Abstract
Background:
We sought to investigate and compare the safety and efficacy of two commonly used antiarrhythmic drugs, Dofetilide (DF) and Sotalol (SL), during inpatient drug initiation in patients with symptomatic atrial fibrillation (AF).
Methods:
We performed a single center retrospective study of consecutive patients, admitted for initiation of either DF or SL, for AF between 2012 and 2015. Rates of successful cardioversion, QT interval prolongation, adverse events and drug discontinuations were calculated and compared. A two-tailed p value less than 0.05 was considered statistically significant.
Results:
Of 378 patients, 298 (78.8%) received DF and 80 (21.2%) SL, mean age was 64 ± 11 years, 90% were Caucasians and 66% were males. Among the patients who remained in AF upon admission (DF: 215/298 (72%) vs. SL: 48/80 (60%)), no significant differences were noted in pharmacological cardioversion rates (DF: 125/215(58%) vs. SL: 30/48 (62.5%); p = 0.58). Baseline QTc was similar between the groups, with higher dose dependent QTc prolongation with DF (472.25± 31.3 vs. 458± 27.03; p = 0.008). There were no significant differences in the rates of adverse events such as bradycardia (7.4% vs. 11.3%; p = 0.26), Torsades de pointes (1.3% vs. 1.2%; p = 1.00), and drug discontinuation (9.0% vs. 5.0%; p = 0.47) between the two groups.
Keywords: Atrial fibrillation, Antiarrhythmic Drug, Normal sinus rhythm, QT interval corrected for heart rate, Dofetilide, Sotalol
Introduction
Advances in catheter ablation have revolutionized the management of symptomatic atrial fibrillation (AF). Despite current progress in ablation strategies, its invasive nature and risk of rare but life-threatening complications limits its use to patients with drug refractory symptomatic AF. Antiarrhythmic drugs (AAD) are proven to be more effective than placebo in conversion and maintenance of normal sinus rhythm (NSR) in AF[1]. They also alleviate symptoms, facilitate electrical cardioversion and decrease the risk of recurrence of AF [2][4]. Class I AADs though effective, have high proarrhythmic potential in patients with structural heart disease [5],[6]. Amiodarone is the most effective AAD, but its use is limited by a myriad of non-cardiac side effects [7],[10]. Dofetilide (DF) and Sotalol (SL) on contrary have minimal non-cardiac side effects. DF, a class III antiarrhythmic agent, acts by blocking rapid component of delayed rectifier potassium current (IKr). It increases action potential duration due to delayed repolarization and prolongs QT interval because of prolongation of effective and functional refractory period of the His-Purkinje system and the ventricles. Sotalol on the other hand exhibits both class II and class III antiarrhythmic effects by non-selective beta blockade and blocking rapid component of delayed rectifier potassium channels respectively. It shows class II properties at relatively low doses (as low as 25mg/day) and class III effects at higher doses (>160mg/day).
In view of QT prolongation and proarrhythmogenic potential of Dofetilide 2014 ACC/AHA guidelines recommend inpatient drug initiation with close electrocardiographic monitoring [11]. Earlier recommendations for Sotalol loading favored inpatient initiation if risk factors for arrhythmias were present[2]. Newer guidelines however are not quite clear on this [11]. As a result of lack of data supporting the safety of outpatient initiation of Sotalol some experts prefer inpatient initiation with electrocardiographic monitoring.
Currently there is limited data directly comparing these two agents in AF populations. We sought to investigate the safety and efficacy of inpatient initiation of Dofetilide compared to Sotalol for symptomatic AF.
METHODS:
Study Population
We retrospectively screened consecutive patients with symptomatic AF who were admitted to our institution for initiation of Dofetilide or Sotalol for rhythm control from 01/2012 to 12/2015. Patients were included if they underwent initiation of oral formulation of either Dofetilide or Sotalol, had an electrocardiogram at baseline and 2-3 hours after each dose of AAD. Patients with cardiac implantable electronic device (CIED), prior bradyarrhythmias, prior ventricular arrhythmias requiring AAD, and atrial arrhythmias who failed either prior AAD or catheter ablation were excluded from the final analysis.
