Abstract
Background:
Reduced P-wave voltage in lead 1 (PVL1) has been associated with atrial fibrillation (AF) recurrence.This study sought to determine the association between reduced PVL1 and AF in the NSTEMI population and the correlation between reduced PVL1 and interatrial block (IAB)/coronary artery disease (CAD).
Methods:
Data were recorded for clinical, echocardiographic, angiographic, electrocardiographic and outcome variables. Patients were followed for a minimum of one year. Chi-square tests, independent samples t-tests and one-way ANOVA were used for the analysis, which was done using IBM SPSS
Results:
A total of 322 consecutive patients were included in the analysis. Patients with new-onset AF had a significantly lower PVL1 (0.085 ± 0.030mV vs. 0.103 ± 0.037mV; p=0.007). There was a significant difference in mean PVL1 between those with no IAB, partial IAB and advanced IAB (p = <0.001). Those with any type of IAB had a significantly lower mean PVL1 than those without (0.094 ± 0.032 mV vs. 0.106 ± 0.038 mV; p=0.005). Patients who developed AF had a significantly longer P-wave duration (126 ± 20ms vs. 119 ± 17ms; p=0.022). Patients with IAB were more likely to develop new-onset AF (15.4% versus 7.5%, p=0.025). There were significant co-linear associations between reduced PVL1 and IAB (p=0.005); reduced PVL1 and diffuse CAD (p=0.031) and IAB and diffuse CAD (p=0.022)
Keywords: Interatrial block,; atrial fibrillation,; P-wave voltage,; NSTEMI.
Introduction
Reduced P-wave amplitude in lead I (PVL1) has recently been shown to be associated with recurrence of atrial fibrillation (AF) [1]. In this study, conduction was shown to be displaced in the Bachmann region in patients with lower P-wave voltages using left atrial voltage and activation maps. A possible mechanism for the higher rates of AF recurrence in patients with reduced PVL1 was proposed to be abnormal interatrial conduction along the Bachmann region, the same mechanism as believed to underlie interatrial block (IAB).[1] Interatrial block has previously been shown to be associated with atrial fibrillation in multiple cardiac populations. [2-13] The P-wave represents atrial depolarization and as such is an indirect measure of atrial conduction.[14] With normal anatomy, in sinus rhythm, the P-wave initiates at the sino-atrial node and travels inferiorly through the right atrium via the intra-atrial conduction pathways and most commonly crosses the interatrial septum superiorly via the Bachmann region, a broad muscular set of fibers.[15-18] Partial interatrial block (IAB) results from a delay of conduction on this interatrial pathway at the Bachmann region. When this pathway is completely blocked, the right atrium is activated cranio-caudally; however, the left atrium is depolarized from the level of the coronary sinus to the posterior and superior region (retrograde activation) producing the classic biphasic P-wave of advanced IAB.[19] IAB is clinically important due to its correlation with the development or recurrence of AF in various cardiac populations.[2-13] While the exact pathology underlying the conduction abnormalities seen in IAB have not yet fully been determined it has been hypothesized that electrical remodeling and fibrotic atrial remodeling due to reduction of the blood supply to the Bachmann region may play a key role.[20-23] In support of this, IAB has been shown to be associated with diffuse coronary artery disease (CAD).[11] This study sought to determine the association of reduced PVL1 with development of AF in a population of patients with NSTEMI and its correlation with IAB and diffuse CAD.
Material and Methods
Patient Selection
Electronic records of a consecutive cohort of patients at Kingston General Hospital who had presented with a NSTEMI between November 2013 and August 2015 and had an ECG completed in-hospital as part of their work-up were retrospectively reviewed. Exclusion criteria were (i) prior history of AF (ii) lack of at least one significant coronary artery lesion (>70% occlusion) (iii) any STEMI within 90 days prior to the NSTEMI, (iv) significant valvular disease or cardiomyopathy and (v) any device pacing the atrium (vi) active hyperthyroidism.
