Abstract
The presence of aflatoxin, a carcinogenic and toxigenic secondary metabolite produced by Aspergillus species, in food matrix has been a major worldwide problem for years now. Food processing methods such as roasting, extrusion, etc. have been employed for effective destruction of aflatoxins, which are known for their thermo-stable nature. The high temperature treatment, adversely affects the nutritive and other quality attributes of the food, leading to the necessity of application of non-thermal processing techniques such as ultrasonication, gamma irradiation, high pressure processing, pulsed electric field (PEF), etc. The present study was focused on analysing the efficacy of the PEF process in the reduction of the toxin content, which was subsequently quantified using HPLC. The process parameters of different pH model system (potato dextrose agar) artificially spiked with aflatoxin mix standard was optimized using the response surface methodology. The optimization of PEF process effects on the responses aflatoxin B1 and total aflatoxin reduction (%) by pH (4–10), pulse width (10–26 µs) and output voltage (20–65%), fitted 2FI model and quadratic model respectively. The response surface plots obtained for the processes were of saddle point type, with the absence of minimum or maximum response at the centre point. The implemented numerical optimization showed that the predicted and actual values were similar, proving the adequacy of the fitted models and also proved the possible application of PEF in toxin reduction.
Keywords: Potato dextrose agar (PDA), Aflatoxin (AFB1), Pulsed electric field (PEF), Response surface methodology (RSM), HPLC
Introduction
Aflatoxins (AFs) are the most toxic class of mycotoxins naturally produced by Aspergillus species (i.e. Aspergillus flavus and Aspergillus parasiticus, rarely by Aspergillus nomius) during pre or postharvest of different crops (Iqbal et al. 2012; Iqbal and Asi 2013). The structures of more than 18 different types of AFs have been identified (Bhat et al. 2010), but the most prevalent and toxic forms are B1 (AFB1), B2, G1 and G2 (Idris et al. 2010). Among these toxins, AFB1 was classified as a group I carcinogen by the International Agency for Research on Cancer (IARC 1993). The regulatory limit of 2 or 4 µg/kg for AFB1 and 4–20 µg/kg for total aflatoxin has been applied by most of the countries in wide range of products (FAO 2004).
Aflatoxins being the major crisis for years, the effect of various physical, chemical and biological methods on the detoxification of aflatoxin content has been studied (Magan and Aldred 2007; BozoÏlu 2009; Velazhahan et al. 2010, Jalili et al. 2010). Food processing such as sorting, cleaning, brewing, cooking, baking, frying, roasting, canning, flaking, nixtamalization, extrusion, etc., has an impact on mycotoxins destruction (BozoÏlu 2009). The aflatoxins known for their thermal stability generally decompose at higher temperatures (230–300 °C) (IARC 2002). Treatment at this higher range temperature would possibly affect the quality of the food product. Non-thermal processing techniques, newer alternative processing methods, performed at ambient or sub lethal temperatures, have been proved to induce minimal damage to nutritional and sensorial attributes while providing a microbialy safe product.
The non-thermal methods such as gamma irradiation, ozonation, high pressure processing, cold plasma, pulse light processing, etc., were also studied for their efficacy in the degradation of aflatoxin (BozoÏlu 2009). Pulsed electric field processing, one of the non-thermal processing method, has been proven to be an effective alternative to thermal processing (Kumar et al. 2015). The possible application of PEF on liquid foods has been well ascertained, wherein its application on solid matrix has become the current area of research with more prospective applications such as solid–liquid extraction (El Belghiti and Vorobiev 2004), mass transfer in plant and meat processing (Toepfl et al. 2006; Toepfl and Heinz 2010), for desired textural changes (Kumar et al. 2014) and for inhibition of fungal growth and toxin production in synthetic media and corn grains (Eisa et al. 2003). Though the effect of PEF on microbes has been established, their effects on toxins produced by the microbes are yet to be investigated.
Though, the PEF treatment effect on microbial toxin as such has not been studied, its application on the degradation of toxic pesticide compounds was established. Chen et al. (2009) and Zhang et al. (2012) reported that the successful degradation of pesticides spiked into apple juice. With the reports on successful application of degradation of toxic pesticides, the same kind of degradation effect can also be expected for the toxin produced by the microorganisms present in the food matrix like peanut, corns, etc.