Dosing:
Patients were dosed according to University of Kansas Medical Center protocol for inpatient initiation of Dofetilide and Sotalol. Patients CrCl and QTc (or QT interval if heart rate is <60 beats/minute) were checked ≤24 hours prior to first dose. If baseline QTc is >480 msec (>500 msec in patients with ventricular conduction abnormalities) drugs were contraindicated. If baseline QTc is between 440- 480 msec, physician was notified and medication was dispensed based on his/her discretion.
Dofetilide (Tikosyn®):
Patients with QTc <440 msec, CrCl > 60 mL/min were started on Dofetilide 500mcg BID. For patients with CrCl between 40 mL/min - 60 mL/min and 20 mL/min - 39 mL/min, initial Dofetilide dose was reduced to 250 mcg BID and 125 mcg BID respectively. If CrCl < 20 ml/min, Dofetilide was contraindicated. A 12 lead EKG was repeated 2-3 hrs after each dose. If QTc increases to more than 15% above baseline QTc or if the QTc is >500 msec (>550 msec in patients with ventricular conduction abnormalities), Dofetilide dose was reduced by 50%. If QTc is still excessively prolonged after one dose reduction, Dofetilide therapy was discontinued. Telemetry monitoring was continued for a minimum of three days (at least 5 doses) or 12 hours after conversion to normal sinus rhythm, whichever is longer.
Sotalol (Betapace®):
Patients with QTc <450, CrCl > 60 were started on Sotalol 80 mg twice daily. If the initial dose does not reduce frequency or relapse of AF and excessive QTc prolongation did not occur after 3 days, the dose was increased to 120 mg twice daily; may further increase to a maximum dose of 160 mg twice daily if response is inadequate and QTc prolongation is not excessive. If CrCl 40-59 ml/min dosing is changed to every 24hrs. If CrCl<40 ml/min, Sotalol is contraindicated. A 12 lead EKG was repeated 2-3 hrs after each dose. If the QTc increases to more than 15% above baseline or if the QTc is >500 msec (>550 msec in patients with ventricular conduction abnormalities), Sotalol dose was reduced to the next dosage down. Telemetry was continued for a minimum of three days (at least 5 doses).
Study Outcomes
The primary safety endpoints of the study include QTc Interval prolongation with each dose, rates of adverse events (Bradycardia, Sinus node arrest (SNA), High grade AV block (AVB), New onset premature ventricular contractions (PVCs), Nonsustained ventricular tachycardia (NSVT), Torsades de pointes (TdP), Ventricular fibrillation (VF)) and drug discontinuation during hospitalization.
Bradycardia was defined as ventricular rate less than 60 beats per minute (bpm) while awake, SNA was defined as sinus pauses of 2 seconds or more while awake, High grade AVB was defined as either Mobitz type 2 second degree AVB or third degree AVB (complete heart block). Indication for drug discontinuation was left to discretion of the treating physician and is usually due to QTc interval exceeding 500 milliseconds despite dosage adjustments, significant bradyarrhythmias either resulting in symptoms or requiring pacemaker, or new onset ventricular arrhythmias as detailed above.
The primary efficacy end point of the study was rate of chemical cardioversion, defined as conversion of underlying AF to NSR after at least two doses of AAD.
Data measurements
Baseline demographics; medical history; medication use, renal function, baseline potassium, magnesium levels, left ventricle ejection fraction (LVEF) and left atrial size on 2D transthoracic echocardiography were obtained from the electronic medical records. Data on QT interval prolongation, adverse events as detailed above, and successful chemical cardioversion were obtained from the serial electrocardiograms performed during hospitalization.
Statistical Analysis
Continuous variables are expressed as mean ±standard deviation (SD) if variables are normally distributed, and median (interquartile range) when deviations from normality were present. Categorical variables are expressed as counts and percentages. Categorical variables were compared between the groups using chi-squared test or fisher's exact test. Continuous variables were compared using independent sample t test. A two tailed p value less than 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics version 23.0 (IBM, Armonk, New York).