Electrocardiogram, echocardiogram and angiogram parameters
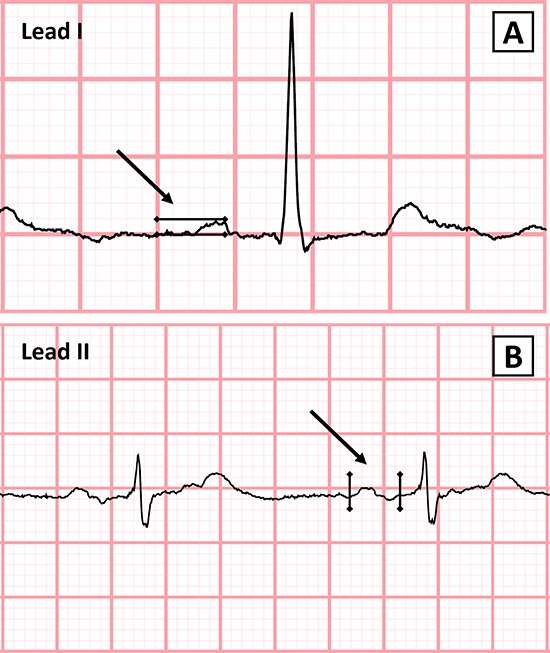
ECGs were scanned at 300 dpi and blindly analyzed using ICONICO semi-automatic calipers. PVL1 was measured from the peak of the P-wave to the isoelectric line of the TP interval (Figure 1A). This method has been previously described and validated with high levels of agreement in both interobserver and intraobserver variability.[1],[24] P-wave onset was defined as the first upward or downward deflection from the isoelectric baseline and the P-wave offset as the return of the waveform to the baseline (Figure 1B). P-wave duration measurement has been previously described and validated.[25] Partial IAB was defined as a P-wave ≥120ms while advanced IAB was defined as a P-wave ≥120ms with biphasic (±) morphology in the inferior leads (II, III and aVF) according to the most recent consensus definition.[19] Reduced PVL1 was defined as a P-wave voltage in lead I less than 0.10 mV. Echocardiographic and angiographic data were taken from clinical reports. Atrial fibrillation was evaluated through review of medical records, EGG’s and holter monitors. AF ≥ 6 minutes’ duration was considered as outcome.[26]
Figure 1A,1B. .Method for the Measurement of P-Wave Duration and Amplitude.
Statistical Methods
Data were collected in Excel and imported into IBM SPSS (version 24.0 for Windows) for statistical analysis. Data were initially described using means and standard deviations for continuous data, and frequencies and percentages for categorical data. This was followed by a univariate analysis to assess the association of the collected data with the outcome, using one-way ANOVA and independent sample t-tests for the continuous data and chi-square tests (Pearson or Fisher's Exact as appropriate) for the categorical data.
Results
Population Demographics
A total of 322 consecutive patients were included in the analysis. The population was 72.3% male, the mean age was 65.4 ± 11.9 years, the mean ejection fraction was 55.2 ± 12.7% and the mean left atrial diameter was 38.7 ± 6.0mm. Population characteristics are presented in [Table 1]. The prevalence of PVL1 less than 0.10 mV (reduced PVL1) was 50.3%, PVL1 between 0.10 and 0.20 mV was 48.8% and PVL1 > 0.20 mV was 0.9%. The prevalence of partial IAB was 31.7% and the prevalence of advanced IAB was 6.5%. The incidence of new onset AF within one year was 10.6%. The population was normally distributed in terms of P-wave voltage and duration.
