Abstract
The effect of ultrasound pretreatment using Single Frequency Counter Current Ultrasound (SFCCU) on the enzymolysis of tea residue protein (TRP) extracted with sodium hydroxide was investigated. The concentration of TRP hydrolysate, enzymolysis kinetics and thermodynamic parameters after SFCCU pretreatment were determined and compared with traditional enzymolysis. The results indicated that both ultrasound assisted and traditional enzymolysis conformed to first-order kinetics within the limits of the studied parameters. Temperature and sonication had affirmative effect on the enzymolysis of TRP with temperature yielding greater impact. Michaelis constant (KM) in ultrasonic pretreated enzymolysis decreased by 32.7% over the traditional enzymolysis. The highest polypeptide concentration of 24.12 mg ml−1 was obtained with the lowest energy requirement at improved conditions of 50 g L−1 of TRP, alcalase concentration of 2000 U g−1, time of 10 min and temperature of 50 °C for the ultrasonic treated enzymolysis. The values of reaction rate constant (k) for TRP enzymolysis increased by 78, 40, 82 and 60% at 20, 30, 40 and 50 °C, respectively. The thermodynamic properties comprising activation energy (Ea), change in enthalpy (∆H) and entropy (∆S) were reduced by ultrasound pretreatment whereas Gibbs free energy (∆G) was increased.
Keywords: Enzymolysis, Kinetics, Tea residue, Ultrasound, Protein
Introduction
Tea has high patronage with a high consumption rate in China, Africa and other parts of the world. In similitude to the increase in patronage and consumption, a lot of research is on-going on the nutritional composition as well as the health benefits associated with the consumption of same (Khan and Mukhtar 2007; Shi et al. 2011). Therapeutically, tea has been studied due to its richness in phenolic compounds and its acclaimed health benefits to the neglect of other vital constituents such as proteins, carbohydrates and oils (Savić et al. 2014; Zaveri 2006). The frequency and quantity of waste generated in the form of residue (after infusion of tea leaves) from the tea industry is high (Zhang et al. 2014), extensively unexploited and uncharacterized. With current level of underutilization of tea residue, its main application is primarily for energy generation through combustion. However, proteins can be extracted from it for other uses such as generation of peptides with bioactive properties. Despite the fact that not so much research has been channeled towards exploiting tea residue protein, literature is also bereft of information on its enzymolysis kinetics and thermodynamic properties.
Studies have shown that enzymatic hydrolysates of some plants have exhibited biological effects such as hypocholesterolemia, anti-atherosclerosis, antitumor and ACE inhibitory properties against certain malignant cells (Qu et al. 2010; Hartman and Meisel 2007; Korhonen and Pihlanto 2006). Peptides from these products could therefore theoretically be incorporated as functional food in food product development (Qu et al. 2013; Zhao et al. 2012). Currently, there is a rising interest about exploitation of protein hydrolysates for the treatment of neural disorders and prevention of age-dependent dementia (Keilhoff et al. 2014) and other terminal diseases. There is therefore the possibility of deriving peptides with certain bioactivities after hydrolyzing tea proteins. However, the degree of hydrolysis and the peptides generated will depend on factors such as the enzyme in use, experimental conditions such as temperature, enzyme–substrate ratio and sample pretreatment prior to hydrolysis. Tea is already noted to have biological activities such antioxidant effects (Anesini et al. 2008), anticarcinogenic potentials (Khan and Mukhtar 2007) and antitumour activities (Guruvayoorappan and Kuttan 2008). These activities are however attributed to the presence of polyphenols and catechins. Studies on biological activities of tea proteins and their diverse contributions have been inadequate if not absent in the promotion of health. Understanding the kinetics of the enzymolysis and thermodynamic processes is inevitable and hugely beneficial to the cause of future researches on tea proteins.
The use of ultrasound has been extensively researched as a pretreatment step in modifying plant cell material by enhancing extraction and improving physicochemical properties of food. Several authors have reported that sonication can improve the efficiency of enzymolysis (Ma et al. 2011), degrade polysaccharides (Ying et al. 2011), and cause impairment to DNA (Furusawa et al. 2012). Ultrasound pretreatment together with enzymatic hydrolysis has also been established as an effective tool in modifying the functionality of protein (Jia et al. 2010). Ultrasonic waves generates forces perpendicular to extractible components resulting in shear strain leading to an increase in mass transfer of extractible components (Jian-Bing et al. 2006). In furtherance to this, the collapse of empty bubbles in sonicated mixture generates chaotic changes in pressure and flow velocity (Jian-Bing et al. 2006). This might lead to high-velocity resulting in intensive collision between sample matrices. This is likely to re-orient the tiny particular pores of matrices due to eddy motion and internal diffusion which will result in surface abrasion, dissolution and sample breakdown (Vilkhu et al. 2008). The use of ultrasound may therefore disrupt chemical bonds and increase the substrate surface area to the action of enzymes (Yachmenev et al. 2009). The objective of this research was to investigate the influence of single frequency ultrasound pretreatments on the enzymolysis reaction kinetics and thermodynamic parameters of ultrasonic pretreated tea residue proteins. This work intends to contribute to the hypothetical basis and technological backing for more research in polypeptide production using tea.
Materials and methods
Materials
The brown tea residue (a product obtained after fermentation, drying and steaming of tea leaves) was obtained from Apogee Foods Co., Ltd. (Nanjing, China) and had a protein content of 21.1% determined by Kjeldahl method (AOAC 1990). Alcalase with the activity of 200 U mg−1 was bought from Wuxi Xuemei Enzyme Preparation Co., Ltd. (Wuxi, China). Sodium hydroxide (NaOH), hydrochloric acid (HCl), sodium carbonate (Na2CO3), copper sulfate pentahydrate (CuSO4·5H2O), sodium potassium tartrate (C4H4O6KNa·4H2O), Folin-phenol reagent B and bovine serum albumin (BSA) were purchased from Xiaoshan Chemicals (Hangzhou, China). All the reagents were of analytical grade. Folin-phenol reagent A comprised of 0.185 M Na2CO3, 0.098 M NaOH, 0.393 mM CuSO4·5H2O and 0.694 mM C4H4O6KNa·4H2O, and it was freshly prepared prior to the test (Huang et al. 2015).
