Abstract
Starch is an attractive raw material as ingredient for edible film manufacture because of its low cost, abundant availability, renewability, and biodegradability. Nevertheless, starch based films exhibit several disadvantages such as brittleness and poor mechanical and barrier properties, which restrict its application for food packaging. The use of the extrusion technology as a pretreatment of the casting technique to change the starch structure in order to obtain edible films, may constitute an alternative to generate coatings with good functional properties and maintain longer the postharvest quality and shelf life of fruits. For this reason, the objective of this study was to optimize the conditions of an extrusion process to obtain a formulation of modified starch to elaborate edible films with good functional properties using the casting technique and assess the effect during the storage when applied on a model fruit. The best conditions of the extrusion process and concentration of plasticizers were obtained using response surface methodology. From optimization study, it was found that appropriate conditions to obtain starch edible films with the best mechanical and barrier properties were an extrusion temperature of 100 °C and a screw speed of 120 rpm, while the glycerol content was 16.73%. Also, once applied in fruit, the loss of quality attributes was diminished.
Keywords: Casting technique, Optimization, Extrusion temperature, Glycerol content and postharvest quality
Introduction
Edible films (EF) are thin layers of edible materials that when applied on food products play an important role in their preservation during their distribution and marketing (Fakhouri et al. 2015). EF can be used to control the permeability to water, oxygen and carbon dioxide, as well as lipid permeability in a food system. Particularly, starch has been the focus of research because it is renewable, inexpensive, edible and readily available (Singh et al. 2009; Gutiérrez et al. 2015; Li et al. 2015). Usually starch based EF are prepared by the casting technique, which consists in dehydrating a filmogenic solution, applied on a mold. The amylose present in the starch is responsible for film forming capacity (Fakhouri et al. 2013; Maran et al. 2013). In particular, corn starch is promising for formation of edible films. However, its native structure is sometimes inefficient because certain conditions of technological processes may reduce their use for industrial applications. Typically, it must be modified or blended with other materials to achieve a more appropriate balance of properties. High amylose starch is a good source for film formation (Fakhouri et al. 2013; Gutiérrez et al. 2015). The addition of plasticizing agents to edible films is required to overcome film brittleness caused by extensive intermolecular forces. Plasticizers reduce these forces and increase the mobility of polymer chains, thereby improving flexibility and extensibility of the film. Hydrophilic compounds such as polyols (sorbitol, glycerol, xylitol, sugar, and maltitol) are used to improve the properties of starch-based edible films (Maran et al. 2013).
In order to overcome the limitations of the starches, a number of techniques for its modification have been developed. A common technique is the extrusion process. The extruders can be used to produce modified starches in a continuous process with a more consistent quality of the products. The extruder has the advantage of being an excellent mixing device and is particularly suitable for processing highly viscous fluids. The extruder also displays good heat transfer. Variations in screw speed offer good control over residence times, and provide opportunities for adding (or removing) reagents and additives such as processing aids and stabilizers during the process (Moad 2011). These process factors can be studied in EF made of starch modified by extrusion process.
Mango (Mangifera indica L.) is a tropical fruit and contains vitamins, minerals and fiber which are essential for human health and make this fruit very attractive for the export market. Mango is classified as climacteric fruit and shows rapidly deterioration after harvest due to ripening and senescence. Additionally, desiccation of mango during transportation, storage and the shelf life period causes its shriveling and reduces the market value of the fruit. In this regard, development of edible coatings may have the potential to inhibit the rapid quality degradation of harvested mango. The determination of postharvest quality of mango can be done by measuring firmness, fruit color (both peel and flesh), total soluble solids, titratable acidity and aromatic compounds. These measurements are the most common to give a first assessment of the quality of the fruit and is used regularly by consumers; it can even give an idea of the sensory and nutritional quality of the mango during its storage (Bibi and Baloch 2014; Rungpichayapichet et al. 2016). Therefore, the objective of this research was to obtain a formulation of high amylose corn starch and glycerol using the extrusion process (applied as a pretreatment) for the preparation of edible films with good mechanical and barrier properties using the casting technique and assess the effect of application on postharvest quality characteristics in mango (Mangifera indica L.) cv. Tommy Atkins fruit.
Materials and methods
Materials
High amylose corn starch (AmilogelTM 03003, Makymat, SA de CV Mexico) and glycerol (JT Baker®, Center Valley, USA) were used to obtain the EF.
Methods
This research was divided into two stages. The first stage pursued the optimization of the glycerol content and the extrusion process conditions in order to obtain a formulation to prepare EF with good mechanical and barrier properties. The second stage pursued the assessment of the postharvest quality in mangoes (Mangifera indica L.) cv. Tommy Atkins treated with the optimized formulation obtained in the first stage.
First stage
Film preparation
The extrusion process was employed to generate a physical modification in the starch, taking into account the conditions given by the experimental design (Table 1), where the study factors were: extrusion temperature (ET), screw speed (SS) and glycerol content (GC). An extruder of single screw, model 8-235-00 (Brabender OHG Duisburg, Germany) with three heating zones, L/D ratio of 20:1, compression 1:1 and a circular die of 2.8 mm diameter was used. The feed rate was kept constant at 35 g/min, while the moisture content was 20 ± 1%. The sample obtained by extrusion process was dissolved in distilled water (1:4). The product obtained was named extruded formulation.
Table 1.