Table 1. Population characteristics.
| Clinical Variable | Value (n = 322) |
|---|---|
| Age (years) ± SD | 65.4 ± 11.9 |
| Male sex | 233 (72.3%) |
| BMI (kg/m2) | 29.8 ± 6.6 |
| Partial interatrial block | 102 (31.9%) |
| Advanced interatrial block | 21 (6.5%) |
| Prior smoker | 204 (63.4%) |
| Hypertension | 232 (72.0%) |
| Dyslipidemia | 182 (56.5%) |
| Diabetes | 104 (32.3%) |
| Prior transient ischemia attack or stroke | 35 (10.9%) |
| Obstructive sleep apnea | 37 (11.4%) |
| Pulmonary disease | 49 (15.2%) |
| Prior known coronary artery disease | 118 (36.6%) |
| Congestive heart failure | 15 (4.7%) |
| Previous cardiac surgery | 43 (13.4%) |
| Prior atrial flutter | 3 (1.0%) |
| Left ventricular ejection fraction (%) | 55.2 ± 12.7 |
| Body surface area (m2) | 1.97 ± 0.22 |
| Left atrial diameter (mm) | 38.7 ± 6.0 |
| Left atrial volume indexed to BSA (ml/m2) | 31.6 ± 21.1 |
| Right atrial volume indexed to BSA (ml/m2) | 22.6 ± 15.6 |
Associations with Atrial Fibrillation
Participants who developed new-onset AF within one year had a significantly lower PVL1 (0.085 ± 0.030mV vs. 0.103 ± 0.037mV; p=0.007) and significantly longer P-wave duration (126 ± 20ms vs. 119 ± 17ms, p=0.022) than those who did not develop AF ([Table 2]). Multivariate logistic regression analysis was not completed due to substantial co-linearity between the three variables in the model (IAB, reduced PVL1 and diffuse CAD defined as the presence of two or more significant coronary artery lesions in the same patient). There were significant co-linear associations between reduced PVL1 and IAB (p=0.005); reduced PVL1 and diffuse CAD (p=0.031) and IAB and diffuse CAD (p=0.022).
Table 2. Difference in P-Wave Voltages between IAB Categories and AF Development.
| Any IAB (either partial or advanced) | |||
|---|---|---|---|
| Absent (n = 199) | Present (n = 123) | P-Value | |
| P-Wave Voltage (mV) | 0.106 ± 0.038 | 0.094 ± 0.032 | 0.005 |
| Advanced IAB | |||
| Absent (n =301) | Present (n = 21) | ||
| P-Wave Voltage (mV) | 0.103 ± 0.362 | 0.074 ± 0.029 | <0.001 |
| New Onset Atrial Fibrillation | |||
| Absent (n =288) | Present (n = 34) | ||
| Mean P-Wave Voltage (mV) | 0.103 ± 0.037 | 0.085 ± 0.030 | 0.007 |
| Mean P-Wave duration (ms) | 119 ± 17 | 126 ± 20 | 0.022 |
Correlation of P-Wave Voltage with P-Wave Duration
There was a significant difference of mean P-wave duration between PVL1 categories (<0.10mV, 0.10-0.20mV and >0.20mV) (p = 0.009) ([Table 3]). This difference favored increased P-wave duration with decreased PVL1 category. There was also a significant difference in the presence of advanced IAB between the PVL1 categories (p = 0.014) and in the prevalence of any IAB (p = 0.035) ([Table 4]).
Table 3. Difference in P-Wave Voltage and Duration by IAB and Voltages Categories.
| Inter Atrial Block Category | Mean P-wave Voltage (mV) | P-Value |
|---|---|---|
| No interatrial block | 0.106 ± 0.038 | |
| Partial interatrial block | 0.098 ± 0.031 | <0.001 |
| Advanced interatrial block | 0.074 ± 0.029 | |
| Voltage Category | Mean P-wave Duration (ms) | P-Value |
| <0.10 mV | 122.1 ± 18.1 | |
| 0.10 - 0.20 mV | 117.0 ± 16.0 | 0.009 |
| >0.20 mV | 105.0 ± 2.6 |
Table 4. Difference in IAB Categories between P-Wave Voltage Categories.