Ultrasound pretreatment of tea residue
Pulverized and dehydrated brown tea residue were screened with 80 μm mesh to produce brown tea residue powder. Preliminary investigations revealed optimal process conditions of 13 min ultrasonic time, 0.13 M Sodium hydroxide concentration, 377 W L−1 ultrasound power and a solid–liquid ratio of 51.5 g L−1 using Single Frequency Counter Current Ultrasound (SFCCU) and Box–Behnken design to give a validated prediction and combination of factors for optimum protein extraction from the tea residue. The 51.5 g L−1 tea residue solutions were sonicated in a restructured SFCCU processor, which was equipped with a 2 cm flat tip probe (Fanbo Biological Engineering Co., Ltd., Wuxi, China; Model FBTQ 2000) at a fixed frequency of 20 kHz, circulating pump speed of 300 r min−1 and pulsed on and off-time of 3 and 2 s respectively. The control was subjected to extraction conditions of 0.13 M sodium hydroxide concentration, time of 1 h and temperature of 60 °C prior to protein extraction.
Enzymolysis reaction of brown tea protein
After sonication or otherwise, the Brown tea solution was centrifuged at 5000 g. Single factor experiments indicated that varying pH had no effect on solubility except at extreme pH less than 4 and greater than 10. Therefore, the supernatant was rapidly adjusted to pH 7 (which had the highest solubility) using drops of 1 N HCl. The supernatant was then freeze dried. The freeze dried samples were then hydrolyzed by alcalase at substrate concentrations of 20, 30, 40 and 50 g L−1, respectively. The tea protein without ultrasonic pretreatment was hydrolyzed at the same substrate concentration. The hydrolysis of tea protein suspensions were carried out by alcalase (2000 U g−1) with the hydrolysis time of 120 min. During the hydrolysis, pH of suspensions was maintained at 8.0 by addition of 1.0 M NaOH using an automatic potentiometric titrator (ZDJ-4A, Instrument and Electronics Science Co., Ltd, Shanghai, China) at pH–stat mode of 20, 30, 40 and 50 °C. The tea protein hydrolysate was withdrawn from the broths at different hydrolysis time, and the pH of tea protein hydrolysate was adjusted to 7.0 followed by boiling the hydrolysate for 10 min to inactivate the enzymes. The hydrolysates were then centrifuged at 9000 rpm for 10 min and the supernatant was stored at − 80 °C for further analysis. The enzymolysis of the freeze dried tea protein without ultrasonic pretreatment was performed using the same protocol.
Initial reaction rate
In determining the initial reaction rate of enzymolysis, the polypeptide concentration (g mL−1) was calculated at enzymolysis time of 5 min after confirming that both the ultrasonic pretreated and traditional enzymolysis displayed first-order reaction kinetics at short enzymolysis period of 5 min. Folin-phenol colorimetric method was used to determine polypeptide concentration (Chen et al. 2003). A volume of 4 mL Folin-phenol reagent A was mixed with 0.5 mL sample in a glass tube. The solution was incubated for 10 min at 25 °C, after which 0.5 mL Folin-phenol reagent B was added to each glass tube, and the absorbance was read at 500 nm on a spectrophotometer (UV-1000 Shanghai, China) after 30 min incubation. A blank was prepared by using distilled water in place of the sample. BSA (0–1000 μg mL−1) was assayed as standard for the calculation of polypeptide concentrations of samples. The initial reaction rate was calculated as:
| 1 |
where Ri is initial reaction rate (g mls−1), Pc is polypeptide concentration (g ml−1) and t is enzymolysis time (s).
Kinetic equation
The classic Michaelis–Menten equation was adopted to study the effect of ultrasonic pretreatment on enzymolysis kinetic constants of brown tea residue protein. In order to estimate the two constants KM and Vmax, the experimental data was plotted according to the double-reciprocal transformation (Eq. 2):
| 2 |
where V is the initial reaction rate (g mL−1 s), [S] is the initial protein concentration (g mL−1), KM is Michaelis constant, and Vmax is the maximum initial velocity (g mL−1 s). The KM and Vmax values were determined as the slope and intercept of Eq. (1) after plotting 1/V against 1/[S].
Enzymolysis reaction kinetics
The first-order kinetic model reported by Takahashi et al. (2002) was applied in the enzymolysis kinetics study of TPH. The kinetic model was written as
| 3 |
The Eq. (3) was integrated and rearranged to its linear form:
| 4 |
where Co and Ct indicate the initial concentration of tea residue protein (g L−1) and concentration of tea residue protein at a certain time during hydrolysis (g L−1) respectively; t indicates the hydrolysis time (min); k indicates the reaction rate constant. As the concentration of leftover substrate is difficult to obtain, it was determined using the amount of peptides released from tea protein (Qu et al. 2013). In the condition of experiment, Co was substituted with and Eq. (4) rearranged to:
| 5 |
where Ct indicates the concentration of tea protein hydrolysate at a certain time during hydrolysis (g L−1); (ultimate polypeptide concentration) indicates the concentration of tea protein hydrolysate (g L−1) that was prepared by hydrolyzing tea protein at pH 8.0 and 50 °C for 10 h using alcalase (Qu et al. 2013). After 10 h of hydrolysis, no further addition of sodium hydroxide was observed and the ultimate concentration of tea protein hydrolysate was assumed to be equal to the concentration of tea protein that was hydrolyzed (Jin et al. 2015). The reaction rate constant k was hence obtained from the slope of the straight line by plotting against t.