Experimental design and results of the response variables evaluated at edible films from different formulations of high amylose corn starch and glycerol with different extrusion conditions
| Treatment | ET (°C) | SS (rpm) | GC (%) | PS (N) | D (mm) | WPV (g m Pa−1 s−1 m−2) | S (%) |
|---|---|---|---|---|---|---|---|
| 1 | 112.16 | 140.27 | 19.05 | 10.59 | 9.74 | 2.91 × 10−11 | 76.30 |
| 2 | 147.84 | 140.27 | 19.05 | 10.61 | 9.44 | 3.04 × 10−11 | 77.72 |
| 3 | 112.16 | 199.73 | 19.05 | 10.54 | 9.95 | 2.80 × 10−11 | 76.65 |
| 4 | 147.84 | 199.73 | 19.05 | 10.83 | 9.67 | 2.81 × 10−11 | 77.36 |
| 5 | 112.16 | 140.27 | 30.95 | 5.62 | 11.36 | 3.93 × 10−11 | 61.74 |
| 6 | 147.84 | 140.27 | 30.95 | 5.85 | 11.20 | 3.91 × 10−11 | 66.50 |
| 7 | 112.16 | 199.73 | 30.95 | 5.37 | 11.35 | 3.80 × 10−11 | 63.68 |
| 8 | 147.84 | 199.73 | 30.95 | 5.56 | 11.20 | 3.99 × 10−11 | 66.69 |
| 9 | 100 | 170 | 25 | 6.77 | 11.13 | 3.62 × 10−11 | 70.62 |
| 10 | 160 | 170 | 25 | 7.93 | 10.62 | 3.82 × 10−11 | 76.65 |
| 11 | 130 | 120 | 25 | 7.31 | 11.06 | 3.63 × 10−11 | 71.39 |
| 12 | 130 | 220 | 25 | 7.80 | 10.80 | 3.57 × 10−11 | 74.30 |
| 13 | 130 | 170 | 15 | 15.02 | 8.73 | 2.68 × 10−11 | 82.51 |
| 14 | 130 | 170 | 35 | 5.03 | 11.68 | 4.15 × 10−11 | 56.34 |
| 15 | 130 | 170 | 25 | 7.42 | 10.95 | 3.55 × 10−11 | 68.66 |
| 16 | 130 | 170 | 25 | 7.59 | 10.85 | 3.47 × 10−11 | 72.21 |
| 17 | 130 | 170 | 25 | 7.04 | 10.95 | 3.56 × 10−11 | 71.91 |
| 18 | 130 | 170 | 25 | 7.44 | 10.72 | 3.81 × 10−11 | 70.72 |
| 19 | 130 | 170 | 25 | 6.87 | 10.94 | 3.75 × 10−11 | 72.07 |
| 20 | 130 | 170 | 25 | 7.17 | 10.86 | 3.54 × 10−11 | 75.72 |
ET extrusion temperature, SS screw speed, GC glycerol content, PS puncture strength, D deformation, WVP water vapor permeability, S water solubility
Casting technique was used to produce EF. Briefly, 300 mL of the extruded formulation were collected and heated for 10 min on a plate (Fisher Scientific, Waltham, MA, USA) at 80 °C, for the preparation of EF or application on a model fruit used in the second stage. For the formation of EF, 25 mL of the gelled formulation were poured into acrylic, and then were placed in an oven at 60 °C for 2 h. The thickness of the films was measured using a digital micrometer (Digital Insize, Model 3109-25A, Spain), obtaining values of 50 ± 5 µm. Finally, films were conditioned in a desiccator with a saturated solution of Mg(NO3)2·6H2O (JT Baker®, Center Valley, USA) to maintain 53% of relative humidity.
Mechanical properties
Puncture strength (PS) and deformation (D) of EF were evaluated using the methodology described by Fitch-Vargas et al. (2016) with an universal texture analyzer (INSTRON 3342, Norwood, MA, USA). Changes of maximum force just before the break, measured in Newtons (N), and the cutting distance from the contact with the sample until the break, measured in millimeters (mm), were evaluated. Twenty samples of EF from each treatment were used for measuring mechanical properties.
Barrier properties
Water vapor permeability
The water vapor permeability (WVP) of EF was determined in accordance to Fitch-Vargas et al. (2016). Films were placed on the top of glass containers with 15 g of calcium chloride (JT Baker®, Center Valley, USA). Subsequently, these glass containers were placed in a desiccator (Dry Keeper, Sanplatec Corp., Osaka, Japan) with a saturated solution of sodium chloride to generate a relative humidity of 75%. The weight gain of the glass container with calcium chloride was registered by quintuplicate every 12 h during 4 days; these data generated a graph of weight gain versus time. WVP was determined according to the next equation:
where: Mp = absorbed moisture mass (g), E = film thickness (m), A = exposed film area (m2), t = time (s) and Δp = partial pressure difference through the film (Pa).
Water solubility
The water solubility (S) was determined according to the methodology reported by Chiumarelli and Hubinger (2012) as percentage of disintegrated material, as expressed in the following equation:
where: % S = water solubility percentage, wi = initial weight of sample and wf = final weight of sample.
Experimental design
A central composite rotable model with α of 1.6817 and three numerical factors: Extrusion Temperature (ET, 100–170 °C), Screw Speed (SS, 120–220 rpm) and Glycerol Content (GC, 15–35%) were used. The three independent variable levels used were selected based on preliminary experiments and technical limitations of the study. The factorial design included 20 experiments: eight data-points (extremes) at levels (− 1) and (+ 1); six axial points outside of the factorial matrix, but inside the experimental domain which corresponded to the values − 1.6818 and 1.6818 and a third set composed by the replicates of the points at the origin of the reference system (central-points), coded as (0, 0). All assays were performed randomly (Table 1). A second order polynomial was used to predict the experimental behavior:
where: yi = generic response; b1…12 = regression coefficients; X1 = Extrusion Temperature, X2 = Screw Speed and X3 = Content Glycerol. The numerical method was applied as optimization technique where the main criteria for determining the optimal treatment was to identify the processing conditions (ET and SS) and plasticizer content (GC) that could provide the highest PS and D values and lowest WVP and S values.
Data analysis
The data were analyzed using the surface response methodology with Design Expert ® Software Version 6 (Stat-Ease, Inc., Minn., USA). The significance of the models was tested using variance analysis (P value and F value at 95% confidence level).
Second stage
The optimal treatment was applied in mangoes (Mangifera indica L.) cv. Tommy Atkins to evaluate the effect of EF on postharvest quality characteristics.