| Any IAB (either partial or advanced) | |||
|---|---|---|---|
| P-Wave Voltage | Absent (n = 199) | Present (n = 123) | P-Value |
| <0.10 mV | 90 (45.2%) | 72 (58.5%) | |
| 0.10 - 0.20 mV | 106 (53.3%) | 51 (41.5%) | 0.035 |
| >0.20 mV | 3 (1.5%) | 0 (0.0%) | |
| Advanced IAB | |||
| Absent (n = 301) | Present (n = 21) | ||
| <0.10 mV | 145 (48.2%) | 17 (81.0%) | |
| 0.10 - 0.20 mV | 153 (50.8%) | 4 (19.0%) | 0.014 |
| >0.20 mV | 3 (1.0%) | 0 (0.0%) |
Correlation of IAB Category with P-Wave Voltage
There was a significant difference of mean PVL1 between those with no IAB, partial IAB and advanced IAB (p = <0.001) ([Table 3]). This difference favored decreased PVL1 with increased severity of IAB category. Patients who had advanced IAB had a significantly lower mean PVL1 than those without advanced IAB (0.074 ± 0.029 mV vs. 0.103 ± 0.362 mV; p=<0.001). Patients who had any type of IAB had a significantly lower mean PVL1 than those without IAB (0.094 ± 0.032 mV vs. 0.106 ± 0.038 mV; p=0.005) ([Table 2])
Discussion
Reduced PVL1 was found to be significantly associated with the development of new-onset AF in this population. In addition, reduced PVL1and IAB were found to be significantly correlated with each other. It is plausible that reduced PVL1 and IAB may be associated with the same pathological process leading to increased P-wave duration and reduced voltage, namely atrial fibrosis. Park et al. have recently demonstrated a significant correlation between reduced PVL1 and displaced conduction in the Bachmann region using left atrial voltage and activation maps.[1] Atrial fibrosis delays cardiac electrical conduction and reduces voltage, phenomena which have been well described previously.[27-31] Since the P-wave voltage depends on the direction of electrical propagation relative to the axis of the lead being measured and the myocardial mass and intervening substrates; it has been proposed that reduced P-wave voltage may be a result of an altered atrial conduction pattern and decreased myocardial mass due to atrial fibrotic scarring and increased degree of electro-anatomical remodeling.[1] It has recently been shown that diffuse CAD is associated with IAB and development of AF in the NSTEMI population.[11] In this current study, both reduced PVL1 and IAB are also significantly correlated with diffuse CAD. Therefore it is possible that the mechanism underlying both decreased PVL1 and IAB is fibrosis of the atria, particularly in the Bachmann region.[32],[33]
Limitations
This study was retrospective in nature and as such may present inherent bias. AF was determined by clinical examination, ECG and Holter monitor reports; thus silent AF episodes may not have been recorded.
Conclusions
Reduced PVL1 is associated with new-onset AF in the NSTEMI population. In addition, PVL1 and IAB are significantly correlated with each other and with diffuse CAD. While the exact mechanism responsible for each have yet to be worked out, it is possible that the underlying cause could stem from fibrosis of the atria
References
- 1.Park Jin-Kyu, Park Junbeom, Uhm Jae-Sun, Joung Boyoung, Lee Moon-Hyoung, Pak Hui-Nam. Low P-wave amplitude (<0.1 mV) in lead I is associated with displaced inter-atrial conduction and clinical recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Europace. 2016 Mar;18 (3):384–91. doi: 10.1093/europace/euv028. [DOI] [PubMed] [Google Scholar]