Thermodynamic parameters
Arrhenius equation (Parkin 2007) was given to describe the relationship between reaction rate constant k and temperature T as follow:
| 6 |
The Eq. (6) was transformed and rearranged to:
| 7 |
where k indicates the reaction rate constant (1 min−1); A indicates the pre-exponential factor (min−1); R indicates the universal gas constant (8.314 J mol−1 K−1); T indicates the temperature in Kelvin (K); Ea indicates the activation energy (J mol−1). Also, thermodynamic parameters of the enzymolysis reaction can be obtained by Eyring equations (Parkin 2007), which are expressed as
| 8 |
where k indicates the reaction rate constant (s−1); kB indicates the Boltzman constant (1.381023 J K−1); h indicates Planck’s constant (6.6256 × 1034Js); R indicates the universal gas constant (8.314 J mol−1 K−1); T indicates the Kelvin temperature (K); ∆G indicates Gibbs free energy of activation (J mol−1). The Gibbs free energy of activation (J mol−1) can be calculated using the equation as follows:
| 9 |
where, ∆H is the enthalpy of activation (J mol−1) and ∆S representing the entropy of activation (J mol−1 K−1).
In a solution, the enthalpy of activation can be calculated from the equation as follows:
| 10 |
Entropy of activation is able to be obtained from the linear form of Eyring equations yielded by combining the Eqs. (7) and (8) as follows:
| 11 |
Degree of hydrolysis and concentration of protein hydrolyzed
The degree of hydrolysis (DH) was obtained by the equation developed by Adler-Nissen (1987) as follows:
| 12 |
where Nb indicates the concentration of NaOH (mol L−1), B indicates the volume of sodium hydroxide consumed (mL), Mp is the mass of protein to be hydrolyzed (g), htot is the total number of peptide bonds in the protein substrate, which is 8.0 mmol g−1 (Adler-Nissen 1987); a is the average degree of dissociation of the a-NH2 groups, which is related with the pK of the amino groups at particular pH and temperature, and is 0.871 for alcalase. Concentration of tea protein hydrolyzed was calculated according to the equation proposed by (Qu et al. 2013) as
| 13 |
where C0 and Ct indicate the initial concentration of tea protein (g L−1) and concentration of tea protein hydrolyzed at a certain time during hydrolysis (g L−1) respectively; DH indicates the degree of hydrolysis (%).
Statistical analysis
All experiments were performed in triplicate and the average used in the analysis. Data was subjected to one-way analysis of variance (ANOVA). LSD’s test was used to ascertain the differences (p < 0.05) between means of the traditional and ultrasound assisted enzymolysis. Analysis of data and graphs were plotted using Sigma Plot SPW 11.0 (Systat Software Inc., London, UK, 2008).
Results and discussion
Effect of ultrasonication pretreatment on kinetics of Enzymolysis reaction of tea protein
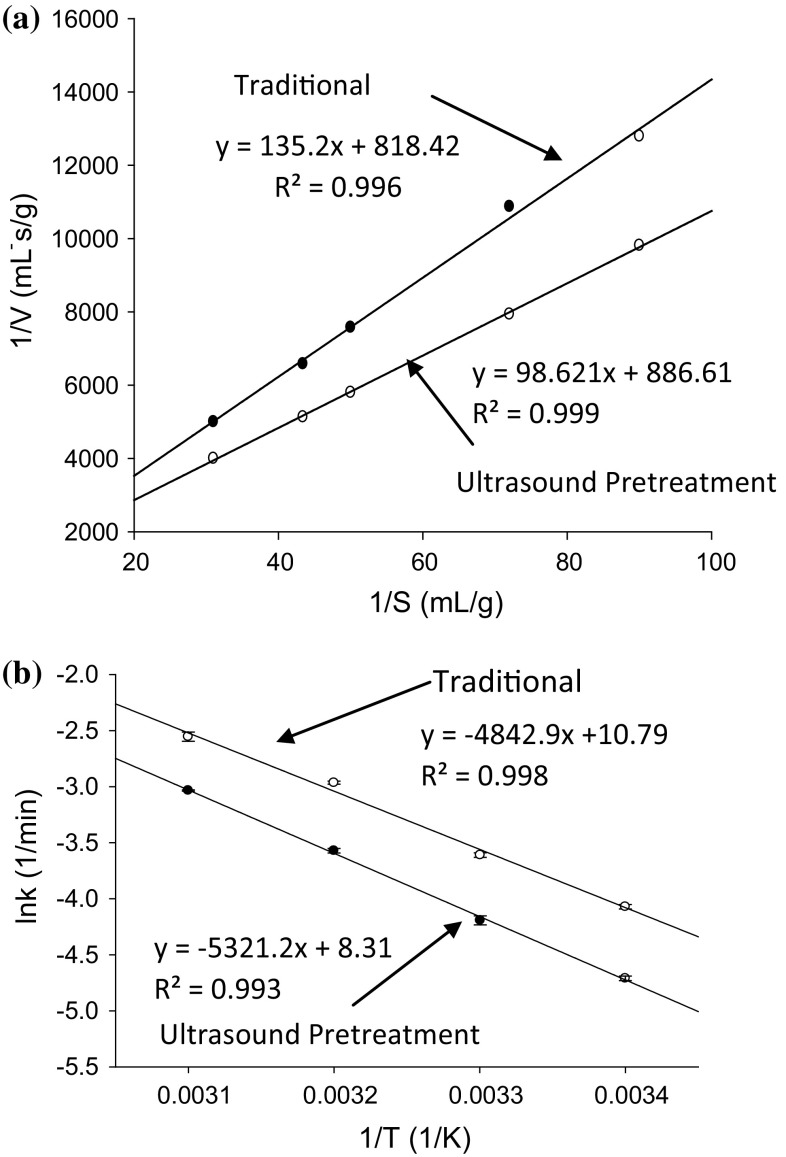
The importance of Michaelis constant (KM) in enzymolysis reaction kinetics can never be underestimated. It is a parameter that is not dependent on the concentration of substrate and it impartially characterizes the affinity between a substrate and enzyme in an enzymolysis reaction. A low KM is indicative of a fast reaction. Another important parameter in reaction kinetics is Vmax which denotes the maximum reaction rate, implying an elevation in binding frequency between substrate and enzyme in a given reaction (Tardioli et al. 2005). Michaelis–Menten kinetic and Lineweaver–Burk equation was adopted to estimate the change of reaction rate constants which was affected by the enzymolysis of ultrasonicated tea residue protein (UTRP) and the plots obtained are presented in Fig. 1a. The change of enzymolysis of tea residue protein affected by ultrasonic pretreatment resulted in a change of rate constant. As was observed, a linear relationship with time at various temperatures revealed by the coefficient of determination greater than 0.99 meant that the traditional and ultrasound assisted enzymolysis process of TRP followed first-order kinetics within the confines of the studied reaction parameters. The reaction constants determined from the slope and intercept of the lines of Fig. 1a were KM of 0.165 ± 0.001 and 0.111 ± 0.007 for Traditional and SFCCU, and Vmax of 1.221 ± 0.001 and 1.128 ± 0.001 respectively for Traditional and SFCCU pretreatment. The rate constant KM of UTRP enzymolysis decreased by 32.7% over the traditional enzymolysis. Because KM usually represents the superficial affinity of substrate to the enzyme, the decrease of KM validated the upsurge in affinity between tea proteins with alcalase. The value of VMax for UTRP enzymolysis was 7.6% less than the traditional enzymolysis. The decrease in KM and VMax value for UTRP enzymolysis might possibly be attributed to ultrasound pretreatment which had partially transformed the conformation of tea protein by weakening hydrogen bonds, Van der Waals forces, hydrophobicity and electrostatic interaction thereby loosening the protein tissue (Zhang et al. 2015; Jin et al. 2016). In line with Yachmenev et al. (2009) the use of ultrasound may break chemical bonds thereby increasing the substrate surface area for the action of enzymes. Sonication may thus have resulted in intensive collision between particles of tea residue protein and sodium hydroxide during the ultrasound pretreatment. The reaction might have re-oriented the tiny particular pores of sample mixture due to eddy motion and internal diffusion resulting in surface abrasion, dissolution and sample breakdown (Vilkhu et al. 2008). The minimal difference between VMax of the traditional and ultrasound assisted enzymolysis indicates that highest binding frequency was obtained when the alcalase had been thoroughly saturated by substrate (Zou et al. 2016).