Evaluation of postharvest quality characteristics
Mature-green mango fruits (Mangifera indica L.) cv. Tommy Atkins were harvested and sorted based on size uniformity, color, freedom of physical and microbial injury and immediately transported to the laboratory. Mangoes were washed with sodium hypochlorite solution (100 ppm) and kept at room temperature until superficially dry. Fruits were randomized into four groups: control (uncoated fruit), EF1 (fruit coated with the edible films modified by extrusion and prepared from the optimized formulation of high amylose corn starch and glycerol), EF2 (fruit coated with carnauba wax) and EF3 (fruit coated with edible films unmodified by extrusion and prepared from high amylose corn starch and glycerol). All EF were applied by immersion of the entire fruit at 25 ± 1 °C, simulating the conditions used in packinghouse. Fruits were stored under refrigeration at 12 ± 1 °C for a period of 16 days with a relative humidity of 90 ± 5%. To evaluate the effect of EF in the fruits, the following analysis were performed.
Physical analysis
Weight loss (WL) was determined by weight difference in the different evaluation periods. Data were expressed as a percentage of initial weight (%). A universal texture analyzer (INSTRON 3342, Norwood, MA, USA) fitted with an 11-mm-diameter probe was used to evaluate firmness (Ribeiro et al. 2007). The pericarp at the center of each slice was penetrated (5 mm depth) with a constant speed of 50 mm/min. Results were expressed in Newtons (N).
Chemical analysis
pH and titratable acidity were evaluated following the methodology of the AOAC (2012). 20 g of sample were homogenized with 100 mL of neutral distilled water using an Ultra-Turrax (IKA T18 basic Ultra-Turrax, Germany) and subsequently were filtered. The pH of the homogenized solution was measured with a pH-meter (Orion Research Inc., Beverly, Mass., USA). Titratable acidity (TA) was determined by titration of the homogenized solution with 0.1 N NaOH (to a pH value of 8.1 ± 0.2) and was expressed as percentage of citric acid. Total soluble solids (TSS) were determined using a refractometer (Atago, Fisher Scientific, Ga., USA) and were expressed as ˚Brix. Twelve slices per replicate were evaluated.
Statistical analysis
For data analysis for the second stage, a completely randomized factorial experimental design was used. The factors were type of EF and days of storage at 12 ± 1 °C. The A factor levels were control, EF1, EF2 and EF3, while B factor levels were 0, 4, 8, 12 and 16 days for physical and chemical analysis. Three replicates per treatment were performed in each experimental unit. Statistical analyses of data were performed through analysis of variance (ANOVA) using Statgraphics plus 6.0 (Manugistics, Rockville, MD) and the means were compared using Fisher’s least significant difference (LSD) test (P < 0.05).
Results and discussion
Mechanical properties
Puncture strength
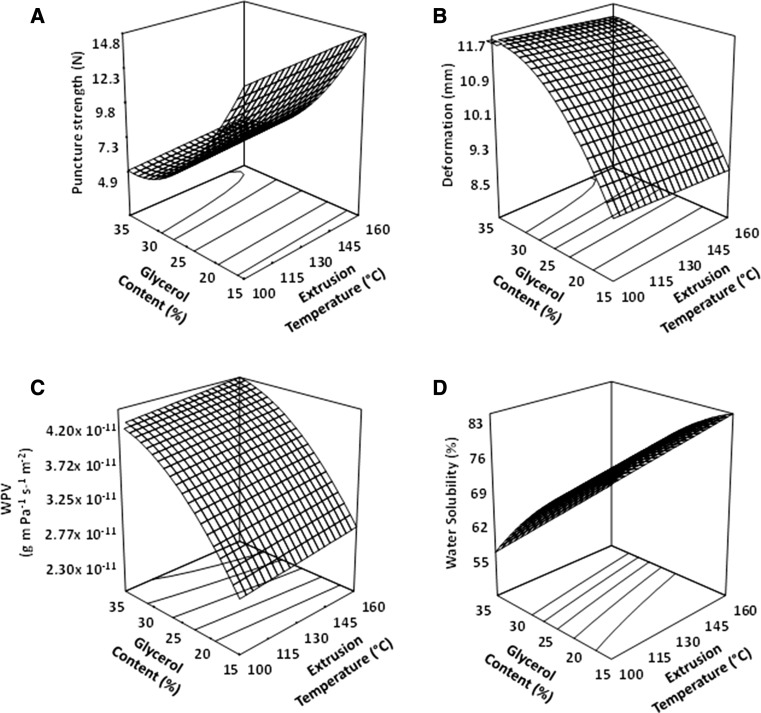
PS is the maximum force required to penetrate through a material. PS showed a significant model of regression with values of R2 = 0.98, coefficient of variation CV = 4.25%, and P of F < 0.01 (Table 2). The linear term of the ET (P < 0.05), and the linear and quadratic terms of the GC (P < 0.01) significantly influenced the PS of films. The SS was not significant for any terms (P > 0.05). Mechanical strength is generally required to maintain the structural integrity and barrier properties of EF. The average of PS value was 15.02 ± 0.34 N and was better than the one reported by Fitch-Vargas et al. (2016) for corn starch EF obtained by a combination of extrusion technology and casting technique (10.39 ± 2.73 N). Figure 1a shows the surface graph of the PS of the edible films. It was observed that when the GC decreased, the highest values of the PS (15.02 ± 3.14 N) were obtained. Also, when the ET increased the PS increased slightly. It has been reported that an increase in the severity of the process increases the degradation of materials made from starch, which could promote the release of short chains with a large number of OH− functional groups, which could interact with each other, resulting in more rigid and less elastic materials, thereby increasing the values of PS (Fakhouri et al. 2013). Chen and Lai (2008) studied the PS of tapioca starch films with glycerol, observing that as glycerol content increased from 25 to 40%, the puncture strength was reduced. This is in accordance with those reported by Yu et al. (1998), who proposed that the effect of glycerol is because it acts as a plasticizer in the starch, suggesting that this molecule being polar and of relatively small size, could penetrate into the starch granule and break the hydrogen internal bonds under high temperature, high pressure and high shear. These results agree with those obtained in this work, since increasing the GC in the mixture, fragile films were generated.
Table 2.