Statistical design tools like RSM have been effectively used in optimizing the processing parameters, using the regression equations that describe the inter-relations between input parameters and product properties. The major constraint on optimizing procedures is that the desired degree of response must be achieved. The present study was focused on optimization of a PEF process using the RSM on the potato dextrose agar (PDA) as the model system to ensure its effectiveness towards the aflatoxin content reduction with minimal matrix interference. The synthetic growth media PDA was selected based on its ability to support Aspergillus growth and aflatoxin production (Horn and Dorner 2001; Abbas et al. 2004; Yousefi et al. 2009). The artificially spiked PDA of varying pH were subjected to treatment based on the experiments designed and were analysed for the reduction in AFB1 and total aflatoxin content.
Materials and methods
PDA (Himedia Pvt. Ltd., Nasik) was used as the model system for the analysis of the effect of the PEF treatment. The pH of the PDA was adjusted using 1 N HCl and 1 N NaOH (Zhang et al. 2014) with the help of the pH meter (Eutech Instruments, Singapore), which was calibrated using 4.00, 7.00 and 10.02 standard buffer solution.
The PEF treatments for the degradation of Aflatoxin content in the model system were optimized using the RSM. The PDA of varying pH was prepared and spiked with Aflatoxin mix standard (Sigma Aldrich), and then it was subjected to varying PEF process. The spiking concentration was fixed to a total aflatoxin concentration of 5.2 µg/kg, comprising of 2 µg/kg AFB1 and AFG1 and 0.6 µg/kg of AFB2 and AFG2 respectively, based on the regulatory limit of 2 µg/kg of AFB1 set by European Union (Commission of the European Communities 2006).
Pulsed electric field processing
PEF treatments were performed using a static batch PEF system (Model: ELCRACK® HVP 5, DIL, German Institute of Food Technologies, Quackenbruck, Germany) with bipolar square-wave pulses (Fig. 1). The maximum voltage was 80 kV, the maximum frequency was 1 kHz and the pulse width was adjustable between 4 and 32 µs. The system consisted of a batch cross field chamber followed by an AKG—cooling system (− 5 °C). The characteristics of the electric pulses delivered across the electrodes and the pulse frequency were monitored using a digital oscilloscope (Model: Digital touch screen oscilloscope Siemens, Made in Denmark). Temperatures were monitored by thermocouples (Testo AG, Lenzkirch, Germany) with a pipe wrap type probe attached to the surface of the treatment chamber. Recorded temperatures did not exceed 35 °C.
Fig. 1.
Schematic PEF—batch system
The PDA (of varying pH) sample of 300 g was spiked with aflatoxin mix standard to obtain a final concentration of 5.2 ppb and filled into the batch chamber of dimension 11 cm × 9 cm × 8 cm; which was further processed by varying the output voltage and pulse width according to the central composite rotatable design (CCRD) (Table 1). The processed samples were then filled in sterile (thermally) pre-fabricated multilayer laminated pouches consisting of 12 μm Polyethylene terephthalate/9 μm Aluminium foil/15 μm Nylon/80 μm cast. Polypropylene (total thickness 116 μm) pouches with a dimension of 15 × 20 cm under sterile conditions and hermetically sealed using impulse sealing machine (Model: HP Impulse Sealer, M/s Sunray Industries Mysore, India). The experiments were performed in triplicate.
Table 1.