Fig. 1.
a Plots of the reciprocal of the initial reaction rate (1/V) versus the reciprocal of substrate concentration (1/S) in ultrasonic pretreated and traditional enzymolysis. b The relationship between ln k and 1/T in traditional and ultrasonic pretreated enzymolysis. The regression coefficients of the curves are 0.998 and 0.993, respectively
Effect of ultrasound pretreatment on reaction rate constant k
It was expected that a change of enzymolysis of TRP affected by ultrasonic pretreatment would result in a change of rate constant. Figure 2 illustrates the plots of against time in the traditional and ultrasound pretreated enzymolysis at different temperatures. It can be seen from Fig. 2a, b that had a good linear relationship with time at various temperatures, as depicted by the coefficient of determination greater than or equal to 0.96. This affirms the position that both the traditional and ultrasound assisted enzymolysis obeyed first order kinetics within the range of parameters considered in the study. The reaction kinetic parameters k and were determined from the slope and intercept by plotting against t. Since it is impossible to determine from the intercept of the graph when is being plotted against t, the ultimate polypeptide concentration must be determined experimentally before can be estimated theoretically (Eq. 6). The ultimate polypeptide was experimentally determined after samples had been hydrolyzed for 10 h at pH 8.0 and 50 °C using alcalase (Jin et al. 2015). The rate constant k, and were then determined from the graph (Fig. 2). The value of k is a vital parameter and can illustrate the extent of ultrasonic assistance to the traditional one, which is temperature of reaction and solvent dependent. UTRP and traditional enzymolysis were investigated by hydrolyzing the protein at substrate concentration of 40.0 g L−1, enzyme–substrate ratio of 2000 U g−1, pH 8.0 and temperature of 20 to 50 °C for 120 min. The rate constants k and ku at different temperatures are summarized in Table 1. In line with expectation, k increased as the temperature increased from 20 to 50 °C. Increment in k with variation in temperature can be associated with a potential boost of frequency of collision between the substrate and alcalase at higher temperatures resulting from increase in pressure following temperature increase. With the value of k in UTRP enzymolysis comparatively higher than the traditional enzymolysis at all temperatures, it can be inferred that the ultrasound pretreatment enhanced the enzymolysis reaction. Reaction rate constant k of ultrasound pretreatment increased by 78, 40, 82 and 60% at the temperature of 20, 30, 40 and 50 °C respectively. This increment cannot be attributed to sonication alone because of the combination of the effect of ultrasonic pretreatment and temperature whereas the traditional enzymolysis only had the temperature effect. Thus, the contribution of ultrasound pretreatment alone was 44, 29, 65 and 38% at respective temperatures of 20, 30, 40 and 50 °C. It can therefore be intimated that temperature played a major role in the enzymolysis process though sonication boosted it. It was further detected that k and kt increased as the temperature increased from 20 to 50 °C. This therefore affirms the fact that the thermal effect positively and linearly influenced the enzymolysis reaction of UTRP. This corroborate the assertion of Kylä-Puhju et al. (2005) that a positive and linear relationship exist between enzyme activity and temperature. The positive thermal effect might be attributed to the attenuation of gas solubility in greater portion of the sample mixture leading to an increase in equilibrium vapour pressure of the system at elevated temperatures (Kylä-Puhju et al. 2005). It can be seen from Table 1 that the value was influenced by ultrasonic pretreatment when compared with traditional enzymolysis. UTRP enzymolysis gave the highest polypeptide concentration of 24.12 ± 0.76 mg mL−1 while the thermal effect gave a polypeptide concentration of 23.59 ± 0.45 mg mL−1 at 50 °C. Ultrasound pretreatment might have weakened electrostatic and hydrophobic interactions between protein molecules resulting in a complimentary breakdown of tea protein by alcalase in the enzymolysis reaction. The alteration of protein structure with free sulfhydryl and disulfide bond contents by ultrasound pretreatments has been reported (Jia et al. 2010; Zhou and Vachet 2012).