Regression coefficients and analysis of variance for the responses of PS, D, WVP and S of high amylose corn starch edible films
| PS | D | WVP | S | |
|---|---|---|---|---|
| Intercept | + 7.36 | + 10.83 | + 3.59E − 11 | + 72.28 |
| ET | − 0.20 *(0.048) | − 0.059 (0.16) | + 6.03 × 10−13 (0.11) | + 1.47 (<0.01) |
| SS | − 0.03 (0.71) | + 2.76 × 10−4 (0.99) | − 3.12 × 10−13 (0.39) | + 0.51 (0.31) |
| GC | − 2.71 (< 0.01) | + 0.83 (< 0.01) | + 4.75 × 10−12 (< 0.01) | − 6.84 (< 0.01) |
| ET2 | NS | NS | NS | NS |
| SS2 | NS | NS | NS | NS |
| GC2 | + 0.92 (< 0.01) | − 0.26 (< 0.01) | − 1.06x10−12 (< 0.01) | − 1.15 (< 0.01) |
| ET-SS | NS | NS | NS | NS |
| TE-GC | NS | NS | NS | NS |
| SS-GC | NS | NS | NS | NS |
| R2 Adjusted | 0.98 | 0.96 | 0.90 | 0.91 |
| CV (%) | 4.25 | 1.39 | 3.73 | 2.53 |
| P of F | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| Lack of fit | 0.27 | 0.08 | 0.53 | 0.88 |
ET extrusion temperature, SS screw speed, GC glycerol content, PS puncture strength, D deformation, WVP water vapor permeability, S water solubility, NS non significative, α < 0.05; *P of F, CV coefficient of variation
Fig. 1.
Effect of extrusion temperature and glycerol content on the response variables, at a screw speed of 170 rpm. Puncture Strength (a); Deformation (b); Water Vapor Permeability (c); Water Solubility (d) of high amylose corn starch edible films
Deformation
The statistical analysis of the deformation data for the EF showed a significant regression model with R2 = 0.96, CV = 1.39%, and P of F < 0.01 (Table 2). GC was significant in the linear and quadratic terms (P < 0.01). ET and SS had no significant effect in any of its terms (P > 0.05). The best value of D in this work was 11.6 ± 1.01 mm was better than the one Zhong and Li (2014) obtained a value of 4.11 ± 0.02 mm in kudzu starch EF with 20% of glycerol obtained by casting technique. Figure 1b shows an increment for D of EF when GC, was increased. Tapia-Blácido et al. (2005) in biofilms based on amaranth flour and Fakhouri et al. (2012) in films of manioc starch and gelatin reported that the elongation values in EF increased when the GC was increased. According to Maran et al. (2013), the puncture deformation of tapioca starch films was increased due to the presence of glycerol, because it increased the mobility of polymer chains. The glycerol could decrease the interactions among the starch molecules, because of the formation of hydrogen bonds between the hydroxyl groups of both molecules. These interactions could have favored the movement and rearrangement of the starch macromolecular chains, which cause the increase in the flexibility of the starch films. Yan et al. (2012) reported a similar behavior in corn starch-based films prepared by the casting technique. Therefore, plasticizer modifies the interactions between the macromolecules, resulting in an increase in chain mobility.
Barrier properties
Water vapor permeability
Water vapor permeability (WVP) showed a significant model of regression with values of R2 = 0.90, CV = 3.73%, and P of F < 0.01 (Table 2). The GC in both the linear and quadratic terms was significant in this parameter (P < 0.01). The ET and SS had no significant effect on any of its terms (P > 0.05). Figure 1c shows that the WPV of the EF increased in proportion to the GC. Since the major purpose of EF is often minimize moisture transfer between the food and the atmosphere, or between two constituents of a food product, WVP should be as low as possible. In this work the minimum value of WVP was 2.68 × 10−11 g m Pa−1 s−1 m−2; and it was obtained when the GC was lower. Ryu et al. (2002) reported in high amylose corn starch films, values of WVP from 1.17 × 10−11 to 1.47 × 10−11 g m Pa−1 s−1 m−2. Fakhouri et al. (2015) obtained a value of 4.37 ± 0.16 × 10−7 g m Pa−1 s−1 m−2 for EF of corn starch, gelatin and glycerol, while Pagno et al. (2016) elaborated films of cassava starch, glycerol and bixin nanocapsules and reported values of WVP from 5.61 × 10−11 to 7.58 × 10−11 g m Pa−1 s−1 m−2. The results found on this work were better than those reported by the mentioned references. The behavior presented in this work can be attributed to the fact that glycerol reduces interactions (hydrogen bonds, ionic and Van der Waals forces) between biopolymers, increasing the free space between the chains. This in turn promotes water diffusion into the film matrix and, consequently, an increase in the WVP of the plasticized films (Sanyang et al. 2016). Furthermore, the hydrophilic nature of glycerol favors the adsorption of water molecules (Maran et al. 2013).
Water solubility
Water solubility (S) is an essential property of starch-based films. Potential functions may need water insolubility to increase product integrity and water resistance. The linear term of the ET (P < 0.01) and the linear and quadratic terms of the GC (P < 0.01) influenced significantly the S of EF. GC was the variable that mostly affected this response. The SS not presented significant effect (P > 0.05). S showed a significant model of regression with values of R2 = 0.91, CV = 2.53%, and P of F < 0.01 (Table 2). Figure 1d shows the surface graph for the S of the EF with respect to the ET and GC. It was observed that when the ET increased and the GC decreased, the S increases significantly. The highest value of S (82.51% ± 3.63) was obtained at low GC and high ET. Furthermore, the lowest S value (56.34% ± 3.52) was obtained when the GC was high and the ET was low. Different authors have studied the extrusion process effects on the S of the starch, their results have indicated that at high ET the solubility was increased (Mehyar and Han 2004; Fakhouri et al. 2013). This is consistent with the results of this work, when the severity of the process was higher (high ET and low GC), causing starch gelatinization. An increase in the starch gelatinization may cause the opening and breaking of its granular structure, resulting in high levels of absorption and solubility. However, when GC is increased, it acts as lubricant that decreases the processing severity and thus the granule integrity is maintained (Sagar and Merrill 1995).