Design of experiments for the PEF processing of spiked PDA
| Run order | pH | Pulse width (µs) | Output voltage (%) | Aflatoxin B1 (%) | Total aflatoxin (%) |
|---|---|---|---|---|---|
| 1 | 7.00 | 18.00 | 42.50 | 88.8681 | 86.6297 |
| 2 | 4.00 | 26.00 | 20.00 | 83.5831 | 82.0046 |
| 3 | 10.00 | 26.00 | 20.00 | 86.8422 | 87.2716 |
| 4 | 1.95 | 18.00 | 42.50 | 85.9023 | 86.2588 |
| 5 | 7.00 | 31.45 | 42.50 | 87.8315 | 86.8175 |
| 6 | 4.00 | 26.00 | 65.00 | 79.9717 | 77.4581 |
| 7 | 12.05 | 18.00 | 42.50 | 94.5815 | 94.9695 |
| 8 | 4.00 | 10.00 | 65.00 | 90.9803 | 88.8385 |
| 9 | 10.00 | 10.00 | 20.00 | 85.9898 | 90.1337 |
| 10 | 7.00 | 18.00 | 42.50 | 88.8681 | 86.6297 |
| 11 | 7.00 | 18.00 | 42.50 | 88.8681 | 86.6297 |
| 12 | 7.00 | 18.00 | 42.50 | 88.8681 | 86.6297 |
| 13 | 7.00 | 18.00 | 42.50 | 88.8681 | 86.6297 |
| 14 | 7.00 | 4.55 | 42.50 | 87.5531 | 82.9254 |
| 15 | 7.00 | 18.00 | 80.34 | 86.6791 | 83.9892 |
| 16 | 7.00 | 18.00 | 4.66 | 87.9155 | 84.3232 |
| 17 | 7.00 | 18.00 | 42.50 | 88.8681 | 86.6297 |
| 18 | 10.00 | 26.00 | 65.00 | 96.3973 | 97.2287 |
| 19 | 4.00 | 10.00 | 20.00 | 89.3436 | 85.3842 |
| 20 | 10.00 | 10.00 | 65.00 | 85.4908 | 89.3243 |
The experiments were conducted as one block of experiments (block 1)
Experimental design using RSM
A face-centered central composite response surface analysis was used to determine the effect of PEF processing on the aflatoxin B1 and total aflatoxin content in the spiked PDA samples. The independent variables were pH (4–10), pulse width (10–26 µs) and output voltage (20–65%) for PEF treatment. The experiments were conducted as one block of experiments. The order of assays within the block was randomised and performed in triplicates. Experimental data obtained were fitted into a polynomial response surface function. The second-order response function was predicted by the following Eq. (1):
| 1 |
where β0 was the value of the fitted response at the center point of the design, i.e., point (0, 0, 0) in case pH—pulse width—output voltage for PEF processing; βi, βii and βij were the linear, quadratic and cross product (interaction effect) regression terms respectively and n denoted the number of independent variables.
The factor, pH of PDA was varied from 4 to 10 as from literature it was observed that the pH had a great influence on the aflatoxin degradation and moreover low acid pH foods were also observed to be contaminated with aflatoxin. The factors, pulse width and output voltage, the PEF process parameters were fixed based on the instrument limitation.
Statistical analysis
Analysis of variance (ANOVA) was performed to obtain the coefficients of the final equation for better accuracy. Design Expert 7.1 software (Stat Ease Inc., Minneapolis, MN) was used to generate a second order polynomial models that fitted the experimental data, the response surface plots for the interaction effect and to optimize PEF treatment. In the present study, desirability functions were developed in order to obtain a maximum degradation of aflatoxin content in the PDA sample. All the variables of polynomial regression at a significance level of p < 0.05 were included in the model, and the coefficient of determination (R2) was generated in order to assess the adequacy of the model. The response surfaces were generated from the equations of the second order polynomial, using the values of each independent variable giving the maximum response (Montgomery 2001; Sin et al. 2006). Numerical optimization procedure was also implemented for the optimized variable levels by desirable maximization of the necessary response (Wadikar et al. 2010).
Aflatoxin analysis
Standard preparation
Aflatoxin mix standard of 2.6 ppm concentration (1 ppm of each B1 and G1 and 0.3 ppm of B2 and G2 respectively), was used for the study. The standard vial was diluted to a total concentration of 260 ppb with Methanol (Lichlorsov®, HPLC grade, Merck Specialities Pvt. Ltd., Mumbai).
Sample preparation
The samples extraction and clean-up procedure were adapted from the AflaCLEAN user manual (LCTech 2012), with minor modifications. The 25 ml of the binary solvent, methanol: water (80:20 v/v) was added to 25 g of sample, which was priorly homogenised with 2.5 g of NaCl (Himedia Pvt Ltd., Nasik) and kept in a rotary shaker (Orbitek, Scigenics Biotech Pvt. Ltd., Chennai) for 30 min at 190 rpm. The mixture was then centrifuged (Remi R23, Remi Elektrochnik Ltd. Vasai) at 3000 rpm for 10 min and filtered. The filtrate volume of 14 ml was made up to 100 ml with phosphate buffer saline (pH 7.3), which was again filtered with Whatmann filter paper no. 44 and 0.45 µm nylon syringe filter (Acrodisc, Waters).