RESULTS:
Out of 378 patients, 298 (78.8%) received Dofetilide, and 80 (21.2%) received Sotalol. The cohort had mean age of 64 ± 11 years; 90% were Caucasians; 66% were males; 61.6% had paroxysmal AF and 41.1% were on AV nodal blockers for rate control. Patients who received Sotalol had higher proportion of patients with coronary artery disease (DF vs. SL: 31.2% vs. 51.2%; p = 0.001), ACE inhibitor/ARB use (DF vs. SL: 55.5% vs. 70%; p = 0.01) and NSR up on admission (DF vs. SL: 27.8% vs. 40%; p = 0.03). No other significant differences were observed in the baseline characteristics between the two groups [Table 1].
Table 1. Baseline Characteristics between Dofetilide and Sotalol groups (n = 378).
Data were represented as mean ± standard deviation or counts (percentage)
| Characteristics | Dofetilide (n = 298) | Sotalol (n = 80) | p value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 64 ± 10.3 | 64 ± 11.6 | 0.46 |
| Gender: Male (%) | 205 (68.8%) | 46 (57.5%) | 0.06 |
| Race: Caucasian (%) | 270 (90.6%) | 71 (88.7%) | 0.14 |
| Body Mass Index (BMI) | 33 ± 7.4 | 33 ± 7.8 | 0.90 |
| Co morbidities | |||
| Paroxysmal AF | 183 (61.4%) | 50 (62.5%) | 0.22 |
| Hypertension | 252 (84.5%) | 67 (83.7%) | 1.00 |
| Diabetes mellitus | 63 (21.1%) | 20 (25%) | 0.45 |
| Hyperlipidemia | 218 (73.1%) | 66 (82.5%) | 0.08 |
| Coronary artery disease | 93 (31.2%) | 41 (51.2%) | 0.001 |
| Stroke/Transient ischemic attack | 25 (8.4%) | 10 (12.5%) | 0.10 |
| Medication | |||
| Beta Blockers | 89 (29.0%) | 28 (35.0%) | 0.57 |
| Calcium Channel Blocker | 165 (55.5%) | 56 (70.0%) | 0.01 |
| ACE Inhibitor/ARB | 89 (29.0%) | 28 (35.0%) | 0.57 |
| Labs | |||
| Creatinine Clearance (ml/min) | 105.2 ± 41.2 | 102.3 ± 37.4 | 0.58 |
| Baseline Potassium level (mEq/L) | 4.12 ± 0.41 | 4.17 ± 0.36 | 0.90 |
| Baseline Magnesium level (mEq/L) | 2.11 ± 1.1 | 2.0 ± 0.18 | 0.38 |
| EKG/Echo | |||
| Sinus rhythm on arrival | 83 (27.8%) | 32 (40%) | 0.03 |
| Baseline QTc (msec) | 448.9 ± 33.2 | 444 ± 42.6 | 0.35 |
| Left Atrial Size (cm) | 4.53 ± 0.8 | 5.45 ± 0.60 | 0.73 |
| Left ventricle Ejection Fraction (%) | 51.7 ± 12 | 52.5 ± 12.6 | 0.65 |
Dosing
Of the 298 patients who received DF, 200 were on a stable dose during admission. Of these, 158 patients received 500mcg BID, 40 received 250mcg BID and 2 received 125mcg BID. Of the 80 patients on Sotalol, 49 were on a stable dose. Of these 30 patients received 120mg BID, 15 received 80mg BID and 4 received 160mg BID.
Safety
Mean baseline QTc intervals were similar between the groups (DF vs. SL: 448.9 ±33.2 vs. 444± 42.6 msec; p = 0.35). Dose dependent QTc prolongation was noted in both the groups but was significantly higher in Dofetilide compared to Sotalol with each dose and at discharge (DF vs. SL: 472.25± 31.3 vs. 458± 27.03 msec; p = 0.008) ( [Table 2], [Figure 1]).
Table 2. Safety outcomes between Dofetilide and Sotalol groups - QTc Intervals (n = 378).