Numerical optimization
A numerical optimization was performed to determine the best extrusion process conditions and GC with the aim to obtain EF with the highest PS and D values and lowest WVP and S values. The numerical method was used for this procedure and different criteria for each of the response variables were established. According to this optimization, the best process conditions were: ET of 100 °C and SS of 120 rpm, while the GC was 16.73%. With these optimum conditions were obtained the following values predicted by each of the corresponding mathematical models: PS = 12.46 ± 0.34 N, D = 9.28 ± 0.15 mm, WVP = 2.68 × 10−11 ± 1.35 × 10−12 g m Pa−1 s−1 m−2 and S = 76.23 ± 1.81%. To verify experimentally the model used, the edible films were prepared under predicted optimal conditions and their properties were validated. The following average values and standard deviations were obtained: PS = 12.46 ± 3.92 N, D = 9.25 ± 1.30 mm, WVP = 2.78 × 10−11 ± 0.38 × 10−11 g m Pa−1 s−1 m−2 and S = 75.97 ± 5.52%. By comparing the experimental values with the values predicted by the mathematical models, it was observed no significant differences between them (P > 0.05). Therefore, the model experimentally used demonstrated a good fit to find the best conditions of extrusion process and glycerol content in the manufacture of edible films with good mechanical and barrier properties.
Postharvest assessment in coated fruits
Weight loss
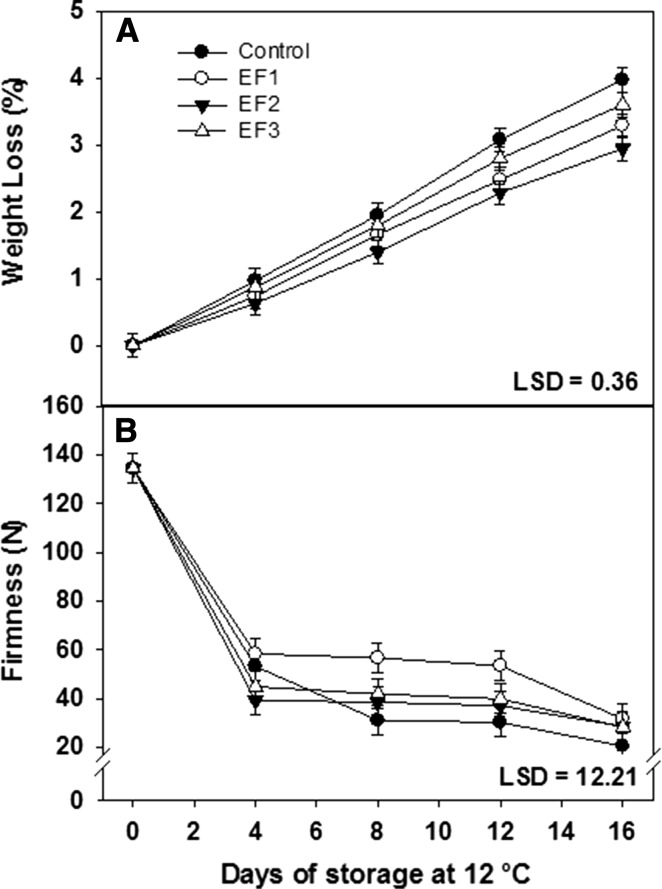
Weight loss (WL) is one of the main problems which affect quality attributes in mango fruits. The symptoms of WL are noticed at values greater than 10% (Vázquez-Celestino et al. 2016). The percentage of WL in mangoes evaluated every 4 days stored for 16 days at 12 ± 1 °C is shown in Fig. 2a. WL increased over time for all treatments as a result of the process of ripening and fruit transpiration (Sothornvit and Rodsamran 2008). The fruits of treatment EF2 had the lowest WL and showed significant differences (P < 0.05) throughout the storage period in comparison to the other treatments (EF1, EF3 and control), with a WL of around 2.3%. This can be attributed to the material hydrophobicity which provides a good barrier against water loss (Pérez-Rivero et al. 2003; Ribeiro et al. 2007). A similar behavior has been reported by Vázquez-Celestino et al. (2016) who observed changes in weight loss of Manila mangoes coated with wax during 22 days of storage at 13 °C; they also obtained similar values in comparison to this work (3.8%). Moreover, EF1 and EF3 treatments resulted with no significant differences (P > 0.05) during the 16 days of storage. On the other hand, EF1 showed significant differences (P < 0.05) respect to control fruits (uncoated). The results obtained in this study are in agreement with those reported by Garcia et al. (2011) who observed that cassava starch-based edible coatings were efficient in reducing weight loss of strawberries during 15 days of cold storage. This reduction can be attributed to the higher difficulty of water to diffuse because of the barrier properties of the coating applied on the strawberry surface. In accordance, Chiumarelli et al. (2010) observed a reduction on weight loss of fresh-cut mangos treated with cassava starch coating.
Fig. 2.
Weight loss (a) and firmness (b) of mangoes cv. Tommy Atkins stored for 16 days at 12 ± 1 °C. Vertical bars indicate LSD (P ≤ 0.05)
Firmness
Mango is a soft fruit that suffers a rapid loss of firmness during ripening which contributes greatly to its short postharvest life. Figure 2b shows the effect exerted by the EF in reducing fruit firmness, especially in the EF1 and EF2 treatments. In general, firmness decreased during the 16 days of storage, falling down from an initial value of 134.5 N to values of 20.7, 28.8 and 28.4 N for the control, EF2 and EF3 treatments, respectively; while EF1 treatment had values with an average of 31.8 N. These results indicated that fruit firmness was preserved by the edible coatings. Tissue softening is attributed to degradation of cell wall components, mainly pectins, as a result of the activity of enzymes such as pectinesterase and polygalacturonase. These cell modifications result in loss of water, which has also been considered an important factor for texture changes in fruits and vegetables (Aguilar-Mendez et al. 2008). EF1 was the most effective treatment in reducing the softening of mango fruits during the 16 days of storage, showing significant difference (P < 0.05) with the control. It is known that edible coatings made from starch exhibit excellent gas barrier properties which could generate atmospheres with low O2 and high CO2 concentrations reducing the fruit enzymatic activity, resulting in better retention of firmness. Similar tendencies have been reported by some investigators in different fruits coated with edible starch films (Chiumarelli and Hubinger 2014; Aguilar-Mendez et al. 2008). Garcia et al. (1998) observed no changes in the firmness of strawberries due to the use of starch based coatings, during the storage, an inhibition of texture loss was also noticed when compared to control sample. Ribeiro et al. (2007) reported that the coefficient of cohesion of starch films is high, resulting in high forces of attraction between molecules of the polymers, thereby forming a good gas barrier. Furthermore, the low permeability to oxygen by starch films can reduce the availability of oxygen in the respiratory activity, and consequently a slow respiration, one of the factors attributed to the firmness retention in fruits.