The filtered extract was cleaned up using Aflaclean immunoaffinity column (LcTech, GmBH, Germany). A filtered extract of 25 ml was passed through a preconditioned immunoaffinity column and washed with one volume of milli-Q water before elution of the toxin. The toxin was then eluted using 1.5 ml Methanol, followed by 1.5 ml of water, which was then, filtered using a 0.2 µm PVDF syringe filter (Cole Parmer, Mumbai).
The eluted toxin was pre-derivatized using trifluoroacetic acid (Merck Specialities Pvt. Ltd., Mumbai), prior to injection into the HPLC system. Pre-derivatization was performed to increase the intensity of aflatoxin B1 and G1. The derivatized sample was made up to 1 ml using water: acetonitrile (9/1 v/v) as diluant (El-Desouky et al. 2012). A volume of 10 µl of this final derivatized and diluted sample was injected for HPLC analysis.
HPLC analysis
The aflatoxin content was quantified using reverse phase HPLC that comprises of a pump (Waters 515 HPLC pump), a pump controller (Water Pump Control Module II), a gradient mixer (600 Gradient mixer WAT051518) and a manual injector port (Rheodyne 7725i). The detector used for the purpose was a Multi-λ Fluorescence detector (Waters 2475) was used for the estimation under the following conditions: 360 nm excitation, 440 nm emission and EUFS 10000. The samples were analysed using a C18 analytical column i.e. Waters Spherisorb® 5 µm ODS2 of dimension 250 mm × 4.6 mm id, 5 µm particle size. All the HPLC analysis were performed under isocratic conditions using a mobile phase of water: methanol: acetonitrile (60/20/20 v/v/v) (Lichlorsolv® HPLC Grade Merck solvents), priorly filtered using 0.45 µm Nylon membrane filter (Pall Corporation, Waters) and degassed using a ultrasonic bath (Elmasonic P 120H, Elma-Hans Schmidbauer, GmbH & Co.KG, Germany) for 25 min, at a flow rate of 1.0 mL min−1 for a total run time of 30 min in ambient temperature condition.
Results and discussion
Prior to the optimization experimental analysis, the reverse phased HPLC system was standardized and calibrated with aflatoxin standard. The aflatoxin spiked PDA was subjected to various processing parameter combination as per the experimental design and then the respective samples were extracted and analysed for the reduction in concentration (AFB1 and total aflatoxin).
HPLC analysis: aflatoxin standardization and calibration
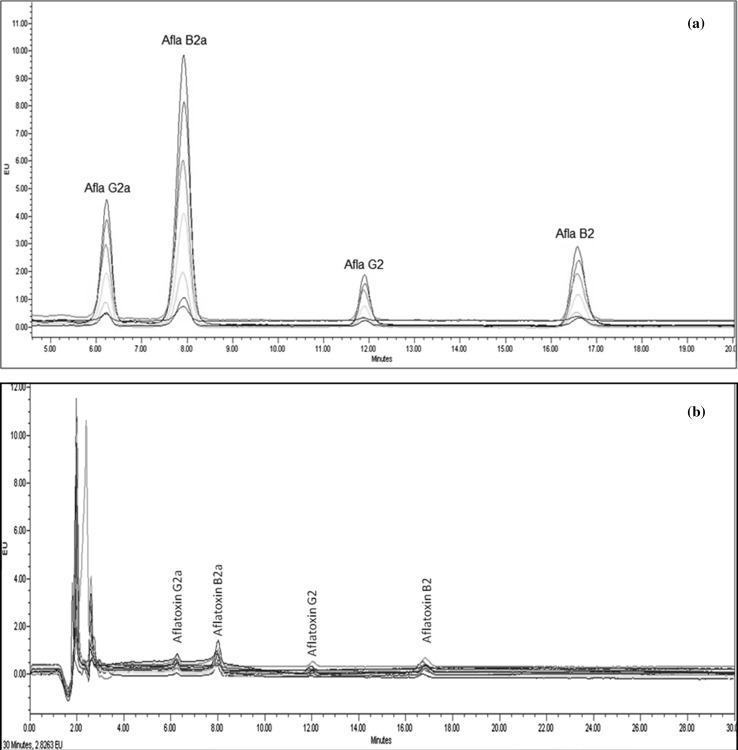
The Aflatoxin mix (27.049 ppm) standard was diluted with methanol to achieve a concentration of 27.49 ppb. The standard of varying concentration (0.13745, 0.2749, 0.5498, 1.0996, 1.6494, 2.1992 and 2.749 ppb) was prepared with Methanol: Water (1:1 v/v) solvent. The volume of 10 μl of each TFA derivatized standard was injected in the HPLC system and the respective chromatogram was obtained. Figure 2a represents the combined chromatogram of all the seven standards. The standard chromatograms were used for the calibration of each Aflatoxin (B1, B2, G1, and G2) individually. The R2 value for the calibration curve of Aflatoxin B1 (B2a), G1 (G2a), B2 and G2 was > 0.99.