Fig. 2.
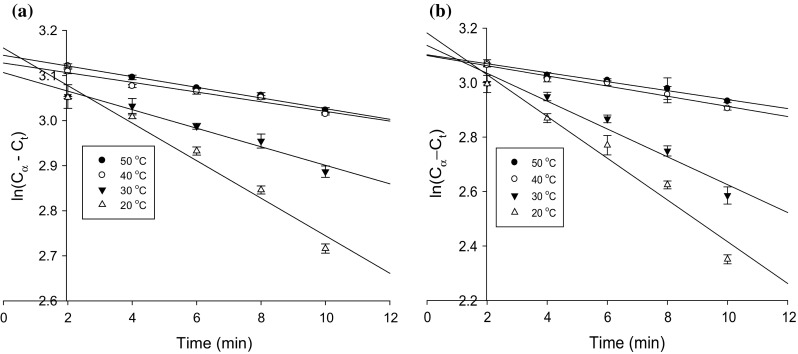
The ln (C∝ − Ct) values versus times in traditional (a) and b ultrasonic pretreated enzymolysis at different temperatures
Table 1.
Reaction rate constants and coefficient of determination in traditional and ultrasonic pretreated enzymolysis at different temperatures
| Type | Temperature (oC) | K (min−1) | Kt (min−1) | Ku (min−1) | C∝ (mg ml−1) | R2 |
|---|---|---|---|---|---|---|
| Traditional | 20 | 0.0093 ± 0.0005a | 0.0093 ± 0.0005a | – | 23.19 ± 0.25bc | 0.991 |
| 30 | 0.0154 ± 0.0015b | 0.0154 ± 0.0015b | – | 22.69 ± 0.38ab | 0.960 | |
| 40 | 0.0281 ± 0.0018c | 0.0281 ± 0.0018c | – | 23.34 ± 0.34ab | 0.975 | |
| 40 | 0.0484 ± 0.0007d | 0.0484 ± 0.0007d | – | 23.59 ± 0.45cd | 0.980 | |
| Ultrasound | 20 | 0.0163 ± 0.0050e | 0.0093 ± 0.0005a | 0.0070 ± 0.0006a | 22.25 ± 0.33a | 0.980 |
| 30 | 0.0214 ± 0.0004f | 0.0154 ± 0.0015b | 0.0060 ± 0.0016a | 22.18 ± 0.02a | 0.960 | |
| 40 | 0.0510 ± 0.0005g | 0.0280 ± 0.0018c | 0.0230 ± 0.0021b | 23.03 ± 0.57bc | 0.960 | |
| 50 | 0.0770 ± 0.0004h | 0.0484 ± 0.0007d | 0.0286 ± 0.0010c | 24.12 ± 0.76d | 0.960 |
k reaction rate constant, kt reaction rate constant for traditional enzymolysis, ku reaction rate constant for ultrasound assisted enzymolysis, C∝ ultimate polypeptide concentration, R2 coefficient of determination. For each column, different letter(s) indicate significant difference in means (p < 0.05)
Effect of ultrasonic pretreatment on enzymolysis thermodynamics
Activation energy is the minimum amount of energy required to initiate a chemical reaction. It is the energy requirement for the conversion of stable molecules into reactive ones and it reflects the speed with which chemical reactions take place (Qu et al. 2010). For a chemical reaction to proceed at a practicable rate, there should exist a considerable number of molecules with translational energy equal to or greater than the activation energy. The activation energy Ea was determined from the slopes and intercepts by plotting ln k against T−1 (Fig. 1b). The thermodynamic parameters ∆G, ∆H and ∆S were determined from Eqs. (8–11). The results of the thermodynamic parameters Ea, ∆G, ∆H and ∆S were presented in Table 2. The Ea values of most reactions ranges from 40 to 400 kJ mol−1. The indication is that the Ea as observed in the study falls within the minimum energy requirements for the reaction between alcalase and TRP to proceed. However the lower Ea (42.950 ± 0.617 kJ mol−1) from the ultrasound pretreated TRP compared with the traditional enzymolysis (46.928 ± 0.468 kJ mol−1) indicated that less energy was needed for the ultrasound assisted enzymolysis than the traditional enzymolysis. When compared with traditional enzymolysis, thermodynamic parameters Ea, ∆H and ∆S of enzymolysis with SDFU pretreatment significantly (p < 0.05) reduced by 8.5, 9.0 and 8.0% respectively (Table 2). The decrease in Ea and ∆H may be as a result of sonication and thermal effect that caused a split of hydrogen bonds that were stabilizing the protein and enzyme at ground state, and the disruption of the internal hydrophobic core, both of which leads to modification in the protein structure (Qu et al. 2013). ∆S represents the variation in the extent of local disordering between transition state and the ground state. The decrease in the ∆S may be as a result of oxidative modification of amino acid residues and initiation of cross linking and accretion which might have led to the increase in enzymolysis activity (Zhou and Vachet 2012).