Data were represented as mean ± standard deviation
| QTc Measurement (msec) | Dofetilide (n = 298) | Sotalol (n = 80) | p value |
|---|---|---|---|
| Baseline QTc | 448.9 ± 33.2 | 444 ± 42.6 | 0.35 |
| QTc Post 1st dose | 464.5 ± 43.1 | 453 ± 32.6 | 0.03 |
| QTc Post 2nd dose | 476.7 ± 37.2 | 476.7 ± 37.2 | 0.02 |
| QTc Post 3rd dose | 474.2 ± 34.8 | 463.5 ± 34 | 0.02 |
| QTc Post 4th dose | 473.5 ± 32.5 | 450 ± 64.8 | 0.01 |
| QTc Post 5th dose | 472.25 ± 31.3 | 458 ± 27.03 | 0.008 |
Figure 1. Dose dependent QTc prolongation associated with Dofetilide and Sotalol (n = 378).
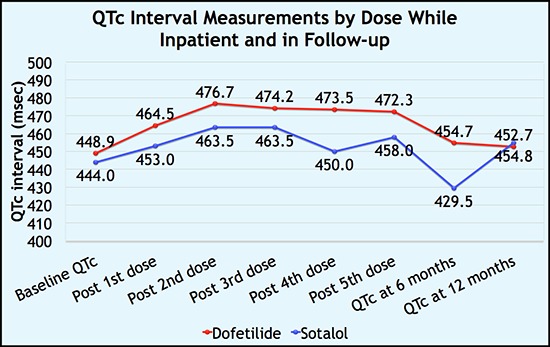
Despite higher QTc prolongation with Dofetilide, there were no statistically significant differences, in the rates of adverse events between the groups during the hospitalization (DF vs. SL: bradycardia – 7.4% vs. 11.3%; p = 0.26, NSVT - 2.3% vs. 1.25%; p = 1.0, TdP - 1.3% vs. 1.2%; p = 1.00) [Table 3].
Table 3. Safety outcomes between Dofetilide and Sotalol groups - Adverse events (n = 378).
Data were represented as counts (percentage)
| Adverse events | Dofetilide (n = 298) | Sotalol (n = 80) | p value |
|---|---|---|---|
| Bradyarrhythmias | |||
| Bradycardia (HR < 60) | 22 (7.4%) | 9 (11.3%) | 0.26 |
| Sinus pause > 2 secs | 0 (0%) | 0 (0%) | - |
| High grade AVB | 6 (2.0%) | 2 (2.5%) | 0.67 |
| Ventricular arrhythmias | |||
| New onset PVCs | 0 (0%)) | 0 (0%) | - |
| Non Sustained VT | 7 (2.3%) | 1 (1.3%) | 1.00 |
| Torsades de pointes (TdP) | 4 (1.3%) | 1 (1.2%) | 1.00 |
| Ventricular fibrillation (VF) | 0 (0%) | 0 (0%) | - |
| Drug Discontinuation | 24 (8.8%) | 4 (5%) | 0.47 |
Overall 28/378 (7.4%) patients had drug discontinuation prior to discharge because of various side effects with most common reason being QT interval exceeding 500 msec. There was no statistically significant difference in the drug discontinuation rates between the groups, (DF vs. SL: 24/298 (9.0%) vs. 4/80 (5.0%); p = 0.47).
Efficacy
In terms of efficacy, among patients who remained in AF upon admission (215/298 (72%) - Dofetilide group; 48/80 (60%) - Sotalol group), no significant differences were noted in pharmacological cardioversion rates between the groups (Dofetilide - 125/215(58%) vs. Sotalol - 30/48 (62.5%); p = 0.58, [Table 4]). Patients who failed pharmacological cardioversion underwent electrical cardioversion subsequently.
Table 4. Efficacy outcomes between Dofetilide and Sotalol groups (n = 263).
Data were represented as counts (percentage)
| Successful Cardioversion | Dofetilide | Sotalol (n = 48) | p value |
|---|---|---|---|
| (n = 215) | |||
| Pharmacological cardioversion (%) | 125 (58%) | 30 (62.5%) | 0.589 |
| Requiring DC cardioversion (%) | 90 (42%) | 18 (37.5%) | 0.578 |
DISCUSSION:
Main findings
We studied the safety and efficacy of inpatient initiation of Dofetilide (DF) compared to Sotalol (SL) in patients with symptomatic AF. Among the patients who remained in AF upon admission, no significant differences were noted in pharmacological cardioversion rates between the two drugs. Baseline QTc was similar between the groups, with higher dose dependent QTc prolongation with DF. There were no significant differences in the rates of adverse events such as bradycardia, Torsades de pointes, and drug discontinuation between the groups.