Fig. 2.
HPLC chromatogram: a standard aflatoxin for calibration and b 15 experimental run samples
LOD and LOQ of each Aflatoxin were calculated using the S/n ratio method. LOD of Aflatoxin B1 (B2a), G1 (G2a), B2 and G2 were 0.005 (s/n = 3.9), 0.005 (s/n = 3.5), 0.008 (s/n = 3) and 0.008 (s/n = 3.6) respectively.
Reduction of aflatoxin content
The PEF batch operation process was performed with fixed process parameters such as pulse frequency (50 Hz), burst (10), energy (1 kJ) and for a time of 10 s. The optimization for the reduction of aflatoxin content was performed with PEF process parameters i.e., pulse width (µs) and output voltage (%) and pH of the PDA, as independent variables. The experiments were performed as per the design of experiments in triplicates and were analysed for its effect on the reduction in toxin content. The overlaid HPLC chromatograms of the 15 experimental runs were represented in Fig. 2b.
Effect of pH on aflatoxin reduction
The pH of the media played a major role in the reduction in the aflatoxin content. On optimization the range of pH used for analysis were 1.95–12.03 (− α and + α). The aflatoxin estimation of the samples revealed that, at acidic pH ≤ 4, there was a significant decrease in the toxin content. At the pH 7, though the toxin was reported to be stable, the decrease in content was evident due to the effect of the process parameters, but were in a closer range. The alkaline pH (10 and 12.03) of the sample resulted in the maximum degradation of the toxin. The results obtained for the effect of pH were in accordance with the results published by Méndez-Albores et al. (2013), significant reduction in the aflatoxin content in cocoa liquors, due to the thermal-alkaline treatment (up to 98%) and also stated that as the alkali concentration increased, lower aflatoxin values were registered. The study by Moreno-Pedraza et al. (2015), where two concentration of AFB1 in buffered solutions (pH of methanol adjusted with NaOH), showed that pH values around neutral were much less effective in destroying aflatoxins than more alkaline conditions. At pH 8.0 only 19.10% of AFB1 was reduced and 80% remained in the solution. However, at pH 11.8 and 12.5, the aflatoxin was reduced 90 and 100% respectively.
Effect of PEF process on aflatoxin content reduction
The reduction in AFB1 and total aflatoxin content was found to range between 77 and 97%, based on the different combination of the variables (pH, pulse width and output voltage). The polynomial regression equation for the AFB1 and total aflatoxin reduction was represented in Eqs. 2 and 3, in terms of coded factors [A—pH, B—pulse width (µs) and C—output voltage (%)], where the presence of both positive and negative signs indicate a saddle type of response.
| 2 |
| 3 |
The polynomial equation obtained for AFB1 reduction symbolised a 2FI model (Eq. 2) and that of the total aflatoxin reduction was of quadratic model (Eq. 3). The normal plot of residuals, drawn between the normal probabilities versus the studentized residual, showed that the 2FI and Quadratic model significantly fitted the effect of pH, pulse width and output voltage on the reduction of AFB1 and total aflatoxin respectively. The response surface plots obtained indicates that the system of contours was a saddle or minimax system, a hyperbolic system of contours, where, in this case the center is neither a maximum nor a minimum point.
The interaction 3D surface plots (Figs. 3a, 4a) for the effect of pH and pulse width on the reduction of AFB1 and total aflatoxin content, showed that the response followed a saddle type response were a maximum or minimum point was absent at the centre. From the plot, it was depicted that maximum reduction of aflatoxin (AFB1 and total aflatoxin) was obtained at a combination of minimum pulse width for an acidic pH and for PDA with alkaline pH processed at maximum pulse width.
Fig. 3.
Response surface plots for the PEF process on aflatoxin B1 reduction. a Effect of pH versus pulse width at constant output voltage of 42.5%, b effect of pH versus output voltage at constant pulse width 18 μs, c effect of pulse width versus output voltage at pH 7.00, d effect of pulse width versus output voltage at pH 4.00 and e effect of pulse width versus output voltage at pH 10.00
Fig. 4.