Table 2.
Thermodynamic parameters in traditional and ultrasonic pretreated enzymolysis
| Type | Ea (× 103 J mol−1) | ∆H (× 103 J mol−1) | ∆S (J mol−1 K) | ∆G (J mol−1 K) |
|---|---|---|---|---|
| Traditional | 46.928 ± 0.468a | 44.240 ± 0.027a | 99.690 ± 0.695a | 30.721 ± 0.582 |
| Ultrasound | 42.950 ± 0.617b | 40.263 ± 0.578b | 107.187 ± 1.551b | 33.230 ± 0.379 |
Ea Activation energy, ∆H Change in enthalpy, ∆S entropy, ∆G Gibbs free energy. For each column, different letter(s) indicate significant difference in means (p < 0.05)
Effect of SFCCU pretreatment on protein hydrolysate release
The application of ultrasound pretreatment in improving the enzymolysis efficiency of plant proteins especially from industrial waste has been widely demonstrated by researchers (Qu et al. 2013; Jin et al. 2015; Zhang et al. 2014). However, at the time of this experiment none has considered tea residue which is major agricultural byproduct in China. The study considered the concentration of protein hydrolysates which is a useful parameter in estimating the solubility of proteins with low solubility such as tea residue protein (Cheng et al. 2017). Identifying the changes in concentration of TRP hydrolysate during the hydrolysis may help to eventually have a better understanding of tea protein enzymolysis. In this research, the concentration of tea protein hydrolysate prepared by alcalase during the hydrolysis time of 120 min in traditional and SFCCU assisted enzymolysis at different temperature and substrate concentration are presented in Figs. 3 and 4. It was observed that increasing the temperature of hydrolysis resulted in an increase in concentration of generated TPH. As higher temperature might result in inactivation of alcalase, tea proteins were hydrolyzed up to a temperature of 50 °C. Benjakul and Morrissey (1997) found that elevated temperatures have deleterious effect on protein hydrolysis catalyzed by alcalase. Ultrasonic pretreatment might have also induced unfolding of TRP resulting in a change in secondary structure of the proteins thereby resulting in a more efficient production of TPH than the traditional method (Jin et al. 2015). Significant relationship (p < 0.05) in terms of concentration of generated TPH existed between variations in same temperature and time of the ultrasound pretreated enzymolysis and the traditional enzymolysis. The conversion rate for tea protein pretreated with ultrasound after 120 min of hydrolysis ranged from 24, 33, 48 and 61% at 20, 30, 40 and 50 °C respectively. However, that of the reaction without ultrasound pretreatment ranged from 22, 30, 45 and 56% at similar respective temperature variations. Comparing the concentration of protein hydrolysates between those with ultrasound pretreatment and those without ultrasound pretreatment depicted a no significant relationship (p > 0.05) between protein hydrolysates generated without ultrasound pretreatment at 60 min and those with ultrasound pretreatment at 50 min. The contribution of the ultrasound pretreatment in terms of polypeptide concentration became more obvious as the hydrolysis time increased. It was also observed that there was no significant relationship (p > 0.05) in hydrolysates concentration between ultrasound pretreated and untreated tea protein at respective times of 80 and 120 min. An assessment of the conversion of proteins to hydrolysates from the traditional enzymolysis revealed that comparable concentration of hydrolysates can be generated at reduced time but with increasing temperature. As it was observed, the concentration of hydrolysates attained after 120 min at 20 °C was comparable to that of 30 °C at 50 min, 40 °C at 15 min and 50 °C at 12 min. A similar trend was observed in ultrasound pretreated samples.
Fig. 3.
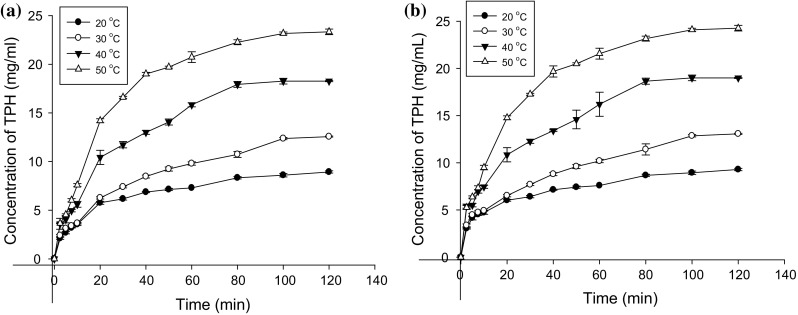
The concentration of tea protein hydrolysate during a traditional and b ultrasound assisted enzymolysis at different temperatures with substrate and enzyme concentration of 40 g L−1 and 2000 U g−1 respectively
Fig. 4.