Titratable acidity
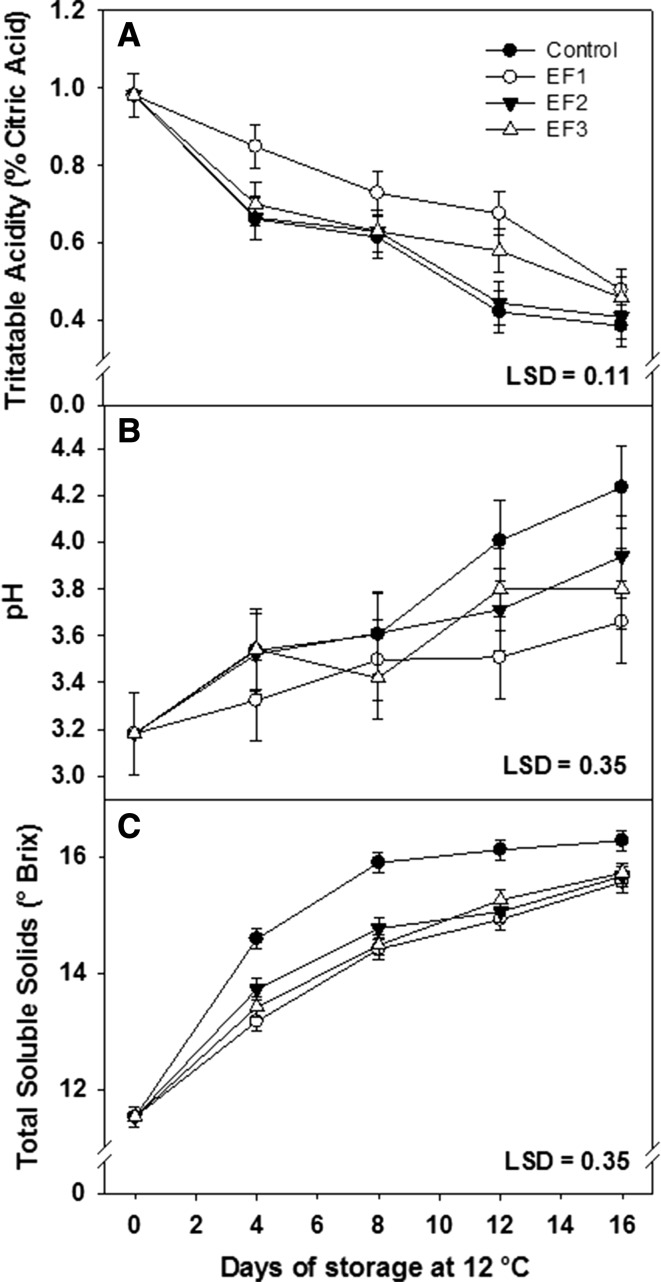
The titratable acidity (TA) has been identified as a quality indicator for mango. In Fig. 3a are presented the results of the TA in mango fruits stored for 16 days at 12 ± 1 °C. This parameter decreased during storage for all treatments, from an initial value of 0.9–0.5% at the end of the evaluations. As expected TA decreased due to a lower content of organic acids as a consequence of their consumption as respiratory substrates or their conversion into sugars (Pérez-Rivero et al. 2003). EF1 and EF2 showed no significant difference between them (P > 0.05), however, presented significant difference with respect to the control during the storage period (P < 0.05). EF3 treatment showed no significant difference both with the control and with EF2 during the storage period (P > 0.05). Nevertheless, EF1 treatment presented the highest values of TA which could be related with a decrease in mangoes respiration rate. According with Rojas-Graü et al. (2008) edible coatings can delay the ripening process because of they generate a semipermeable membrane around the fruit, decreasing the respiration rate, avoiding the organic acids utilization. Similar results were obtained by Bibi and Baloch (2014) who reported that the acidity was significantly lower in mangoes coated with a starch solution as compared with uncoated mangoes. Likewise, Mejia-Torres et al. (2009) applied different waxes on tomato fruit before storage at 20 °C and found a significant effect on chemical composition only in some kinds of waxes when compared with the control.
Fig. 3.
Titratable Acidity (a), pH (b) and total soluble solids (c) of mangoes cv. Tommy Atkins stored for 16 days at 12 ± 1 °C. Vertical bars indicate LSD (P ≤ 0.05)
pH
During the period of ripening of mango fruit, generally is observed a trend of increasing pH. This increase in pH is attributed to the physiological process called breathing, in which organic acids are consumed for use in several biochemical processes. This behavior was observed in the present study during the storage period starting in an initial value of 3.1 until obtaining 4.3 as the highest value at the end of storage (Fig. 3b). On day 4 of storage, the fruits of the EF1, EF2, EF3 and control treatments showed the pH values of 3.3, 3.5, 3.4, and 3.5, respectively, presenting no significant difference between them (P > 0.05). In addition, on day 8 of storage, the four treatments showed the same trend. However, on day 12 and 16 the EF1 y EF2 treatments showed significant difference with the control treatment (P < 0.05). These results show that the carnauba wax and starch extruded EF does not affect the pH increase during ripening. The lowest pH change respect to the initial value was for EF1 treatment which could be due to changes generated in the internal atmosphere of mangoes fruits. This behavior is consistent with previous reports. Bibi and Baloch (2014) studied the effect of a starch based edible coating on the pH of mangoes harvested at the green stage. The results indicated that the pH values were statistically lower in the coated fruit in comparison to controls. Meanwhile, Pérez-Rivero et al. (2003) reported that pH values were not different between control and waxed Tommy Atkins mangoes. On the other hand, Mejia-Torres et al. (2009) in a study of application of carnauba wax in tomato, reported that the application of wax had no significant effect on the pH of the fruits after 15 days of storage.