All AADs are proarrhythmogenic. The most severe pro-arrhythmia comes from drugs that prolong action potential duration. Both Dofetilide and Sotalol by the virtue of their potassium current (IKr) blockade, cause prolongation of action potential duration and effective refractory period, resulting in concentration dependent QT interval prolongation in order to be effective. This is suspected to be high with Sotalol compared to Dofetilide because of its inherent beta blockade properties resulting in simultaneous bradycardia while QT prolongation. In the presence of appropriate substrate (hypokalemia, hypomagnesaemia, bradycardia or genetic predisposition) this QT prolongation may predispose to torsades de pointes. The risk of life threatening ventricular arrhythmias warrants inpatient initiation of both Dofetilide and Sotalol with electrocardiographic monitoring.
At the outset, current practice patterns at our institution reflected a predominant use of Dofetilide (78.8%) over Sotalol (21.2%). In part, this is because of safety data supporting the use of DF in chronic heart failure (CHF). Moreover, In DIAMOND sub study, Dofetilide was found to improve survival in patients with AF and LV dysfunction when sinus rhythm was restored and maintained [12]. Safety and efficacy of Sotalol has not been systematically evaluated in CHF patients, but its use has been associated with a reduction in the risk of death and shocks in patients with implantable cardioverter defibrillators (ICDs), many of whom have LV systolic dysfunction [13].
In our study, 70 %( 215) of patients in the Dofetilide group and 60 %( 48) in the Sotalol group had AF on admission. Of these patients, there was no statistically significant difference in the pharmacological conversion rates between the two groups (Dofetilide- 125/215(58%) vs. Sotalol - 30/48 (62.5%)). Our study contradicts earlier reports which suggest Sotalol is not effective for chemical cardioversion and thus not indicated for this purpose[14]. This discrepancy may be because of the large proportion (approximately 60%) of our study population with paroxysmal AF. There is a possibility that these patients might have spontaneously converted to sinus rhythm.
Our primary safety endpoint was occurrence of adverse events like QT prolongation, bradyarrhythmias and tachyarrhythmias. We did not observe significant differences in the rates of overall adverse events or bradyarrhythmias/tachyarrhythmias individually between the groups. Dosage specific outcome stratification was not relevant as dosages were selected based on calculated creatinine clearance. Serum drug levels would have been the same between groups if adjusted for renal function. In a similar study by Agusala et al, there was no difference in overall adverse events between Sotalol and Dofetilide groups however, DF group had more QT prolongation and ventricular arrhythmias. They also reported higher baseline QTc as an independent predictor of adverse events in patients treated with Dofetilide[15].
We reported dose dependent QTc prolongation in both groups but was significantly higher in Dofetilide compared to Sotalol. Despite Dofetilide being associated with higher QTc prolongation it did not translate into an increase in risk of ventricular arrhythmias including TdP. This observation could have been because of the way both medications are dosed. Dofetilide is usually started at a higher dose and titrated down if patient develops adverse effects. In case of Sotalol, patients are started at a lower dose and then titrated up if tolerating the dose well. Moreover, Sotalol’s class III effects which causes the QT prolongation is only seen with doses ≥160mg.