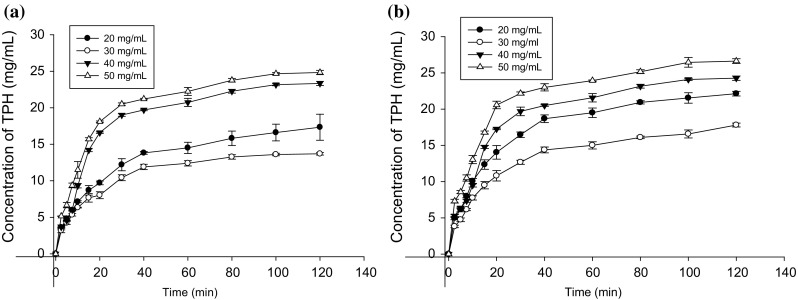
a Concentration of tea protein hydrolysate (TPH) during traditional treatment at different substrate concentrations at 50 °C. b Concentration of tea protein hydrolysate (TPH) during ultrasound assisted enzymolysis at different substrate concentrations at 50 °C
Assessment of the enzymolysis of different substrate concentration revealed that protein hydrolysates prepared by ultrasound assistance was significantly higher (p < 0.05) than those prepared by the traditional enzymolysis at the same hydrolysis time and substrate concentration. It was also observed that the conversion of tea proteins to hydrolysates at lower substrate concentration was higher than that of high substrate concentration for both ultrasound and traditional enzymolysis. However, the concentration of TRP hydrolysates at higher substrate concentration was higher than that at low substrate concentration. This could be attributed to the fact the effectiveness of alcalase was impeded by higher substrate concentration. After 120 min of hydrolysis, ultrasound assisted enzymolysis increased the hydrolysate concentration when compared with the traditional method by 20, 9.7, 2.4 and 3.6% respectively at substrate concentrations of 20, 30, 40 and 50 g L−1. Ultrasound pretreatment increased the hydrolysate concentration by 9.8, 1.7, 0.5 and 2% for respective substrate concentrations of 20, 30, 40 and 50 g L−1 during the first 60 min of hydrolysis. The indication was that ultrasound improved the hydrolysates concentration with time. It was made evident that ultrasound assisted enzymolysis improved the hydrolysis of tea residue protein at the experimented conditions.
Conclusion
The effect of ultrasound pretreatment on the enzymolysis kinetics and thermodynamic properties of tea residue protein hydrolysis using alcalase was assessed. It was found that the initial reaction rate was significantly enhanced by ultrasonic pretreatment. Temperature and sonication had affirmative effect on the enzymolysis of TRP but the effect of temperature was greater. The thermodynamic properties were all reduced by the ultrasonic pretreatment with the exception of Gibbs free energy. It was also observed that increasing the temperature of hydrolysis resulted in an increase in concentration of tea protein hydrolysates. Besides, it was noted that the conversion of tea proteins to hydrolysates at lower substrate concentration was higher than that of high substrate concentration for both ultrasound and traditional enzymolysis. The higher efficiency of SFCCU in assisting the enzymolysis may be correlated with the decrease of Ea and KM by lowering the energy barrier between ground and active state thereby improving the affinity between substrate and enzyme.
Acknowledgements
This research was supported by grants from the 863 Research Program of China (No. 2013AA100203), Key Technology R & D Program of Jiangsu (No. BE2013404), the Key University Science Research Project of Jiangsu Province (No. 16KJA550003), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Province Graduate Student Research and Innovation Project (SJZZ16-0200), Zhenjiang Key R & D Program (NY2016018). We acknowledge the contribution of Prof. Wang Zhenbin who was not fully available for the completion of this work due to ill-health until his sudden demise.
References
- Adler-Nissen J. Newer uses of microbial enzymes in food processing. Trends Biotechnol. 1987;5(6):170–174. doi: 10.1016/0167-7799(87)90091-6. [DOI] [Google Scholar]
- Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agric Food Chem. 2008;56(19):9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- AOAC (1990) Official methods of analysis. In: Association of official analytical chemists, 15th edn. Washington pp 805–845
- Benjakul S, Morrissey MT. Protein hydrolysates from pacific whiting solid wastes. J Agric Food Chem. 1997;45(9):3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Chen JH, Tao L, Li J, Zhu WH, Yuan YS. In: Biochemistry experiment. Chen JH, editor. Beijing: Science Press; 2003. pp. 59–61. [Google Scholar]
- Cheng Y, Liu Y, Wu J, Donkor PO, Li T, Ma H. Improving the enzymolysis efficiency of potato protein by simultaneous dual-frequency energy-gathered ultrasound pretreatment: thermodynamics and kinetics. Ultrason Sonochem. 2017;37:351–359. doi: 10.1016/j.ultsonch.2017.01.034. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Fujiwara Y, Campbell P, Zhao QL, Ogawa R, Hassan MA, Kondo T. DNA double-strand breaks induced by cavitational mechanical effects of ultrasound in Cancer cell lines. PLoS ONE. 2012;7(1):29012–29022. doi: 10.1371/journal.pone.0029012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruvayoorappan C, Kuttan G. (+)-Catechin inhibits tumour angiogenesis and regulates the production of nitric oxide and TNF-α in LPS-stimulated macrophages. Innate immun. 2008;14(3):160–174. doi: 10.1177/1753425908093295. [DOI] [PubMed] [Google Scholar]
- Hartman R, Meisel H. Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotechnol. 2007;18:163–169. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Huang L, Ma H, Peng L, Wang Z, Yang Q. Enzymolysis kinetics of garlic powder with single frequency countercurrent ultrasound pretreatment. Food Bioprod Process. 2015;95:292–297. doi: 10.1016/j.fbp.2014.10.015. [DOI] [Google Scholar]
- Jia J, Ma H, Zhao W, Wang Z, Tian W, Luo WL, He R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010;119:336–342. doi: 10.1016/j.foodchem.2009.06.036. [DOI] [Google Scholar]