Total soluble solids
The total soluble solids (TSS) content is an important postharvest quality parameter and is related to the degree of conservation. Figure 3c shows the changes in the TSS values obtained for mangoes fruits during this study. The fruit shows an increase in TSS during the storage period, reaching values from 11.5 to 16.2 °Brix for the control treatment and values of 15.5, 15.6, and 15.7 °Brix for the EF1, EF2 and EF3 treatments, respectively. This increase in TSS is attributed to starch being hydrolyzed to simple sugars during ripening (Sothornvit and Rodsamran 2008). In accordance to Fig. 3c, at day 4 EF1, EF2 and EF3 showed no significant differences (P > 0.05) between them but showed significant differences with the control fruit (P < 0.05). These results demonstrate that the use of EF reduces the TSS, by decreasing the fruit metabolism, regulating the exchange of gases and reducing the respiration rate, requiring less organic acids for this process. Baldwin et al. (1999) reported similar soluble solids values for the same mango variety used in this work, coated with carnauba wax and stored for 19 days at 15 °C. Also, Bibi and Baloch (2014) reported that the rate of increase of total soluble solids was inhibited in mango fruits coated with a starch solution and was significantly different compared to uncoated mangoes.
Conclusion
The statistical models showed to have a good fit to find the best process conditions for obtaining a formulation of high-amylose corn starch and glycerol using the extrusion process, suitable for the preparation of edible films. Therefore, the combination of extrusion technology with the casting technique allowed to obtain EF of high amylose corn starch and glycerol with greater PS and D and lower WVP than those reported in the literature. EF prepared from an extruded formulation of high amylose corn starch and glycerol was an effective treatment to maintain physical and chemical quality of mangoes cv. Tommy Atkins at 12 °C for 16 days.
Acknowledgements
The authors thank to CONACYT for providing financial support for the development of this work.
References
- Aguilar-Mendez MA, San Martin-Martinez E, Tomas SA, Cruz-Ore A, Jaime-Fonseca MR. Gelatine–starch films: physicochemical properties and their application in extending the postharvest shelf life of avocado (Persea americana) J Sci Food Agric. 2008;88:185–193. doi: 10.1002/jsfa.3068. [DOI] [Google Scholar]
- AOAC . Oficcial methods of analysis. 19. Arlington: Association of Official Analytical Chemists Inc; 2012. [Google Scholar]
- Baldwin EA, Burns JK, Kazokas W, Brecht JK, Hagenmaier RD, Bender RJ, Pesis E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biol Technol. 1999;17(3):215–226. doi: 10.1016/S0925-5214(99)00053-8. [DOI] [Google Scholar]
- Bibi F, Baloch MK. Postharvest quality and shelf life of mango (Mangifera indica L.) fruit as affected by various coatings. J Food Process. 2014;38(1):499–507. doi: 10.1111/j.1745-4549.2012.00800.x. [DOI] [Google Scholar]
- Chen CH, Lai LS. Mechanical and water vapor barrier properties of tapioca starch/decolorized hsian-tsao leaf gum films in the presence of plasticizer. Food Hydrocoll. 2008;22(8):1584–1595. doi: 10.1016/j.foodhyd.2007.11.006. [DOI] [Google Scholar]
- Chiumarelli M, Hubinger MD. Stability, solubility, mechanical and barrier properties of cassava starch-Carnauba wax edible coatings to preserve fresh-cut apples. Food Hydrocoll. 2012;28:59–67. doi: 10.1016/j.foodhyd.2011.12.006. [DOI] [Google Scholar]
- Chiumarelli M, Hubinger MD. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll. 2014;38:20–27. doi: 10.1016/j.foodhyd.2013.11.013. [DOI] [Google Scholar]
- Chiumarelli M, Pereira LM, Ferrari CC, Sarantópoulos CI, Hubinger MD. Cassava starch coating and citric acid to preserve quality parameters of fresh-cut “Tommy Atkins” mango. J Food Sci. 2010;75(5):297–304. doi: 10.1111/j.1750-3841.2010.01636.x. [DOI] [PubMed] [Google Scholar]
- Fakhouri FM, Martelli SM, Bertan LC, Yamashita F, Innocentini LH, Collares-Queiroz FP. Edible films made from blends of manioc starch and gelatin—influence of different types of plasticizer and different levels of macromolecules on their properties. LWT Food Sci Technol. 2012;9(1):149–154. doi: 10.1016/j.lwt.2012.04.017. [DOI] [Google Scholar]
- Fakhouri FM, Costa D, Yamashita F, Martelli SM, Rodolfo C, Alganer K, Collares-Queiroz FP, Innocentini-Mei LH. Comparative study of processing methods for starch/gelatin films. Carbohydr Polym. 2013;95:681–689. doi: 10.1016/j.carbpol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Fakhouri FM, Martelli SM, Caon T, Velasco JI, Mei LHI. Edible films and coatings based on starch/gelatin: film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol Technol. 2015;109:57–64. doi: 10.1016/j.postharvbio.2015.05.015. [DOI] [Google Scholar]
- Fitch-Vargas PR, Aguilar-Palazuelos E, Jesús Zazueta-Morales J, Vega-García MO, Valdez-Morales JE, Martínez-Bustos F, Jacobo-Valenzuela N. Physicochemical and microstructural characterization of corn starch edible films obtained by a combination of extrusion technology and casting technique. J Food Sci. 2016;81(9):E2224–E2232. doi: 10.1111/1750-3841.13416. [DOI] [PubMed] [Google Scholar]
