tomography and optical coherence microscopy imaging of ex vivo human renal tissues. J Urol. 2012;187:691–9. doi: 10.1016/j.juro.2011.09.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su LM, Kuo J, Allan RW, Liao JC, Ritari KL, Tomeny PE, et al. Fiber-Optic Confocal Laser Endomicroscopy of Small Renal Masses: Toward Real-Time Optical Diagnostic Biopsy. J Urol. 2016;195:486–92. doi: 10.1016/j.juro.2015.07.115. [DOI] [PubMed] [Google Scholar]
- 16.Tao YK, Shen D, Sheikine Y, Ahsen OO, Wang HH, Schmolze DB, et al. Assessment of breast pathologies using nonlinear microscopy. Proc Natl Acad Sci U S A. 2014;111:15304–9. doi: 10.1073/pnas.1416955111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abeytunge S, Li Y, Larson B, Peterson G, Seltzer E, Toledo-Crow R, et al. Confocal microscopy with strip mosaicing for rapid imaging over large areas of excised tissue. J Biomed Opt. 2013;18:61227. doi: 10.1117/1.JBO.18.6.061227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JQ, Bydlon TM, Richards LM, Yu B, Kennedy SA, Geradts J, et al. Optical Assesssment of Tumor Resection Margins in the Breast. IEEE Journal of Selected Topics in Quantum Electronics. 2010;16:530–44. doi: 10.1109/jstqe.2009.2033257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Tulman DB, Sholl AB, Kimbrell HZ, Mandava SH, Elfer KN, et al. Gigapixel surface imaging of radical prostatectomy specimens for comprehensive detection of cancer-positive surgical margins using structured illumination microscopy. Sci Rep. 2016;6:27419. doi: 10.1038/srep27419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlichenmeyer TC, Wang M, Elfer KN, Brown JQ. Video-rate structured illumination microscopy for high-throughput imaging of large tissue areas. Biomed Opt Express. 2014;5:366–77. doi: 10.1364/BOE.5.000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elfer KN, Sholl AB, Wang M, Tulman DB, Mandava SH, Lee BR, et al. DRAQ5 and Eosin (‘D&E’) as an Analog to Hematoxylin and Eosin for Rapid Fluorescence Histology of Fresh Tissues. PLoS ONE. 2016;11:e0165530. doi: 10.1371/journal.pone.0165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Wang M, Tulman D, Mandava SH, Elfer KN, Gabrielson A, et al. Nondestructive Diagnosis of Kidney Cancer on 18-gauge Core Needle Renal Biopsy Using Dual-color Fluorescence Structured Illumination Microscopy. Urology. 2016;98:195–9. doi: 10.1016/j.urology.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Kimbrell HZ, Sholl AB, Tulman DB, Elfer KN, Schlichenmeyer TC, et al. High-Resolution Rapid Diagnostic Imaging of Whole Prostate Biopsies Using Video-Rate Fluorescence Structured Illumination Microscopy. Cancer Res. 2015;75:4032–41. doi: 10.1158/0008-5472.CAN-14-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddox M, Mandava S, Liu J, Boonjindasup A, Lee BR. Robotic partial nephrectomy for clinical stage T1b tumors: intermediate oncologic and functional outcomes. Clinical genitourinary cancer. 2015;13:94–9. doi: 10.1016/j.clgc.2014.07.011. [DOI] [PubMed] [Google Scholar]