- 2.Enriquez Andres, Conde Diego, Hopman Wilma, Mondragon Ignacio, Chiale Pablo A, de Luna Antoni Bayés, Baranchuk Adrian. Advanced interatrial block is associated with recurrence of atrial fibrillation post pharmacological cardioversion. Cardiovasc Ther. 2014 Apr;32 (2):52–6. doi: 10.1111/1755-5922.12063. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell Jane, Koppikar Sahil, Barake Walid, Redfearn Damian, Michael Kevin, Simpson Christopher, Hopman Wilma, Baranchuk Adrian. Prolonged P-wave duration is associated with atrial fibrillation recurrence after successful pulmonary vein isolation for paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2014 Mar;39 (2):131–8. doi: 10.1007/s10840-013-9851-1. [DOI] [PubMed] [Google Scholar]
- 4.Enriquez Andres, Sarrias Axel, Villuendas Roger, Ali Fariha Sadiq, Conde Diego, Hopman Wilma M, Redfearn Damian P, Michael Kevin, Simpson Christopher, De Luna Antoni Bayés, Bayés-Genís Antoni, Baranchuk Adrian. New-onset atrial fibrillation after cavotricuspid isthmus ablation: identification of advanced interatrial block is key. Europace. 2015 Aug;17 (8):1289–93. doi: 10.1093/europace/euu379. [DOI] [PubMed] [Google Scholar]
- 5.Tekkesin Ahmet Ilker, Çinier Göksel, Cakilli Yasin, Hayıroğlu Mert İlker, Alper Ahmet Taha. Interatrial block predicts atrial high rate episodes detected by cardiac implantable electronic devices. J Electrocardiol. 2016 Sep 20;50 (2):234–237. doi: 10.1016/j.jelectrocard.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Enriquez Andres, Conde Diego, Femenia Francisco, de Luna Antoni Bayés, Ribeiro Antonio, Muratore Claudio, Valentino Mariana, Retyk Enrique, Galizio Nestor, Hopman Wilma M, Baranchuk Adrian. Relation of interatrial block to new-onset atrial fibrillation in patients with Chagas cardiomyopathy and implantable cardioverter-defibrillators. Am. J. Cardiol. 2014 May 15;113 (10):1740–3. doi: 10.1016/j.amjcard.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Alexander Bryce, Rodriguez Claudia, de la Isla Leopoldo Perez, Islas Fabian, Quevedo Pilar Jimenez, Nombela-Franco Luis, Hopman Wilma, Malik Paul, Baranchuk Adrian. The impact of advanced Interatrial block on new-onset atrial fibrillation following TAVR procedure. Int. J. Cardiol. 2016 Nov 15;223 ():672–673. doi: 10.1016/j.ijcard.2016.08.083. [DOI] [PubMed] [Google Scholar]
- 8.Baranchuk Adrian, Parfrey Brendan, Lim Leonard, Morriello Florence, Simpson Christopher S, Hopman Wilma M, Redfearn Damian P, Fitzpatrick Michael. Interatrial block in patients with obstructive sleep apnea. Cardiol J. 2011;18 (2):171–5. [PubMed] [Google Scholar]
- 9.Sadiq Ali Fariha, Enriquez Andres, Conde Diego, Redfearn Damian, Michael Kevin, Simpson Christopher, Abdollah Hoshiar, Bayés de Luna Antoni, Hopman Wilma, Baranchuk Adrian. Advanced Interatrial Block Predicts New Onset Atrial Fibrillation in Patients with Severe Heart Failure and Cardiac Resynchronization Therapy. Ann Noninvasive Electrocardiol. 2015 Nov;20 (6):586–91. doi: 10.1111/anec.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbosa-Barros Raimundo, Alexander Bryce, Baranchuk Adrian. Interatrial Block in Brugada Syndrome. Rev Esp Cardiol (Engl Ed) 2017 Nov;70 (11) doi: 10.1016/j.rec.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Alexander Bryce, MacHaalany Jimmy, Lam Brandon, van Rooy Henri, Haseeb Sohaib, Kuchtaruk Adrian, Glover Benedict, Bayés de Luna Antoni, Baranchuk Adrian. Comparison of the Extent of Coronary Artery Disease in Patients With Versus Without Interatrial Block and Implications for New-Onset Atrial Fibrillation. Am. J. Cardiol. 2017 Apr 15;119 (8):1162–1165. doi: 10.1016/j.amjcard.