Response surface plots for the effect of PEF process on total aflatoxin reduction. a Effect of pH versus pulse width at constant output voltage of 42.5%, b effect of pH versus output voltage at constant pulse width 18 μs, c effect of pulse width versus output voltage at pH 7.00, d effect of pulse width versus output voltage at pH 4.00 and e effect of pulse width versus output voltage at pH 10.00
The increase in degradation of aflatoxin by the interaction effect of output voltage and pH, represented in the Figs. 3b and 4b, was a minimax type plot. The increase in pH from the acidic range to the alkaline range significantly increased the aflatoxin reduction percentage. The effect of output voltage was observed to have a different pattern when compared to pH. The maximum output voltage for a product at pH 10 had a maximum reduction in both AFB1 and total aflatoxin content, whereas for pH 4 product, the maximum reduction was depicted to be when treated with a minimum output voltage for AFB1 and at the mean value for total aflatoxin content.
The response surface plots for the variables pulse width and output voltage on the AFB1 reduction at varying pH (4, 7 and 10) were also of saddle type and were represented in Figs. 3c, d, e and 4c, d, e. At pH 7, maximum reduction of AFB1 content was observed at two regions, where a combination of pulse width and output voltage was both minimum and maximum respectively (Fig. 3c). Whereas, the maximum reduction of the total aflatoxin content was depicted to lie almost in the central region of the graph, but with a slight shift towards region of maximum pulse width and output voltage (Fig. 4c). At pH 4, it was observed that the increase in pulse width negatively affected both AFB1 and total aflatoxin reduction percentage and also the effects of output voltage variation were minimal (Figs. 3d, 4d). The maximum reduction region for a pH of 10 was observed to lie at the maximum pulse width and output voltage range, wherein the effect of pulse width is more evident than of output voltage in AFB1 reduction (Fig. 3e); unlike the total aflatoxin reduction, where both the variables have a significant effect (Fig. 4e).
ANOVA analysis
The multiple regression and statistical analysis for the effect of the variables on AFB1 was observed to follow a response surface 2FI model. The ANOVA analysis proved that the 2FI model fitted significantly with a p > F (0.0031) which was less than 0.05 (p < 0.05), R2 and adjusted R2 value of 0.7390 and 0.6185 respectively. Whereas, the ANOVA analysis for the effect on the total aflatoxin reduction percentage revealed to best fit the quadratic model significantly with p > F value 0.0197, i.e., p < 0.05 and with a maximum R2 (0.785) and the adjusted R2 (0.592) value. The R2 of predicted and observed values were in close agreement. The adequate precision values of both the responses (AFB1 and total aflatoxin were observed to greater than 4, which proved the model’s adequacy to navigate within the design space.
Numerical optimization
The numerical optimization, being one of the best techniques to minimize the time and effort involved in the optimization of multifactor and multi-response systems, was performed to find out the optimum combination processing conditions such as pulse width and output voltage for varying pH conditions. The optimization of pulse width and output voltage was implemented for the maximum reduction in AFB1 and total aflatoxin content (total AF). The Table 2 represents the predicted and the actual responses obtained for the analysis performed for the optimized process conditions. Since these values were almost similar, the fitted models were found to be suitable for predicting the response.
Table 2.
Numerical optimization: predicted and actual values for AFB1 and total aflatoxin (total AF) reduction
| pH | Pulse width (µs) | Output voltage (%) | AFB1 (%) | Total AF (%) | ||
|---|---|---|---|---|---|---|
| Predicted | Actual | Predicted | Actual | |||
| 4 | 20 | 10 | 91.763 | 91.928 | 87.436 | 83.374 |
| 7 | 51 | 26 | 89.532 | 92.248 | 87.667 | 85.906 |
| 10 | 65 | 26 | 95.553 | 94.879 | 94.646 | 90.303 |
Conclusion
The study for the optimization of the pulse electric field process proved that the treatments were suitable for the reduction of aflatoxin B1 and total aflatoxin content in potato dextrose agar. The major effect of reduction was due to the influence of the pH of the model system when compared to the process parameters. The PEF optimization revealed that the effect of the factors on the response aflatoxin B1 and total aflatoxin reduction fitted the 2FI polynomial model and quadratic model respectively. As it was stated by Rustom et al. (1993), the rate of degradation of aflatoxin increases with an increase in moisture content of heated food, the potato dextrose agar, used as a model system for the study was of high moisture content. Hence when the optimized parameters were adapted to the real food matrix the degradation percentage of toxin may vary with its moisture content.