Future directions
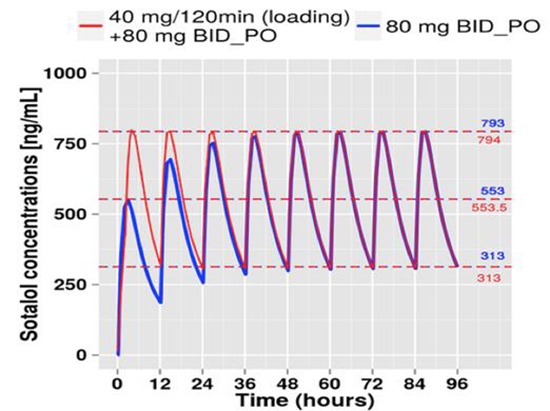
As these two drugs clearly cause dose dependent QTc prolongation, in-hospital monitoring is required to avoid rare but life-threatening arrhythmias. This incurs a high cost burden of nearly 3,400 $ per patient and the major brunt of this cost is due to hospital room and overhead costs, while the pharmacy costs account for only $200-$230 per patient[16]. Attempts to identify low risk population that can safely undergo outpatient initiation of AAD were unsuccessful so far [15]. Unlike Dofetilide, Sotalol is available in both oral and intravenous formulation and the overall risk of torsades in patients treated with a single infusion of IV Sotalol is low compared with that of patients given chronic oral Sotalol therapy [17]. A consideration would be to use IV Sotalol loading followed by transition to PO medication thereby considerably reducing the length of hospitalization. The center for translational medicine at the University of Maryland, Baltimore, USA developed a dosing strategy for patients with postoperative AF based on body weight with 40 mg IV Sotalol infusion for 2 hours followed by 80 mg PO bid immediately after the infusion. With this strategy, the target dose was achieved in less than 6 hours vs. ~36 hours in standard oral dose (80 mg bid) [Figure 2]. Development of a standard intravenous to oral switch regimen for inpatient initiation of Sotalol for symptomatic AF, to reach target maintenance dose faster may cut down the cost associated with the Sotalol initiation and expedite discharge on a stable oral regimen.
Figure 2. Intravenous versus oral Sotalol loading for postoperative atrial fibrillation. Notice that the target dose was achieved in less than 6 hours with intravenous regimen compared with ~36 hours in standard oral regimen (80 mg po bid) (Courtesy: Center for translational medicine - University of Maryland Baltimore, USA.).
Limitations
Our study presents the obvious limitations of a retrospective observational study. Predetermined selection criteria were not used prior to initiation of either Dofetilide or Sotalol thus leading to the possibility of selection bias. Specific drug titration and discontinuation parameters could not be enforced due to the retrospective nature of the study. Despite higher proportion of patients receiving Dofetilide at our institution, we did not notice any statistically significant differences in the safety and efficacy profiles of Dofetilide and Sotalol.
CONCLUSION:
In our large, single center experience, we did not observe significant differences in the safety or efficacy between inpatient initiations of Dofetilide and Sotalol in AF patients. The use of Dofetilide resulted in significantly higher QTc when compared to that of Sotalol however, it did not translate to a difference in pharmacological cardioversion rates, tolerance, or adverse events.
References
- 1.Lafuente-Lafuente Carmelo, Mouly Stéphane, Longás-Tejero Miguel Angel, Mahé Isabelle, Bergmann Jean-François. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch. Intern. Med. 2006 Apr 10;166 (7):719–28. doi: 10.1001/archinte.166.7.719. [DOI] [PubMed] [Google Scholar]
- 2.Fuster Valentin, Rydén Lars E, Cannom David S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Le Heuzey Jean-Yves, Kay G Neal, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann Samuel, Smith Sidney C, Jacobs Alice K, Adams Cynthia D, Anderson Jeffery L, Antman Elliott M, Halperin Jonathan L, Hunt Sharon Ann, Nishimura Rick, Ornato Joseph P, Page Richard L, Riegel Barbara, Priori Silvia G, Blanc Jean-Jacques, Budaj Andrzej, Camm A John, Dean Veronica, Deckers Jaap W, Despres Catherine, Dickstein Kenneth, Lekakis John, McGregor Keith, Metra Marco, Morais Joao, Osterspey Ady, Tamargo Juan Luis, Zamorano José Luis. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006 Aug 15;114 (7):e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 3.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife José, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9 (4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Nattel Stanley, Opie Lionel H. Controversies in atrial fibrillation. Lancet. 2006 Jan 21;367 (9506):262–72. doi: 10.1016/S0140-6736(06)68037-9. [DOI] [PubMed] [Google Scholar]