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Sellés Manuel, Baranchuk Adrian, Elosua Roberto, de Luna Antonio Bayés. Rationale and design of the BAYES (Interatrial Block and Yearly Events) registry. Clin Cardiol. 2017 Apr;40 (4):196–199. doi: 10.1002/clc.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gul Enes E, Pal Raveen, Caldwell Jane, Boles Usama, Hopman Wilma, Glover Benedict, Michael Kevin A, Redfearn Damian, Simpson Chris, Abdollah Hoshiar, Baranchuk Adrian. Interatrial block and interatrial septal thickness in patients with paroxysmal atrial fibrillation undergoing catheter ablation: Long-term follow-up study. Ann Noninvasive Electrocardiol. 2017 Jul;22 (4) doi: 10.1111/anec.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersson Richard, Mosén Henrik, Steding-Ehrenborg Katarina, Carlson Jonas, Faxén Lisa, Mohtadi Alan, Platonov Pyotr G, Holmqvist Fredrik. Physiological variation in left atrial transverse orientation does not influence orthogonal P-wave morphology. Ann Noninvasive Electrocardiol. 2017 Mar;22 (2) doi: 10.1111/anec.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemery Robert, Guiraudon Gerard, Veinot John P. Anatomic description of Bachmann's bundle and its relation to the atrial septum. Am. J. Cardiol. 2003 Jun 15;91 (12):1482–5, A8. doi: 10.1016/s0002-9149(03)00405-3. [DOI] [PubMed] [Google Scholar]
- 16.Ariyarajah Vignendra, Spodick David H. The Bachmann Bundle and interatrial conduction. Cardiol Rev. 2006 Jun 22;14 (4):194–9. doi: 10.1097/01.crd.0000195221.26979.2b. [DOI] [PubMed] [Google Scholar]
- 17.Platonov Pyotr G, Mitrofanova Lubov, Ivanov Vitaly, Ho Siew Yen. Substrates for intra-atrial and interatrial conduction in the atrial septum: anatomical study on 84 human hearts. Heart Rhythm. 2008 Aug;5 (8):1189–95. doi: 10.1016/j.hrthm.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Magnani Jared W, Zhu Lei, Lopez Faye, Pencina Michael J, Agarwal Sunil K, Soliman Elsayed Z, Benjamin Emelia J, Alonso Alvaro. P-wave indices and atrial fibrillation: cross-cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. Am. Heart J. 2015 Jan;169 (1):53–61.e1. doi: 10.1016/j.ahj.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayés de Luna Antonio, Platonov Pyotr, Cosio Francisco G, Cygankiewicz Iwona, Pastore Carlos, Baranowski Rafa, Bayés-Genis Antoni, Guindo Josep, Viñolas Xavier, Garcia-Niebla Javier, Barbosa Raimundo, Stern Shlomo, Spodick David. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol. 2012 Sep;45 (5):445–51. doi: 10.1016/j.jelectrocard.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Saremi Farhood, Channual Stephanie, Krishnan Subramaniam, Gurudevan Swaminatha V, Narula Jagat, Abolhoda Amir. Bachmann Bundle and its arterial supply: imaging with multidetector CT--implications for interatrial conduction abnormalities and arrhythmias. Radiology. 2008 Aug;248 (2):447–57. doi: 10.1148/radiol.2482071908. [DOI] [PubMed] [Google Scholar]
- 21.Ariyarajah Vignendra, Fernandes Jaxon, Apiyasawat Sirin, Spodick David H. Angiographic localization of potential culprit coronary arteries in patients with interatrial block following a positive exercise tolerance test. Am. J. Cardiol. 2007 Jan 01;99 (1):58–61. doi: 10.1016/j.amjcard.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 22.Bayés de Luna Antoni, Baranchuk Adrian, Martínez-Sellés Manuel, Platonov Pyotr G. Anticoagulation in patients at high risk of stroke without documented atrial fibrillation. Time for a paradigm shift? Ann Noninvasive Electrocardiol. 2017 Jan;22 (1) doi: 10.1111/anec.