- Jian-Bing J, Xiang-hong L, Mei-qiang C, Zhi-chao X. Improvement of leaching process of geniposide with ultrasound. Ultrason Sonochem. 2006;13:455–462. doi: 10.1016/j.ultsonch.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Jin J, Ma H, Qu W, Wang K, Zhou C, He R, Luo L, Owusu J. Effects of multifrequency power ultrasound on the enzymolysis of corn gluten meal: kinetics and thermodynamics Study. Ultrason Sonochem. 2015;27:46–53. doi: 10.1016/j.ultsonch.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Jin J, Ma H, Wang BAE, Yagoub GA, Wang K, He R, Zhou C. Effects and Mechanism of dual-frequency power ultrasound on the molecular weight distribution of corn gluten meal hydrolysates. Ultrason Sonochem. 2016;30:44–51. doi: 10.1016/j.ultsonch.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Lucas B, Pinkernelle J, Steiner M, Fansa H. Effects of cerebrolysin on motor-neuron-like NSC-34 cells. Exp Cell Res. 2014;327:234–255. doi: 10.1016/j.yexcr.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81(7):519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen H, Pihlanto A. Bioactive peptides: production and functionality. Int Dairy J. 2006;16:945–960. doi: 10.1016/j.idairyj.2005.10.012. [DOI] [Google Scholar]
- Kylä-Puhju M, Ruusunen M, Puolanne E. Activity of porcine muscle glycogen debranching enzyme in relation to pH and temperature. Meat Sci. 2005;69:143–149. doi: 10.1016/j.meatsci.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Ma H, Huang L, Jia J, He R, Luo L, Zhu W. Effect of energy-gathered ultrasound on alcalase. Ultrason Sonochem. 2011;18:419–424. doi: 10.1016/j.ultsonch.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Parkin KL. Enzymes. In: Damodaran S, Parkin KL, Fennema OR, editors. Fennema’s food chemistry. Boca Raton: CRC Press; 2007. pp. 331–436. [Google Scholar]
- Qu W, Ma H, Pan Z, Luo L, Wang Z, He R. Preparation and antihypertensive activity of peptides from Porphyra Yezoensis. Food Chem. 2010;123(1):14–20. doi: 10.1016/j.foodchem.2010.03.091. [DOI] [Google Scholar]
- Qu W, Ma H, Liu B, He R, Pan Z, Abano EE. Enzymolysis reaction kinetics and thermodynamics of defatted wheat germ protein with ultrasonic pretreatment. Ultrason Sonochem. 2013;20:1408–1413. doi: 10.1016/j.ultsonch.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Savić IM, Nikolić VD, Savić IM, Nikolić LB, Jović MD, Jović MD. The qualitative analysis of the green tea extract using ESI-MS method. Savrem tehnol. 2014;3(1):30–37. doi: 10.5937/savteh1401030S. [DOI] [Google Scholar]
- Shi CY, Yang H, Wei CL, Yu O, Zhang ZZ, Jiang CJ, Sun J, Li YY, Chen Q, Xia T, Wan XC. Deep sequencing of the Camellia sinensis transcriptome revealed candidate genes for major metabolic pathways of tea-specific compounds. BMC Genom. 2011;12(1):131. doi: 10.1186/1471-2164-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Hasegawa K, Yamaguchi I, Okada H, Ueda T, Gejyo F, Naiki H. Establishment of a first-order kinetic model of light chain-associated amyloid fibril extension in vitro. Biochim Biophys Acta (BBA)-Prot Prot. 2002;1601(1):110–120. doi: 10.1016/S1570-9639(02)00435-1. [DOI] [PubMed] [Google Scholar]
- Tardioli PW, Sousa RJ, Giordano RC, Giordano RLC. Kinetic model of the hydrolysis of polypeptides catalyzed by alcalase immobilized on 10% glyoxyl-agarose. Enzyme Microb Technol. 2005;36:555–564. doi: 10.1016/j.enzmictec.2004.12.002. [DOI] [Google Scholar]
- Vilkhu K, Mawson R, Simons L, Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry: a review. Innov Food Sci Emerg Technol. 2008;9(2):161–169. doi: 10.1016/j.ifset.2007.04.014. [DOI] [Google Scholar]
- Yachmenev V, Condon B, Klasson T, Lambert A. Acceleration of the enzymatic hydrolysis of corn stover and sugar cane bagasse celluloses by low intensity uniform ultrasound. J Biobased Mater Bioenergy. 2009;3:25–31. doi: 10.1166/jbmb.2009.1002. [DOI] [Google Scholar]
- Ying Z, Han X, Li J. Ultrasound-assisted extraction of polysaccharides from mulberry Leaves. Food Chem. 2011;127:1273–1279. doi: 10.1016/j.foodchem.2011.01.083. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang C, Sanders JPM, Bruins ME. Critical parameters in cost-effective alkaline extraction for high protein yield from leaves. Biomass Bioenergy. 2014;67:466–472. doi: 10.1016/j.biombioe.2014.05.020. [DOI] [Google Scholar]
- ZhangY Ma H, Wang B, Qu W, Li Y, He R, Wali A. Effects of ultrasound pretreatment on the enzymolysis and structural characterization of wheat gluten. Food Biophys. 2015;10:385–395. doi: 10.1007/s11483-015-9393-4. [DOI] [Google Scholar]
- Zhao Q, Xiong H, Selomulya C, Chen XD, Zhong H, Wang S, Sun W, Zhou Q. Enzymatic hydrolysis of rice dreg protein: effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012;134:1360–1367. doi: 10.1016/j.foodchem.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Vachet RW. Increased protein structural resolution from diethylpyrocarbonate-based covalent labeling and mass spectrometric detection. J Am Soc Mass Spectrom. 2012;23:708–717. doi: 10.1007/s13361-011-0332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Ding Y, Feng W, Wang W, Li Q, Chen Y, Wu H, Wang X, Yang L, Wu X. Enzymolysis kinetics, thermodynamics and model of porcine cerebral protein with single-frequency countercurrent and pulsed ultrasound-assisted processing. Ultrason Sonochem. 2016;28:294–301. doi: 10.1016/j.ultsonch.2015.08.006. [DOI] [PubMed] [Google Scholar]