Acknowledgements
SV greatly acknowledges University Grants Commission (UGC), Govt of India for awarding Senior Research Fellowship (SRF). The authors also express deep gratitude to The Director, Defence Food Research Laboratory, Mysore, for his constant support and encouragement.
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported by the authors.
References
- Abbas HK, Zablotowicz RM, Weaver MA, Horn BW, Xie W, Shier WT. Comparison of cultural and analytical methods for determination of aflatoxin production by Mississippi Delta Aspergillus isolates. Can J Microbiol. 2004;50:193–199. doi: 10.1139/w04-006. [DOI] [PubMed] [Google Scholar]
- Bhat R, Rai RV, Karim AA. Mycotoxins in food and feed: present status and future concerns. Compr Rev Food Sci Food Saf. 2010;9:57–81. doi: 10.1111/j.1541-4337.2009.00094.x. [DOI] [PubMed] [Google Scholar]
- BozoÏlu F. Different mycotoxin inactivation applications and their inactivation mechanisms. Proc Nat Sci Matica Srpska Novi Sad. 2009;117:27–35. doi: 10.2298/ZMSPN0917027B. [DOI] [Google Scholar]
- Chen F, Zeng L, Zhang Y, Liao X, Ge Y, Hu X, Jiang L. Degradation behaviour of methamidophos and chlorpyrifos in apple juice treated with pulsed electric fields. Food Chem. 2009;112:956–961. doi: 10.1016/j.foodchem.2008.07.016. [DOI] [Google Scholar]
- Commission of the European Communities Commission regulation (EC) no. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union. 2006;L364:5–24. [Google Scholar]
- Eisa NA, Ali FM, El-Habbaa GM, Abdel-Reheem SK, Abou-El-Ella MF. Pulsed electric field technology for checking aflatoxin production in cultures and corn grains. Egypt J Phytopathol. 2003;31(1–2):75–86. [Google Scholar]
- El Belghiti K, Vorobiev E. Mass transfer of sugar from beets enhanced by pulsed electric field. Food Bioprod Process. 2004;82(3):226–230. doi: 10.1205/fbio.82.3.226.44187. [DOI] [Google Scholar]
- El-Desouky TA, Sharoba AMA, El-Desouky AI, El-Mansy HA, Naguib K. Effect of ozone gas on degradation of aflatoxin B1 and Aspergillus Flavus fungal. J Environ Anal Toxicol. 2012;2:128. [Google Scholar]
- FAO . Worldwide regulations for mycotoxins in food and feed in 2003. Rome: Food and Agriculture Organization of the United Nations; 2004. [Google Scholar]
- Horn BW, Dorner JW. Effect of competition and adverse culture conditions on aflatoxin production by Aspergillus flavus through successive generations. Mycologia. 2001;94(5):741–751. doi: 10.1080/15572536.2003.11833167. [DOI] [PubMed] [Google Scholar]
- Idris YMA, Mariod AA, Elnour IA, Mohamed AA. Determination of aflatoxin levels in Sudanese edible oils. Food Chem Toxicol. 2010;48:2539–2541. doi: 10.1016/j.fct.2010.05.021. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) (1993) Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. Monographs on the evaluation of carcinogenic risks of chemicals to humans World Health Organization, vol 56, pp 245–540
- International Agency for Research on Cancer (IARC) (2002) Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC monographs on the evaluation of carcinogenic risks to humans, vol 82, p 174 [PMC free article] [PubMed]
- Iqbal SZ, Asi MR. Assessment of aflatoxin M1 in milk and milk products from Punjab. Pak Food Control. 2013;30:235–239. doi: 10.1016/j.foodcont.2012.06.026. [DOI] [Google Scholar]
- Iqbal SZ, Asi MR, Arino A, Akram N, Zuber M. Aflatoxin contamination in different fractions of rice from Pakistan and estimation of dietary intakes. Mycotoxin Res. 2012;28:175–180. doi: 10.1007/s12550-012-0131-1. [DOI] [PubMed] [Google Scholar]