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander Bryce, Sadiq Fariha, Azimi Kousha, Glover Benedict, Antiperovitch Pavel, Hopman Wilma M, Jaff Zardasht, Baranchuk Adrian. Reverse atrial electrical remodeling induced by cardiac resynchronization therapy. J Electrocardiol. 2017 May 19;50 (5):610–614. doi: 10.1016/j.jelectrocard.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Kizilirmak Filiz, Demir Gultekin Gunhan, Gokdeniz Tayyar, Gunes Haci Murat, Cakal Beytullah, Guler Ekrem, Karaca İbrahim Oguz, Omaygenç Mehmet Onur, Yılmaz Fatih, Olgun Fatih Erkam, Kilicaslan Fethi. Changes in Electrocardiographic P Wave Parameters after Cryoballoon Ablation and Their Association with Atrial Fibrillation Recurrence. Ann Noninvasive Electrocardiol. 2016 Nov;21 (6):580–587. doi: 10.1111/anec.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dilaveris P, Batchvarov V, Gialafos J, Malik M. Comparison of different methods for manual P wave duration measurement in 12-lead electrocardiograms. Pacing Clin Electrophysiol. 1999 Oct;22 (10):1532–8. doi: 10.1111/j.1540-8159.1999.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 26.Hohnloser Stefan H, Capucci Alessandro, Fain Eric, Gold Michael R, van Gelder Isabelle C, Healey Jeff, Israel Carsten W, Lau Chu P, Morillo Carlos, Connolly Stuart J. ASymptomatic atrial fibrillation and Stroke Evaluation in pacemaker patients and the atrial fibrillation Reduction atrial pacing Trial (ASSERT). Am. Heart J. 2006 Sep;152 (3):442–7. doi: 10.1016/j.ahj.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Akoum Nazem, Fernandez Genaro, Wilson Brent, Mcgann Christopher, Kholmovski Eugene, Marrouche Nassir. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2013 Oct;24 (10):1104–9. doi: 10.1111/jce.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrouche Nassir F, Wilber David, Hindricks Gerhard, Jais Pierre, Akoum Nazem, Marchlinski Francis, Kholmovski Eugene, Burgon Nathan, Hu Nan, Mont Lluis, Deneke Thomas, Duytschaever Mattias, Neumann Thomas, Mansour Moussa, Mahnkopf Christian, Herweg Bengt, Daoud Emile, Wissner Erik, Bansmann Paul, Brachmann Johannes. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014 Feb 05;311 (5):498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 29.Everett Thomas H, Olgin Jeffrey E. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007 Mar;4 (3 Suppl):S24–7. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burstein Brett, Nattel Stanley. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 2008 Feb 26;51 (8):802–9. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 31.de Jong Sanne, van Veen Toon A B, van Rijen Harold V M, de Bakker Jacques M T. Fibrosis and cardiac arrhythmias. J. Cardiovasc. Pharmacol. 2011 Jun;57 (6):630–8. doi: 10.1097/FJC.0b013e318207a35f. [DOI] [PubMed] [Google Scholar]
- 32.Benito Eva M, Carlosena-Remirez Alicia, Guasch Eduard, Prat-González Susana, Perea Rosario J, Figueras Rosa, Borràs Roger, Andreu David, Arbelo Elena, Tolosana J Maria, Bisbal Felipe, Brugada Josep, Berruezo Antonio, Mont Lluis. Left atrial fibrosis quantification by late gadolinium-enhanced magnetic resonance: a new method to standardize the thresholds for reproducibility. Europace. 2017 Aug 01;19 (8):1272–1279. doi: 10.1093/europace/euw219. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Hui, Wang Weizong, Wang Cong, Xie Xinxing, Hou Yinglong. Association of pre-ablation level of potential blood markers with atrial fibrillation recurrence after catheter ablation: a meta-analysis. Europace. 2017 Mar 01;19 (3):392–400. doi: 10.1093/europace/euw088. [DOI] [PubMed] [Google Scholar]