- 5.Ruskin J N. The cardiac arrhythmia suppression trial (CAST). N. Engl. J. Med. 1989 Aug 10;321 (6):386–8. doi: 10.1056/NEJM198908103210608. [DOI] [PubMed] [Google Scholar]
- 6.Siebels J, Cappato R, Rüppel R, Schneider M A, Kuck K H. Preliminary results of the Cardiac Arrest Study Hamburg (CASH). CASH Investigators. Am. J. Cardiol. 1993 Nov 26;72 (16):109F–113F. doi: 10.1016/0002-9149(93)90973-g. [DOI] [PubMed] [Google Scholar]
- 7.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg M J, Green M, Kus T, Lambert J, Dubuc M, Gagné P, Nattel S, Thibault B. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N. Engl. J. Med. 2000 Mar 30;342 (13):913–20. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 8.Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J. Am. Coll. Cardiol. 2003 Jul 02;42 (1):20–9. doi: 10.1016/s0735-1097(03)00559-x. [DOI] [PubMed] [Google Scholar]
- 9.Singh Bramah N, Singh Steven N, Reda Domenic J, Tang X Charlene, Lopez Becky, Harris Crystal L, Fletcher Ross D, Sharma Satish C, Atwood J Edwin, Jacobson Alan K, Lewis H Daniel, Raisch Dennis W, Ezekowitz Michael D. Amiodarone versus sotalol for atrial fibrillation. N. Engl. J. Med. 2005 May 05;352 (18):1861–72. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 10.Jong Gwo-ping, Chang Mu-hsin, Chang Ting-chuan, Chou Pesus, Fu Chong-yau, Tien Li-yun, Chen Chung-yin, Ma Tso-chiang. Long-term efficacy and safety of very-low-dose amiodarone treatment for the maintenance of sinus rhythm in patients with chronic atrial fibrillation after successful direct-current cardioversion. Chin. Med. J. 2006 Dec 20;119 (24):2030–5. [PubMed] [Google Scholar]
- 11.January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cigarroa Joaquin E, Cleveland Joseph C, Conti Jamie B, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Murray Katherine T, Sacco Ralph L, Stevenson William G, Tchou Patrick J, Tracy Cynthia M, Yancy Clyde W. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014 Dec 02;64 (21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen O D, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001 Jul 17;104 (3):292–6. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 13.Pacifico A, Hohnloser S H, Williams J H, Tao B, Saksena S, Henry P D, Prystowsky E N. Prevention of implantable-defibrillator shocks by treatment with sotalol. d,l-Sotalol Implantable Cardioverter-Defibrillator Study Group. N. Engl. J. Med. 1999 Jun 17;340 (24):1855–62. doi: 10.1056/NEJM199906173402402. [DOI] [PubMed] [Google Scholar]
- 14.Slavik R S, Tisdale J E, Borzak S. Pharmacologic conversion of atrial fibrillation: a systematic review of available evidence. Prog Cardiovasc Dis. 2001 Sep 25;44 (2):121–52. doi: 10.1053/pcad.2001.26966. [DOI] [PubMed] [Google Scholar]
- 15.Agusala Kartik, Oesterle Adam, Kulkarni Chiraag, Caprio Timothy, Subacius Haris, Passman Rod. Risk prediction for adverse events during initiation of sotalol and dofetilide for the treatment of atrial fibrillation. Pacing Clin Electrophysiol. 2015 Apr;38 (4):490–8. doi: 10.1111/pace.12586. [DOI] [PubMed] [Google Scholar]
- 16.Kim Michael H, Klingman David, Lin Jay, Pathak Prathamesh, Battleman David S. Cost of hospital admission for antiarrhythmic drug initiation in atrial fibrillation. Ann Pharmacother. 2009 May;43 (5):840–8. doi: 10.1345/aph.1L698. [DOI] [PubMed] [Google Scholar]
- 17.Marill K A, Runge T. Meta-analysis of the Risk of Torsades de Pointes in patients treated with intravenous racemic sotalol. Acad Emerg Med. 2001 Feb;8 (2):117–24. doi: 10.1111/j.1553-2712.2001.tb01275.x. [DOI] [PubMed] [Google Scholar]