- Garcia MA, Martino MN, Zaritzky NE. Plasticized starch-based coatings to improve strawberry (Fragaria × Ananassa) quality and stability. J Agric Food Chem. 1998;46:3758–3767. doi: 10.1021/jf980014c. [DOI] [Google Scholar]
- Garcia LC, Pereira LM, Sarantópoulos L, Claire IG, Hubinger MD. Effect of antimicrobial starch edible coating on shelf-life of fresh strawberries. Packag Technol Sci. 2011;7:413–425. [Google Scholar]
- Gutiérrez TJ, Morales NJ, Tapia MS, Pérez E, Famá L. Corn starch 80: 20 “waxy”: regular, “native” and phosphated, as bio-matrixes for edible films. Procedia Mater Sci. 2015;8:304–310. doi: 10.1016/j.mspro.2015.04.077. [DOI] [Google Scholar]
- Li X, Qiu C, Ji N, Sun C, Xiong L, Sun Q. Mechanical, barrier and morphological properties of starch nanocrystals-reinforced pea starch films. Carbohydr Polym. 2015;121:155–162. doi: 10.1016/j.carbpol.2014.12.040. [DOI] [PubMed] [Google Scholar]
- Maran JP, Sivakumar V, Thirugnanasambandham K, Sridhar R. Response surface modeling and analysis of barrier and optical properties of maize starch edible films. Int J Biol Macromol. 2013;60:412–421. doi: 10.1016/j.ijbiomac.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Mehyar GF, Han JH. Physical and mechanical properties of high-amylose rice and pea starch films as affected by relative humidity and plasticizer. J Food Sci. 2004;69(9):E449–E454. doi: 10.1111/j.1365-2621.2004.tb09929.x. [DOI] [Google Scholar]
- Mejia-Torres S, Vega-García M, Valverde-Juárez J, López-Valenzuela JA, Caro-Corrales JJ. Effect of wax application on the quality, lycopene content and chilling injury of tomato fruit. J Food Qual. 2009;32:735–746. doi: 10.1111/j.1745-4557.2009.00284.x. [DOI] [Google Scholar]
- Moad G. Chemical modification of starch by reactive extrusion. Prog Polym Sci. 2011;36(2):218–237. doi: 10.1016/j.progpolymsci.2010.11.002. [DOI] [Google Scholar]
- Pagno CH, de Farias YB, Costa TMH, de Oliveira Rios A, Flôres SH. Synthesis of biodegradable films with antioxidant properties based on cassava starch containing bixin nanocapsules. J Food Sci Technol. 2016;53(8):3197–3205. doi: 10.1007/s13197-016-2294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Rivero B, Bringas E, Cruz L, Báez S. Aplicación de cera comestible en mango. Parte i: efecto en las características físico-químicas durante el almacenamiento comercial. Rev Iberoam Tecnol Postcosecha. 2003;5:100–112. [Google Scholar]
- Ribeiro C, Vicente AA, Teixeira JA. Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol Technol. 2007;44:63–70. doi: 10.1016/j.postharvbio.2006.11.015. [DOI] [Google Scholar]
- Rojas-Graü MA, Tapia MS, Martín-Belloso O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT Food Sci Technol. 2008;41(1):139–147. doi: 10.1016/j.lwt.2007.01.009. [DOI] [Google Scholar]
- Rungpichayapichet P, Mahayothee B, Nagle M, Khuwijitjaru P, Müller J. Robust NIRS models for non-destructive prediction of postharvest fruit ripeness and quality in mango. Postharvest Biol Technol. 2016;111:31–40. doi: 10.1016/j.postharvbio.2015.07.006. [DOI] [Google Scholar]
- Ryu SY, Rhim JW, Roh HJ, Kim SS. Preparation and physical properties of zein-coated high-amylose corn starch film. LWT Food Sci Technol. 2002;35(8):680–686. doi: 10.1006/fstl.2002.0929. [DOI] [Google Scholar]
- Sagar AD, Merrill EW. Starch fragmentation during extrusion processing. Polymer. 1995;36(9):1883–1886. doi: 10.1016/0032-3861(95)90935-U. [DOI] [Google Scholar]
- Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (Arenga pinnata) starch for food packaging. J Food Sci Technol. 2016;53(1):326–336. doi: 10.1007/s13197-015-2009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Belton PS, Georget DM. The effects of iodine on kidney bean starch: films and pasting properties. Int J Biol Macromol. 2009;45(2):116–119. doi: 10.1016/j.ijbiomac.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Sothornvit R, Rodsamran P. Effect of a mango film on quality of whole and minimally processed mangoes. Postharvest Biol Technol. 2008;47(3):407–415. doi: 10.1016/j.postharvbio.2007.08.005. [DOI] [Google Scholar]
- Tapia-Blácido D, Sobral PJ, Menegalli FC. Development and characterization of biofilms based on amaranth flour (Amaraanthus caudatus) J Food Eng. 2005;67:215–223. doi: 10.1016/j.jfoodeng.2004.05.054. [DOI] [Google Scholar]
- Vázquez-Celestino D, Ramos-Sotelo H, Rivera-Pastrana DM, Vázquez-Barrios ME, Mercado-Silva EM. Effects of waxing, microperforated polyethylene bag, 1-methylcyclopropene and nitric oxide on firmness and shrivel and weight loss of ‘Manila’mango fruit during ripening. Postharvest Biol Technol. 2016;111:398–405. doi: 10.1016/j.postharvbio.2015.09.030. [DOI] [Google Scholar]
- Yan Q, Hanxue H, Pei G, Haizhou D. Effects of extrusion and glycerol content on properties of oxidized and acetylated corn starch-based films. Carbohydr Polym. 2012;87:707–712. doi: 10.1016/j.carbpol.2011.08.048. [DOI] [PubMed] [Google Scholar]
- Yu J, Chen S, Gao J, Zheng H, Zhang J, Lin T. A study on properties of starch/glycerin blend. Starch/Stärke. 1998;50(6):246–250. doi: 10.1002/(SICI)1521-379X(199806)50:6<246::AID-STAR246>3.0.CO;2-7. [DOI] [Google Scholar]
- Zhong Y, Li Y. Effects of glycerol and storage relative humidity on the properties of kudzu starch-based edible films. Starch/Stärke. 2014;66(5):524–532. doi: 10.1002/star.201300202. [DOI] [Google Scholar]