- Jalili M, Jinap S, Noranizan A. Effect of gamma radiation on reduction of mycotoxins in black pepper. Food Control. 2010;21:1388–1393. doi: 10.1016/j.foodcont.2010.04.012. [DOI] [Google Scholar]
- Kumar R, Vijayalakshmi S, Kathiravan T, Nadanasabapathi S. Effect of thermal and pulsed electric field (PEF) processing on structural changes in food matrix. Indian Food Industry. 2014;33(4):31–37. [Google Scholar]
- Kumar R, Bawa AS, Kathiravan T, Nadanasabapathi S. Optimization of pulsed electric fields parameters of mango nectar processing using response surface methodology. Int Food Res J. 2015;22(4):1353–1360. [Google Scholar]
- LCTech (2012) AflaCLEAN user manual, LCTech sample preparation and analysis. LCTech GmbH, Dorfen (Germany), pp 1–32
- Magan N, Aldred D. Post-harvest control strategies: minimizing mycotoxins in the food chain. Int J Food Microbiol. 2007;119:131–139. doi: 10.1016/j.ijfoodmicro.2007.07.034. [DOI] [PubMed] [Google Scholar]
- Méndez-Albores A, Campos-Aguilar AZ, Moreno-Martínez E, Vázquez- Durán A. Physical and chemical degradation of B-aflatoxins during the roasting and dutching of cocoa liquor. J Agric Sci Technol. 2013;15:557–567. [Google Scholar]
- Montgomery DC. Design and analysis of experiments. New York: Wiley; 2001. [Google Scholar]
- Moreno-Pedraza A, Valdés-Santiago L, Hernández-Valadez LJ, Rodríguez-Sixtos HA, Winkler R, Guzmán-de Peña DL (2015) Reduction of aflatoxin B1 during tortilla production and the identification of degradation by-products by direct-injection electrospray mass spectrometry (DIESI-MS). Salud Publica Mex 57:50–57. http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S003636342015000100008&lng=en&nrm=iso&tlng=en [DOI] [PubMed]
- Rustom IYS, Lopez-Leiva MH, Nair BM. Effect of pH and heat treatment on the mutagenic activity of peanut beverage contaminated with aflatoxin B1. Food Chem. 1993;46:37–42. doi: 10.1016/0308-8146(93)90072-N. [DOI] [Google Scholar]
- Sin HN, Yusof S, Abdul HNS, Rahman AR. Optimization of hot water extraction for sapodilla juice using response surface methodology. J Food Eng. 2006;74:352–358. doi: 10.1016/j.jfoodeng.2005.03.005. [DOI] [Google Scholar]
- Toepfl S, Heinz V (2010) Role for pulsed electric fields. Industry innovation, The World of Food Ingredients, p 65
- Toepfl S, Heinz V, Knorr D (2006) Pulsed electric fields (PEF) processing of meat. IUFOST2006/591
- Velazhahan R, Vijayanandraj S, Vijayasamundeeswari A, Paranidharan V, Samiyappan R, Iwamoto T, Friebe B, Muthukrishnan S. Detoxification of aflatoxins by seed extracts of the medicinal plant, Trachyspermum ammi (L.) Sprague ex Turrill—structural analysis and biological toxicity of degradation product of aflatoxin G1. Food Control. 2010;21:719–725. doi: 10.1016/j.foodcont.2009.10.014. [DOI] [Google Scholar]
- Wadikar DD, Nanjappa C, Premavalli KS, Bawa AS. Development of ginger based ready-to-eat appetizers by response surface methodology. Appetite. 2010;55:76–83. doi: 10.1016/j.appet.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Yousefi S, Dadgar S, Safara M, Zaini F. Aflatoxin production by Aspergillus flavus isolates from green–tiger shrimps (Penaeus semisulcatus) Iran J Microbiol. 2009;1(4):18–22. [Google Scholar]
- Zhang Y, Hou Y, Zhang Y, Chen J, Chen F, Liao X, Hu X. Reduction of diazinon and dimethoate in apple juice by pulsed electric field treatment. J Sci Food Agric. 2012;92:743–750. doi: 10.1002/jsfa.4636. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xue B, Li M, Mu Y, Chen Z, Li J, Shan A. Screening a strain of Aspergillus niger and optimization of fermentation conditions for degradation of aflatoxin B1. Toxins. 2014;6:3157–3172. doi: 10.3390/toxins6113157. [DOI] [PMC free article] [PubMed] [Google Scholar]